Enhanced Fire Safety of Energy-Saving Foam by Self-Cleavage CO2 Pre-Combustion and Phosphorus Release Post-Combustion
Abstract
1. Introduction
2. Results
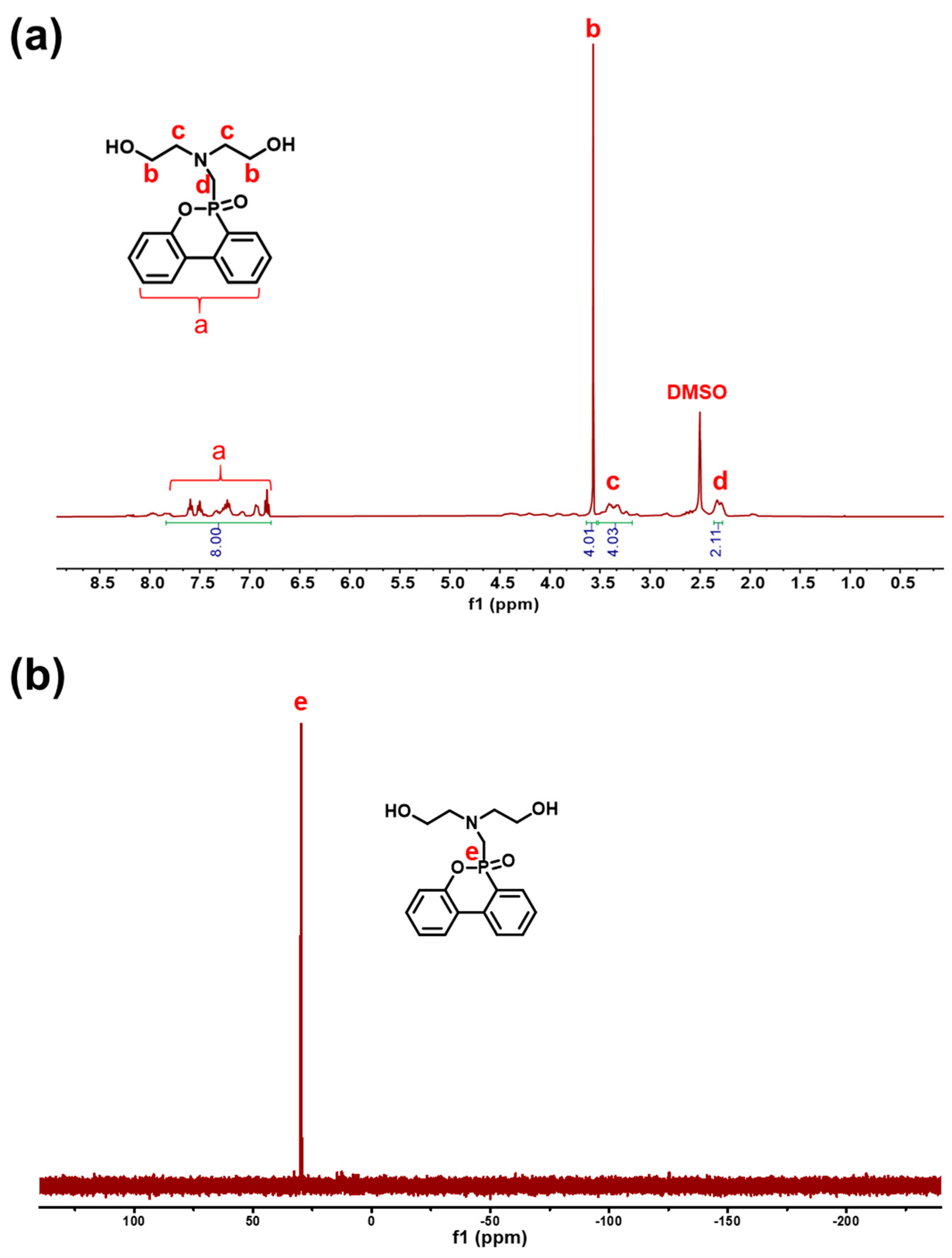
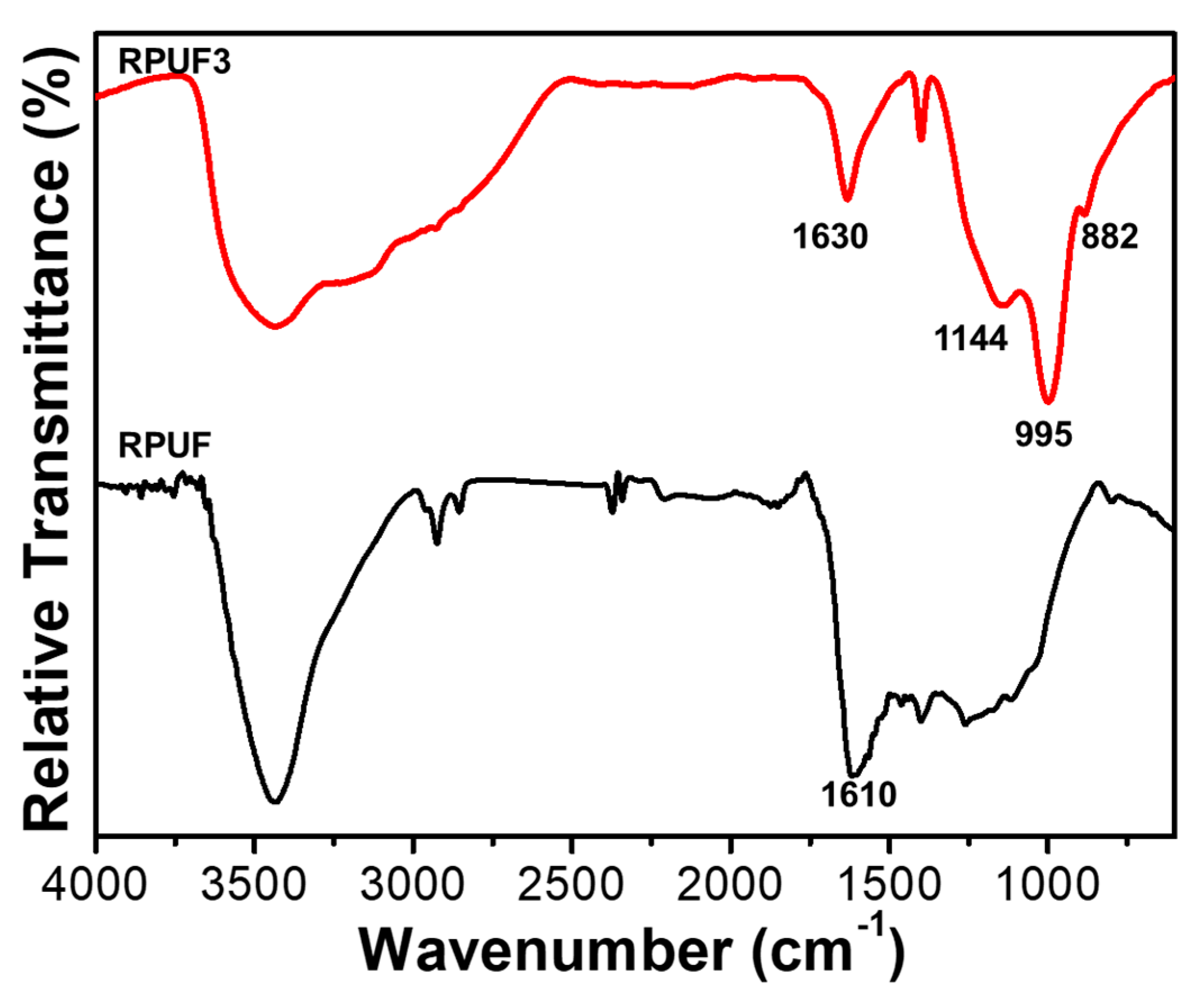
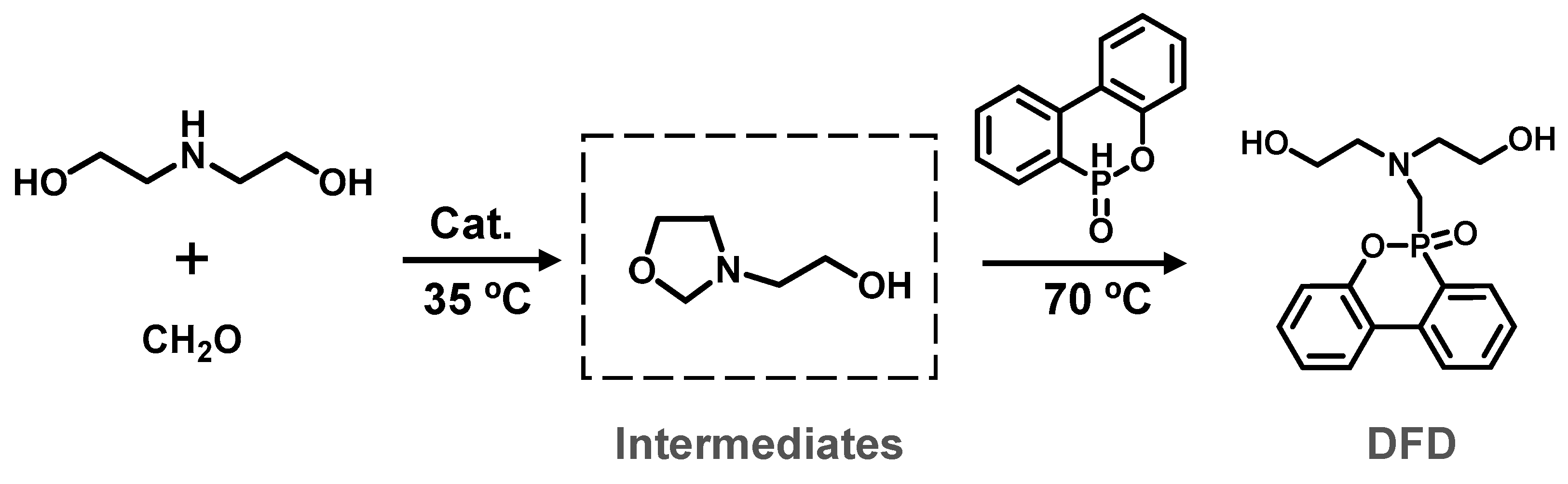
2.1. Characterization of Reactive FRs
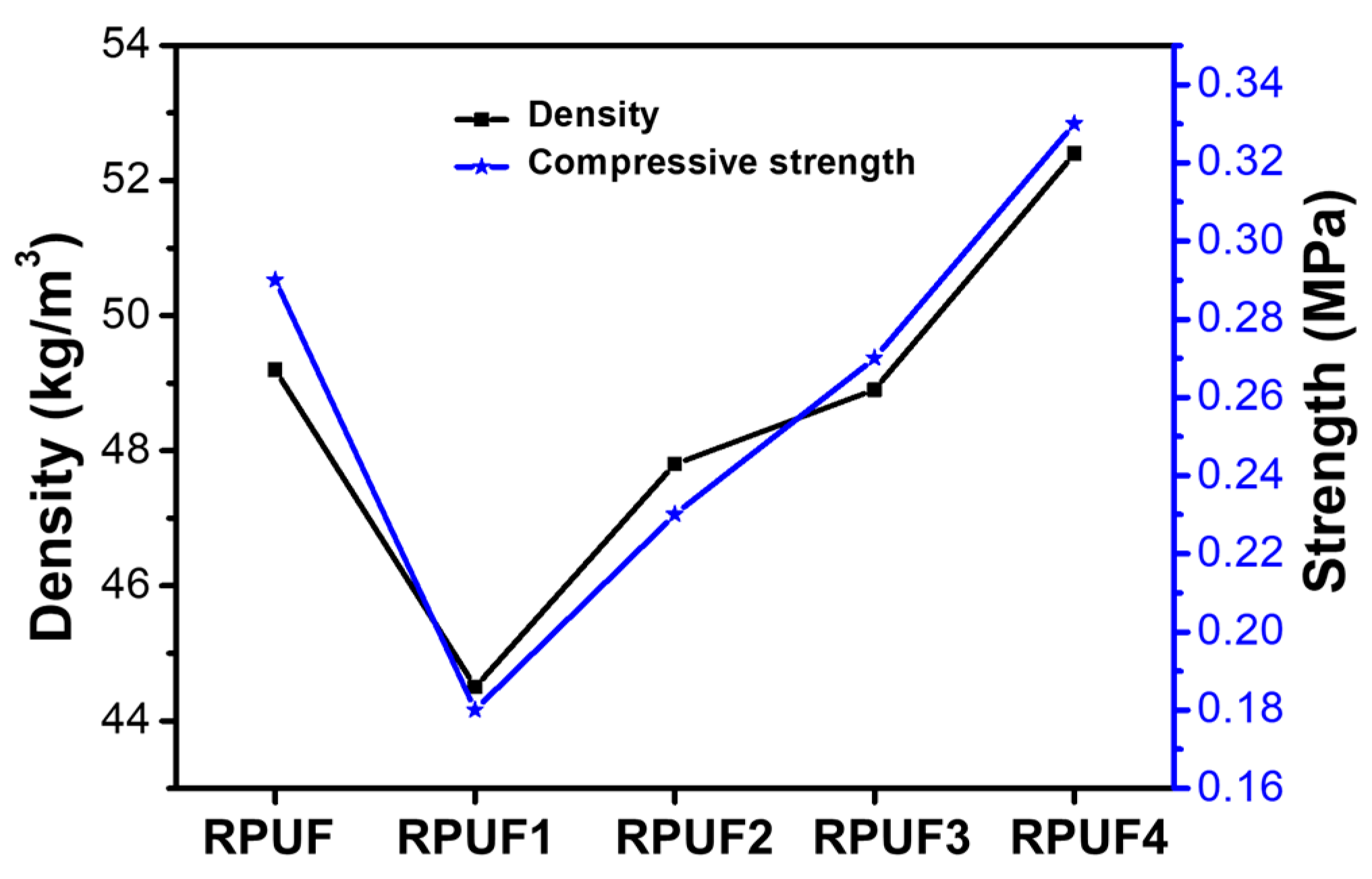
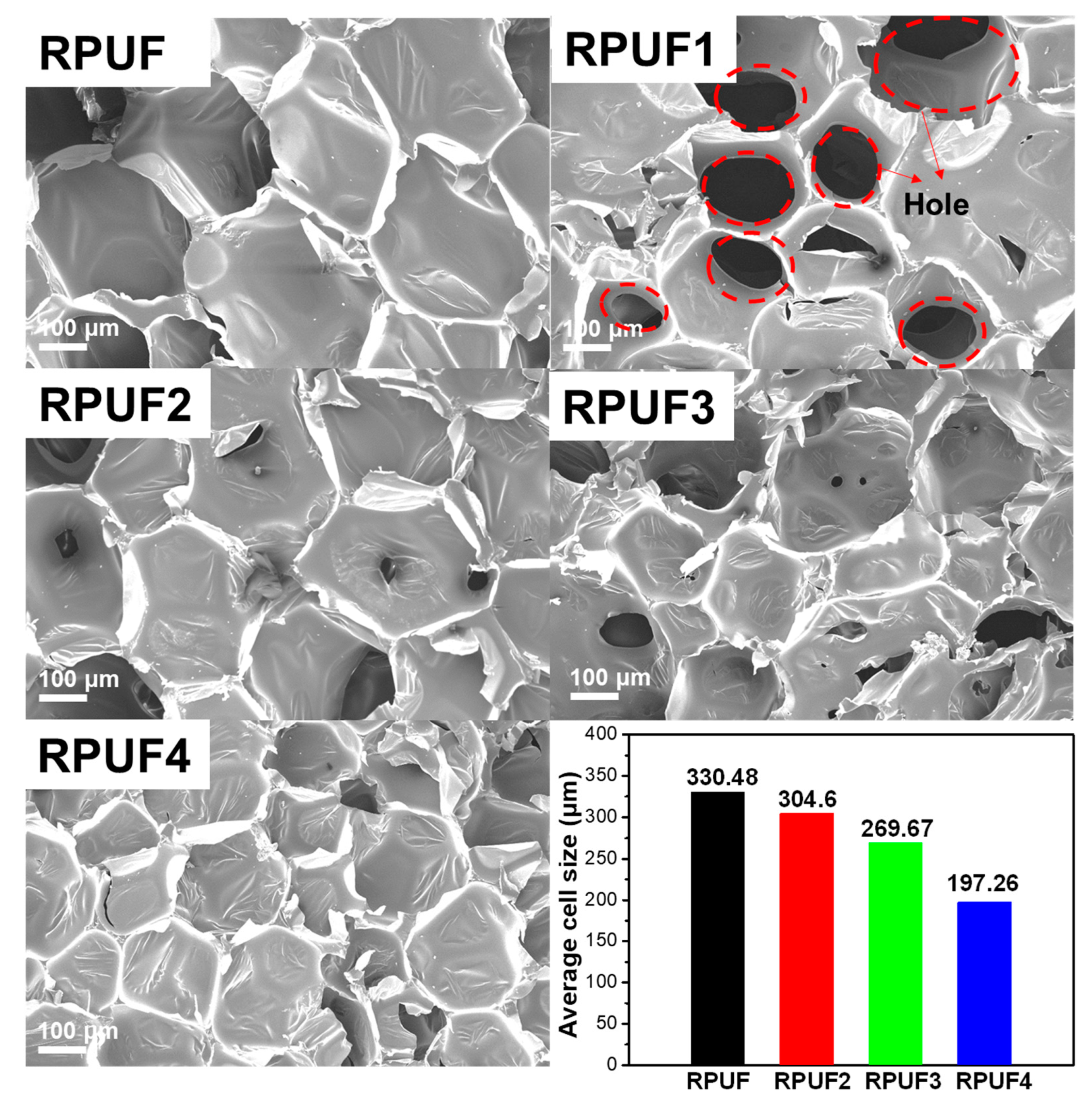
2.2. Physical Properties of RPUFs and Flame-Retardant RPUFs
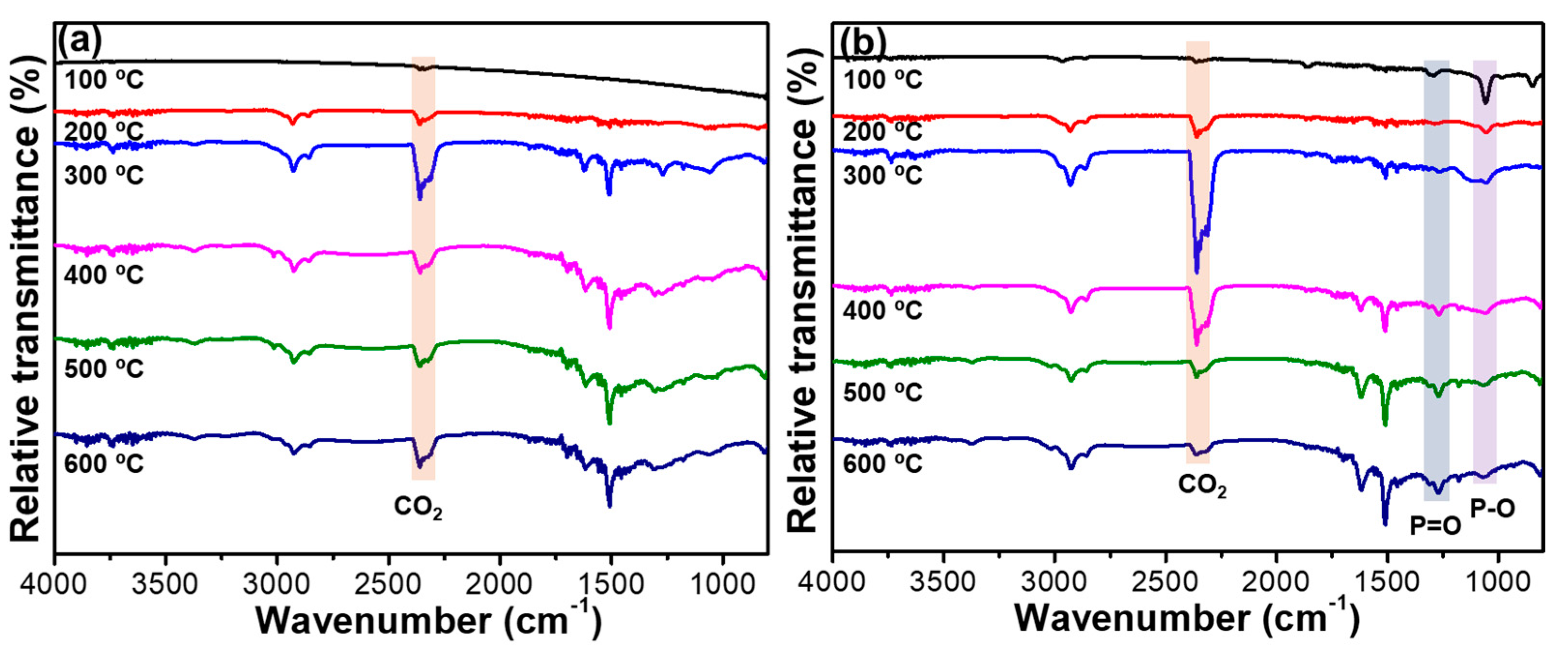
2.3. Thermal Stability of RPUFs and Flame-Retardant RPUFs
2.4. Flame Retardancy of RPUF and Flame-Retardant RPUFs
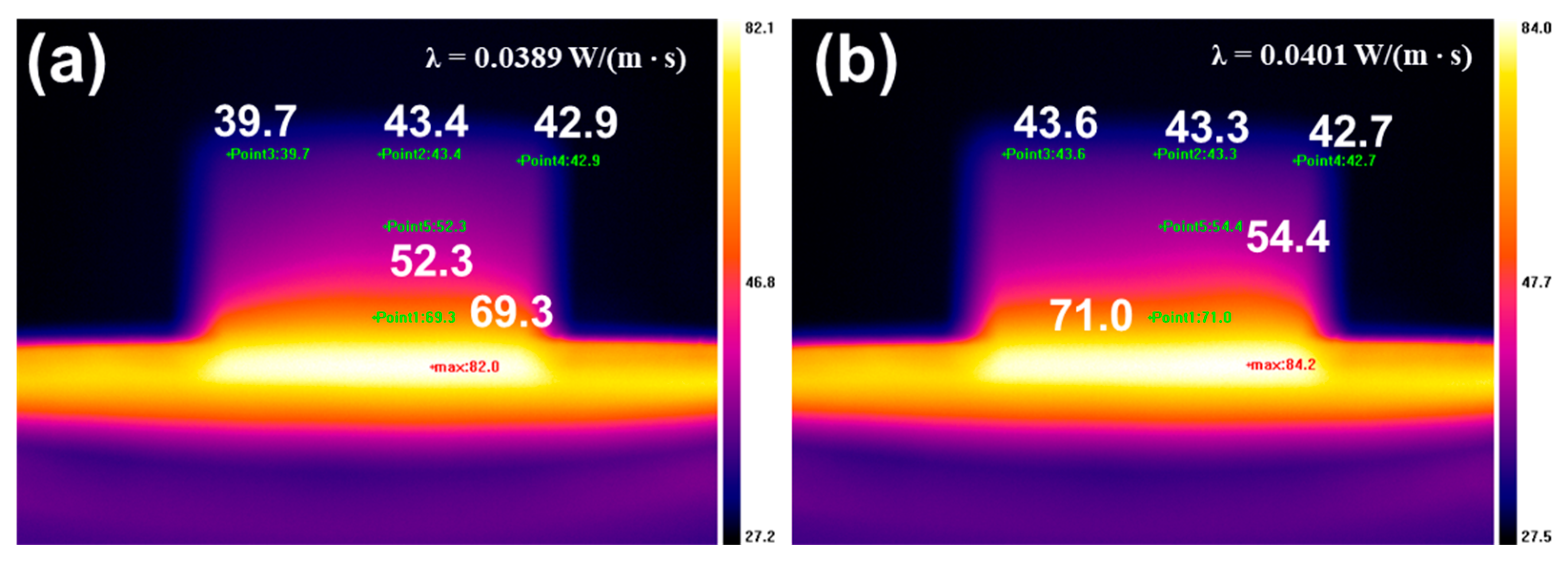
2.5. Thermal Insulation of RPUFs and Flame-Retardant RPUFs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Reactive FRs
3.3. The Prepared of RPUF and Flame-Retardant RPUFs
