Zirconium Phosphate-Pillared Zeolite MCM-36 for Green Production of γ-Valerolactone from Levulinic Acid via Catalytic Transfer Hydrogenation
Abstract
1. Introduction
2. Results and Discussion
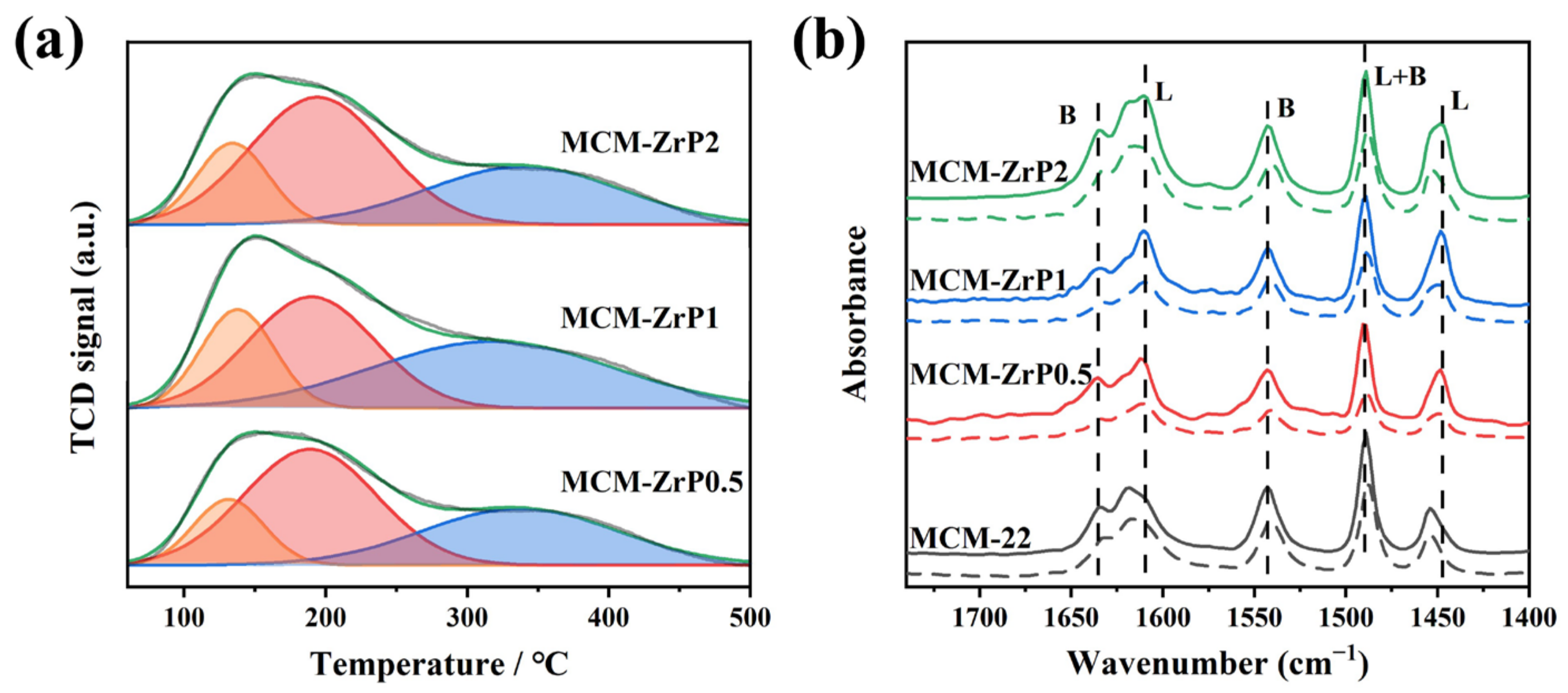
2.1. Characterization of Catalysts
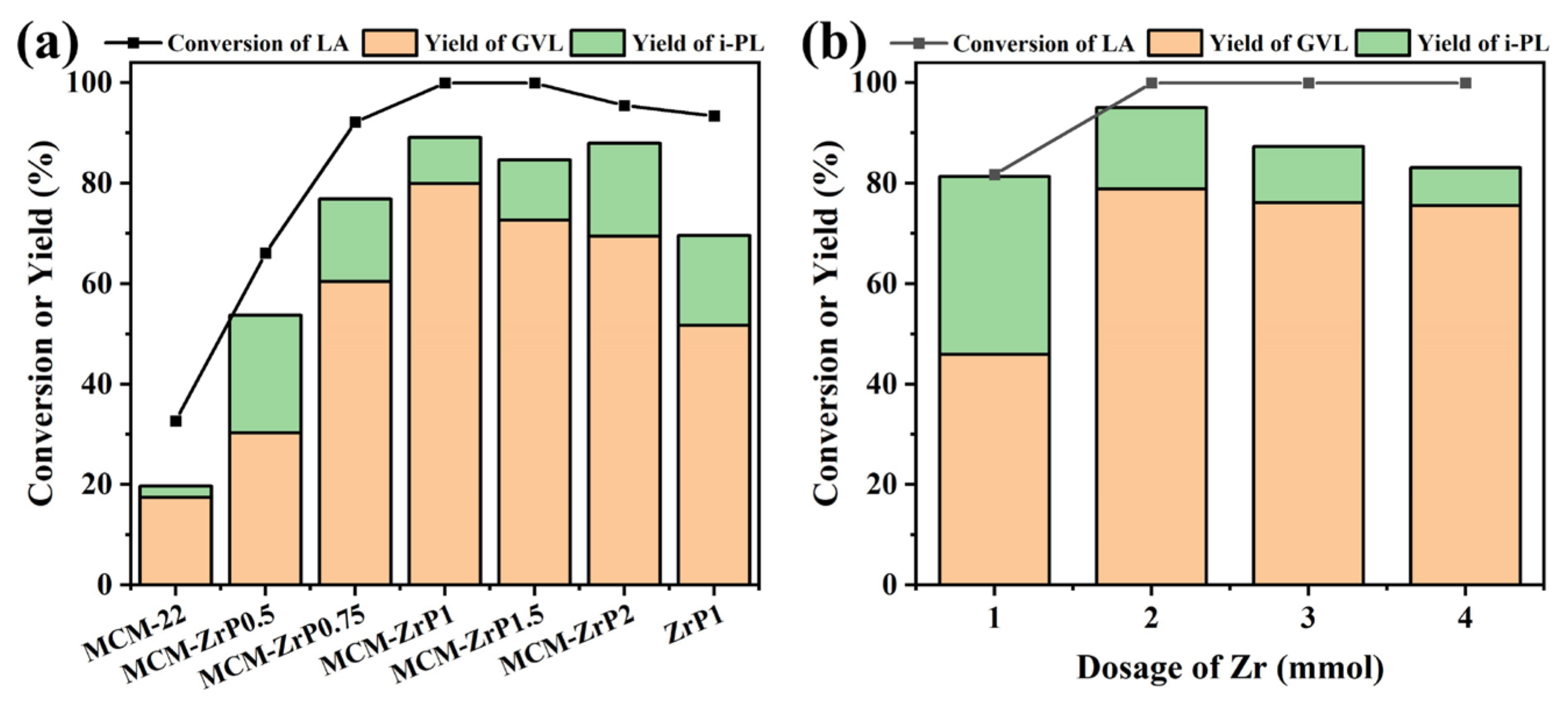
2.2. Screening of Catalysts
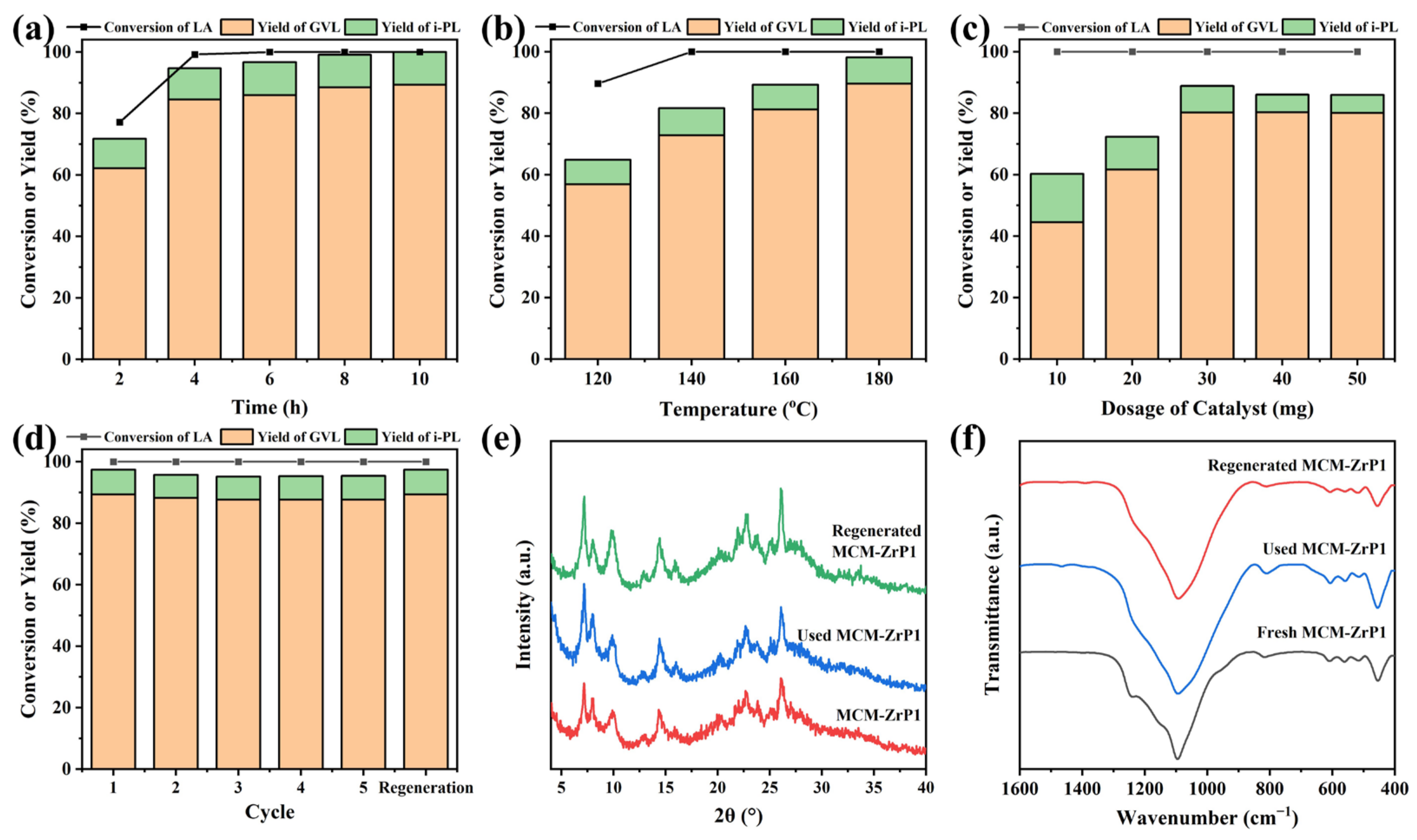
2.3. Catalytic Performance of Catalysts
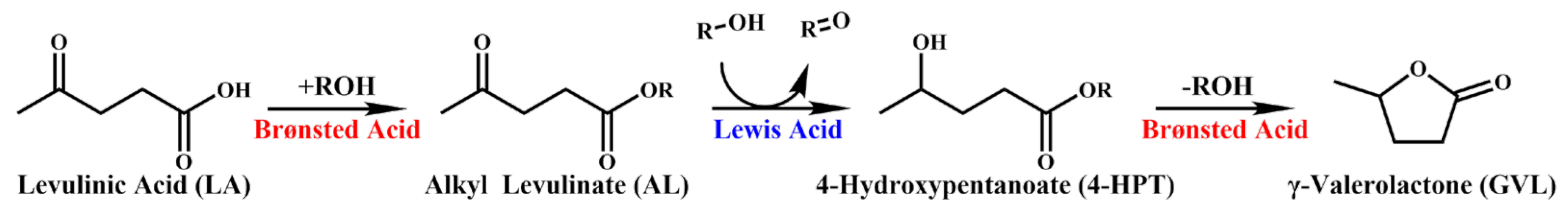
2.4. Discussion of Catalytic Mechanism
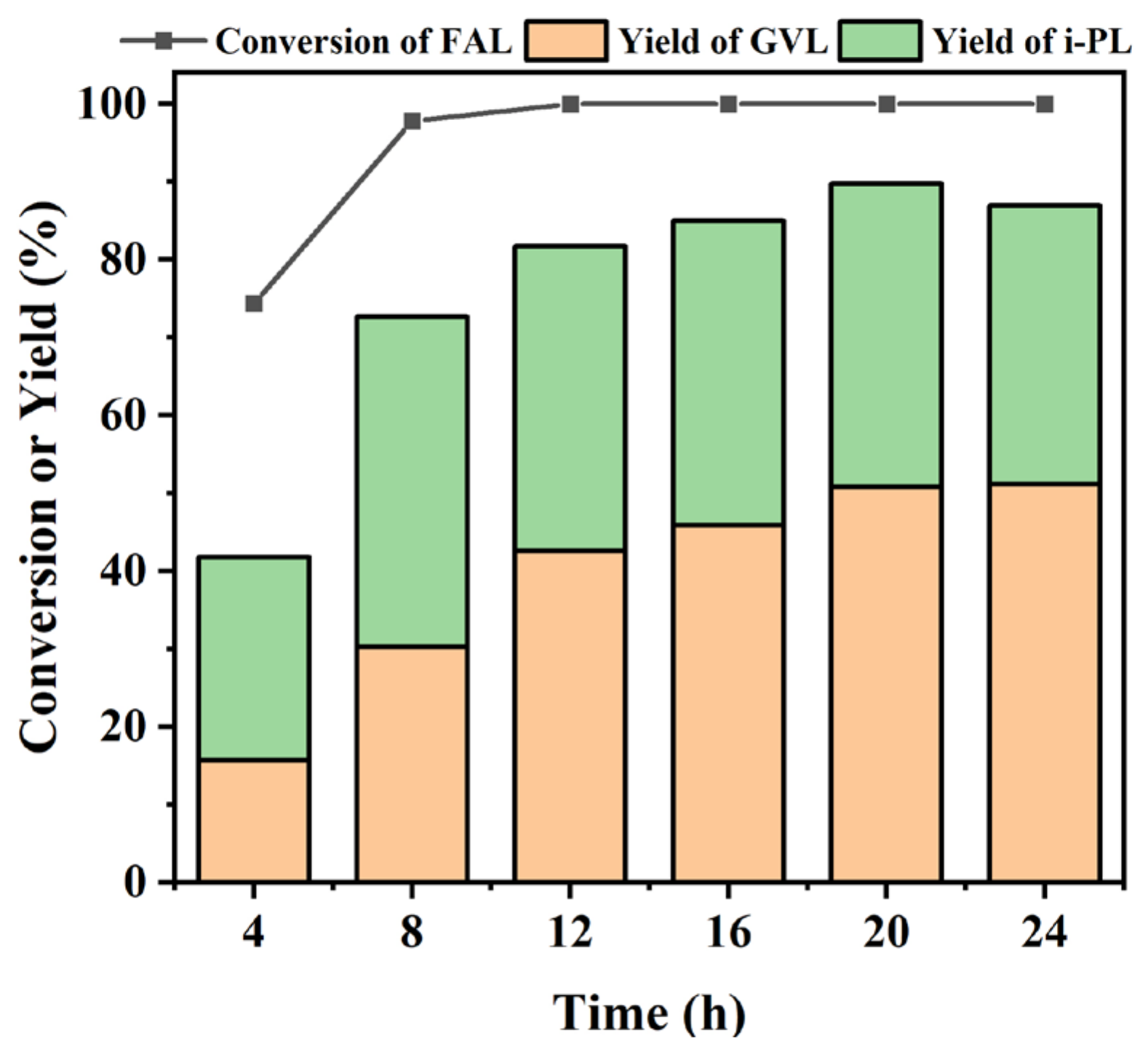
2.5. Extended Applications in Cascade Biomass Conversions
3. Materials and Methods
3.1. Materials
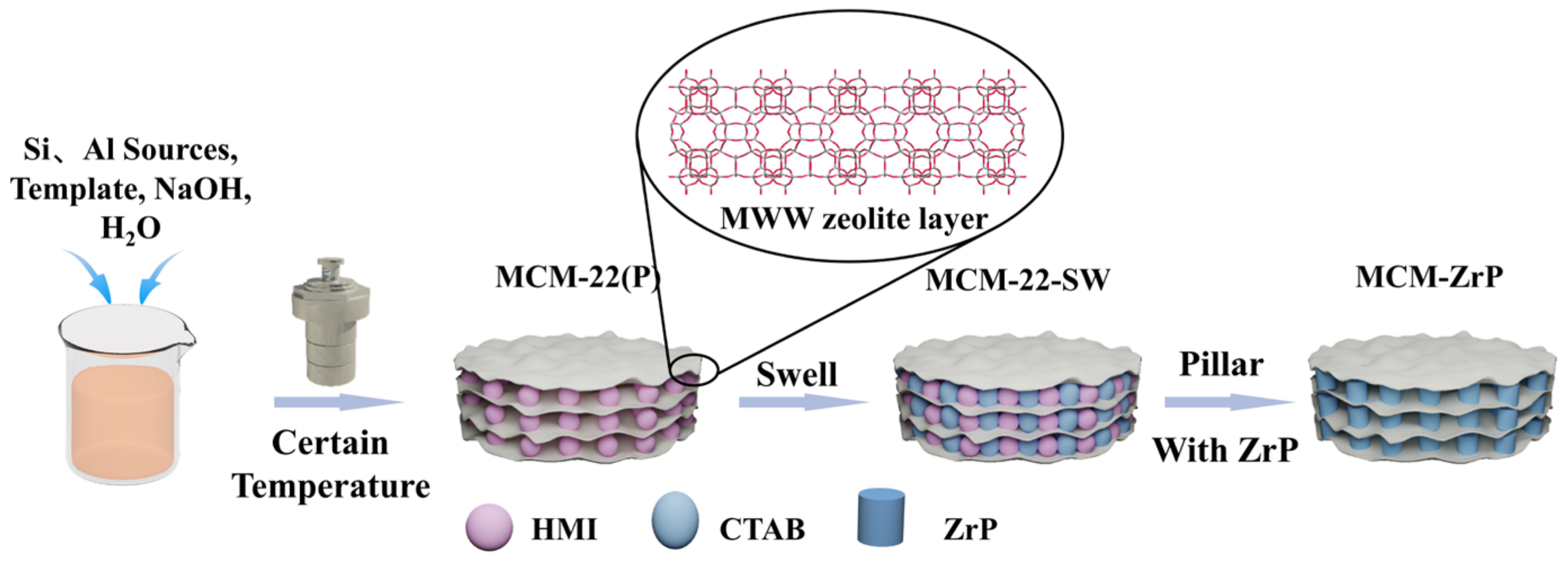
3.2. Catalyst Preparation
3.2.1. Synthesis of MCM-22 (P)
3.2.2. Swelling and Pillaring
3.3. Characterization
3.4. Experiment of Hydrogen Transfer Reactions of Biomass-Derived Carbon Oxides and Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manal, A.K.; Advani, J.H.; Srivastava, R. Bifunctional Acid-Base Zirconium Phosphonate for Catalytic Transfer Hydrogenation of Levulinic Acid and Cascade Transformation of Furfural to Biofuel Molecules. ChemCatChem 2022, 14, e202200576. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Chen, M.; Lv, W.; Meng, P.; Gao, W.; Meng, X.; Fan, W.; Xu, J.; Yan, W.; et al. Low-silica Cu-CHA Zeolite Enriched with Al Pairs Transcribed from Silicoaluminophosphate Seed: Synthesis and Ammonia Selective Catalytic Reduction Performance. Angew. Chem. Int. Ed. Engl. 2023, 62, e202306174. [Google Scholar] [CrossRef] [PubMed]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. Engl. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of γ-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Xu, R.; Liu, K.; Du, H.; Liu, H.; Cao, X.; Zhao, X.; Qu, G.; Li, X.; Li, B.; Si, C. Falling Leaves Return to Their Roots: A Review on the Preparation of γ-Valerolactone from Lignocellulose and Its Application in the Conversion of Lignocellulose. ChemSusChem 2020, 13, 6461–6476. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Chang, G.; Zhou, W. Enhancing Reductive Conversion of Levulinic Acid and Levulinates to Gamma-Valerolactone: Role of Oxygen Vacancy in MnOx Catalysts. Bioresour Technol. 2024, 406, 131001. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and Catalytic Transformation of Levulinic Acid: A Platform for Speciality Chemicals and Fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Gurbuz, E.I.; Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Reactive Extraction of Levulinate Esters and Conversion to γ-Valerolactone for Production of Liquid Fuels. ChemSusChem 2011, 4, 357–361. [Google Scholar] [CrossRef]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angew. Chem. Int. Ed. Engl. