Synthetic and Natural Antifungal Substances in Cereal Grain Protection: A Review of Bright and Dark Sides
Abstract
1. Introduction
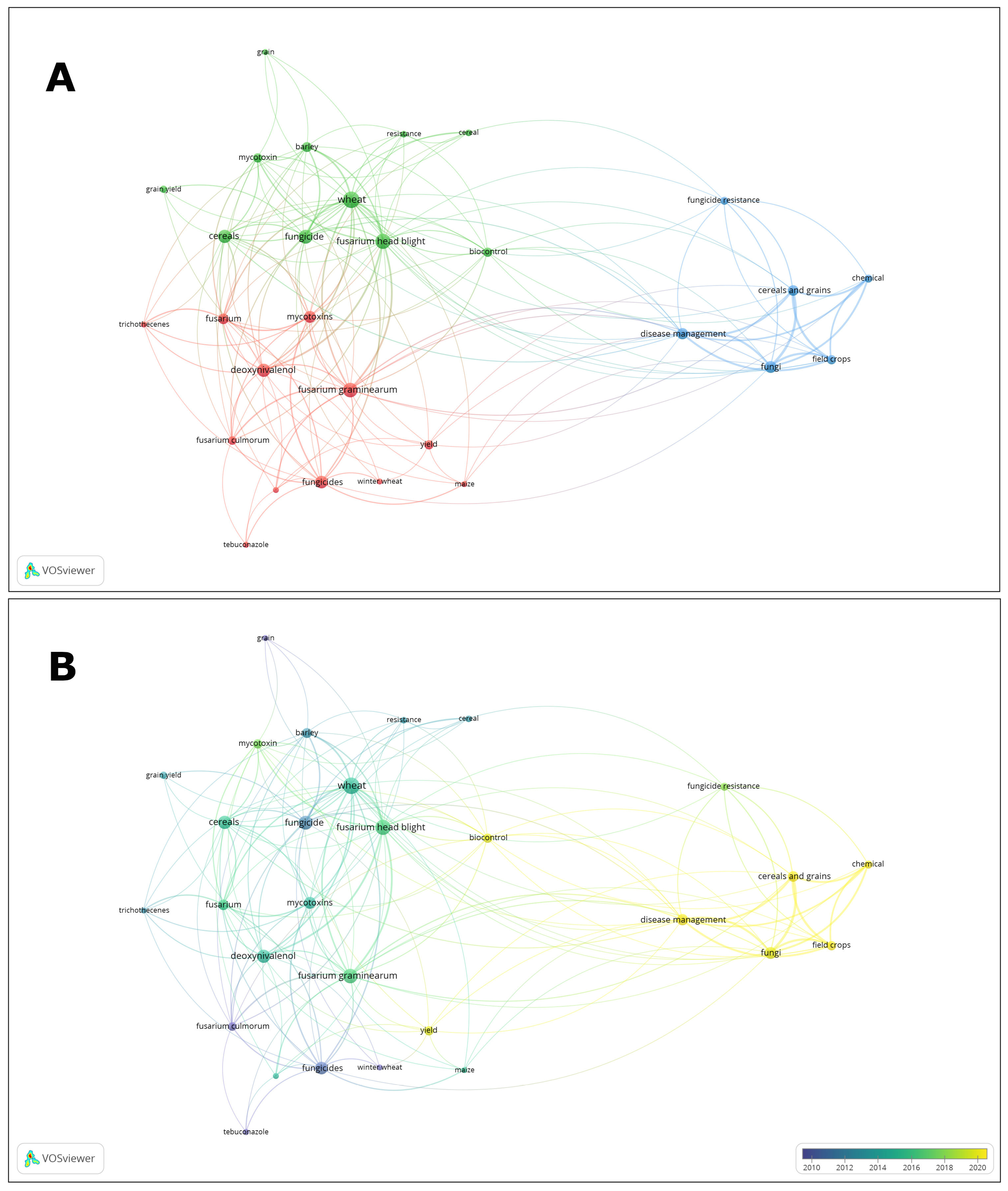
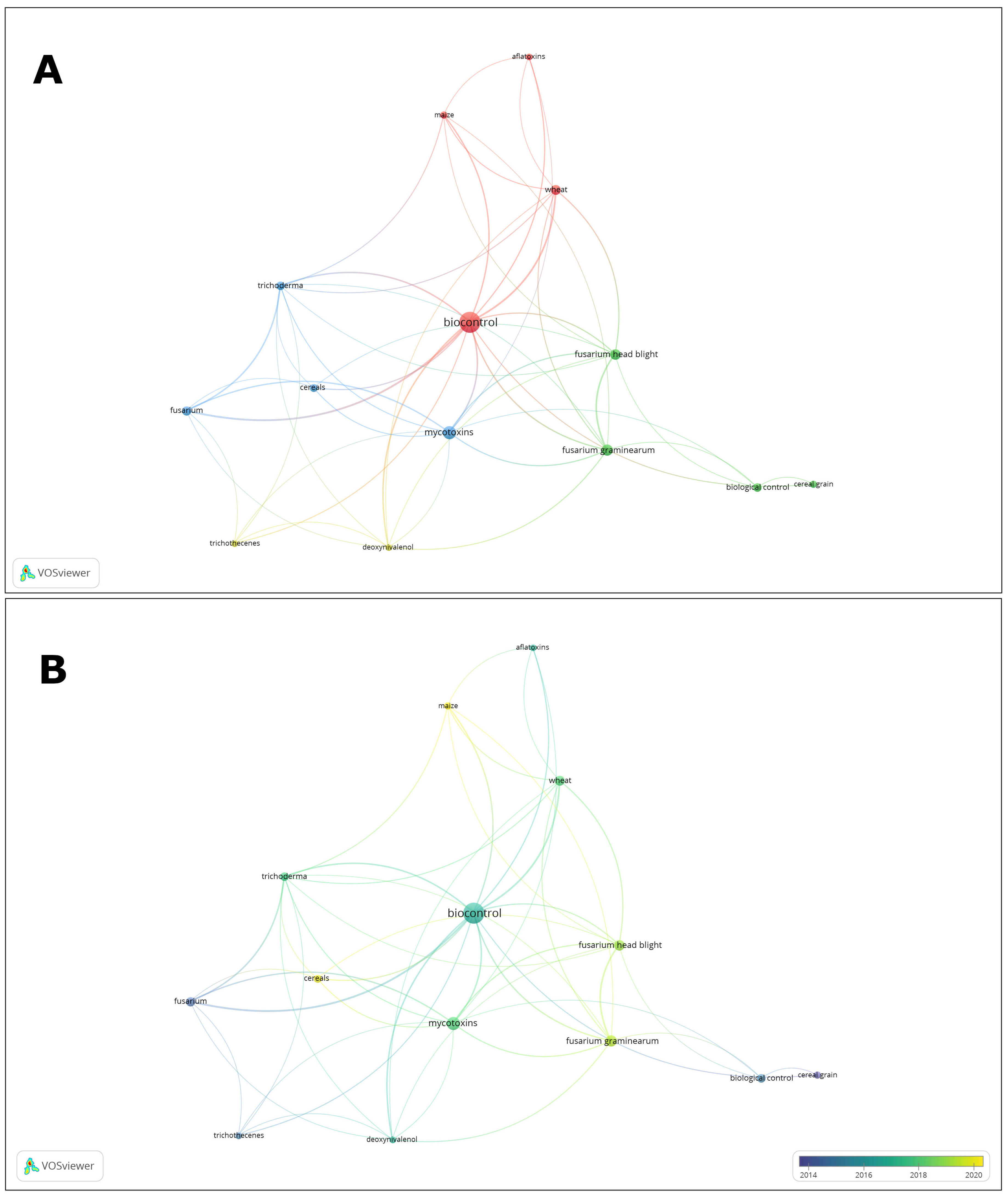
2. Bibliometric Data Mapping and Clustering
3. Synthetic Fungicides
3.1. Short History
3.2. Cereal Crops around the Globe
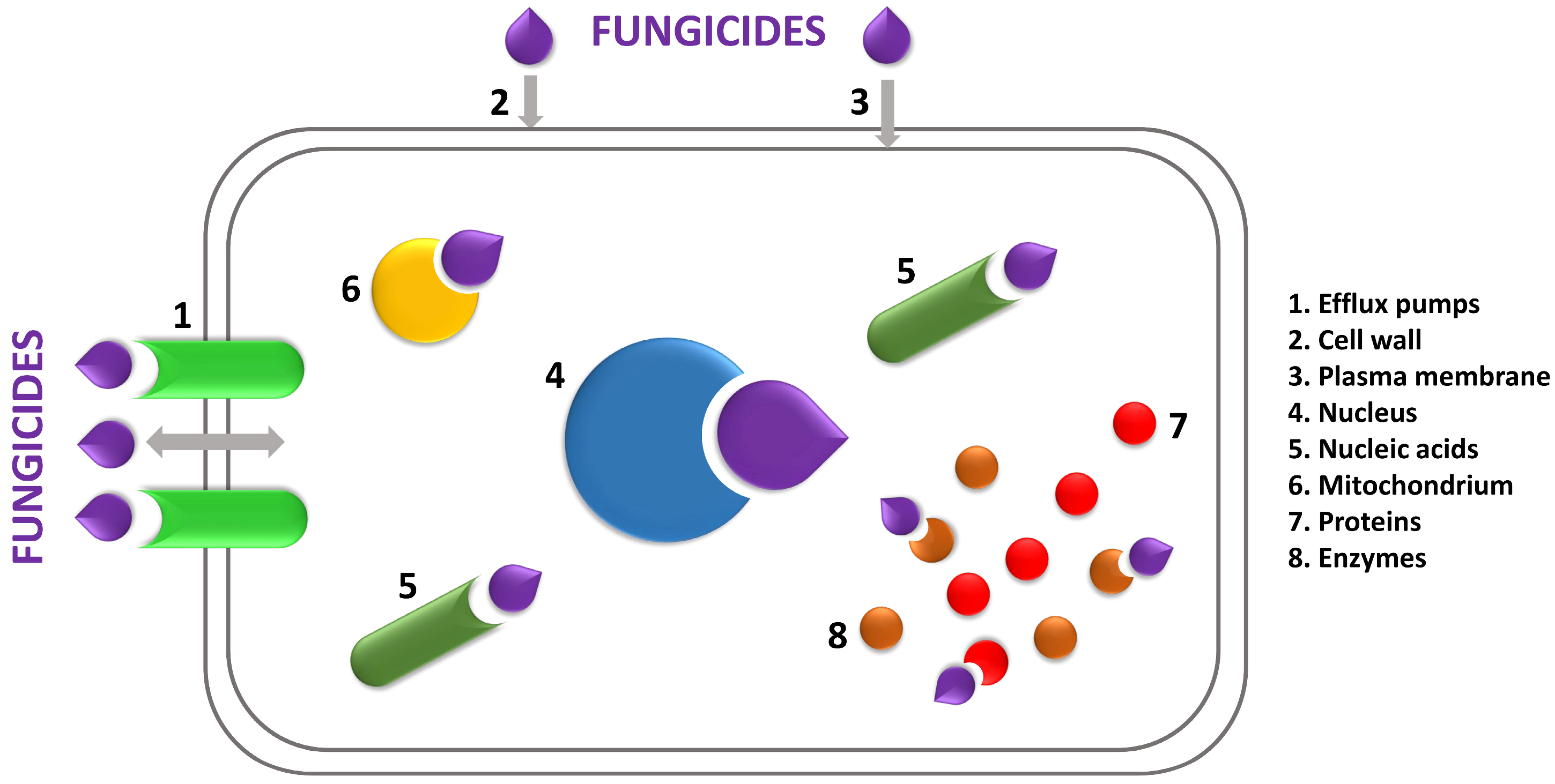
3.3. Areas of Fungicide Action in Mold Cells
3.4. Fungicide Groups According to Their Mode of Action (MOA) in the Biosynthetic Pathways of Plant Pathogens
- MBI-R (Melanin Biosynthesis Inhibitors—Reductase; 16.1 FRAC group) hydroxynaphthalene reductase inhibitors;
- MBI-D (Melanin Biosynthesis Inhibitors—Dehydratase; 16.2 FRAC group) inhibiting scytalone dehydratase;
- MBI-P (Melanin Biosynthesis Inhibitors—Polyketide synthase; 16.3 FRAC group) interfering with the activity of polyketide synthase.
