Shear Thickening, Star-Shaped Polymer Electrolytes for Lithium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
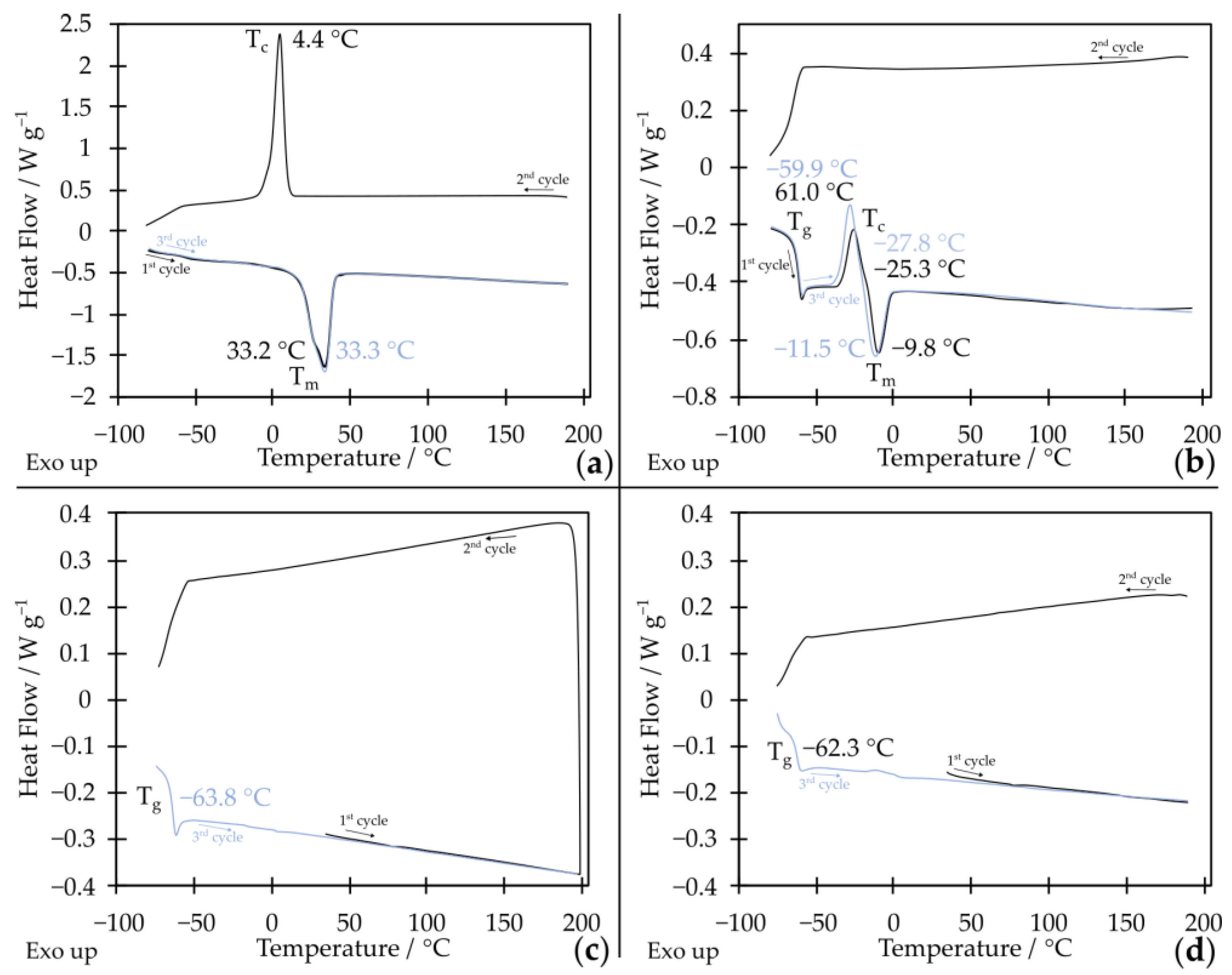
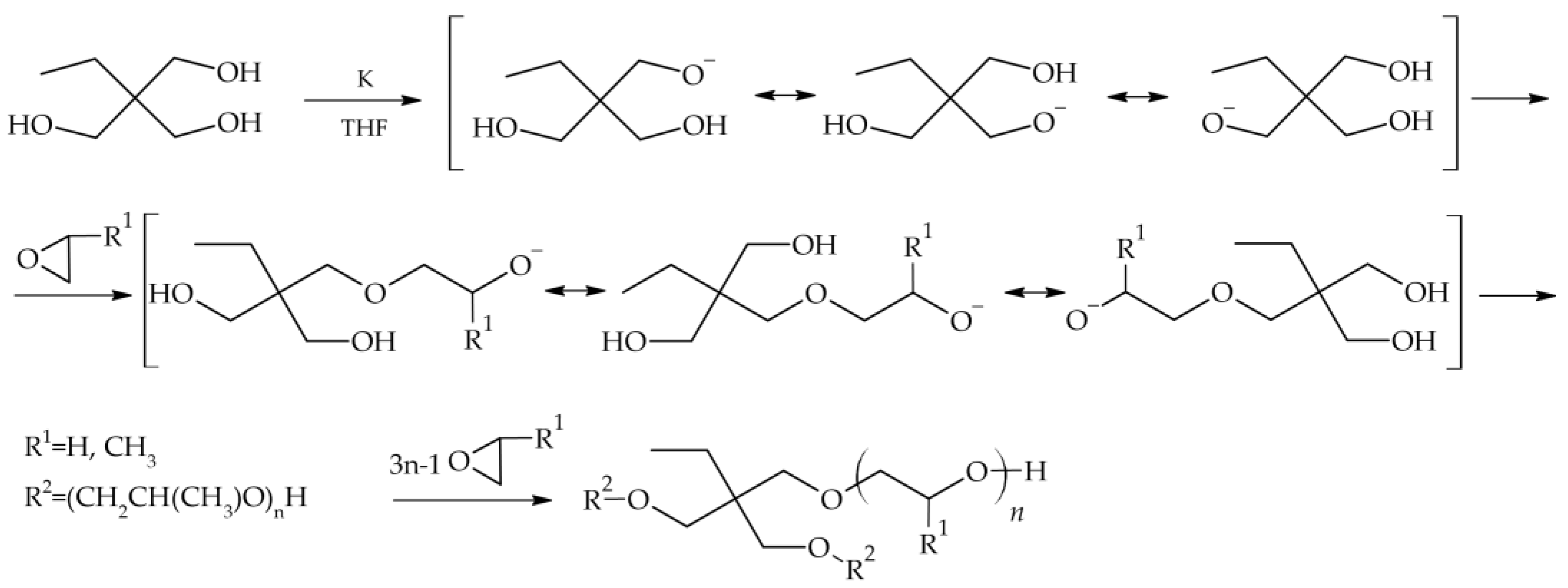
2.1. Star-Shaped Oxyethylene and Oxypropylene Glycols
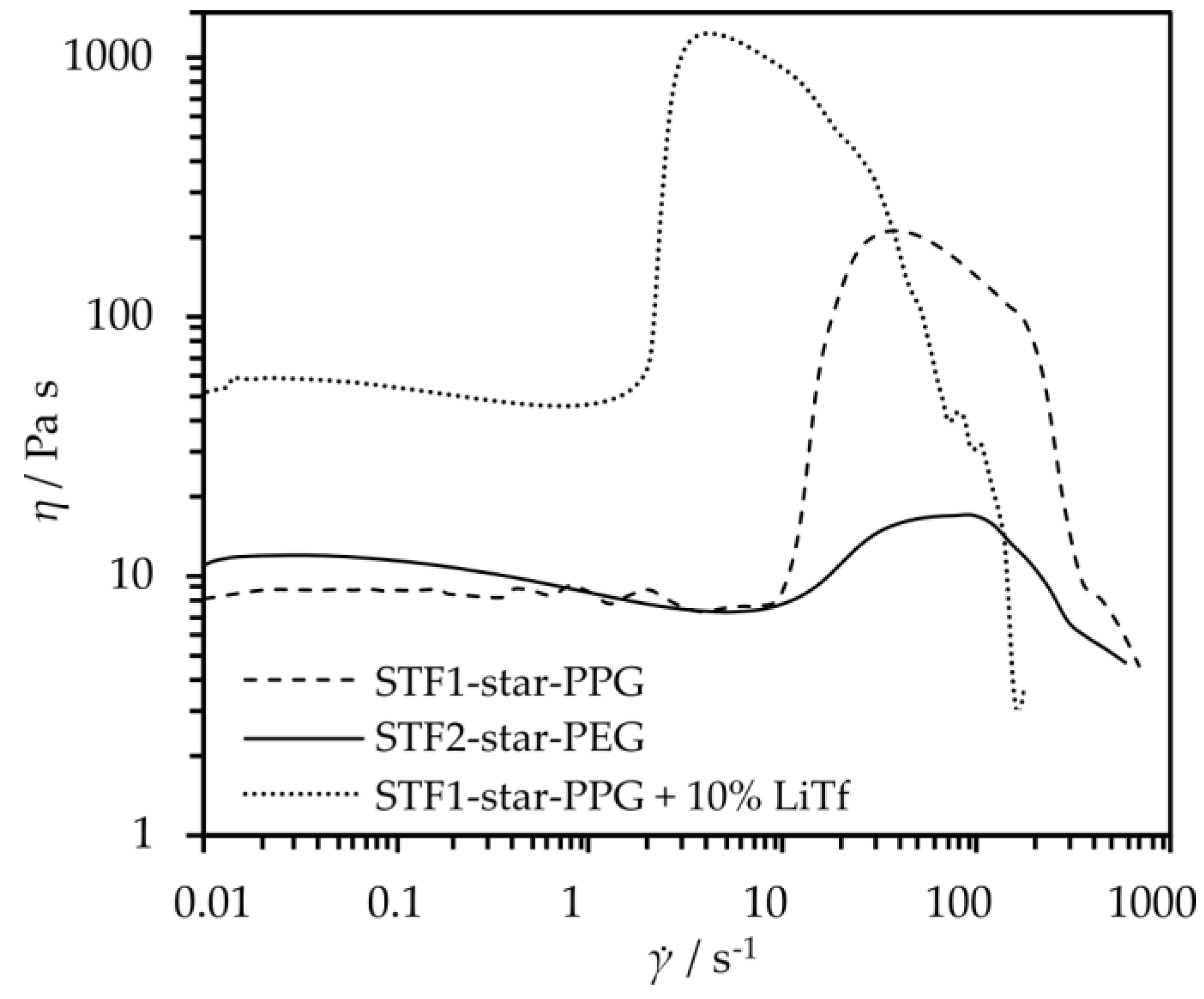
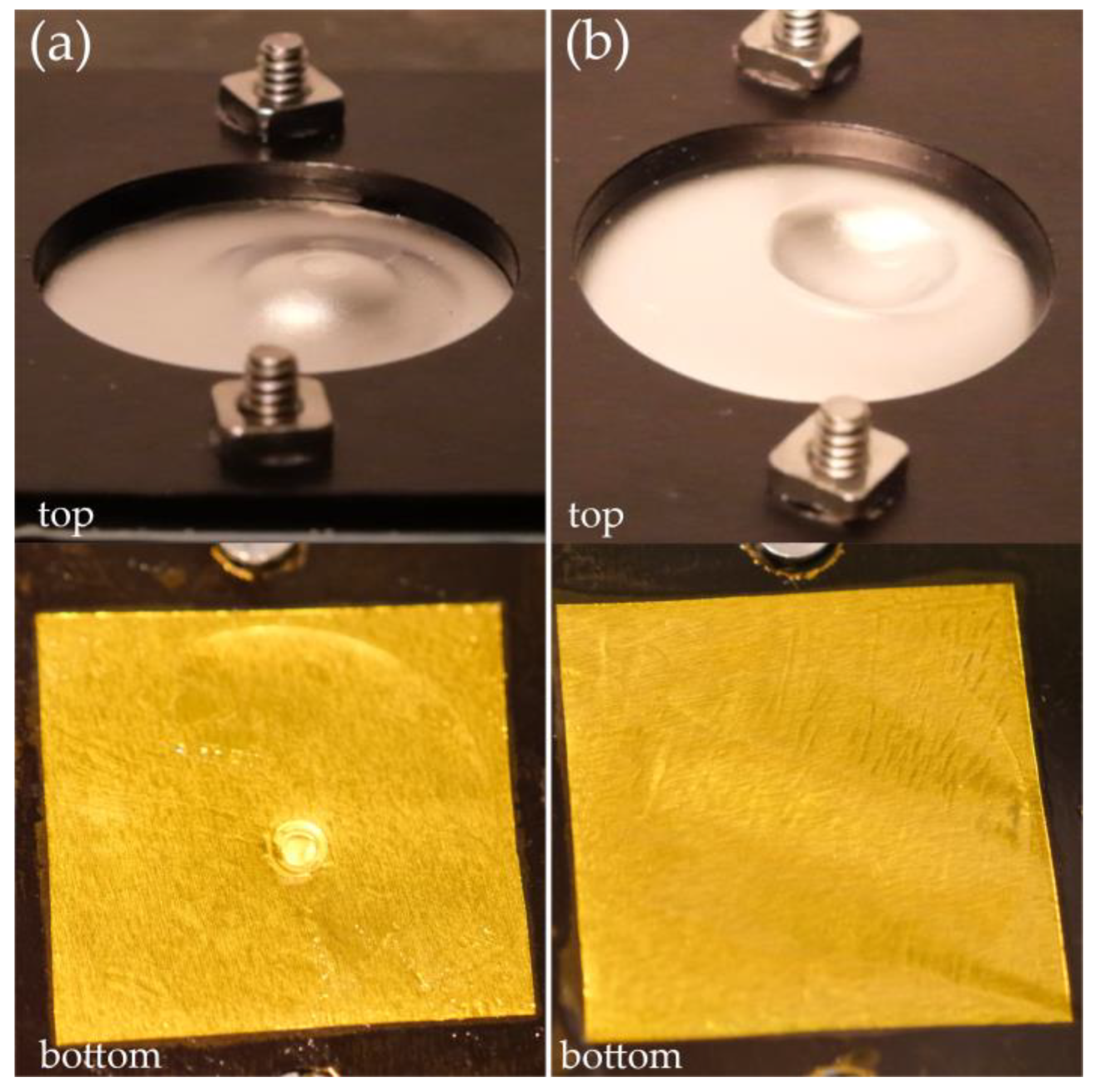
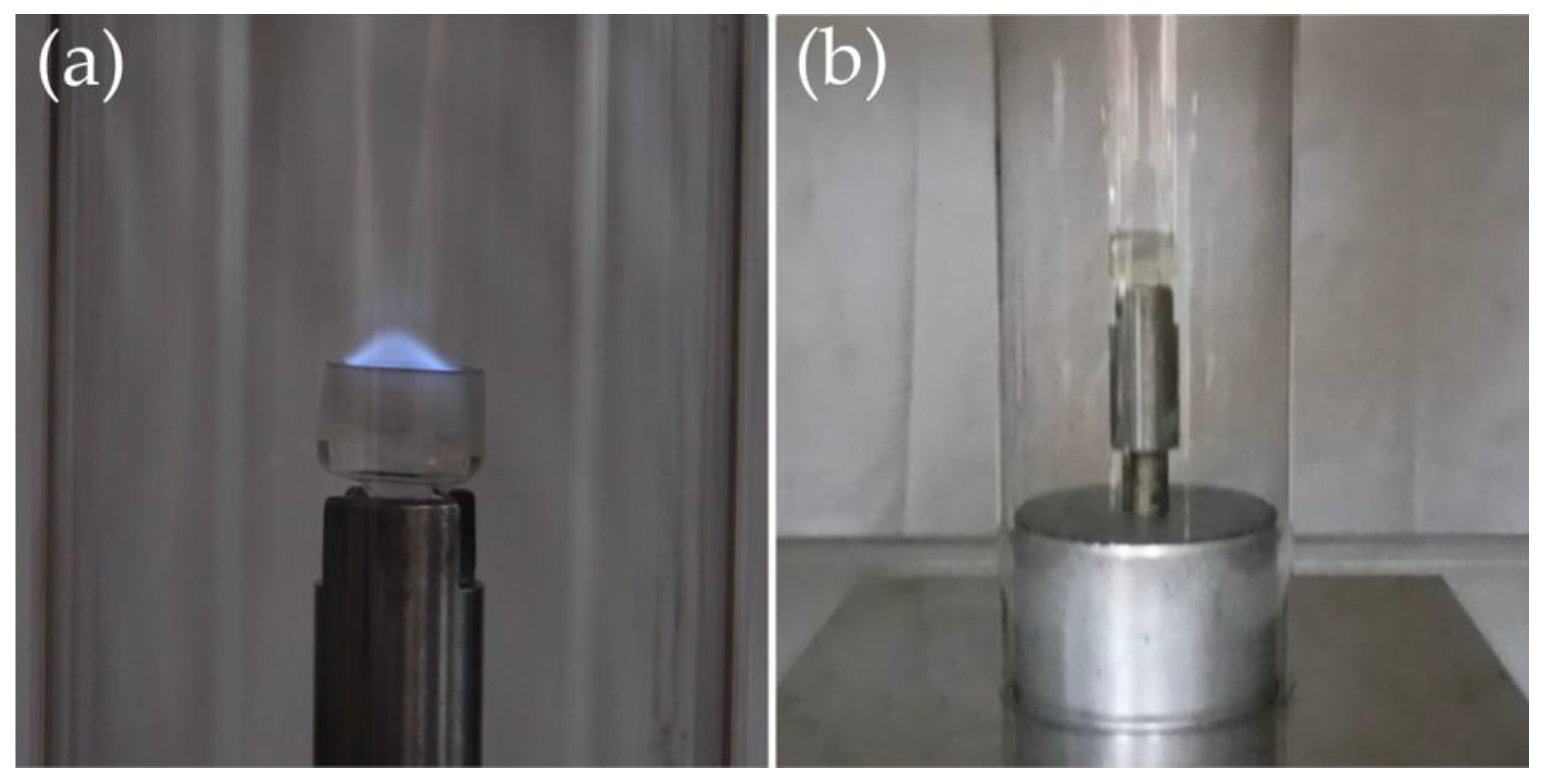
2.2. Shear Thickening Fluids
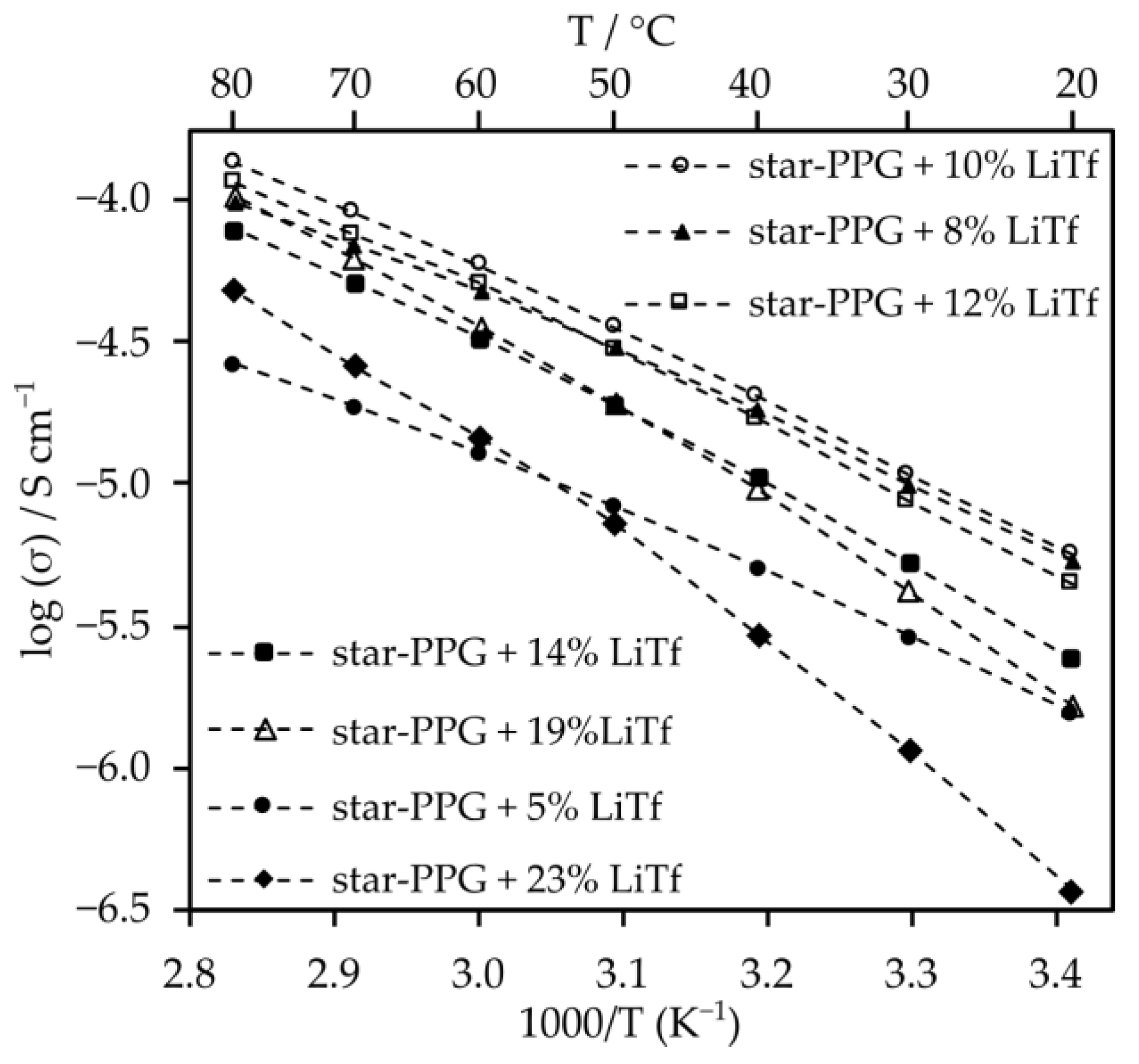
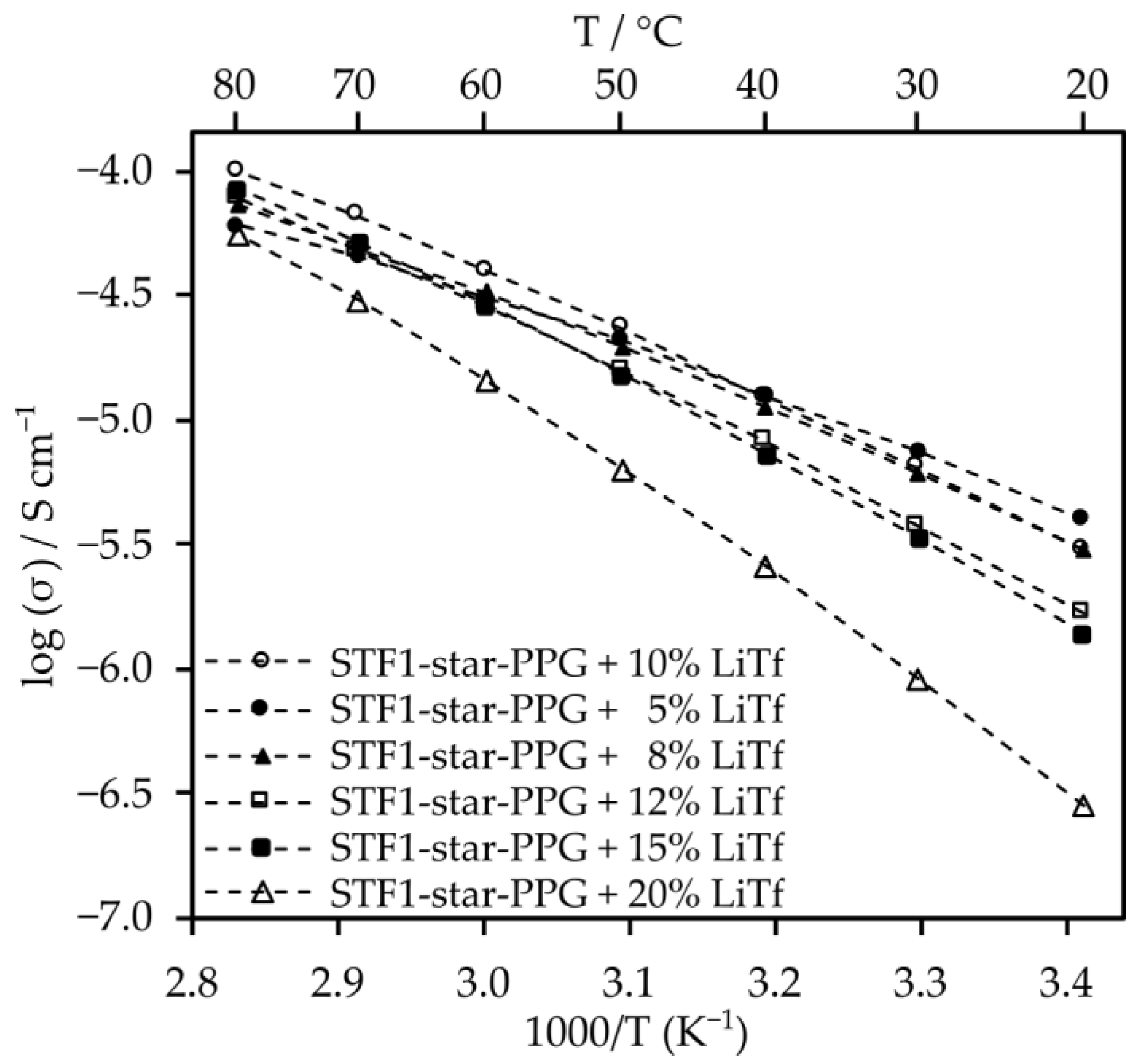
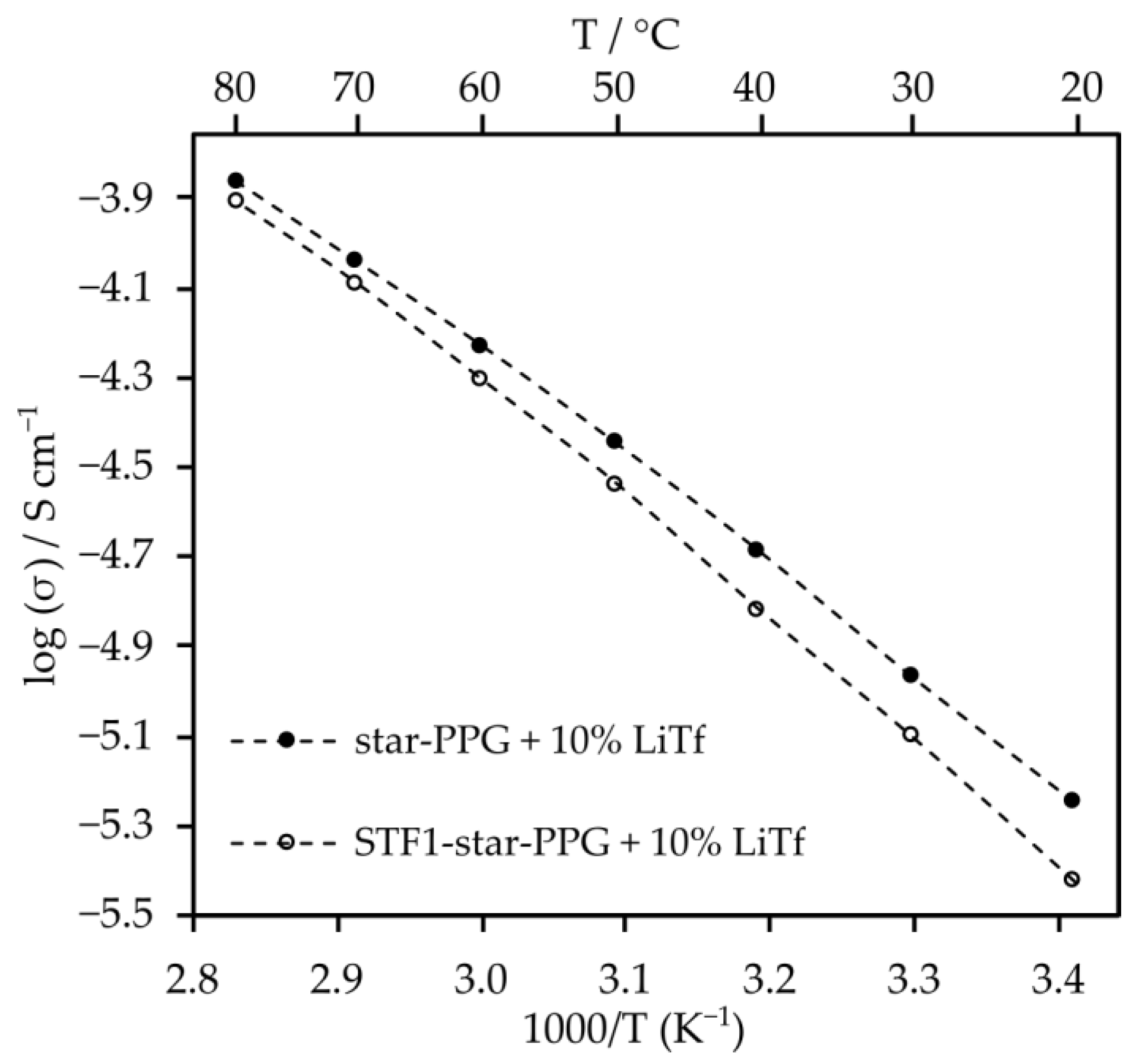
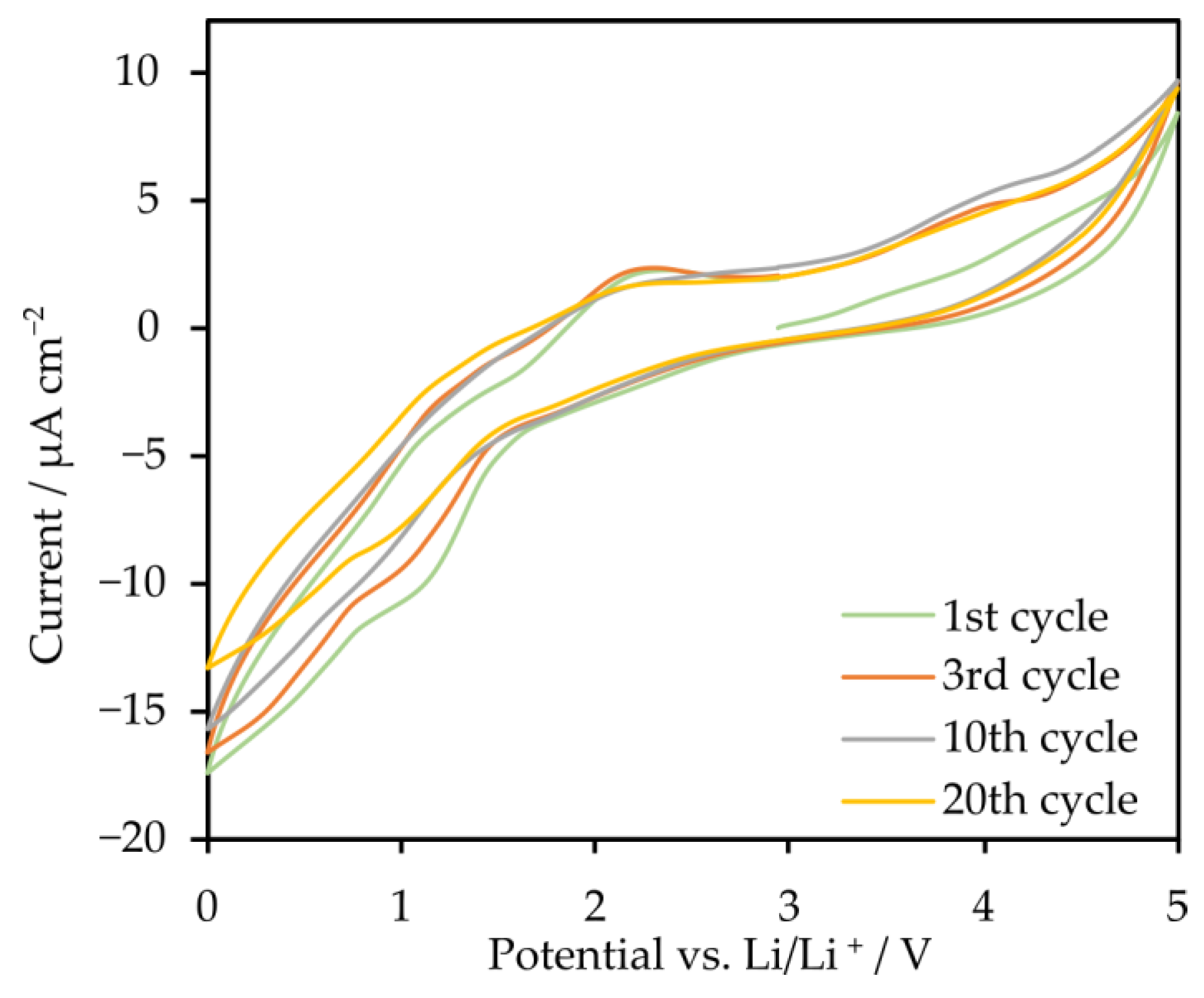
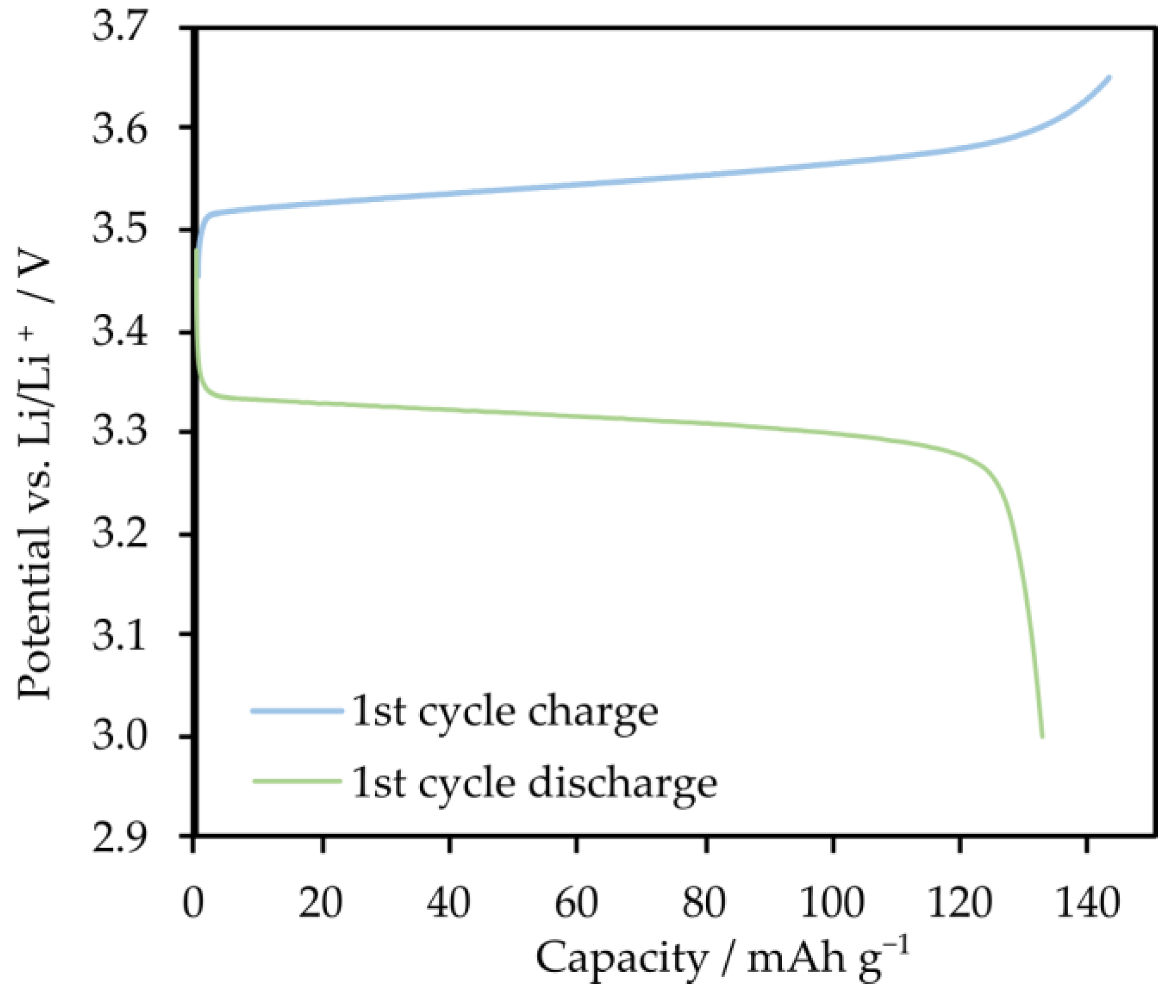
2.3. Electrolytes Based on Star-Shaped Glycols
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Synthesis of Star-Shaped Oxyethylene and Oxypropylene Glycols
3.3. Preparation of Electrolytes
3.4. Experimental Techniques