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.A.; Stevens, J.G.; Ke, J.; Poliakoff, M. Maximising Opportunities in Supercritical Chemistry: The Continuous Conversion of Levulinic Acid to γ-Valerolactone in CO2. Chem. Commun. 2007, 4632–4634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xue, Y.; Guo, J.; Cen, Y.; Wang, J.; Fan, W. Integrated Conversion of Hemicellulose and Furfural into γ-Valerolactone over Au/ZrO2 Catalyst Combined with ZSM-5. ACS Catal. 2016, 6, 2035–2042. [Google Scholar] [CrossRef]
- Gowda, R.R.; Chen, E.Y. Recyclable Earth-Abundant Metal Nanoparticle Catalysts for Selective Transfer Hydrogenation of Levulinic Acid to Produce γ-Valerolactone. ChemSusChem 2016, 9, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Z.; Zeng, X.; Jiang, Y.; Liu, S.; Lei, T.; Sun, Y.; Lin, L. In Situ Catalytic Hydrogenation of Biomass-Derived Methyl Levulinate to γ-Valerolactone in Methanol. ChemSusChem 2015, 8, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.-T.; Lavrič, Ž.; Kostyniuk, A.; Dražić, G.; Grilc, M.; Likozar, B.; Zabukovec Logar, N.; Djinović, P.; Novak Tušar, N. Innovative Microkinetic Modelling-Supported Structure–Activity Analysis of Ni/ZSM-5 during Vapor-Phase Hydrogenation of Levulinic Acid. Chem. Eng. J. 2024, 495, 153456. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Blair Vásquez, P.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer Hydrogenation of Methyl and Ethyl Levulinate Promoted by a ZrO2 Catalyst: Comparison of Batch vs. Continuous Gas-Flow Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Winoto, H.P.; Fikri, Z.A.; Ha, J.-M.; Park, Y.-K.; Lee, H.; Suh, D.J.; Jae, J. Heteropolyacid Supported on Zr-Beta Zeolite as an Active Catalyst for One-Pot Transformation of Furfural to γ-Valerolactone. Appl. Catal. B 2019, 241, 588–597. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Lai, D.M.; Fu, Y.; Guo, Q.X. Catalytic Conversion of Biomass-Derived Carbohydrates into γ-Valerolactone without Using an External H2 Supply. Angew. Chem. Int. Ed. Engl. 2009, 48, 6529–6532. [Google Scholar] [CrossRef]
- Liu, M.; Chen, G.; Song, Z.; He, Z.; Zhong, A.; Cui, M. Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid. Catalysts 2024, 14, 424. [Google Scholar] [CrossRef]