- AP (Anilino-Pyrimidines; FRAC group 9), which is capable of secreting hydrolases and inhibiting methionine biosynthesis;
- enopyranuronic acid antibiotic (FRAC group 23), the active ingredient of which is blasticidin-S, which is capable of inhibiting protein biosynthesis by affecting ribosomal peptidyl transfer;
- kasugamycin, the active ingredient of hexopyranosyl antibiotic (24 FRAC group, which inhibits translation initiation and thus inhibits protein synthesis;
- glucopyranosyl antibiotics (FRAC group 25), which inhibit the initiation stage;
3.5. Fungicides in Crop Protection
- A
- Stage of application:
- Preventive: These fungicides protect plants by preemptively guarding against pathogenic attacks;
- Interventional: Applied immediately after the onset of infection, these fungicides aim to halt further proliferation of the pathogen;
- Destructive: Designed to eradicate spores and mycelium, thereby curbing the spread of the disease.
- B
- Effect of active substances of plants:
- Contact: These fungicides act on the plant’s surface, inhibiting spore germination and other vital processes;
- Translaminar: This type of fungicide penetrates and moves within the leaf tissue, providing protection throughout the leaf structure;
- Systemic: These fungicides penetrate plant tissues and distribute within them, creating a barrier against infection and fungal growth;
- Local: These fungicides exert their effects by penetrating only a few cell layers at the site of application
- C
- Methods/places of application:
- Seed dressing: Application of finely ground solid particles dusted onto the seeds surface;
- Foliar fungicides: Sprayed onto the foliage of plants at different growth stages;
- Root/soil application: Soil drenching or injection into the soil underneath it near the roots.
- D
- Type of active substance used in fungicides.
- E
- Mechanisms of action of active substances on fungal cells.
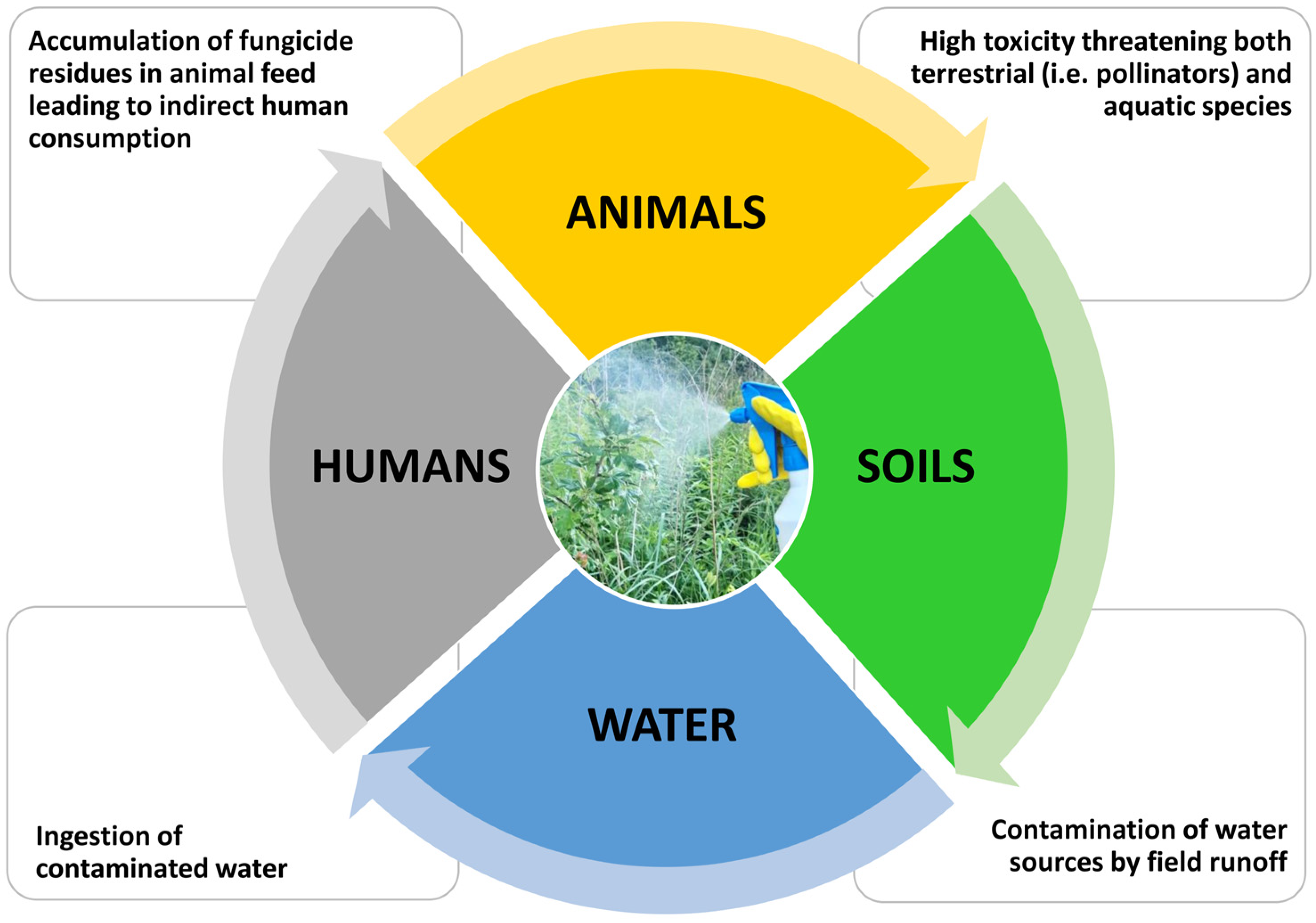
3.6. Synthetic Antifungals and the Environment
4. Biological Methods of Cereal Preservation
4.1. Bacteria, Yeast, and Molds as Biocontrol Agents
4.2. Antifungal Activities of Plant Extracts and Essential Oils on Grains
5. Integrated Control
6. Summary
- Registration procedures and regulatory guidelines;
- Research tools that monitor and evaluate the risk of long-term use, in terms of maintaining the biodiversity of natural environments and, above all, the safety of people and animals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baibakova, E.V.; Nefedjeva, E.E.; Suska-Malawska, M.; Wilk, M.; Sevriukova, G.A.; Zheltobriukhov, V.F. Modern fungicides: Mechanisms of action, fungal resistance and phytotoxic effects. Annu. Res. Rev. Biol. 2019, 32, 1–16. [Google Scholar] [CrossRef]
- Dias, M. Phytotoxicity: An overview of the physiological responses of plants exposed to fungicides. J. Bot. 2012, 135479. [Google Scholar] [CrossRef]
- Finch, S.; Samuel, A.; Lane, G.P. Lockhart and Wiseman’s Crop Husbandry Including Grassland, 9th ed.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2014; pp. 119–157. [Google Scholar]
- Beck, B.D.; Matzen, N.; Madsen, H.P.; Kirkegaard, S.S.; Nielsen, C.A.S.; Nørholm, S.R.; Almskou-Dahlgaard, A.; Meyer, A.; Jørgensen, L.N. Disease control in wheat. In Applied Crop Protection; DCA-Nationalt Center for Fødevarer og Jordbrug: Tjele, Denmark, 2024; Volume 226, pp. 17–38. [Google Scholar]
- Köycü, N.D.; Sukut, F. Effect of the timing of fungicide application on yield and quality parameters of wheat infected with Fusarium crown rot disease. Black Sea J. Agric. 2024, 7, 113–120. [Google Scholar] [CrossRef]
- Oerke, E.C.; Dehne, H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Thind, T.S. Changing trends in discovery of new fungicides: A perspective. Indian Phytopathol. 2021, 74, 875–883. [Google Scholar] [CrossRef]
- Russell, P.E. A Century of Fungicide Evolution. J. Agric. Sci. 2005, 143, 11–25. [Google Scholar] [CrossRef]
- Lundgren, K.D.; Swensson, A. A survey of results of investigations on some organic mercury compounds used as fungicides. Am. Ind. Hyg. Assoc. J. 1960, 21, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Qadri, R.; Azam, M.; Khan, M.I.; Yang, Y.; Ejaz, S.; Akram, M.T.; Khan, M.A. Conventional and modern technologies for the management of post-harvest diseases. In Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches; Springer: Cham, Switzerland, 2020; Volume 13, pp. 137–172. [Google Scholar] [CrossRef]
- Horsfal, J.G. Fungi and fungicides: The story of a nonconformist. Annu. Rev. Phytopathol. 1975, 13, 1–14. [Google Scholar] [CrossRef]
- Klittich, C.J. Milestones in fungicide discovery: Chemistry that changed agriculture. Plant Health Prog. 2008, 9, 31. [Google Scholar] [CrossRef]
- A Short History of Fungicides. Available online: https://www.apsnet.org/edcenter/apsnetfeatures/Pages/Fungicides.aspx/ (accessed on 16 December 2023).
