Elevating Skincare Science: Grape Seed Extract Encapsulation for Dermatological Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass
2.2. Preparation of Grape Seed Extracts (GSEs)
2.3. Antioxidant Activity
2.3.1. ABTS Radical Cation Decolorization Assay
2.3.2. DPPH Radical Cation Decolorization Assay
2.3.3. Folin–Ciocalteau Colorimetric Method
2.4. Antimicrobial Activity
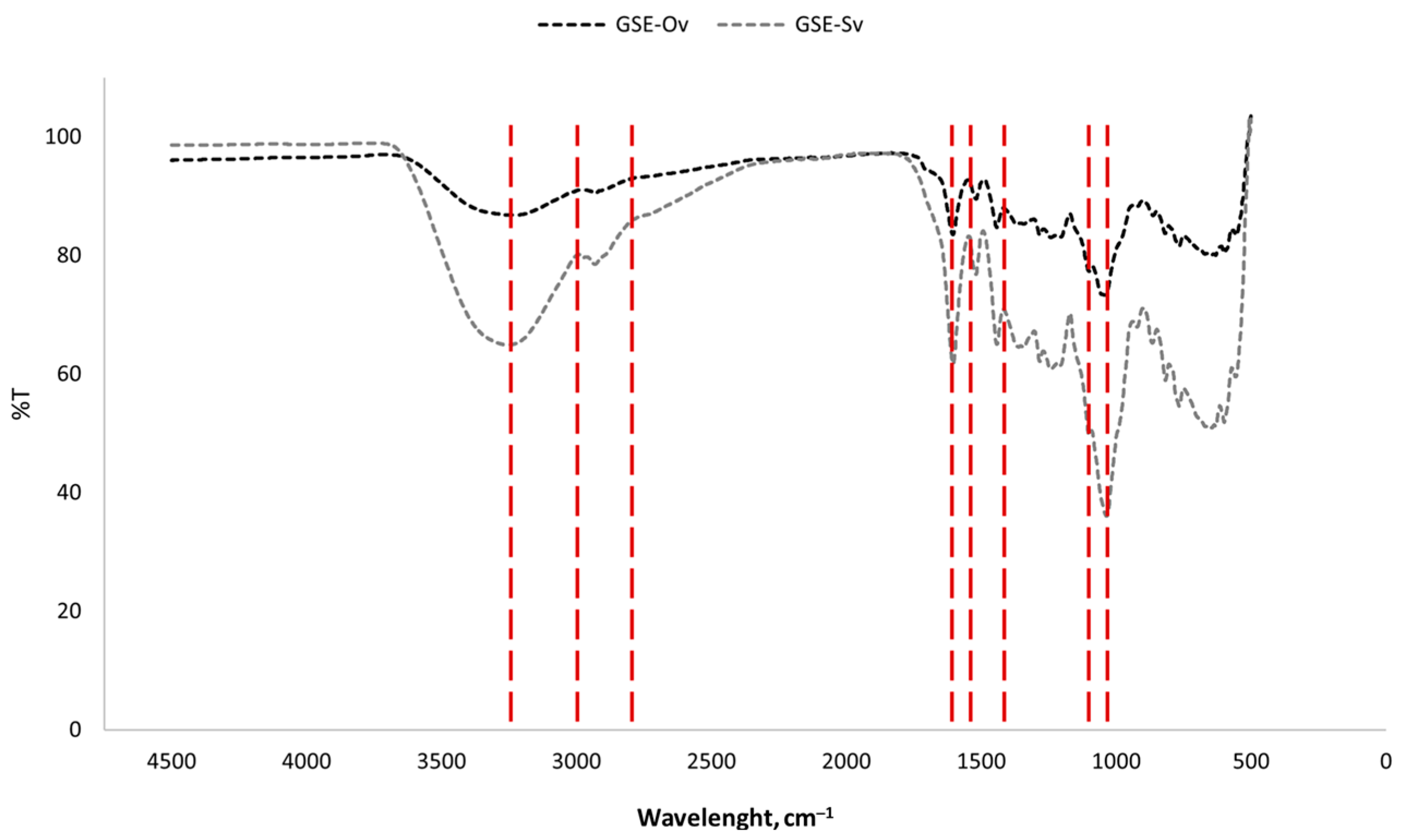
2.5. Fourier-Transform Infrared (FTIR) Analysis
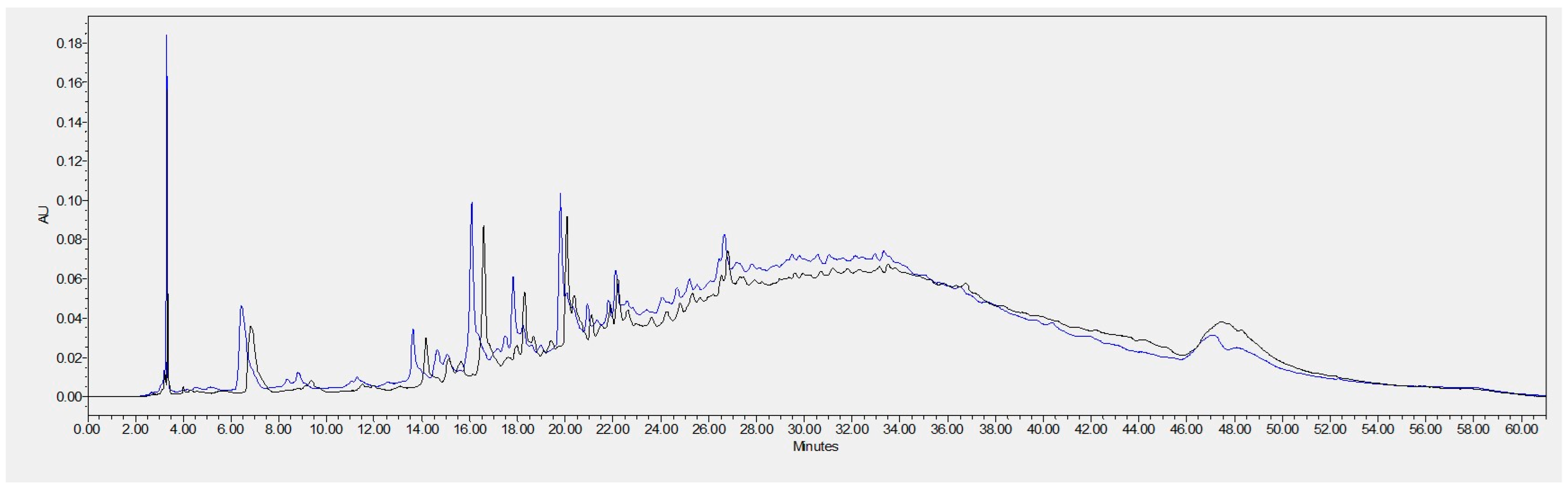
2.6. GSEs Composition Analysis by High-Performance Liquid Chromatography (HPLC)
2.7. GSE-Ov Encapsulation with Microdispersions
2.8. Microdispersion Characterization
2.8.1. Zeta Potential
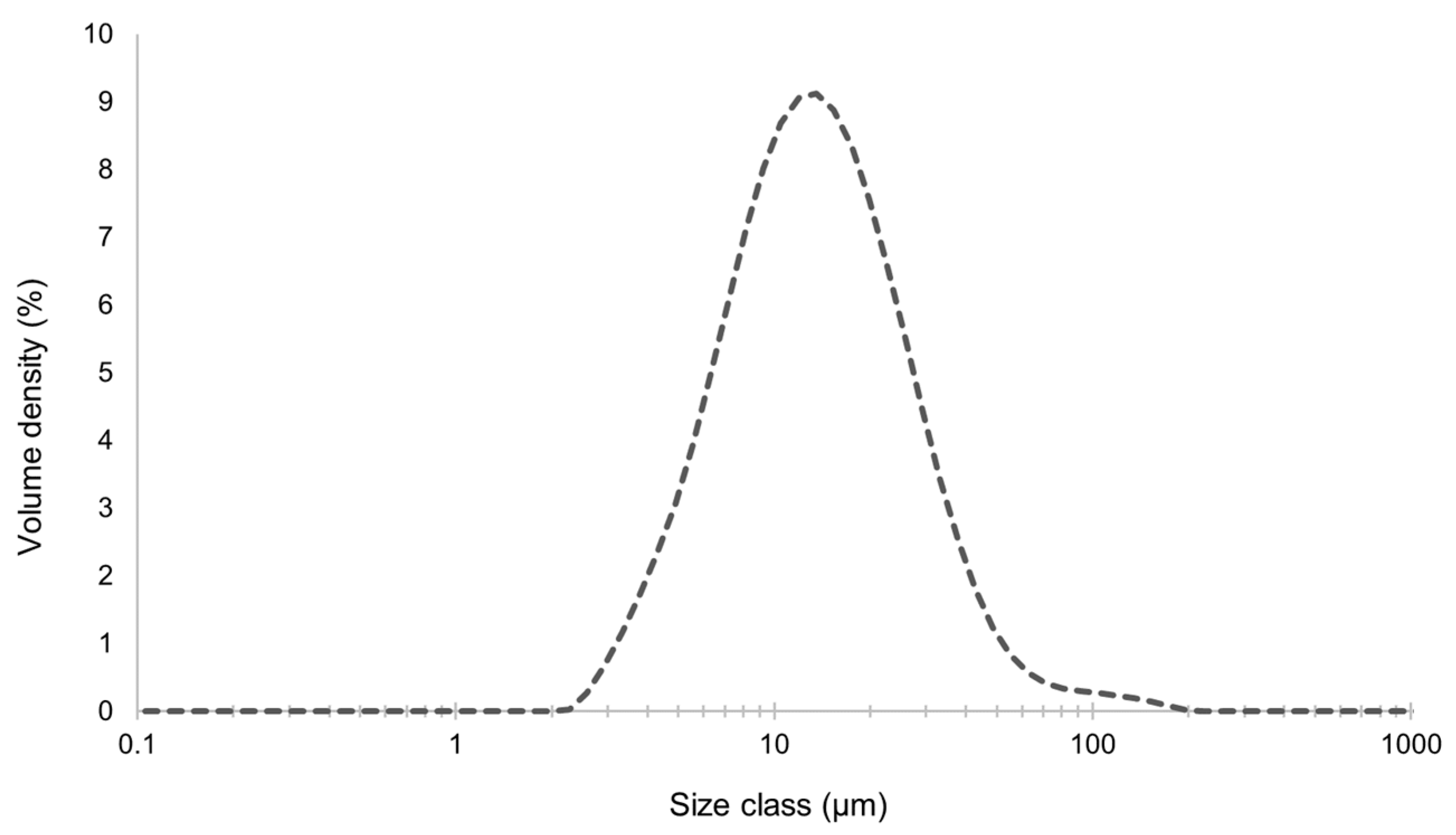
2.8.2. Particle Size Distribution
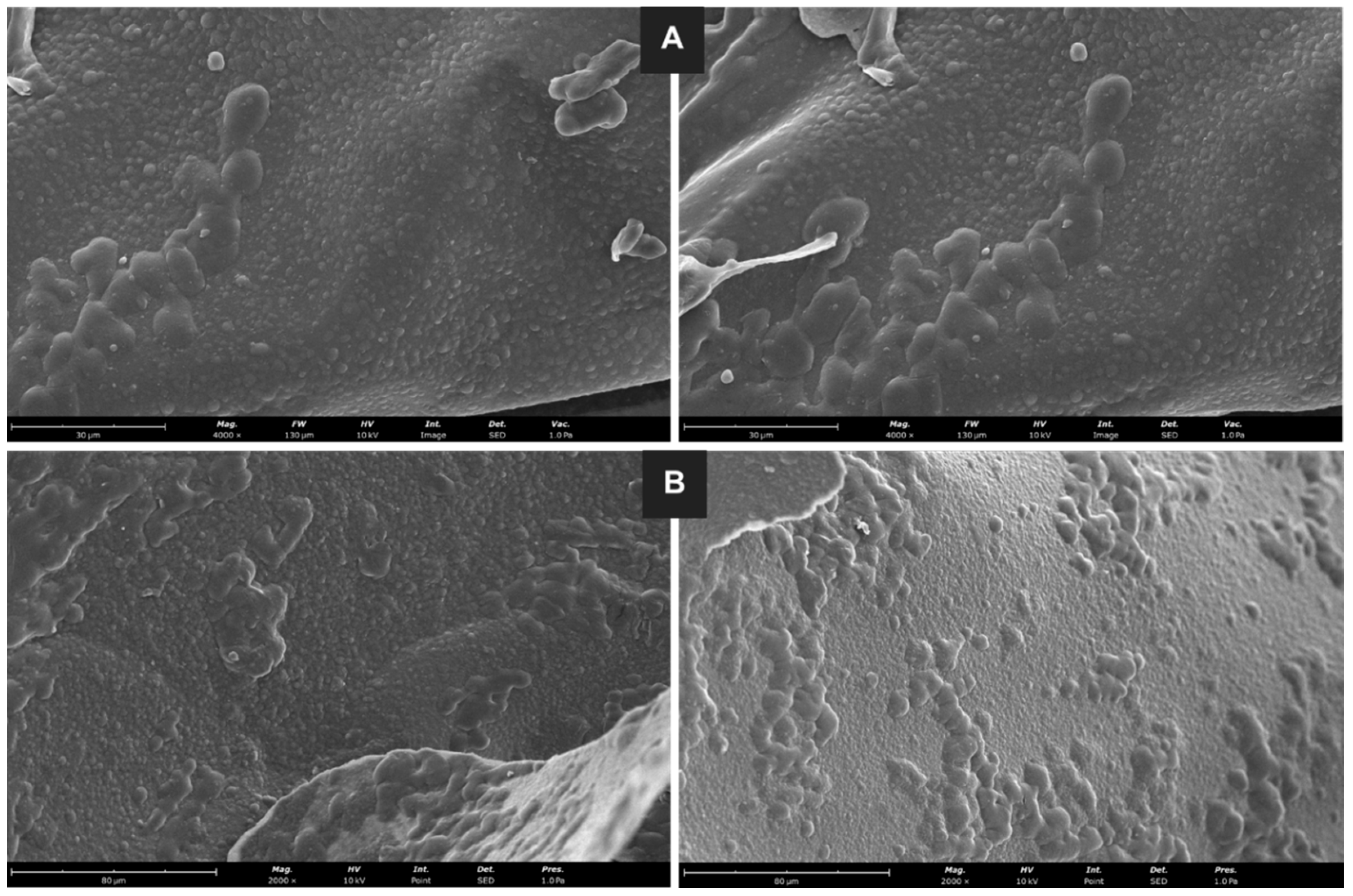
2.8.3. Morphology
2.8.4. FTIR Analysis
2.8.5. Encapsulation Efficiency
2.8.6. In Vitro Phenolic Compounds Release Assay
2.9. Cell Culture
2.10. Cytotoxicity
2.11. Quantification of Pro-Inflammatory Cytokines
2.12. Human Pro-Collagen I α1 Quantification
2.13. Statistical Analysis
3. Results and Discussion
3.1. Initial Screening of GSE Potential and Characterization
3.1.1. Antioxidant Activity
3.1.2. Antimicrobial Activity
3.1.3. Individual Phenolic Compound Identification by HPLC
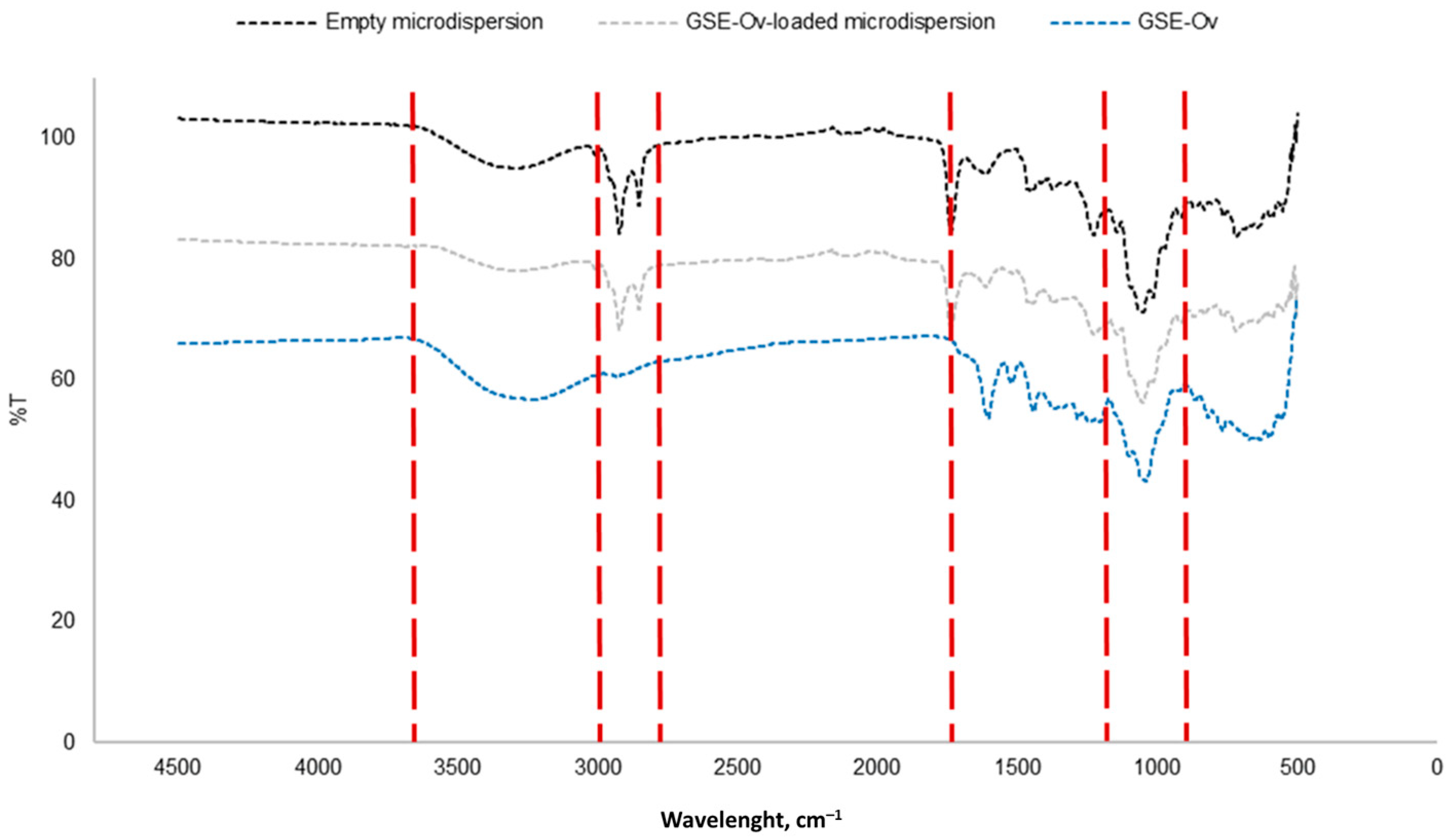
3.1.4. Fourier Transform Infrared Analysis
3.2. GSE-Ov Encapsulation Microdispersion
3.3. Optimization of the Encapsulation Process