- He, J.; Schill, L.; Yang, S.; Riisager, A. Catalytic Transfer Hydrogenation of Bio-Based Furfural with NiO Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 17220–17229. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-Phase Catalytic Transfer Hydrogenation and Cyclization of Levulinic acid and Its Esters to γ-Valerolactone over Metal Oxide Catalysts. Chem. Commun. 2011, 47, 12233–12235. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, H.; Hu, L.; Hao, W.; Sun, Y.; Zeng, X.; Lin, L.; Liu, S. Conversion of Biomass to γ-Valerolactone by Catalytic Transfer Hydrogenation of Ethyl Levulinate over Metal Hydroxides. Appl. Catal. B 2014, 147, 827–834. [Google Scholar] [CrossRef]
- Song, J.; Zhou, B.; Zhou, H.; Wu, L.; Meng, Q.; Liu, Z.; Han, B. Porous Zirconium-Phytic Acid Hybrid: A Highly Efficient Catalyst for Meerwein-Ponndorf-Verley Reductions. Angew. Chem. Int. Ed. Engl. 2015, 54, 9399–9403. [Google Scholar] [CrossRef] [PubMed]
- Valekar, A.H.; Cho, K.-H.; Chitale, S.K.; Hong, D.-Y.; Cha, G.-Y.; Lee, U.-H.; Hwang, D.W.; Serre, C.; Chang, J.-S.; Hwang, Y.K. Catalytic Transfer Hydrogenation of Ethyl Levulinate to γ-Valerolactone over Zirconium-Based Metal–Organic Frameworks. Green Chem. 2016, 18, 4542–4552. [Google Scholar] [CrossRef]
- Song, S.; Di, L.; Wu, G.; Dai, W.; Guan, N.; Li, L. Meso-Zr-Al-Beta Zeolite as a Robust Catalyst for Cascade Reactions in Biomass Valorization. Appl. Catal. B 2017, 205, 393–403. [Google Scholar] [CrossRef]
- Chen, X.; Deng, L.; Zhou, S.; Qiao, C.; Tian, Y. Alkaline Modified Zr-Loading on Nanosheet Zeolite towards Production of γ-Valerolactone from Levulinic Acid through Catalytic Transfer Hydrogenation. Appl. Catal. A 2024, 682, 119816. [Google Scholar] [CrossRef]
- Mi, Z.; Lu, T.; Zhang, J.-N.; Xu, R.; Yan, W. Synthesis of Pure Silica Zeolites. Chem. Res. Chin. Univ. 2021, 38, 9–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Catalytic Hydration of Aromatic Alkynes to Ketones over H-MFI Zeolites. Chem. Res. Chin. Univ. 2022, 38, 173–180. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Zhou, X.; Hao, W.; Zhang, S.; Lan, C.; Wang, X.; Wang, Z.; Xu, J.; Zhang, J.-N.; et al. Facile Activation of Lithium slag for the Hydrothermal Synthesis of Zeolite A with Commercial Quality and High Removal Efficiency for the Isotope of Radioactive 90Sr. Inorg. Chem. Front. 2022, 9, 468–477. [Google Scholar] [CrossRef]