- Krämer, W.; Schirmer, U. Modern crop protection compounds. In Crop Protection; Krämer, W., Schirmer, U., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 415–746. [Google Scholar]
- Food Safety Commission of Japan. Dichlobentiazox (pesticides). Food Saf. 2020, 8, 6–7. [Google Scholar] [CrossRef]
- Tian, Z.; Li, J.; Zhang, F.; Xu, L.; Zhou, F.; Zhou, L.; Wang, H.; Liu, R. Effects of mixtures containing physcion and several fungicides on the yield of wheat by seed coating and its potential mechanisms. Agriculture 2024, 14, 237. [Google Scholar] [CrossRef]
- Leadbeater, A. Recent developments and challenges in chemical disease control. Plant Prot. Sci. 2015, 51, 163–169. [Google Scholar] [CrossRef]
- Mahapatra, S.; Chakraborty, S.; Debnath, D.; Roy, C. Insights into wheat blast: Its epidemiology, recent advances and managements strategies. J. Crop Health 2024, 76, 397–409. [Google Scholar] [CrossRef]
- Fungicide Resistance Management; Fact Sheet EPP. Available online: https://shareok.org/bitstream/handle/11244/334679/oksa_EPP-7663_2009-04.pdf?sequence=1 (accessed on 16 December 2023).
- De Mio, L.L.M.; Peres, N.A.; Schnabel, G.; Isshi, H. A special isssue on fungicide resistance and management strategies. Trop. Plant Pathol. 2024, 49, 1–4. [Google Scholar] [CrossRef]
- Matelionienė, N.; Žvirdauskienė, R.; Kadžienė, G.; Zavtrikovienė, E.; Supronienė, S. In Vitro sensitivity test of Fusarium species from weeds and non-gramineous plants to triazole fungicides. Pathogens 2024, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ullah, Z.; Ullah, R.; Ahmad, H. Late blight of potato (Phytophthora infestans) I: Fungicides application and associated challenges. Turk. J. Agric. Food Sci. Technol. 2017, 5, 261–266. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Conventional pesticides in agriculture: Benefits versus risks. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef]
- Jess, S.; Kildea, S.; Moody, A.; Rennick, G.; Murchie, A.K.; Cooke, L.R. European Union policy on pesticides: Implications for agriculture in Ireland. Pest Manag. Sci. 2014, 70, 1646–1654. [Google Scholar] [CrossRef]
- Suleiman, R.; Rosentrater, K.A. Current maize production, postharvest losses and the risk of mycotoxins contamination in Tanzania. In Proceedings of the ASABE International Meeting Presentation, New Orleans, LA, USA, 26–29 July 2015. [Google Scholar] [CrossRef][Green Version]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Crop Protection Research and Reports. Available online: https://www.mordorintelligence.com/market-analysis/crop-protection (accessed on 16 April 2024).
- Statistics Division Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 16 December 2023).
- Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 16 December 2023).
- Crop Yields. Available online: https://ourworldindata.org/crop-yields#explore-data-on-crop-yields (accessed on 18 February 2024).
- Denyer, S.P. Mechanisms of action of biocides. Int. Biodeterior. 1990, 26, 89–100. [Google Scholar] [CrossRef]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- FRAC Code List 2024: Fungal Control Agents Sorted by Cross Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2024.pdf?sfvrsn=52e14e9a_2 (accessed on 4 June 2024).
- Chen, Q.; Zhang, J.W.; Chen, L.L.; Yang, J.; Yang, X.L.; Ling, Y.; Yang, Q. Design and synthesis of chitin synthase inhibitors as potent fungicides. Chin. Chem. Lett. 2017, 28, 1232–1237. [Google Scholar] [CrossRef]
- Kurland, C.; Olsen, G. Current opinion in microbiology: Editorial overview. Curr. Opin. Microbiol. 2002, 5, 497–498. [Google Scholar] [CrossRef]
- Banba, S.; Hamada, T.; Araki, N.; Ebihara, K. Synthesis and activities of tolprocarb derivatives against Pyricularia oryzae: Relationships among the activities for polyketide synthase, melanin biosynthesis, and rice blast. J. Pestic. Sci. 2017, 42, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Fukuchi, A. Management of melanin biosynthesis dehydratase inhibitor (MBI-D)-resistance in Pyricularia oryzae using a non-MBI-D fungicidal application program for nursery boxes and a diclocymet and ferimzone mixture for field foliar applications. J. Pestic. Sci. 2018, 43, 287–292. [Google Scholar] [CrossRef]
- Lee, J.K.; Jung, H.M.; Kim, S.Y. 1,8-Dihydroxynaphthalene (DHN)-Melanin Biosynthesis increase erythritol production in Torula corallina, and DHN-melanin inhibits erythrose reductase. Appl. Environ. Microbiol. 2003, 69, 3427–3434. [Google Scholar] [CrossRef][Green Version]
- Motoyama, T.; Yamaguchi, I. Fungicides, Melanin Biosynthesis Inhibitors. In Encyclopedia of Agrochemicals; Plimmer, J.R., Gammon, D.W., Ragsdale, N.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Pan, J.; Hu, C.; Yu, J.H. Lipid biosynthesis as an antifungal target. J. Fung. 2018, 4, 50. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Vujanovic, V.; Gan, Y. Fungicide: Modes of Action and Possible Impact on Nontarget Microorganisms; John Wiley & Sons: Hoboken, NJ, USA, 2011; 8p. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef] [PubMed]
- Umate, P. Comparison of Genes encoding enzymes of sterol biosynthesis from plants to orthologs in yeast. Rice Res. 2016, 4, 160. [Google Scholar] [CrossRef]
- Opalski, K.S.; Tresch, S.; Kogel, K.-H.; Grossmann, K.; Köhle, H.; Hückelhoven, R. Metrafenone: Studies on the mode of action of a novel cereal powdery mildew fungicide. Pest Manag. Sci. 2006, 62, 393–401. [Google Scholar] [CrossRef]
- Rogalska, A.; Miśkiewicz, K.; Marczak, A. Inhibitors of microtubule polymerization- new natural compounds as potential anti-cancer drugs. Postepy Hig. Med. Dosw. 2015, 69, 571–585. [Google Scholar] [CrossRef]
- Vela-Corcía, D.; Romero, D.; De Vicente, A.; Pérez-García, A. Analysis of β-tubulin-carbendazim interaction reveals that binding site for MBC fungicides does not include residues involved in fungicide resistance. Sci. Rep. 2018, 8, 7161. [Google Scholar] [CrossRef] [PubMed]
- Wildman, H.G.; Lyr, H.; Russell, P.E.; Sisler, H.D. Modern fungicides and antifungal compounds. Mycologia 1997, 89, 820. [Google Scholar] [CrossRef]
- Calderone, R.; Li, D.; Traven, A. System-level impact of mitochondria on fungal virulence: To metabolism and beyond. FEMS Yeast Res. 2015, 15, fov027. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microb. 2010, 76, 6615–6630. [Google Scholar] [CrossRef]
- Ujváry, I. Pest control agents from natural products. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Elsevier: New York, NY, USA, 2010; pp. 119–229. [Google Scholar]
- Zhang, Y.; Lamm, R.; Pillonel, C.; Lam, S.; Xu, J.-R. Osmoregulation and fungicide resistance: The Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 2002, 68, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Nehra, B.; Kumar, S.; Monga, V. Recent advancements in the medicinal chemistry of bacterial type II topoisomerase inhibitors. Bioorg. Chem. 2020, 104, 104266. [Google Scholar] [CrossRef]
- Ypema, H. Fungicides, Hymexazol. In Encyclopedia of Agrochemicals; Plimmer, J.R., Gammon, D.W., Ragsdale, N.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Poole, N.F.; Arnaudin, M.E. The role of fungicides for effective disease management in cereal crops. Can. J. Plant Path. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Ayesha, M.; Suryanarayanan, T.; Nataraja, K.; Prasad, S.; Shaanker, R. Seed treatment with systemic fungicides: Time for review. Front. Plant Sci. 2021, 12, 654512. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kumar, S. Seed coating with fungicides and various treatments for protection of crops: A review. Int. J. Agric. Environ. Sustain. 2020, 2, 6–13. [Google Scholar]
- Pesticides. Available online: https://ourworldindata.org/pesticides (accessed on 18 February 2024).
- PAN International Consolidated List of Banned Pesticides. Available online: https://pan-international.org/pan-international-consolidated-list-of-banned-pesticides/ (accessed on 16 December 2023).
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC; European Union: Maastricht, The Netherlands, 2009; Available online: https://eur-lex.europa.eu/eli/reg/2009/1107/oj (accessed on 4 August 2024).
- NPRO (NPIC, 2024. Product Research Online). 2024. Available online: http://npic.orst.edu/NPRO (accessed on 24 March 2024).
- EPA United States Environmental Protection Agency. Central Data Exchange. Available online: https://cdx.epa.gov (accessed on 24 March 2024).