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhang, C.; Deng, Y.; Zhang, P. A Review of Research on the Effect of Temperature on the Properties of Polyurethane Foams. Polymers 2022, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yin, C.; Fernandez, C.; Fu, L.; Lin, C.-T. A Systematic Review and Bibliometric Analysis of Flame-Retardant Rigid Polyurethane Foam from 1963 to 2021. Polymers 2022, 14, 3011. [Google Scholar] [CrossRef] [PubMed]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane foams from vegetable oil-based polyols: A review. Polym. Bull. 2023, 80, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Y. Panel estimation for urbanization, energy consumption and CO2 emissions: A regional analysis in China. Energy Policy 2012, 49, 488–498. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Gao, J.; Zhou, Z.; Tang, G.; Wang, C. Research on two sides horizontal flame spread over rigid polyurethane with different flame retardants. J. Therm. Anal. Calorim. 2021, 146, 2141–2150. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Ye, L.; Zhang, X.-G.; Tang, P.-M.; Tang, J.-H.; Ji, X.; Li, Z.-M. Effects of Expandable Graphite and Ammonium Polyphosphate on the Flame-Retardant and Mechanical Properties of Rigid Polyurethane Foams. J. Appl. Polym. Sci. 2009, 114, 853–863. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Xu, D.; Yu, K.; Qian, K. Thermal degradation study of rigid polyurethane foams containing tris(1-chloro-2-propyl)phosphate and modified aramid fiber. Polym. Test. 2018, 67, 159–168. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Yu, K.; Feng, S.; Zhang, H.; Zhao, J. Synergistic flame retardancy of piperazine pyrophosphate/magnesium hydroxide/fly ash cenospheres-doped rigid polyurethane foams. Constr. Build. Mater. 2023, 408, 133670. [Google Scholar] [CrossRef]
- Vo, D.K.; Do, T.D.; Nguyen, B.T.; Tran, C.K.; Nguyen, T.A.; Nguyen, D.M.; Pham, L.H.; Nguyen, T.D.; Nguyen, T.D.; Hoang, D.Q. Effect of metal oxide