- Yu, X.; Liu, J.; Ru, C.; Cai, S.; Wang, J.; Liu, M.; Zhang, D.; Shen, J.; Jin, X.; Yang, C. Lattice Expansion and Electronic Reconfiguration of MnCu Oxide Catalysts for Enhanced Transfer Hydrogenation of Levulinate. ACS Sustain. Chem. Eng. 2022, 10, 13402–13414. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over Sulfonic Acid-Functionalized UiO-66. ACS Sustain. Chem. Eng. 2017, 5, 1141–1152. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Roman-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Bronsted and Lewis Acid Sites for the Production of γ-Valerolactone from Furfural. Angew. Chem. Int. Ed. Engl. 2013, 52, 8022–8025. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Song, W.-C.; Li, Y.; Yang, E.-C. One-Pot Transformation of Furfural into γ-Valerolactone Catalyzed by a Hierarchical Hf-Al-USY Zeolite with Balanced Lewis and Brønsted Acid Sites. Sustain. Energy Fuels 2021, 5, 4724–4735. [Google Scholar] [CrossRef]
- Zones, S.I. New zeolite SSZ-25. E.U. Patent EP0231860A2, 12 January 1987. [Google Scholar]
- Bellussi, G.; Perego, G.; Clerici, M.G.; Giusti, A. Synthetic, Crystalline, Porous Material Containing Oxides of Silicon and Boron. E.U. Patent EP0293032, 5 November 1988. [Google Scholar]
- Camblor, M.A.; Corell, C.; Corma, A.; Díaz-Cabañas, M.-J.; Nicolopoulos, S.; González-Calbet, J.M.; Vallet-Regí, M. A New Microporous Polymorph of Silica Isomorphous to Zeolite MCM-22. Chem. Mater. 1996, 8, 2415–2417. [Google Scholar] [CrossRef]
- Ji, Y.-J.; Xu, H.; Wang, D.-R.; Xu, L.; Ji, P.; Wu, H.; Wu, P. Mesoporus MCM-22 Zeolites Prepared through Organic Amine-Assisted Reversible Structural Change and Protective Desilication for Catalysis of Bulky Molecules. ACS Catal. 2013, 3, 1892–1901. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Y.; Ma, H.; Xu, H.; Jiang, J.G.; Lu, H.; Wu, P. Hydrothermal Synthesis of Boron-Free Zr-MWW and Sn-MWW Zeolites as Robust Lewis acid Catalysts. Chem. Commun. 2020, 56, 4696–4699. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Nivarthy, G.S.; Eder, F.; Seshan, K.; Lercher, J.A. Synthesis, Characterization and Catalytic Activity of the Pillared Molecular Sieve MCM-36. Microporous Mesoporous Mater. 1998, 25, 207–224. [Google Scholar] [CrossRef]
- Barth, J.-O.; Jentys, A.; Kornatowski, J.; Lercher, J.A. Control of Acid-Base Properties of New Nanocomposite Derivatives of MCM-36 by Mixed Oxide Pillaring. Chem. Mater. 2004, 16, 724–730. [Google Scholar] [CrossRef]
- Barth, J.-O.; Kornatowski, J.; Lercher, J.A. Synthesis of New MCM-36 Derivatives Pillared with Alumina or Magnesia–Alumina. J. Mater. Chem. 2002, 12, 369–373. [Google Scholar] [CrossRef]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe Modified Derivatives of 2D MWW-Type Zeolites (MCM-22, ITQ-2 and MCM-36) as New Catalysts for DeNOx Process. Appl. Catal. B 2015, 168–169, 531–539. [Google Scholar] [CrossRef]