- Donley, N. The USA lags behind other agricultural nations in banning harmful pesticides. Environ. Health 2019, 18, 44. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of fungicides. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 569–580. [Google Scholar] [CrossRef]
- Shen, C.; Tang, C.; Zhu, K.; He, C.; Yang, C.; Zuo, Z. Toxicity and ecological risk assessment for two AhR agonistic pesticides mepanipyrim and cyprodinil and their metabolites. Environ. Sci. Pollut. Res. 2023, 30, 58944–58955. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, W.; Li, X.; Wang, D. Photodegradation and oxidation of fungicide cyprodinil by advanced oxidation processes (AOPs): Transformation products and toxicity assessment. J. Hazard. Mater. 2021, 416, 125933. [Google Scholar] [CrossRef]
- Bharathi, V.S.K.; Jayas, D.S. Ethyl formate: A comprehensive review on its Function as a fumigant for stored products. J. Stored Prod. Res. 2024, 106, 102280. [Google Scholar] [CrossRef]
- Kiefer, K.; Bader, T.; Minas, N.; Salhi, E.; Janssen, E.M.-L.; von Gunten, U.; Hollender, J. Chlorothalonil transformation products in drinking water resources: Widespread and challenging to abate. Water Res. 2020, 183, 116066. [Google Scholar] [CrossRef]
- Carneiro, L.S.; Martínez, L.C.; Gonçalves, W.G.; Santana, L.M.; Serrão, J.E. The fungicide iprodione affects midgut cells of non-target honey bee Apis mellifera workers. Ecotoxicol. Environ. Saf. 2020, 189, 109991. [Google Scholar] [CrossRef]
- Oiki, S.; Yaguchi, T.; Urayama, S.-I.; Hagiwara, D. Wide distribution of resistance to the fungicides fludioxonil and iprodione in Penicillium species. PLoS ONE 2022, 17, e0262521. [Google Scholar] [CrossRef] [PubMed]
- Dall’Agnol, J.C.; Ferri Pezzini, M.; Suarez Uribe, N.; Joveleviths, D. Systemic effects of the pesticide mancozeb—A literature review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Quds, R.; Hashmi, M.A.; Iqbal, Z.; Mahmood, R. Interaction of mancozeb with human hemoglobin: Spectroscopic, molecular docking and molecular dynamic simulation studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 280, 121503. [Google Scholar] [CrossRef] [PubMed]
- Kaikai, N.-E.; Ba-M’hamed, S.; Ghanima, A.; Bennis, M. Exposure to metam sodium-based pesticide impaired cognitive performances in adult mice: Involvement of oxidative damage and glial activation. Toxicol. Appl. Pharmacol. 2023, 477, 116677. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Iqbal, M.; Tang, Z.; Zhang, H. Thiram exposure in environment: A critical review on cytotoxicity. Chemosphere 2022, 295, 133928. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Xu, X.; Kuang, H.; Liu, L.; Xu, C.; Sun, M. Immunochromatographic visualization detection platform for bitertanol in foods. Food Chem. 2024, 444, 138599. [Google Scholar] [CrossRef]
- He, R.; Fan, J.; Chen, R.; Guo, D.; Zhao, M.; Zhang, Z.; Liang, C.; Chen, M.; Song, H.; Zhang, W. Stereoselective in vitro metabolism of cyproconazole in rat liver microsomes and identification of major metabolites. Chemosphere 2021, 264, 128495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ni, H.; Xu, W.; Wu, B.; Xie, T.; Zhang, C.; Cheng, J.; Li, Z.; Tao, L.; Zhang, Y. Difenoconazole induces oxidative DNA damage and mitochondria mediated apoptosis in SH-SY5Y cells. Chemosphere 2021, 283, 131160. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, J.; Zhang, L.; Feng, T.; Zhang, Z.; Zhang, B. Fungicide Difenoconazole Induced Biochemical and Developmental Toxicity in Wheat (Triticum aestivum L.). Plants 2021, 10, 2304. [Google Scholar] [CrossRef]
- Han, L.; Xu, H.; Wang, Q.; Liu, X.; Li, X.; Wang, Y.; Nie, J.; Liu, M.; Ju, C.; Yang, C. Deciphering the degradation characteristics of the fungicides imazalil and penflufen and their effects on soil bacterial community composition, assembly, and functional profiles. J. Hazard. Mater. 2023, 460, 132379. [Google Scholar] [CrossRef]
- Roman, D.L.; Matica, M.A.; Ciorsac, A.; Boros, B.V.; Isvoran, A. The effects of the fungicide myclobutanil on soil enzyme activity. Agriculture 2023, 13, 1956. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Q.; Yuan, K.; Li, Y.; Shi, M.; Miao, J.; Liu, X. Monitoring and characterization of prochloraz resistance in Fusarium fujikuroi in China. Pestic. Biochem. Physiol. 2022, 187, 105189. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Li, X.; He, S.; Shi, L.; Yu, S.; Wang, F.; Xu, S.; Cao, D.; Fang, H.; Yu, Y. Root uptake of Imidacloprid and Propiconazole is affected by root composition and soil characteristics. J. Agric. Food. Chem. 2020, 68, 15381–15389. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Dong, B. A comprehensive review on toxicological mechanisms and transformation products of tebuconazole: Insights on pesticide management. Sci. Total Environ. 2023, 908, 168264. [Google Scholar] [CrossRef]
- Yu, S.; Li, X.; He, S.; Zhang, H.; Jin, M.; Zheng, Y.; Zhang, L.; Yu, Y. Uptake and translocation of triadimefon by wheat (Triticum aestivum L.) grown in hydroponics and soil conditions. J. Hazard. Mater. 2022, 423, 127011. [Google Scholar] [CrossRef]
- Park, J.; Hong, T.; An, G.; Park, H.; Song, G.; Lim, W. Triadimenol promotes the production of reactive oxygen species and apoptosis with cardiotoxicity and developmental abnormalities in zebrafish. Sci. Total Environ. 2023, 862, 160761. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Matica, M.A.; Baerle, V.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Assessment of the effects of triticonazole on soil and human health. Molecules 2022, 27, 6554. [Google Scholar] [CrossRef]
- Draskau, M.K.; Boberg, J.; Taxvig, C.; Pedersen, M.; Frandsen, H.L.; Christiansen, S.; Svingen, T. In vitro and in vivo endocrine disrupting effects of the azole fungicides triticonazole and flusilazole. Environ. Pollut. 2019, 255, 113309. [Google Scholar] [CrossRef] [PubMed]
- Kirman, C.; Li, A.; Sheehan, P.; Bus, J.; Lewis, R.; Hays, S. Ethylene oxide review: Characterization of total exposure via endogenous and exogenous pathways and their implications to risk assessment and risk management. J. Toxicol. Environ. Health Sci. 2021, 24, 1–29. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Yoon, C.-Y.; Kim, J.; Choi, J.-Y.; Hwang, S.; Park, K.-H. Transcriptome profiling of etridiazole-exposed zebrafish (Danio rerio) embryos reveals pathways associated with cardiac and ocular toxicities. Int. J. Mol. Sci. 2023, 24, 15067. [Google Scholar] [CrossRef]
- Ullah, R.; Salcedo, Y.E.; Mukesh Mehta, J.; Al Balushi, J.; Khariwal, M.; Patel, N. Exploring the protective role of G6PD deficiency in aluminum phosphide poisoning: A case report and review of the literature. Cureus 2024, 16, e58888. [Google Scholar] [CrossRef]
- Adel, B.; Elgharbawy, N.M.; Shahin, M.M.; Abo-Elfadl, A.A.; Saad, K.M. Insulin-euglycemia therapy in acute aluminum phosphide poisoning: A randomized clinical trial. Clin. Toxicol. 2023, 61, 1032–1039. [Google Scholar] [CrossRef]
- Yang, Y.; Bustani, G.S.; Alawsi, T.; Altalbawy, F.M.A.; Kareem, A.K.; Gupta, J.; Zhu, P.; Hjazi, A.; Alawadi, A.H.; Mustafa, Y.F. The cardioprotective effects of cerium oxide nanoparticles against the poisoning generated by aluminum phosphide pesticide: Controlling oxidative stress and mitochondrial damage. Pestic. Biochem. Phys. 2023, 197, 105701. [Google Scholar] [CrossRef] [PubMed]
- Liman, R.; Ali, M.M.; Ciğerci, İ.H.; İstifli, E.S.; Sarıkurkcu, C. Cytotoxic and genotoxic evaluation of copper oxychloride through Allium test and molecular docking studies. Environ. Sci. Pollut. Res. Int. 2021, 28, 44998–45008. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, A.; Blavi, L.; Abdelli, N.; Melo-Duran, D.; Vidal, A.; Rodríguez, M.; Monteiro, A.N.T.R.; Perez, J.F.; Darwich, L.; Solà-Oriol, D. Effects of dicopper oxide and copper sulfate on growth performance and gut microbiota in broilers. Poult. Sci. 2021, 100, 101224. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shi, X.; Wu, Z.; Zhang, J.; Hao, J.; Liu, P.; Liu, X. Carboxylesterase and cytochrome P450 confer metabolic resistance simultaneously to azoxystrobin and some other fungicides in Botrytis cinerea. J. Agric. Food Chem. 2024, 72, 9680–9690. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Li, F. Research progress on benzimidazole fungicides: A review. Molecules 2024, 29, 1218. [Google Scholar] [CrossRef]
- Zhou, T.; Guo, T.; Wang, Y.; Wang, A.; Zhang, M. Carbendazim: Ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere 2023, 314, 137723. [Google Scholar] [CrossRef]
- Park, J.; An, G.; Park, H.; Hong, T.; Lim, W.; Song, G. Developmental defects induced by thiabendazole are mediated via apoptosis, oxidative stress and alteration in PI3K/Akt and MAPK pathways in zebrafish. Environ. Int. 2023, 176, 107973. [Google Scholar] [CrossRef]
- Jia, K.; Cheng, B.; Huang, L.; Xiao, J.; Bai, Z.; Liao, X.; Cao, Z.; Shen, T.; Zhang, C.; Hu, C.; et al. Thiophanate-methyl induces severe hepatotoxicity in zebrafish. Chemosphere 2020, 248, 125941. [Google Scholar] [CrossRef]