3.4. Characterization of the Selected Microdispersion Preparation
3.4.1. Zeta Potential, Size Distribution, and Morphology
3.4.2. FTIR Analysis
3.5. Encapsulation Efficiency and Phenolic Compound Release Profile
3.6. Safety and Skincare Potential of Encapsulated GSE-Ov
3.6.1. Cytotoxicity
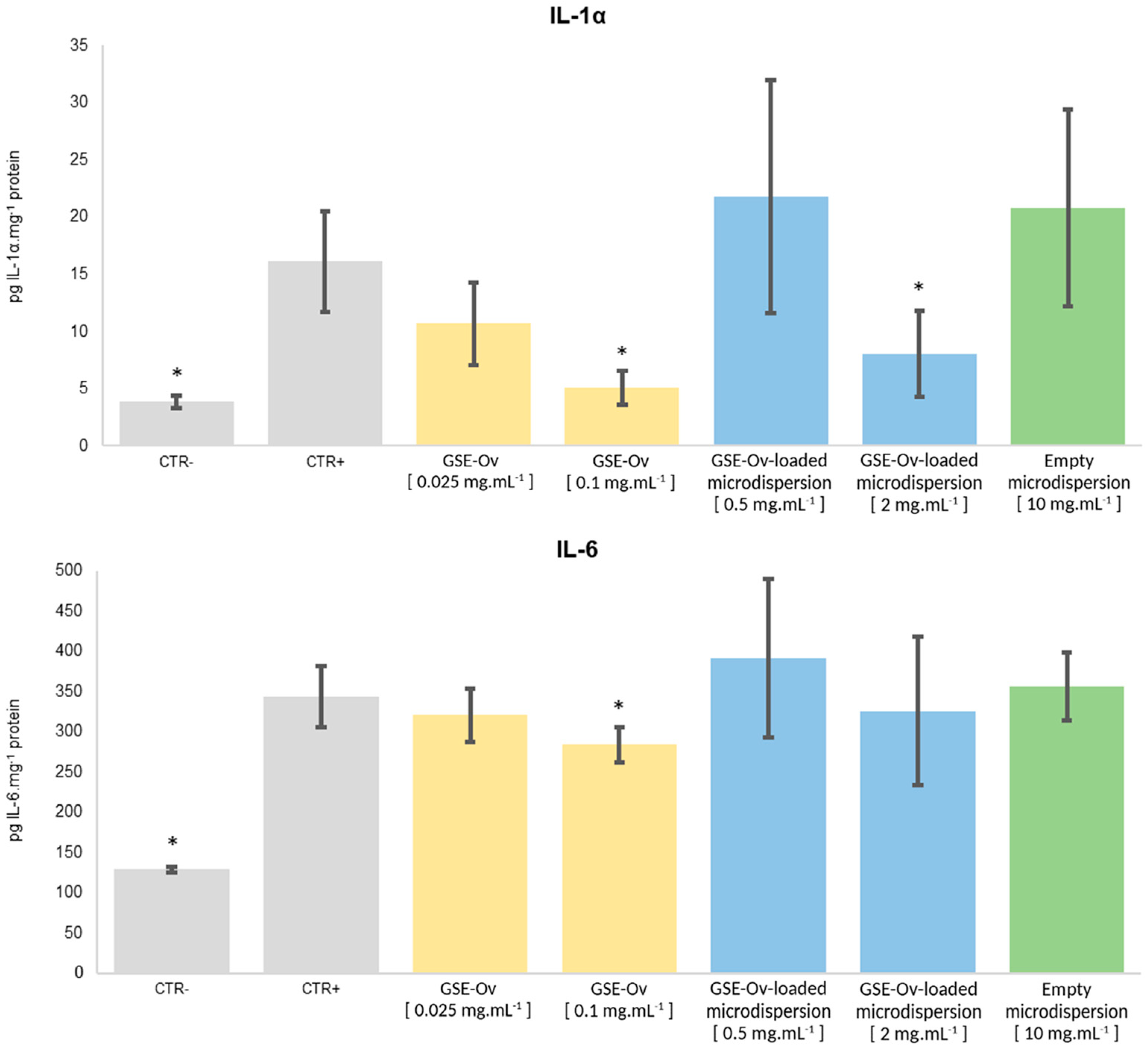
3.6.2. Anti-Inflammatory Activity
3.6.3. Human Pro-Collagen I α1 Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M., Avci, P., Prow, T., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 1–14. [Google Scholar] [CrossRef]
- Walters, K.A.; Roberts, M.S. The structure and function of skin. In Dermatological and Transdermal Formulations; Walters, K.A., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 19–58. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Carvalho, M.J.; de Carvalho, N.M.; Azevedo-Silva, J.; Mendes, A.; Ribeiro, I.P.; Fernandes, J.C.; Oliveira, A.L.S.; Oliveira, C.; Pintado, M.; et al. Skincare potential of a sustainable postbiotic extract produced through sugarcane straw fermentation by Saccharomyces cerevisiae. BioFactors 2023, 49, 1038–1060. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. Toll-like receptors in skin infections and inflammatory diseases. Infect. Disord.-Drug Targets (Former. Curr. Drug Targets-Infect. Disord.) 2008, 8, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from Human Skin Commensal Bacteria Protect against Staphylococcus aureus and Are Deficient in Atopic Dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [PubMed]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000 Res. 2018, 7, 1953. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Chen, X.Y.; Li, X.; Xue, F.; Chen, L.H.; Liu, N.; Wang, B.; Wang, L.Q.; Wang, X.P.; Yang, H.; et al. Psoriatic lesions are characterized by higher bacterial load and imbalance between Cutibacterium and Corynebacterium. J. Am. Acad. Dermatol. 2019, 82, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Rozas, M.; de Ruijter, A.H.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From dysbiosis to healthy skin: Major contributions of Cutibacterium acnes to skin homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-De la Paz, S. Grape (Vitis vinifera L.) seed oil: A functional food from the winemaking industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P. Grape processing by-products as active ingredients for cosmetic proposes. In Handbook of Grape Processing by-Products; Galanakis, C.M., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 267–292. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Franjieh, D.; Hoss, I.; Manca, M.L.; Manconi, M.; Castangia, I.; Perra, M.; Maroun, R.G.; Louka, N. Stability and antioxidant activity of hydro-glyceric extracts obtained from different grape seed varieties incorporated in cosmetic creams. Antioxidants 2022, 11, 1348. [Google Scholar] [CrossRef] [PubMed]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT-Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Hoss, I.; Rajha, H.N.; El Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of Wine-Making By-Products’ Extracts in Cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. A potential valorization strategy of wine industry by-products and their application in cosmetics—Case study: Grape pomace and grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.M.; Bajenaru, L.; Berger, D.; Mitran, R.A.; Deaconu, M.; Lincu, D.; Guzun, A.S.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of nanoconfinement of polyphenolic extract from grape pomace into functionalized mesoporous silica on its biocompatibility and radical scavenging activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Oliveira, A.L.; Pedrosa, S.S.; Pintado, M.; Madureira, A.R. Potential of sugarcane extracts as cosmetic and skincare ingredients. Ind. Crops Prod. 2021, 169, 113625. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Castangia, I.; Rajha, H.N.; Manca, M.L.; Manconi, M. An outlook on modern and sustainable approaches to the management of grape pomace by integrating green processes, biotechnologies and advanced biomedical approaches. J. Funct. Foods 2022, 98, 105276. [Google Scholar] [CrossRef]
- Castro, M.L.; Ferreira, J.P.; Pintado, M.; Ramos, O.L.; Borges, S.; Baptista-Silva, S. Grape by-Products in Sustainable Cosmetics: Nanoencapsulation and Market Trends. Appl. Sci. 2023, 13, 9168. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C. Impact of Nanotechnology in the Modern World. Ann. Clin. Case Stud. 2023, 5, 1082. [Google Scholar]