- Szymaszek-Wawryca, A.; Díaz, U.; Samojeden, B.; Motak, M. Catalytic Performance of One-Pot Synthesized Fe-MWW Layered Zeolites (MCM-22, MCM-36, and ITQ-2) in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. Molecules 2022, 27, 2983. [Google Scholar] [CrossRef]
- Korzeniowska, A.; Grzybek, J.; Kałahurska, K.; Kubu, M.; Roth, W.J.; Gil, B. The Structure-Catalytic Activity Relationship for the Transient Layered Zeolite MCM-56 with MWW Topology. Catal. Today 2020, 345, 116–124. [Google Scholar] [CrossRef]
- Ogorzały, K.; Jajko, G.; Korzeniowska, A.; Mazur, M.; Li, A.; Roth, W.J.; Gil, B.; Makowski, W. The Effect of Amorphous Silica Support on the Catalytic Activity of Liquid-Exfoliated Monolayered MCM-56 Zeolite. J. Porous Mater. 2023, 30, 1459–1468. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, G.; Liu, N.; Xu, J.; Shen, Q.; Li, Y. Highly Selective Synthesis of Para-Diethylbenzene by Alkylation of Ethylbenzene with Diethyl Carbonate over Boron Oxide Modified HZSM-5. J. Mol. Catal. A Chem. 2014, 395, 384–391. [Google Scholar] [CrossRef]
- Jankowska, A.; Kowalczyk, A.; Rutkowska, M.; Mozgawa, W.; Gil, B.; Chmielarz, L. Silica and Silica–Titania Intercalated MCM-36 Modified with Iron as Catalysts for Selective Reduction of Nitrogen Oxides—The Role of Associated Reactions. Catal. Sci. Technol. 2020, 10, 7940–7954. [Google Scholar] [CrossRef]
- Zhang, Q.; Ming, W.; Ma, J.; Zhang, J.; Wang, P.; Li, R. De Novo Assembly of a Mesoporous Beta Zeolite with Intracrystalline Channels and Its Catalytic Performance for Biodiesel Production. J. Mater. Chem. A 2014, 2, 8712–8718. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Mart´ınez-Triguero, J.; Pergher, S.B. Delaminated Zeolites: Combining the Benefits of Zeolites and Mesoporous Materials for Catalytic Uses. J. Catal. 1999, 186, 57–63. [Google Scholar] [CrossRef]
- Wei, H.; Xie, S.; Gao, N.; Liu, K.; Liu, X.; Xin, W.; Li, X.; Liu, S.; Xu, L. Alkali Treatment upon MCM-49 Zeolite with Various Contents of HMI in the Presence of CTAB and Application in Anisole Acylation with Acetic Anhydride. Appl. Catal. A 2015, 495, 152–161. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Bai, H.; Lu, X. HZ-ZrP Catalysts with Adjustable Ratio of Brønsted and Lewis Acids for the One-Pot Value-Added Conversion of Biomass-Derived Furfural. ACS Sustain. Chem. Eng. 2020, 8, 7403–7413. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Song, W.-C.; Yang, E.-C.; Zhao, X.-J.; Guan, N.; Li, L. Hierarchical FAU-Type Hafnosilicate Zeolite as a Robust Lewis Acid Catalyst for Catalytic Transfer Hydrogenation. ACS Sustain. Chem. Eng. 2019, 7, 16329–16343. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Gao, L.; Yan, T.; Yan, Y.; Zhang, Y.; Tang, Y. Direct Grafting Synthesis of bi-Functional Zr-Al-MWW Zeolites and Their Catalytic Characteristics in Lewis-Brønsted Cascade Reaction. Microporous Mesoporous Mater. 2022, 341, 112110. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic Transfer Hydrogenation of Butyl Levulinate to γ-Valerolactone over Zirconium Phosphates with Adjustable Lewis and Brønsted Acid Sites. Appl. Catal. B 2017, 214, 67–77. [Google Scholar] [CrossRef]
- Kasipandi, S.; Cho, J.M.; Park, K.S.; Shin, C.-H.; Wook Bae, J. Unprecedented Activity and Stability on Zirconium Phosphates Grafted Mesoporous Silicas for Renewable Aromatics Production from Furans. J. Catal. 2020, 385, 10–20. [Google Scholar] [CrossRef]
- Wan, F.; Yang, B.; Zhu, J.; Jiang, D.; Zhang, H.; Zhang, Q.; Chen, S.; Zhang, C.; Liu, Y.; Fu, Z. The Transfer Hydrogenation of High Concentration Levulinic Acid to γ-Valerolactone Catalyzed by Glucose Phosphate Carbamide Zirconium. Green Chem. 2021, 23, 3428–3438. [Google Scholar] [CrossRef]
- Xie, C.; Song, J.; Zhou, B.; Hu, J.; Zhang, Z.; Zhang, P.; Jiang, Z.; Han, B. Porous Hafnium Phosphonate: Novel Heterogeneous Catalyst for Conversion of Levulinic Acid and Esters into γ-Valerolactone. ACS Sustain. Chem. Eng. 2016, 4, 6231–6236. [Google Scholar] [CrossRef]
- Liu, W.; Song, Z.; Ikegawa, T.; Nishiguchi, H.; Ishihara, T.; Takita, Y. Two Step Synthesis and Characterization of Thermally Stable Hexagonal Zirconium Phosphate. Mater. Lett. 2004, 58, 3328–3331. [Google Scholar] [CrossRef]
- Sun, Y.; Afanasiev, P.; Vrinat, M.; Coudurier, G. Porous zirconium Phosphates Prepared by Surfactant-Assisted Precipitation. J. Mater. Chem. 2000, 10, 2320–2324. [Google Scholar] [CrossRef]
- Spielbauer, D.; Mekhemer, G.A.H.; Riemer, T.; Zaki, M.I.; Knözinger, H. Structure and Acidic Properties of Phosphate-Modified Zirconia. J. Phys. Chem. B 1997, 101, 4681–4688. [Google Scholar] [CrossRef]