- Ghodke, P.P.; Gonzalez-Vasquez, G.; Wang, H.; Johnson, K.M.; Sedgeman, C.A.; Guengerich, F.P. Enzymatic bypass of an N6-deoxyadenosine DNA–ethylene dibromide–peptide cross-link by translation DNA polymerases. J. Biol. Chem. 2021, 296, 100444. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-G.; Choi, J.; Hong, Y.-S.; Park, C.G.; Kim, B.-G.; Lee, S.-Y.; Lim, H.J.; Mo, H.H.; Lim, E.; Cha, W. Negative effect of methyl bromide fumigation work on the central nervous system. PLoS ONE 2020, 15, e0236694. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Vähäkangas, K. Chloropicrin-induced toxicity in the respiratory system. Toxicol. Let. 2020, 323, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cao, A.; Wu, J.; Fang, W.; Huang, B.; Yan, D.; Wang, Q.; Li, Y. Effects of chloropicrin fumigation combined with biochar on soil bacterial and fungal communities and Fusarium oxysporum. Ecotoxicol. Environ. Saf. 2021, 220, 112414. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Huang, B.; Song, Z.; Ren, L.; Hao, B.; Liu, J.; Zhu, J.; Fang, W.; Yan, D.; et al. Organic fertilizer improves soil fertility and restores the bacterial community after 1,3-dichloropropene fumigation. Sci. Total Environ. 2020, 738, 140345. [Google Scholar] [CrossRef]
- Moreau, M.; Fisher, J.; Andersen, M.E.; Barnwell, A.; Corzine, S.; Ranade, A.; McMullen, P.D.; Slattery, S.D. NAM-based prediction of point-of-contact toxicity in the lung: A case example with 1,3-dichloropropene. Toxicology 2022, 481, 153340. [Google Scholar] [CrossRef]
- Pandey, J.K. Use of hazardous chemical pesticides in India: A review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 523–537. [Google Scholar] [CrossRef]
- Saquib, Q.; Siddiqui, M.A.; Ansari, S.M.; Alwathnani, H.A.; Musarrat, J.; Al-Khedhairy, A.A. Cytotoxicity and genotoxicity of methomyl, carbaryl, metalaxyl, and pendimethalin in human umbilical vein endothelial cells. J. Appl. Toxicol. 2021, 41, 832–846. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, A.M. Microbial bioremediation of fungicides. In Nanohybrid Fungicides; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 441–474. [Google Scholar] [CrossRef]
- Zia, R.; Taj, A.; Younis, S.; Bukhari, S.Z.; Latif, F.; Feroz, Y.; Midrarullah Imran, A.; Bajwa, S.Z. Application of nanosensors for pesticide detection. In Nanosensors for Smart Agriculture. Micro and Nano Technologies; Denizli, A., Nguyen, A., Nadda, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 259–302. [Google Scholar] [CrossRef]
- Hamed, S.M.; Okla, M.K.; Al-Saadi, L.S.; Hozzein, W.N.; Mohamed, H.S.; Selim, S.; AbdElgawad, H. Evaluation of the phycoremediation potential of microalgae for captan removal: Comprehensive analysis on toxicity, detoxification and antioxidants modulation. J. Hazard. Mater. 2022, 427, 128177. [Google Scholar] [CrossRef]
- Sułowicz, S.; Markowicz, A.; Dulski, M.; Nowak, A.; Środek, D.; Borymski, S. Assessment of the ecotoxicological impact of captan@ZnO35–45nm and captan@SiO2 20–30 nm nanopesticide on non-target soil microorganisms—A 100-day case study. Appl. Soil Ecol. 2023, 184, 104789. [Google Scholar] [CrossRef]
- Zhu, Y.; Ke, M.; Yu, Z.; Lei, C.; Liu, M.; Yang, Y.; Lu, T.; Zhou, N.-Y.; Peijnenburg, W.J.G.M.; Tang, T.; et al. Combined effects of azoxystrobin and oxytetracycline on rhizosphere microbiota of Arabidopsis thaliana. J. Environ. Int. 2024, 186, 108655. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, Y.; Zhang, C.; Liu, X.; Fang, N.; Wang, X.; Zhao, X.; Jiang, J. Trifloxystrobin induced developmental toxicity by disturbing the ABC transporters, carbohydrate and lipid metabolism in adult zebrafish. Chemosphere 2024, 349, 140747. [Google Scholar] [CrossRef]
- Man, Y.; Wu, C.; Yu, B.; Mao, L.; Zhu, L.; Zhang, L.; Zhang, Y.; Jiang, H.; Yuan, S.; Zheng, Y.; et al. Abiotic transformation of kresoxim-methyl in aquatic environments: Structure elucidation of transformation products by LC-HRMS and toxicity assessment. Water Res. 2023, 233, 119723. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Bian, J.; Han, S.; Zhang, C.; Xu, W.; Tao, L.; Li, Z.; Zhang, Y. Characterization of hepatotoxic effects induced by pyraclostrobin in human HepG2 cells and zebrafish larvae. Chemosphere 2023, 340, 139732. [Google Scholar] [CrossRef]
- Xu, G.; Jia, X.; Wu, C.; Liu, X.; Dong, F. Chiral fungicide famoxadone: Stereoselective bioactivity, aquatic toxicity, and environmental behavior in soils. J. Agric. Food Chem. 2021, 69, 8530–8535. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Geng, Y.; Bao, C.; Mei, Q.; Shi, T.; Ma, X.; Hua, R.; Fang, L. Complete biodegradation of fungicide carboxin and its metabolite aniline by Delftia sp. HFL-1. Sci. Total Environ. 2024, 912, 168957. [Google Scholar] [CrossRef]
- Balasubramanya, R.H.; Patil, R.B. Degradation of carboxin and oxycarboxin in different soils. Plant Soil. 1980, 57, 195–201. [Google Scholar] [CrossRef]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides; European Union: Maastricht, The Netherlands, 2009; Available online: http://data.europa.eu/eli/dir/2009/128/oj (accessed on 4 August 2024).
- Regulation 2023/114. In EU Pesticide Approvals, Renewals, and Extensions in 2023; European Union: Maastricht, The Netherlands, 2023; Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023 (accessed on 27 May 2024).
- Regulation 2023/689. In EU Pesticide Approvals, Renewals, and Extensions in 2023; European Union: Maastricht, The Netherlands, 2023; Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023 (accessed on 27 May 2024).
- Regulation 2023/918. In EU Pesticide Approvals, Renewals, and Extensions in 2023; European Union: Maastricht, The Netherlands, 2023; Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023 (accessed on 27 May 2024).
- Regulation 2023/1446. In EU Pesticide Approvals, Renewals, and Extensions in 2023; European Union: Maastricht, The Netherlands, 2023; Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023 (accessed on 27 May 2024).
- Regulation 2023/2592. In EU Pesticide Approvals, Renewals, and Extensions in 2023; European Union: Maastricht, The Netherlands, 2023; Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023 (accessed on 27 May 2024).
- Fang, W.; Peng, Y.; Muir, D.; Lin, J.; Zhang, X. A critical review of synthetic chemicals in surface waters of the US, the EU and China. Environ. Int. 2019, 131, 104994. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, Q.; Cai, H.; Li, S.; Shen, Z.; Song, T. Fungicides reduce soil microbial diversity, network stability and complexity in wheat fields with different disease resistance. Appl. Soil Ecol. 2024, 201, 105513. [Google Scholar] [CrossRef]
- Pimentão, A.R.; Cuco, A.P.; Pascoal, C.; Cássio, F.; Castro, B.B. Current trends and mismatches on fungicide use and assessment of the ecological effects in freshwater ecosystems. Environ. Pollut. 2024, 347, 123678. [Google Scholar] [CrossRef] [PubMed]
- Pyatina, S.A.; Shishatskaya, E.I.; Dorokhin, A.S.; Menzyanova, N.G. Border cell population size and oxidative stress in the root apex of Triticum aestivum seedlings exposed to fungicides. Environ. Sci. Pollut. Res. 2024, 31, 25600–25615. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An overview of strobilurin fungicide degradation: Current status and future perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole resistance in Aspergillus fumigatus: A consequence of antifungal use in agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Singh, R.; Kumar, P.; Shukla, G. Pros and cons of fungicides: An overwiev. Int. J. Eng. Sci. Res. Technol. 2018, 6, 112–117. [Google Scholar] [CrossRef]
- Wang, M.; Duan, H.; Zhou, C.; Yu, L.; Meng, X.; Lu, W.; Yu, H. Synergistic effects of chemical fungicides with crude extracts from Bacillus amyloliquefaciens to control northern corn leaf blight. Agriculture 2024, 14, 606. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Robin, D.C.; Marchand, P.A. Evolution of the biocontrol active substances in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2019, 75, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Marchand, P.A. BioControl Agents in Europe: Substitution plant protection active substances or a new paradigm? Agrochemicals 2023, 2, 538–550. [Google Scholar] [CrossRef]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. Biocontrol 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Mills, N.J. Biological Control—Ecology and Applications; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Galli, M.; Feldmann, F.; Vogler, U.K.; Kogel, K.H. Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J. Plant Dis. Prot. 2024, 131, 265–291. [Google Scholar] [CrossRef]
- Fuerst, E.P.; James, M.S.; Pollard, A.T.; Okubara, P.A. Defense enzyme responses in dormant wild oat and wheat caryopses challenged with a seed decay pathogen. Front. Plant Sci. 2018, 8, 2259. [Google Scholar] [CrossRef]
- Bailly, C.; Bousquet, A.; Braun, V.; Buitink, J.; Desbois-Vimont, C.; Durand Tardif, M.; Fougereux, J.A.; Gaillard, A.; Gouleau, A.; Grappin, P.; et al. Towards Seed Protection Using Biocontrol Strategies; hal-02931599; HAL Open Science: Paris, France, 2019; Available online: https://hal.science/hal-02931599 (accessed on 24 May 2024).