- Ngwuluka, N.C.; Abu-Thabit, N.Y.; Uwaezuoke, O.J.; Erebor, J.O.; Ilomuanya, M.O.; Mohamed, R.R.; Soliman, S.M.A.; Elella, M.H.A.; Ebrahim, N.A.A. Natural Polymers in Micro- and Nanoencapsulation for Therapeutic and Diagnostic Applications: Part I: Lipids and Fabrication Techniques. In Nano- and Microencapsulation—Techniques and Applications; Abu-Thabit, N., Ed.; InTech: London, UK, 2021; pp. 3–54. [Google Scholar] [CrossRef]
- Perra, M.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Segura-Carretero, A.; Pedraz, J.L.; Bacchetta, G.; Muntoni, A.; Gioannis GDe Manca, M.L.; Manconi, M. Extraction of the antioxidant phytocomplex from wine-making by-products and sustainable loading in phospholipid vesicles specifically tailored for skin protection. Biomed. Pharmacother. 2021, 142, 111959. [Google Scholar] [CrossRef] [PubMed]
- INdermal. 2022. Available online: https://indermal.com/en/index-english/ (accessed on 30 April 2023).
- Bucić-Kojić, A.; Sovová, H.; Planinić, M.; Tomas, S. Temperature-dependent kinetics of grape seed phenolic compounds extraction: Experiment and model. Food Chem. 2013, 136, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Malpiedi, L.P.; Nerli, B.B. Micellar systems of aliphatic alcohol ethoxylates as a sustainable alternative to extract soybean isoflavones. Food Chem. 2018, 264, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Fuente, B.D.L.; Rodrigues, M.; Pires, T.C.; Mandim, F.; Almeida, A.; Dias, M.I.; Caleja, C.; Barros, L. Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction. Biomolecules 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.A.; Misra, S.S. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Campos, D.; Oliveira, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Study of olive pomace antioxidant dietary fibre powder throughout gastrointestinal tract as multisource of phenolics, fatty acids and dietary fibre. Food Res. Int. 2021, 142, 110032. [Google Scholar] [CrossRef]
- Machado, A.R.; Assis, L.M.; Costa, J.A.V.; Badiale-Furlong, E.; Motta, A.S.; Micheletto, Y.M.S.; Souza-Soares, L.A. Application of sonication and mixing for nanoencapsulation of the cyanobacterium Spirulina platensis in liposomes. Int. Food Res. J. 2014, 21, 2201. [Google Scholar]
- ISO 10993-5; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2009.
- Mandić, A.I.; Đilas, S.M.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Vulić, J.J. Antioxidant activity of white grape seed extracts on DPPH radicals. Acta Period. Technol. 2009, 40, 53–61. [Google Scholar] [CrossRef]
- Tournour, H. Re: How Does the Difference Happen between ABTS and DPPH Radical Scavenging Activity? 2016. Available online: https://www.researchgate.net/post/How-does-the-difference-happen-between-ABTS-and-DPPH-radical-scavenging-activity/5774553aed99e1e7a531f53a/citation/download (accessed on 28 November 2023).