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Z.; Zhang, X.; Wang, W.; Chen, Y.; Shan, T. Collision-Caused thermal runaway investigation of li-ion battery in Real-World electric vehicles. Appl. Therm. Eng. 2024, 236, 121901. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium-ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Tran, M.K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A review of lithium-ion battery thermal runaway modeling and diagnosis approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green. Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer electrolytes for lithium-ion batteries: State of the art and perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Warner, J.T. Lithium-Ion Battery Chemistries: A Primer, 1st ed; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Hyperbranched phosphorus flame retardants: Multifunctional additives for epoxy resins. Polym. Chem. 2019, 10, 4346–4358. [Google Scholar] [CrossRef]
- An, K.; Tran, Y.H.T.; Kwak, S.; Han, J.; Song, S.W. Design of fire-resistant liquid electrolyte formulation for safe and long-cycled lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2106102. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef]
- Cavers, H.; Molaiyan, P.; Abdollahifar, M.; Lassi, U.; Kwade, A. Perspectives on Improving the Safety and Sustainability of High Voltage Lithium-Ion Batteries Through the Electrolyte and Separator Region. Adv. Energy Mater. 2022, 12, 2200147. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. Solid Polymer Electrolytes for Lithium Batteries: A Tribute to Michel Armand. Inorganics 2022, 10, 110. [Google Scholar] [CrossRef]
- Barnes, H.A. Shear-Thickening (“Dilatancy”) in Suspensions of Nonaggregating Solid Particles Dispersed in Newtonian Liquids. J. Rheol. 1989, 33, 329–366. [Google Scholar] [CrossRef]
- Wagner, N.; Wetzel, E. Advanced Body Armor Utilizing Shear Thickening Fluids. US20060234577A1, 19 October 2006. [Google Scholar]