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Kaufman, G. Seed coating: A tool for stand establishment; a stimulus to seed quality. HortTechnology 1991, 1, 98–102. [Google Scholar] [CrossRef]
- Bakker, P.A.; Pieterse, C.M.; Van Loon, L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens 2019, 8, 78. [Google Scholar] [CrossRef]
- Chan, Y.K.; Savard, M.E.; Reid, L.M.; Cyr, T.; McCormick, W.A.; Seguin, C. Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. BioControl 2009, 54, 567–574. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Schisler, D.A.; Price, N.P.; Vaughn, S.F. Cyclic lipopeptide profile of three Bacillus subtilis strains, antagonists of Fusarium head blight. J. Microbiol. 2011, 49, 603–609. [Google Scholar] [CrossRef]
- Zanon, M.S.A.; Cavaglieri, L.R.; Palazzini, J.M.; Chulze, S.N.; Chiotta, M.L. Bacillus velezensis RC218 and emerging biocontrol agents against Fusarium graminearum and Fusarium poae in barley: In vitro, greenhouse and field conditions. Int. J. Food Microbiol. 2024, 413, 110580. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mionetto, A.; Tiscornia, S.; Bettucci, L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015, 31, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Q.; Wang, K.; Brian, K.; Liu, C.; Gu, Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour. Technol. 2010, 101, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus species: Excellent biocontrol agents against tomato fiseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Baffoni, L.; Gaggia, F.; Dalanaj, N.; Prodi, A.; Nipoti, P.; Pisi, A.; Biavati, B.; Di Gioia, D. Microbial inoculants for the biocontrol of Fusarium spp. in durum wheat. BMC Microbiol. 2015, 15, 242. [Google Scholar] [CrossRef]
- Zhao, H.; Vegi, A.; Wolf-Hall, C. Screening of lactic acid bacteria for anti-Fusarium activity and optimization of incubation conditions. J. Food Prot. 2017, 80, 1648–1656. [Google Scholar] [CrossRef]
- Rajendran, K.; Krishnamoorthy, M.; Karuppiah, K.; Ethiraj, K.; Sekar, S. Chitinase from Streptomyces mutabilis as an effective eco-friendly biocontrol agent. Appl. Biochem. Biotechnol. 2024, 196, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Song, W.; Wang, J.; Cao, Y.; Han, X.; Xu, C.; Wang, F.; Ge, B. Biocontrol of Botrytis cinerea by Streptomyces noursei C27 and preliminary identification of antimicrobial metabolites. Biol. Contr. 2024, 196, 105561. [Google Scholar] [CrossRef]
- Matić, S.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Antagonistic yeasts and thermotherapy as seed treatments to control Fusarium fujikuroi on rice. Biol. Control 2014, 73, 59–67. [Google Scholar] [CrossRef]
- Druvefors, A.U.; Schnürer, J. Mold-inhibitory activity of different yeast species during airtight storage of wheat grain. FEMS Yeast Res. 2005, 5, 373–378. [Google Scholar] [CrossRef]
- Sun, Z.; Li, S.; Ren, Q.; Xu, J.; Lu, X.; Sun, M. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef]
- Jensen, B.; Knudsen, I.M.B.; Jensen, D.F. Biological seed treatment of cereals with fresh and long-term stored formulations of Clonostachys rosea: Biocontrol efficacy against Fusarium culmorum. Eur. J. Plant Pathol. 2000, 106, 233–242. [Google Scholar] [CrossRef]
- Shi, C.; Yan, P.; Li, J.; Wu, H.; Li, Q.; Guan, S. Biocontrol of Fusarium graminearum growth and deoxynivalenol production in wheat kernels with bacterial antagonists. Int. J. Environ. Res. Public Health 2014, 11, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Boland, G.J.; Zhou, T. Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium head blight and deoxynivalenol in wheat. J. Appl. Microbiol. 2009, 106, 1805–1817. [Google Scholar] [CrossRef]
- Nourozian, J.; Etebarian, H.R.; Khodakaramian, G. Biological control of Fusarium graminearum on wheat by antagonistic bacteria. Songklanakarin J. Sci. Technol. 2006, 28 (Suppl. S1), 29–38. [Google Scholar]
- Johnsson, L.; Hökeberg, M.; Gerhardson, B. Performance of the Pseudomonas chlororaphis biocontrol agent MA 342 against cereal seed-borne diseases in field experiments. Eur. J. Plant Pathol. 1998, 104, 701–711. [Google Scholar] [CrossRef]
- Mattei, V.; Motta, A.; Saracchi, M.; Kunova, A.; Cortesi, P.; Pizzatti, C.; Pasquali, M. Wheat seed coating with Streptomyces sp. strain DEF39 spores protects against Fusarium head blight. Microorganisms 2022, 10, 1536. [Google Scholar] [CrossRef]
- Nagaraja, H.; Chennappa, G.; Rakesh, S.; Naik, M.K.; Amaresh, Y.S.; Sreenivasa, M.Y. Antifungal activity of Azotobacter nigricans against trichothecene-producing Fusarium species associated with cereals. Food Sci. Biotechnol. 2016, 25, 1197–1204. [Google Scholar] [CrossRef]
- Schisler, D.A.; Core, A.B.; Boehm, M.J.; Horst, L.; Krause, C.; Dunlap, C.A.; Rooney, A.P. Population dynamics of the Fusarium head blight biocontrol agent Cryptococcus flavescens OH 182.9 on wheat anthers and heads. Biol. Contr. 2014, 70, 17–27. [Google Scholar] [CrossRef]
- Xue, A.G.; Guo, W.; Chen, Y.; Siddiqui, I.; Marchand, G.; Liu, J.; Changzhong, R. Effect of seed treatment with novel strains of Trichoderma spp. on establishment and yield of spring wheat. Crop Prot. 2017, 96, 97–102. [Google Scholar] [CrossRef]
- Petros Kubheka, B.; Weldegabir Ziena, L. production. In Trichoderma—Technology and Uses; Juliatti, F.C., Ed.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Ferrigo, D.; Mondin, M.; Edith, L.; Fabio, F.; Causin, R.; Raiola, A. Effect of seed biopriming with Trichoderma harzianum strain INAT11 on Fusarium ear rot and Gibberella ear rot diseases. Biol. Contr. 2020, 147, 104286. [Google Scholar] [CrossRef]
- Modrzewska, M.; Bryła, M.; Kanabus, J.; Pierzgalski, A. Trichoderma as a biostimulator and biocontrol agent against Fusarium in the production of cereal crops: Opportunities and Possibilities. Plant Pathol. 2022, 71, 1471–1485. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Kumari, R.; Kumar, V.; Arukha, A.P.; Rabbee, M.F.; Ameen, F.; Koul, B. Screening of the biocontrol efficacy of potent Trichoderma strains against Fusarium oxysporum f. sp. ciceri and Scelrotium rolfsii causing wilt and collar rot in chickpea. Microorganisms 2024, 12, 1280. [Google Scholar] [CrossRef]
- Bharti, L.; Yadav, K.; Kumar Chaubey, A. Trichoderma spp.: Approach for bio-control agent. In Challenges in Plant Disease Detection and Recent Advancements; Bahadur, A., Ed.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Natsiopoulos, D.; Topalidou, E.; Mantzoukas, S.; Eliopoulos, P.A. Endophytic Trichoderma: Potential and prospects for plant health management. Pathogens 2024, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, U.; Packa, D.; Wiwart, M. Microbial inhibition of Fusarium pathogens and biological modification of trichothecenes in cereal grains. Toxins 2017, 9, 408. [Google Scholar] [CrossRef]
- Larrea, M.; Nicole, C.; Benítez Rodas, G.A.; Sandoval-Espínola, W.J.; Arrúa, P.D.; Lopez-Nicora, H.Q.; Arrúa, S.A.; Fernández Rios, D.; Arrúa, A.A. Trichoderma as biocontrol agent—In focus. Rev. Soc. Cient. Parag. 2024, 29, 137–171. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- BIOMIN Science & Solutions; BIOMIN Holding GmbH: Herzogenburg, Austria, 2015.
- Ndiaye, S.; Zhang, M.; Fall, M.; Ayessou, N.M.; Zhang, Q.; Li, P. Current review of mycotoxin biodegradation and bioadsorption: Microorganisms, mechanisms, and main important applications. Toxins 2022, 14, 729. [Google Scholar] [CrossRef]
- Harkai, P.; Szabó, I.; Cserháti, M.; Krifaton, C.; Risa, A.; Radó, J.; Balázs, A.; Berta, K.; Kriszt, B. Biodegradation of aflatoxin-B1 and zearalenone by Streptomyces sp. collection. Int. Biodeterior. Biodegrad. 2016, 108, 48–56. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Basińska-Barczak, A.; Ćwiek-Kupczyńska, H.; Gromadzka, K.; Popiel, D.; Stępień, L. Suppressive effect of Trichoderma spp. on toxigenic Fusarium species. Pol. J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Yan, Z.; Liao, Y.; Chen, J.; De Boevre, M.; De Saeger, S.; Wu, A. Antagonistic and detoxification potentials of Trichoderma isolates for control of zearalenone (ZEN) producing Fusarium graminearum. Front. Microbiol. 2018, 8, 2710. [Google Scholar] [CrossRef] [PubMed]
- Galletti, S.; Paris, R.; Cianchetta, S. Selected isolates of Trichoderma gamsii induce different pathways of systemic resistance in maize upon Fusarium verticillioides challenge. Microbiol. Res. 2020, 233, 126406. [Google Scholar] [CrossRef]
- Dini, I.; Alborino, V.; Lanzuise, S.; Lombardi, N.; Marra, R.; Balestrieri, A.; Ritieni, A.; Woo, S.L.; Vinale, F. Trichoderma enzymes for degradation of aflatoxin B1 and ochratoxin A. Molecules 2022, 7, 3959. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological detoxification of mycotoxins: Current status and future advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Ritieni, A. Role of probiotics against mycotoxins and their deleterious effects. J. Food Res. 2014, 4, 10–21. [Google Scholar] [CrossRef]
- Sangsila, A.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Itsaranuwat, P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Contr. 2016, 62, 187–192. [Google Scholar] [CrossRef]
- Tinyiro, S.E.; Wokadala, C.; Xu, D.; Yao, W. Adsorption and degradation of zearalenone by Bacillus strains. Folia Microbiol. 2011, 56, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, M.; Shi, Z.-Q.; Ghassempour, A.; Sedaghat, N.; Ahmadzadeh, M.; Mirabolfathy, M.; Javan-Nikkhah, M. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Contr. 2021, 23, 100–106. [Google Scholar] [CrossRef]