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J.M.; Brüggemann, H. Bacterial skin commensals and their role as host guardians. Benef. Microbes 2014, 5, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial potential, antioxidant activity, and phenolic content of grape seed extracts from four grape varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y.C. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–270. [Google Scholar] [CrossRef]
- Monga, J.; Aggarwal, V.; Suthar, S.K.; Nongalleima, K.; Sharma, M. Topical (+)-catechin emulsified gel prevents DMBA/TPA-induced squamous cell carcinoma of the skin by modulating antioxidants and inflammatory biomarkers in BALB/c mice. Food Funct. 2014, 5, 3197–3207. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Pinent, M.; Ardévol, A.; Blay, M. Procyanidin B2 inhibits inflammasome-mediated IL-1β production in lipopolysaccharide-stimulated macrophages. Mol. Nutr. Food Res. 2014, 59, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier transform infrared (FTIR) spectroscopy to analyse human blood over the last 20 years: A review towards lab-on-a-chip devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Günter, E.A.; Popeyko, O.V. Delivery system for grape seed extract based on biodegradable pectin-Zn-alginate gel particles. Int. J. Biol. Macromol. 2022, 219, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Byrne, H.J.; Heredia, F.J. Study of phenolic extractability in grape seeds by means of ATR-FTIR and Raman spectroscopy. Food Chem. 2017, 232, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Nicoli, S.F.; Gabrielli, P.; et al. Grape seeds: Chromatographic profile of fatty acids and phenolic compounds and qualitative analysis by FTIR-ATR spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef]
- Vovesná, A.; Zhigunov, A.; Balouch, M.; Zbytovská, J. Ceramide liposomes for skin barrier recovery: A novel formulation based on natural skin lipids. Int. J. Pharm. 2021, 596, 120264. [Google Scholar] [CrossRef] [PubMed]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of nanomaterials: Tools and challenges. In Nanomaterials for Food Applications; Rubio, A.L., Rovira, M.J.F., Sanz, M.M., Gómez-Mascaraque, L.G., Eds.; Elsevier: Oxford, UK, 2019; pp. 313–353. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-based nanoparticles for drug delivery systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Oxford, UK, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, J.; Dutta, P.K.; Koh, J. A systematic study on chitosan-liposome based systems for biomedical applications. Int. J. Biol. Macromol. 2020, 160, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lim, S.J.; Lee, M.K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. Structural, functional and physicochemical properties of pectin from grape pomace as affected by different extraction techniques. Int. J. Biol. Macromol. 2023, 224, 739–753. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Assis, L.M.D.; Machado, A.R.; Motta, A.d.S.d.; Costa, J.A.V.; Souza-Soares, L.A. Development and characterization of nanovesicles containing phenolic compounds of microalgae Spirulina strain LEB-18 and Chlorella pyrenoidosa. Adv. Mater. Phys. Chem. 2014, 4, 6–12. [Google Scholar] [CrossRef]
- Machado, A.R.; Pinheiro, A.C.; Vicente, A.A.; Souza-Soares, L.A.; Cerqueira, M.A. Liposomes loaded with phenolic extracts of Spirulina LEB-18: Physicochemical characterization and behavior under simulated gastrointestinal conditions. Food Res. Int. 2019, 120, 656–667. [Google Scholar] [CrossRef]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.S.; Jung, H.S. Precise control of liposome size using characteristic time depends on solvent type and membrane properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Production of Nanoliposomes by a Supercritical CO2 Assisted Process: Application to Cosmetics. Chem. Eng. Trans. 2023, 101, 61–66. [Google Scholar] [CrossRef]
- Merino, D.; Ollier, R.; Lanfranconi, M.; Alvarez, V. Preparation and characterization of soy lecithin-modified bentonites. Appl. Clay Sci. 2016, 127, 17–22. [Google Scholar] [CrossRef]
- Santos, E.E.; Amaro, R.C.; Bustamante, C.C.C.; Guerra, M.H.A.; Soares, L.C.; Froes, R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Whittinghill, J.M.; Norton, J.; Proctor, A. Stability determination of soy lecithin-based emulsions by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 37–42. [Google Scholar] [CrossRef]
- Pezeshky, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Moghadam, M.; Babazadeh, A. Vitamin A palmitate-bearing nanoliposomes: Preparation and characterization. Food Biosci. 2016, 13, 49–55. [Google Scholar] [CrossRef]
- Lu, Q.; Li, D.C.; Jiang, J.G. Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J. Agric. Food Chem. 2011, 59, 13004–13011. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021, 176, 113870. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 1–15. [Google Scholar] [CrossRef]