- Rao, K.N.; Sridhar, A.; Lee, A.F.; Tavener, S.J.; Young, N.A.; Wilson, K. Zirconium Phosphate Supported Tungsten Oxide Solid Acid Catalysts for the Esterification of Palmitic Acid. Green Chem. 2006, 8, 790–797. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Sutradhar, N.; Roy, B.; Tarafdar, A.; Bajaj, H.C.; Panda, A.B. Mesoporous Zirconium Phosphate Catalyzed Reactions: Synthesis of Industrially Important Chemicals in Solvent-Free Conditions. Appl. Catal. A 2010, 385, 22–30. [Google Scholar] [CrossRef]
- Zhao, G.L.; Yuan, Z.Y.; Chen, T.H. Synthesis of Amorphous Supermicroporous Zirconium Phosphate Materials by Nonionic Surfactant Templating. Mater. Res. Bull. 2005, 40, 1922–1928. [Google Scholar] [CrossRef]
- Weingarten, R.; Kim, Y.T.; Tompsett, G.A.; Fernández, A.; Han, K.S.; Hagaman, E.W.; Conner, W.C.; Dumesic, J.A.; Huber, G.W. Conversion of Glucose into Levulinic Acid with Solid Metal(IV) Phosphate catalysts. J. Catal. 2013, 304, 123–134. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ivanova, I.I. With Open Arms: Open Sites of ZrBEA Zeolite Facilitate Selective Synthesis of Butadiene from Ethanol. ACS Catal. 2015, 5, 4833–4836. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, A.; Di Iorio, J.R.; Paris, C.; Boronat, M.; Corma, A.; Roman-Leshkov, Y.; Moliner, M. Selective Active Site Placement in Lewis Acid Zeolites and Implications for Catalysis of Oxygenated Compounds. Chem. Sci. 2020, 11, 10225–10235. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, X.; Bai, L.; Liu, S.; Song, K.; Zhou, X.; Guo, J.; Meng, X.; Li, C. Effective One-Pot Tandem Synthesis of Γ-Valerolactone from Biogenic Furfural over Zirconium Phosphate Catalyst. Biofuels Bioprod. Biorefin. 2022, 16, 1613–1626. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Liu, H.; Jia, W.; Yu, X.; Wang, S.; Zeng, X.; Sun, Y.; Wei, J.; Tang, X.; et al. Domino Transformation of Furfural to Γ-Valerolactone over Sapo-34 Zeolite Supported Zirconium Phosphate Catalysts with Tunable Lewis and Brønsted Acid Sites. Mol. Catal. 2021, 506, 111538. [Google Scholar] [CrossRef]
- Colon, J. X-ray Photoelectron Spectroscopy and Catalytic Activity of α-Zirconium Phosphate and Zirconium Phosphate Sulfophenylphosphonate. J. Catal. 1990, 124, 148–159. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Yu, Z.; Zhang, M.; Xiong, J.; Zhang, R.; Li, X.; Qiao, Y.; Lu, X. Efficient MPV Reductions of Biomass-Derived Aldehydes and Ketones over an Assembling Zirconium-Gallic Acid Hybrid under Mild Condition. Fuel 2022, 328, 125233. [Google Scholar] [CrossRef]
- Lawton, S.L.; Fung, A.S.; Kennedy, G.J.; Alemany, L.B.; Chang, C.D.; Hatzikos, G.H.; Lissy, D.N.; Rubin, M.K.; Timken, H.-K.C.; Steuernagel, S.; et al. Zeolite MCM-49: A Three-Dimensional MCM-22 Analogue Synthesized by In Situ Crystallization. J. Phys. Chem. 1996, 100, 3788–3798. [Google Scholar] [CrossRef]