- Haq, S.; Morley, C.J. Fibrous Armour Material. US9816788B2, 14 November 2017. [Google Scholar]
- Williams, T.H.; Day, J.; Pickard, S. Surgical and Medical Garments and Materials Incorporating Shear Thickening Fluids. US20090255023A1, 15 October 2009. [Google Scholar]
- Seshimo, K. Viscoelastic Damper. US4759428A, 26 July 1988. [Google Scholar]
- Hesse, H. Rotary Shock Absorber. US4503952A, 12 March 1985. [Google Scholar]
- Rosenberg, B. Nonlinear Energy Absorption System. US3833952A, 10 September 1974. [Google Scholar]
- Zhang, X.Z.; Li, W.H.; Gong, X.L. The rheology of shear thickening fluid (STF) and the dynamic per-formance of an STF-filled damper. Smart Mater. Struct. 2008, 17, 035027. [Google Scholar] [CrossRef]
- Szafran, M.; Antosik, A.; Głuszek, M.; Falkowski, P.; Bobryk, E.; Żurowski, R.; Rokicki, G.; Tryznowski, M.; Kaczorowski, M.; Leonowicz, M.; et al. Football Shin Guard with Increased Degree of Energy Absorbing Capacity. PL231757B1, 11 September 2017. [Google Scholar]