- Teixeira, F.S.; Vidigal SS, M.P.; Pimentel, L.L.; Costa, P.T.; Tavares-valente, D.; Azevedo-silva, J.; Pintado, M.E.; Fernandes, J.C.; Rodríguez-alcalá, L.M. Phytosterols and novel triterpenes recovered from industrial fermentation coproducts exert in vitro anti-inflammatory activity in macrophages. Pharmaceuticals 2021, 14, 583. [Google Scholar] [CrossRef]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Galozzi, P.; Bindoli, S.; Doria, A.; Sfriso, P. The revisited role of interleukin-1 alpha and beta in autoimmune and inflammatory disorders and in comorbidities. Autoimmun. Rev. 2021, 20, 102785. [Google Scholar] [CrossRef] [PubMed]
- Iznardo, H.; Puig, L. IL-1 family cytokines in inflammatory dermatoses: Pathogenetic role and potential therapeutic implications. Int. J. Mol. Sci. 2022, 23, 9479. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-rich grape seed extract reduces inflammation and oxidative stress and restores tight junction barrier function in Caco-2 colon cells. Nutrients 2020, 12, 1623. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques AL, P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of Marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Kwatra, B. Collagen Supplementation: Therapy for Skin Disorders: A Review. World J. Pharm. Res. 2020, 9, 2504–2518. [Google Scholar]
- Jhawar, N.; Wang, J.V.; Saedi, N. Oral collagen supplementation for skin aging: A fad or the future? J. Cosmet. Dermatol. 2020, 19, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Akit, H.; Collins, C.; Fahri, F.; Hung, A.; D’Souza, D.; Leury, B.; Dunshea, F. Dietary lecithin decreases skeletal muscle COL1A1 and COL3A1 gene expression in finisher gilts. Animals 2016, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kim, C.I.; Leo, M.A.; Mak, K.M.; Rojkind, M.; Lieber, C.S. Polyunsaturated lecithin prevents acetaldehyde-mediated hepatic collagen accumulation by stimulating collagenase activity in cultured lipocytes. Hepatology 1992, 15, 373–381. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]




| Analysis | GSE-Ov | GSE-Sv | Ascorbic Acid | BHT |
|---|---|---|---|---|
| Total phenolics (mg GA eq g−1 dry extract) | 258.96 ± 30.94 a | 203.72 ± 42.64 | --- | --- |
| ABTS IC50 (mg mL−1) | 0.138 ± 0.034 a | 0.163 ± 0.015 | 0.05 * | 0.13 * |
| DPPH IC50 (mg mL−1) | 0.079 ± 0.003 a | 0.088 ± 0.004 | 0.04 * | 0.28 * |
| Microorganism | GSE-Ov | GSE-Sv |
|---|---|---|
| MBC mg mL−1 | ||
| S. epidermidis | 50 | 50 |
| Methicillin-sensitive S. aureus (MSSA) | 3.125 | 6.25 |
| Methicillin-resistant S. aureus (MRSA) | 6.25 | 6.25 |
| C. acnes | >50 | >50 |
| Polyphenol | Retention Time (min) | GSE-Ov (A1) | GSE-Sv (A2) | A1/A2 |
|---|---|---|---|---|
| Gallic acid | 6.834 | 827,724 | 1,059,774 | 0.78 |
| Catechin | 16.579 | 1,111,979 | 1,333,777 | 0.83 |
| Procyanidin B1 | 14.164 | 339,778 | 225,966 | 1.50 |
| Procyanidin B2 | 17.569 | --- | 572,197 | --- |
| Conditions # | Process Description | ζ (mV) | PDI/Uniformity |
|---|---|---|---|
| I | LFH + 1 min sonication (70% amplitude) | −29.7 ± 2.9 | 0.407 ± 0.008 |
| II | RPE (Reverse Phase Evaporation) method | −14.4 ± 1.3 | 0.432 ± 0.031 |
| III | Condition #I + GSE-Ov | −5.1 ± 1.1 | 0.405 ± 0.014 |
| IV | Condition #III + chitosan | −9.3 ± 0.7 | 1.000 ± 0.000 |
| V (final) | LFH + GSE-Ov + pectin | −20.3 ± 2.4 | 0.637 * |
| Time (h) | Percentage of Released GSE (Mean ± SD) |
|---|---|
| 1 | 12.67 ± 0.46% |
| 2 | 24.95 ± 0.69% |
| 4 | 37.84 ± 0.40% |
| 6 | 48.18 ± 0.75% |
| 24 | 59.42 ± 0.41% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, M.L.; Azevedo-Silva, J.; Valente, D.; Machado, A.; Ribeiro, T.; Ferreira, J.P.; Pintado, M.; Ramos, O.L.; Borges, S.; Baptista-Silva, S. Elevating Skincare Science: Grape Seed Extract Encapsulation for Dermatological Care. Molecules 2024, 29, 3717. https://doi.org/10.3390/molecules29163717
Castro ML, Azevedo-Silva J, Valente D, Machado A, Ribeiro T, Ferreira JP, Pintado M, Ramos OL, Borges S, Baptista-Silva S. Elevating Skincare Science: Grape Seed Extract Encapsulation for Dermatological Care. Molecules. 2024; 29(16):3717. https://doi.org/10.3390/molecules29163717
Chicago/Turabian StyleCastro, Maria Leonor, João Azevedo-Silva, Diana Valente, Adriana Machado, Tânia Ribeiro, João Paulo Ferreira, Manuela Pintado, Oscar L. Ramos, Sandra Borges, and Sara Baptista-Silva. 2024. "Elevating Skincare Science: Grape Seed Extract Encapsulation for Dermatological Care" Molecules 29, no. 16: 3717. https://doi.org/10.3390/molecules29163717
APA StyleCastro, M. L., Azevedo-Silva, J., Valente, D., Machado, A., Ribeiro, T., Ferreira, J. P., Pintado, M., Ramos, O. L., Borges, S., & Baptista-Silva, S. (2024). Elevating Skincare Science: Grape Seed Extract Encapsulation for Dermatological Care. Molecules, 29(16), 3717. https://doi.org/10.3390/molecules29163717