- Zeidan, R.; Ul Hassan, Z.; Ashfaq, M.Y.; Al-Thani, R.; Jaoua, S. Investigation of heat-resistant antifungal agents from Bacillus amyloliquefaciens and Bacillus subtilis for biocontrol of mycotoxigenic fungi. Environ. Technol. Innov. 2024, 36, 103748. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Chen, Z.Y.; Liu, H.; Li, P. Investigation of Pseudomonas fluorescens strain 3JW1 on preventing and reducing aflatoxin contaminations in peanuts. PLoS ONE 2017, 12, e0178810. [Google Scholar] [CrossRef] [PubMed]
- Jakopović, Ž.; Čiča, K.H.; Mrvčić, J.; Pucić, I.; Čanak, I.; Frece, J.; Pleadin, J.; Stanzer, D.; Zjalić, S.; Markov, K. Properties and fermentation activity of industrial yeasts Saccharomyces cerevisiae, S. uvarum, Candida utilis and Kluyveromyces marxianus exposed to AFB1, OTA and ZEA. Food Technol. Biotechnol. 2018, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Ayilara, M.S.; Akinola, S.A.; Babalola, O.O. Biocontrol mechanisms of endophytic fungi. Egypt. J. Biol. Pest Control. 2022, 32, 46. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H. Microbial biofungicides as a substitute for chemical fungicides in the control of phytopathogens: Current perspectives and research directions. Scientifica 2024, 2024, 5322696. [Google Scholar] [CrossRef]
- Goettel, M.S.; Hajek, A.E.; Siegel, J.P.; Evans, H.C. Safety of fungal biocontrol agents. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Butt, T.M., Jackson, C., Magan, N., Eds.; CABI International: Wallingford, UK, 2001; pp. 347–375. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Cabrefiga, J.; Frances, J.; Montesinos, E. Prospects and limitations of microbial pesticides for control of bacterial and fungal pomefruit tree diseases. Trees 2012, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Sal, E.; Stemler, J.; Salmanton-García, J.; Falces-Romero, I.; Kredics, L.; Meyer, E.; Würstl, B.; Lass-Flörl, C.; Racil, Z.; Klimko, N.; et al. Invasive Trichoderma spp. infections: Clinical presentation and outcome of cases from the literature and the FungiScope® registry. J. Antimicrob. Chemother. 2022, 77, 2850–2858. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N.K. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Int. Food Res. 2012, 49, 201–208. [Google Scholar] [CrossRef]
- Taheri, P.; Soweizy, M.; Tarighi, S. Application of essential oils to control some important fungi and bacteria pathogenic on cereals. J. Nat. Pest. Res. 2023, 6, 100052. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Urbaniak, M.; Stępień, Ł.; Gramza-Michałowska, A.; Waśkiewicz, A. Efficacy of Lamium album as a natural fungicide: Impact on seed germination, ergosterol, and mycotoxins in Fusarium culmorum-infected wheat seedlings. Front. Microbiol. 2024, 15, 1363204. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Nouasri, A.; Herrera, M.; Djenane, D.; Ariño, A. Antifungal activity of menthol, eugenol and their combination against Aspergillus ochraceus and Aspergillus niger in vitro and in stored cereals. Foods 2023, 12, 2108. [Google Scholar] [CrossRef]
- Almeida, N.A.; Freire, L.; Carnielli-Queiroz, L.; Bragotto, A.P.A.; Silva, N.C.C.; Rocha, L.O. Essential oils: An eco-friendly alternative for controlling toxigenic fungi in cereal grains. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13251. [Google Scholar] [CrossRef] [PubMed]
- Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal effect of plant extracts on the growth of the cereal pathogen Fusarium spp.—An in vitro study. Agronomy 2022, 12, 3204. [Google Scholar] [CrossRef]
- Gwad, M.M.A.; El-Sayed, A.S.A.; Abdel-Fattah, G.M.; Abdelmoteleb, M.; Abdel-Fattah, G.G. Potential fungicidal and antiaflatoxigenic effects of cinnamon essential oils on Aspergillus flavus inhabiting the stored wheat grains. BMC Plant Biol. 2024, 24, 394. [Google Scholar] [CrossRef]
- Anžlovar, S.; Likar, M.; Koce, J.D. Antifungal potential of thyme essential oil as a preservative for storage of wheat seeds. Acta Bot. Croat. 2017, 761, 64–71. [Google Scholar] [CrossRef]
- Belasli, A.; Miri, Y.B.; Aboudaou, M.; Ouahioune, L.A.; Montañes, L.; Ariño, A.; Djenane, D. Antifungal, antitoxigenic, and antioxidant activities of the essential oil from laurel (Laurus nobilis L.): Potential use as wheat preservative. Food Sci. Nutr. 2020, 8, 4717–4729. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.T.; Martinazzo, A.P.; Garcia, S.A.; Berbet, P.A.; Teodoro, C.E.S. Control of Aspergillus flavus in wheat grains using Cymbopogon flexuosus essential oil. Acta Bras. 2021, 5, 92–96. [Google Scholar] [CrossRef]
- Roselló, J.; Sempere, F.; Berzola-Sanz, I.; Chiralt, A.; Santamarina, M.P. Antifungal activity and potential use of essential oils against Fusarium culmorum and Fusarium verticillioides. J. Essent. Oil-Bear. Plants 2015, 18, 359–367. [Google Scholar] [CrossRef]
- Bocate, K.P.; Evangelista, A.G.; Luciano, F.B. Garlic essential oil as an antifungal and anti-mycotoxin agent in stored corn. LWT—Food Sci. Technol. 2021, 147, 111600. [Google Scholar] [CrossRef]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A.; Lopez-Ruiz, F.; Jayasena, K.; Oliver, R.P. Origin of Fungicide-Resistant Barley Powdery Mildew in Western Australia: Lessons to Be Learned. In Fungicide Resistance in Plant Pathogens; Ishii, H., Hollomon, D., Eds.; Springer: Tokyo, Japan, 2015; pp. 329–340. [Google Scholar] [CrossRef]
- Kang, Z.; Li, X.; Wan, A.; Wang, M.; Chen, X. Differential sensitivity among Puccinia striiformis f. sp. tritici isolates to propiconazole and pyraclostrobin fungicides. . Can. J. Plant Pathol. 2019, 41, 415–434. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.; Qi, A.; Fitt, B.D.L. Control of crop diseases through integrated crop management to deliver climate-smart farming systems for low- and high-input crop production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Leitzke, R.; Thomas, J.E.; O’Driscoll, A.; Klocke, B.; Maumene, C.; Lindell, I.; Wahlquist, K.; Zemeca, L.; et al. Management of rust in wheat using IPM principles and alternative products. Agriculture 2024, 14, 821. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.J.; Giotis, C.; Leifert, C. Potato late blight management in organic agriculture. Pest Manag. 2004, 15, 176–180. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Xue, M.; Liu, Z.; Zhang, Q.; Hou, J.; Xing, M.; Wang, R.; Liu, T. The combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control Fusarium wilt in cowpea. J. Fungi 2021, 7, 685. [Google Scholar] [CrossRef]
- Shashikumar, H.M.; Koulagi, S.; Navyashree, S.E. Compatibility of Trichoderma viride and Trichoderma harzianum with Fungicides against Soil Borne Diseases of Tomato and Cabbage. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1920–1928. [Google Scholar] [CrossRef]
- Saha, S.; Bhosale, S.; Chavan, V.; Thosar, R.; Ranade, Y.; Holkar, S.; Rai, R.; Kulkarni, M.; Kulkarni, Y.; Tiwari, A. Study of endophytes as biocontrol agents vis-a-vis their compatibility to fungicides in grapes. Int. J. Bio-Resour. Stress Manag. 2023, 14, 1539–1549. [Google Scholar] [CrossRef]
- Tomer, A.; Sigh, R.; Prasad, D. Compatibility of Trichoderma harzianum with systemic and two non-systemic fungicides in vitro. Asian J. Crop Sci. 2018, 10, 174–179. [Google Scholar] [CrossRef]
- Madhavi, G.B.; Bhattiprolu, S.L.; Reddy, V.B. Compatibility of biocontrol agent Trichoderma viride with various pesticides. J. Hortic. Sci. 2011, 6, 71–73. [Google Scholar] [CrossRef]
- Escudero-Leyva, E.; Alfaro-Vargas, P.; Muñoz-Arrieta, R.; Charpentier-Alfaro, C.; Granados-Montero, M.M.; Valverde-Madrigal, K.S.; Pérez-Villanueva, M.; Méndez-Rivera, M.; Rodríguez-Rodríguez, C.E.; Chaverri, P.; et al. Tolerance and biological removal of fungicides by Trichoderma species isolated from the endosphere of wild Rubiaceae plants. Front. Agron. 2022, 3, 772170. [Google Scholar] [CrossRef]
- Sunkad, G.; Meghana, P. Compatibility of indigenous Trichoderma asperellum with chemical fungicides for the management of chickpea wilt. J. Biol. Control 2023, 37, 6. [Google Scholar] [CrossRef]
- Lima, G.; Castoria, R.; De Curtis, F.; Raiola, A.; Ritieni, A.; De Cicco, V. Integrated control of blue mould using new fungicides and biocontrol yeasts lowers levels of fungicide residues and patulin contamination in apples. Postharvest Biol. Technol. 2011, 60, 164–172. [Google Scholar] [CrossRef]
- Errampalli, D.; Brubacher, N.R. Biological and integrated control of postharvest blue mold (Penicillium expansum) of apples by Pseudomonas syringae and cyprodinil. Biol. Control. 2006, 36, 49–56. [Google Scholar] [CrossRef]
- Musheer, N.; Jamil, A.; Choudhary, A. Solo and combined applications of fungicides and bio-agents to reduce severity of Fusarium oxysporum and induce antioxidant metabolites in Ocimum tenuiflorum L. J. Plant Pathol. 2023, 105, 237–251. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Torres, A.M.; Chulze, S.N. Tolerance of triazole-based fungicides by biocontrol agents used to control Fusarium head blight in wheat in Argentina. Lett. Appl. Microbiol. 2018, 66, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Curtis, F.D.; De Cicco, V.; Lima, G. Efficacy of biocontrol yeasts combined with calcium silicate or sulphur for controlling durum wheat powdery mildew and increasing grain yield components. Field Crops Res. 2012, 134, 36–46. [Google Scholar] [CrossRef]
- Fielding, B.C.; Knowles, C.; Vries, F.A.; Klaasen, J.A. Testing of eight medicinal plant extracts in combination with kresoxim-methyl for integrated control of Botrytis cinerea in apples. Agriculture 2015, 5, 400–411. [Google Scholar] [CrossRef]
- Adandonon, A.; Aveling, T.A.S.; Labuschagne, N.; Tamo, M. Biocontrol agents in combination with Moringa oleifera extract for integrated control of Sclerotium-caused cowpea damping-off and stem rot. Eur. J. Plant Pathol. 2006, 115, 409–418. [Google Scholar] [CrossRef]