- Ding, J.; Li, W.; Shen, S.Z. Research and applications of shear thickening fluids. Recent. Pat. Mater. Sci. 2011, 4, 43–49. [Google Scholar] [CrossRef]
- Shu, K.; Wang, C.; Li, W.; Bussell, T.; Ding, J. Electrolytes with reversible switch between liquid and solid phases. Curr. Opin. Electrochem. 2020, 21, 297–302. [Google Scholar] [CrossRef]
- Ding, J.; Tian, T.; Meng, Q.; Guo, Z.; Li, W.; Zhang, P.; Ciacchi, F.T.; Huang, J.; Yang, W. Smart Multifunctional Fluids for Lithium Ion Batteries: Enhanced Rate Performance and Intrinsic Mechanical Protection. Sci. Rep. 2013, 3, 2485. [Google Scholar] [CrossRef] [PubMed]
- Veith, G.M.; Armstrong, B.L.; Wang, H.; Kalnaus, S.; Tenhaeff, W.E.; Patterson, M.L. Shear Thickening Electrolytes for High Impact Resistant Batteries. ACS Energy Lett. 2017, 9, 2084–2088. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, X.; Liu, B.; Deng, H. Research Progress of Shear Thickening Electrolyte Based on Liquid–Solid Conversion Mechanism. Batteries 2023, 9, 384. [Google Scholar] [CrossRef]
- Shen, B.H.; Armstrong, B.L.; Doucet, M.; Heroux, L.; Browning, J.F.; Agamalian, M.; Tenhaeff, W.E.; Veith, G.M. Shear Thickening Electrolyte Built from Sterically Stabilized Colloidal Particles. ACS Appl. Mater. Interfaces 2018, 10, 9424–9434. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Baker, G.L.; Colson, S. Composite Polymer Electrolytes Using Fumed Silica Fillers: Rheology and Ionic Conductivity. Chem. Mater. 1994, 6, 2359–2363. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Dealy, J.M.; Read, D.J.; Larson, R.G. Structure and Rheology of Molten Polymers: From Structure to Flow Behavior and Back Again, 2nd ed.; Carl Hanser Verlag: Munich, Germany, 2018. [Google Scholar]