- Rubin, M.K.; Bala Cynwyd, P.; Chu, P.; West Deptford, N.J. Composition of Synthetic Porous Crystalline Material, Its Synthesis and Use. U.S. Patent 4,954,325, 4 September 1990. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquidcrystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Ma, M.; Hou, P.; Zhang, P.; Guo, Q.; Yue, H.; Huang, J.; Tian, G.; Feng, S. Tandem Catalysis of Furfural to γ-Valerolactone over Polyoxometalate-Based Metal-Organic Frameworks: Exploring the Role of Confinement in the Catalytic Process. Renewable Energy 2024, 227, 120474. [Google Scholar] [CrossRef]



| SBET a (m2/g) | Smicro b (m2/g) | Sext b (m2/g) | Vtotal c (cm3/g) | Vmicro b (cm3/g) | |
|---|---|---|---|---|---|
| MCM-22 | 581 | 503 | 78 | 0.796 | 0.199 |
| MCM-ZrP0.5 | 298 | 145 | 153 | 0.635 | 0.064 |
| MCM-ZrP1 | 286 | 108 | 178 | 0.612 | 0.046 |
| MCM-ZrP2 | 306 | 133 | 173 | 0.427 | 0.056 |
| Si/Al a | Si/Zr a | Zr/P a | Acidic Sites (μmol/g) | B/L c | |||
|---|---|---|---|---|---|---|---|
| Weak b | Moderate b | Total b | |||||
| MCM-22 | 12.40 | - | - | 241 | 301 | 543 | 2.76 |
| MCM-ZrP0.5 | 10.42 | 4.75 | 0.63 | 527 | 287 | 815 | 1.85 |
| MCM-ZrP1 | 10.88 | 3.51 | 1.11 | 560 | 417 | 977 | 0.88 |
| MCM-ZrP2 | 12.15 | 15.89 | 1.57 | 634 | 309 | 943 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, P.; Su, H.; Jin, K.; Li, Q.; Yan, W. Zirconium Phosphate-Pillared Zeolite MCM-36 for Green Production of γ-Valerolactone from Levulinic Acid via Catalytic Transfer Hydrogenation. Molecules 2024, 29, 3779. https://doi.org/10.3390/molecules29163779
Hou P, Su H, Jin K, Li Q, Yan W. Zirconium Phosphate-Pillared Zeolite MCM-36 for Green Production of γ-Valerolactone from Levulinic Acid via Catalytic Transfer Hydrogenation. Molecules. 2024; 29(16):3779. https://doi.org/10.3390/molecules29163779
Chicago/Turabian StyleHou, Pan, Haopeng Su, Keyan Jin, Qiang Li, and Wenfu Yan. 2024. "Zirconium Phosphate-Pillared Zeolite MCM-36 for Green Production of γ-Valerolactone from Levulinic Acid via Catalytic Transfer Hydrogenation" Molecules 29, no. 16: 3779. https://doi.org/10.3390/molecules29163779
APA StyleHou, P., Su, H., Jin, K., Li, Q., & Yan, W. (2024). Zirconium Phosphate-Pillared Zeolite MCM-36 for Green Production of γ-Valerolactone from Levulinic Acid via Catalytic Transfer Hydrogenation. Molecules, 29(16), 3779. https://doi.org/10.3390/molecules29163779