- El-kazzaz, M.K.; Salem, E.A.; Ghoneim, K.E.; Elsharkawy, M.M.; El-kot, G.A.E.N. Integrated control of rice kernel smut disease using plant extracts and salicylic acid. Arch. Phytopathol. Plant Prot. 2015, 48, 664–675. [Google Scholar] [CrossRef]
- Panday, D.; Bhusal, N.; Das, S.; Ghalehgolabbehbahani, A. Rooted in nature: The rise, challenges, and potential of organic farming and fertilizers in agroecosystems. Sustainability 2024, 16, 1530. [Google Scholar] [CrossRef]
- Mihelič, R.; Pintarič, S.; Eler, K.; Suhadolc, M. Effects of transitioning from conventional to organic farming on soil organic carbon and microbial community: A comparison of long-term non-inversion minimum tillage and conventional tillage. Biol. Fertil. Soils 2024, 60, 341–355. [Google Scholar] [CrossRef]
- Burandt, Q.C.; Deising, H.B.; von Tiedemann, A. Further limitations of synthetic fungicide use and expansion of Organic Agriculture in Europe Will Increase the Environmental and Health Risks of Chemical Crop Protection Caused by copper-containing fungicides. Environ. Toxicol Chem. 2024, 43, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Soussi, A.; Zero, E.; Sacile, R.; Trinchero, D.; Fossa, M. Smart sensors and smart data for precision agriculture: A Review. Sensors 2024, 24, 2647. [Google Scholar] [CrossRef]
- Salas, B.; Salcedo, R.; Garcia-Ruiz, F.; Gil, E. Design, implementation and validation of a sensor-based precise airblast sprayer to improve pesticide applications in orchards. Precis. Agric. 2024, 25, 865–888. [Google Scholar] [CrossRef]
- Santos, J.L.; Pereira, P.S.; Reis, K.H.B.; Freitas, D.R.; Picanço Filho, M.C.; Peluzio, J.M.; Sarmento, R.A.; Guedes, R.N.C.; Picanço, M.C. Decision-making for thrips control in soybean fields using precision agriculture principles. J. Appl. Entomol. 2024, 148, 140–149. [Google Scholar] [CrossRef]
- Mehedi, I.M.; Hanif, M.S.; Bilal, M.; Vellingiri, M.T.; Palaniswamy, T. Remote sensing and decision support system applications in precision agriculture: Challenges and possibilities. IEEE Access 2024, 12, 44786–44798. [Google Scholar] [CrossRef]
| Region | Annual Production of Cereals * [million tonnes] | Crop Area * [million ha] | Estimated Pesticide Usage [kg/ha] | Estimated Fungicide Usage [kg/ha] |
|---|---|---|---|---|
| World | 2166 | 482.5 | 117.9 | 48.4 |
| EU (27) | 254.3 | 47.5 | 38.8 (EU) | 19.4 (EU) |
| US | 398.6 | 47.9 | 4.3 | 0.3 |
| Brazil | 121.2 | 24.9 | 12.7 | 2.8 |
| China | 418.2 | 67.5 | 1.4 | 0.4 |
| Fungicide | Group Name (FRAC Group) | Examples of Application Area | Permitted Usage in Agriculture EU/USA/CHINA/BRAZIL | References |
|---|---|---|---|---|
| Morpholines | ||||
| Tridemorph | Amines (5) | Foliar spraying | −/+/+/− | [67] |
| Pyrimidines | ||||
| Cyprodinil | AP-fungicides (9) | Foliar spraying, soil fumigant | +/+/+/+ | [68,69] |
| Carboxylic Acid Esters | ||||
| Ethyl formate | Carboxylic Acid Esters (n.a.) | Grain fumigant | −/?/?/? | [70] |
| Phthalonitriles | ||||
| Chlorothalonil | Chloronitriles (M 05) | Foliar spraying | −/+/+/+ | [71] |
| Dicarboximides | ||||
| Iprodione | Dicarboximides (2) | Seed treatment, foliar spraying | −/+/+/+ | [72,73] |
| Dithiocarbamates | ||||
| Mancozeb | Dithiocarbamates (M 03) | Seed treatment | −/+/+/+ | [74,75] |
| Metam-sodium | Soil fumigation | +/+/+/+ | [76] | |
| Thiram | Foliar spraying | −/+/+/+ | [77] | |
| Imidazoles, Triazoles | ||||
| Bitertanol | DMIs (3) | Foliar spraying, seed treatment | −/+/+/− | [78] |
| Cyproconazole | Foliar spraying | −/+/−/+ | [79] | |
| Difenoconazole | Seed treatment | +/+/+/+ | [80,81] | |
| Imazalil | Seed treatment | +/+/+/+ | [82] | |
| Myclobutanil | Seed treatment, foliar spraying | −/+/+/+ | [83] | |
| Prochloraz | Seed treatment, foliar spraying | −/+/+/− | [84] | |
| Propiconazole | Seed treatment, foliar spraying | −/+/+/+ | [85] | |
| Tebuconazole | Foliar spraying | +/+/+/+ | [86,87] | |
| Triadimefon | Seed treatment | −/+/+/+ | [88] | |
| Triadimenol | Foliar spraying | −/+/+/+ | [89] | |
| Triticonazole | Seed treatment | +/+/+/+ | [90,91] | |
| Epoxides | ||||
| Ethylene oxide | Cyclic ethers (n.a.) | Grain fumigant | −/−/−/− | [92] |
| 1,2,4-thiadiazoles | ||||
| Etridiazole | Heteroaromatics (32) | Seed treatment | −/+/−/+ | [93] |
| Inorganic compounds | ||||
| Aluminum phosphide | Aluminum, Copper salts etc. (NC) | Grain fumigant | +/+/−/? | [94,95,96] |
| Copper oxychloride | Foliar spraying, seed treatment | +/+/+/+ | [97] | |
| Copper sulfate | Seed treatment | +/+/−/+ | [98] | |
| Benzimidazoles | ||||
| Benomyl | MBCs (1) | Seed treatment, foliar spraying | −/−/+/− | [99,100] |
| Carbendazim | Foliar spraying, Seed treatment | −/+/+/+ | [101] | |
| Thiabendazole | Seed treatment | +/+/+/+ | [102] | |
| Thiophanates | ||||
| Thiophanate-methyl | MBCs (1) | Seed treatment | −/+/+/+ | [103] |
| Halogenated aliphatics | ||||
| Ethylene dibromide | Organobromine compounds (n.a.) | Grain fumigant | −/−/−/− | [104] |
| Methyl bromide | Soil fumigation | −/−/−/− | [105] | |
| Chloropicrin | Organochlorine compounds (n.a.) | Soil fumigant, grain fumigant | −/+/−/? | [106,107] |
| 1,3-dichloropropene | Soil fumigant | −/+/+/+ | [108,109] | |
| Ethylene dichloride | Grain fumigant | −/−/−/− | [110] | |
| Acylalanines | ||||
| Metalaxyl | Phenylamides (4) | Seed treatment | +/+/−/+ | [111] |
| Phthalimides | ||||
| Captafol | Phthalimides (M 04) | Seed treatment | −/−/−/− | [112,113] |
| Captan | Soil fumigant, seed treatment | +/+/+/+ | [114,115] | |
| Strobilurins | ||||
| Azoxystrobin | QoIs (11) | Foliar spraying | +/+/+/+ | [99,116,117] |
| Trifloxystrobin | Foliar spraying | +/+/+/+ | [117] | |
| Kresoxim methyl | Foliar spraying | +/+/+/+ | [118] | |
| Pyraclostrobin | Foliar spraying | −/+/+/+ | [119] | |
| Oxazoles | ||||
| Famoxadone | QoIs (11) | Foliar spraying | +/+/+/+ | [120] |
| Oxathiins | ||||
| Carboxin | SDHIs (7) | Seed treatment | −/+/+/+ | [121] |
| Oxycarboxin | Seed treatment, foliar spraying | −/+/+/+ | [122] | |
| Microorganisms | Pathogens | Active Compounds | References |
|---|---|---|---|
| Bacteria | |||
| Bacillus subtilis | Fusarium graminearum | surfactin, iturin, fengycin lipopeptides | [151,152,154] |
| Bacillus megaterium | Fusarium graminearum | not studied | [154] |
| Bacillus vallismortis | Fusarium graminearum Alternaria alternata Rhizoctonia solani Cryphonectria parasitica Phytophthora capsici | bacillomycin D | [155] |
| Bacillus amyloliquefaciens | F. graminearum Fusarium spp. | iturin lipopeptide | [157,165] |
| Paenibacillus polymyxa | F. graminearum | not studied | [166] |
| Lacticaseibacillus rhamnosus | F. graminearum | not studied | [158] |
| Lactiplantibacillus plantarum | F. graminearum Fusarium spp. | not studied | [157,158] |
| Pseudomonas fluorescens | F. graminearum | not studied | [167] |
| Pseudomonas chlororaphis | Drechslera graminea D. teres D. avenae Ustilago avenae U. hordei Tilletia caries | [168] | |
| Streptomyces spp. | F. graminearum Rhizoctonia solani | secondary metabolites chitinases volatile organic compounds | [150,167,168,169] |
| Azotobacter nigricans | F. sporotrichioides F. graminearum F. poae F. crookwellense F. equiseti F. sambucinum F. culmorum | not studied | [170] |
| Yeasts | |||
| Sporidiobolus pararoseus | Fusarium fujikuroi | not studied | [161] |
| Pichia guilliermondii | Fusarium fujikuroi Penicillium roqueforti | not studied | [161,162] |
| Metschnikowia pulcherrima | Fusarium fujikuroi | not studied | [161] |
| Cryptococcus flavescens | F. graminearum | not studied | [171] |
| Pichia anomala | Penicillium roqueforti | not studied | [162] |
| Pichia burtonii | |||
| Pichia farinosa | |||
| Pichia membranifaciens | |||
| Candida silvicola | |||
| Candida fennica | |||
| Candida pelliculosa | |||
| Candida silvicultrix | |||
| Molds | |||
| T. asperellum | F. graminearum Pseudomonas syringae | not studied induced resistance | [172] [173] |
| T. citrinoviride | F. graminearum | not studied | [158] |
| T. harzianum | F. verticillioides F. graminearum F. oxysporum B. cinerea | secretion of chitinase, competition for space | [171,172,173,174] |
| T. brevicrasum | Rizoctonia solani | mycoparasitism | [173] |
| Clonostachys rosea | F. culmorum F. graminearum F. verticillioides F. crookwellense Alternaria dauci A. radicina Botrytis cinerea B. aclada Bipolaris sorokiniana Drechslera teres Helminthosporium solani Moniliophthora roreri, Phytophthora palmivora Rhizoctonia solani Rhynchosporium communea Sclerotinia sclerotiorum | secretion cell-wall-degrading enzymes | [163,164] |
| Antifungals | Advantages | Disadvantages |
|---|---|---|
| Natural |
|
|
| Synthetic |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczygieł, T.; Koziróg, A.; Otlewska, A. Synthetic and Natural Antifungal Substances in Cereal Grain Protection: A Review of Bright and Dark Sides. Molecules 2024, 29, 3780. https://doi.org/10.3390/molecules29163780
Szczygieł T, Koziróg A, Otlewska A. Synthetic and Natural Antifungal Substances in Cereal Grain Protection: A Review of Bright and Dark Sides. Molecules. 2024; 29(16):3780. https://doi.org/10.3390/molecules29163780
Chicago/Turabian StyleSzczygieł, Tomasz, Anna Koziróg, and Anna Otlewska. 2024. "Synthetic and Natural Antifungal Substances in Cereal Grain Protection: A Review of Bright and Dark Sides" Molecules 29, no. 16: 3780. https://doi.org/10.3390/molecules29163780
APA StyleSzczygieł, T., Koziróg, A., & Otlewska, A. (2024). Synthetic and Natural Antifungal Substances in Cereal Grain Protection: A Review of Bright and Dark Sides. Molecules, 29(16), 3780. https://doi.org/10.3390/molecules29163780