- Bender, J.; Wagner, N.J. Reversible shear thickening in monodisperse and bidisperse colloidal dispersion. J. Rheol. 1996, 40, 899–916. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Walls, H.J.; Khan, S.A. Rheology of Silica Dispersions in Organic Liquids: New Evidence for Solvation Forces Dictated by Hydrogen Bonding. Langmuir 2000, 16, 7920. [Google Scholar] [CrossRef]
- Zou, J.; Kou, H.; Chang, R.; Zhou, X.; Yang, J.; Tang, J.; Zhang, Y. Hydrogen-Bond-Driven High Ionic Conductivity, Li+ Transfer Number and Lithium Interface Stability of Poly (Vinylidene Fluoride-Hexafluoropropylene) Based Solid-State Electrolytes. J. Power Sources 2024, 614, 235028. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Product Properties (II): Environmental Behaviour and Failure, In Properties of Polymers, 4th ed.; Van Krevelen, D.W., Te Nijenhuis, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 847–873. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słojewska, M.; Czerwiński, A.; Kaczorowski, M.; Zygadło-Monikowska, E. Shear Thickening, Star-Shaped Polymer Electrolytes for Lithium-Ion Batteries. Molecules 2024, 29, 3782. https://doi.org/10.3390/molecules29163782
Słojewska M, Czerwiński A, Kaczorowski M, Zygadło-Monikowska E. Shear Thickening, Star-Shaped Polymer Electrolytes for Lithium-Ion Batteries. Molecules. 2024; 29(16):3782. https://doi.org/10.3390/molecules29163782
Chicago/Turabian StyleSłojewska, Magdalena, Arkadiusz Czerwiński, Marcin Kaczorowski, and Ewa Zygadło-Monikowska. 2024. "Shear Thickening, Star-Shaped Polymer Electrolytes for Lithium-Ion Batteries" Molecules 29, no. 16: 3782. https://doi.org/10.3390/molecules29163782
APA StyleSłojewska, M., Czerwiński, A., Kaczorowski, M., & Zygadło-Monikowska, E. (2024). Shear Thickening, Star-Shaped Polymer Electrolytes for Lithium-Ion Batteries. Molecules, 29(16), 3782. https://doi.org/10.3390/molecules29163782