nanoparticles and aluminum hydroxide on the physicochemical properties and flame-retardant behavior of rigid polyurethane foam. Constr. Build. Mater. 2022, 356, 129268. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Tian, Y.; Ding, N.; Wang, Z. Controllable synthesis of three kinds of zinc borates and flame retardant properties in polyurethane foam. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 274–280. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, G.; Xu, W. Roles of organically-modified montmorillonite and phosphorous flame retardant during the combustion of rigid polyurethane foam. Polym. Degrad. Stab. 2014, 101, 32–39. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X. Mechanical properties and flame retardancy of rigid polyurethane foams containing SiO2 nanospheres/graphene oxide hybrid and dimethyl methylphosphonate. Polym. Plast. Technol. Eng. 2018, 57, 884–892. [Google Scholar] [CrossRef]
- Espadas-Escalante, J.; Aviles, F.; Gonzalez-Chi, P.; Oliva, A. Thermal conductivity and flammability of multiwall carbon nanotube/polyurethane foam composites. J. Cell. Plast. 2017, 53, 215–230. [Google Scholar] [CrossRef]
- Zheng, W.; Du, J.; Wu, Z.; Yin, M.; Liu, W.; Han, S. Efficient and economical synthesis of flame retardant rigid polyurethane nanocomposite foam through phosphorus-based compounds and in-situ exfoliated microcrystal muscovite. Polym. Plast. Technol. Mater. 2024, 63, 385–398. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Wang, Y.; Yuan, H.; Chen, Y.; Luo, B. Effect of potassium on the pyrolysis of biomass components: Pyrolysis behaviors, product distribution and kinetic characteristics. Waste Manag. 2021, 121, 255–264. [Google Scholar] [CrossRef]
- Zhu, H.; Yi, B.; Hu, H.; Fan, Q.; Wang, H.; Yao, H. The effects of char and potassium on the fast pyrolysis behaviors of biomass in an infrared-heating condition. Energy 2021, 214, 119065. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Chi, Z.; Guo, Z.; Xu, Z.; Zhang, M.; Li, M.; Shang, L.; Ao, Y. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Song, L.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Aluminum hypophosphite in combination with expandable graphite as a novel flame retardant system for rigid polyurethane foams. Polym. Adv. Technol. 2014, 25, 1034–1043. [Google Scholar] [CrossRef]
- Han, S.; Zhu, X.; Chen, F.; Chen, S.; Liu, H. Flame-retardant system for rigid polyurethane foams based on diethyl bis(2-hydroxyethyl)aminomethylphosphonate and in-situ exfoliated clay. Polym. Degrad. Stab. 2020, 177, 109178. [Google Scholar] [CrossRef]
- Qian, X.; Liu, Q.; Zhang, L.; Li, H.; Liu, J.; Yan, S. Synthesis of reactive DOPO-based flame retardant and its application in rigid polyisocyanurate-polyurethane foam. Polym. Degrad. Stab. 2022, 197, 109852. [Google Scholar] [CrossRef]
- Wang, S.-X.; Zhao, H.-B.; Rao, W.-H.; Huang, S.-C.; Wang, T.; Liao, W.; Wang, Y.-Z. Inherently flame-retardant rigid polyurethane foams with excellent thermal insulation and mechanical properties. Polymer 2018, 153, 616–625. [Google Scholar] [CrossRef]
- Pang, X.-Y.; Xin, Y.-P.; Shi, X.-Z.; Xu, J.-Z. Effect of different size-modified expandable graphite and ammonium polyphosphate on the flame retardancy, thermal stability, physical, and mechanical properties of rigid polyurethane foam. Polym. Eng. Sci. 2019, 59, 1381–1394. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, B.W.; Zeng, F.-R.; Lin, X.-C.; Zhang, J.-Y.; Wang, X.-L.; Wang, Y.-Z.; Zhao, H.B. Fully recyclable multifunctional adhesive with high durability, transparency, flame retardancy, and harsh- environment resistance. Sci. Adv. 2022, 8, eadd8527. [Google Scholar] [CrossRef]
- Han, S.; Yang, X.; Hu, X.; Wang, Y.; Wu, F. Optimal design of energy-saving foam with multifunctional characteristics: Superb flame retardancy, outstanding hydrophobic, excellent thermal insulation and robust mechanical performances. Polym. Degrad. Stab. 2023, 217, 110518. [Google Scholar] [CrossRef]
- Bifulco, A.; Varganici, C.-D.; Rosu, L.; Mustata, F.; Rosu, D.; Gaan, S. Recent advances in flame retardant epoxy systems containing non-reactive DOPO based phosphorus additives. Polym. Degrad. Stab. 2022, 200, 109962. [Google Scholar] [CrossRef]
- Hirsch, C.; Striegl, B.; Mathes, S.; Adlhart, C.; Edelmann, M.; Bono, E.; Gaan, S.; Salmeia, K.A.; Hoelting, L.; Krebs, A.; et al. Multiparameter toxicity assessment of novel DOPO-derived organophosphorus flame retardants. Arch. Toxicol. 2017, 91, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, D.; Korwitz, A.; Komber, H.; Jehnichen, D.; Haeussler, L.; Scheibner, H.; Liebmann, M.; Jaehnichen, K.; Voit, B. Biobased Aliphatic Polyesters with DOPO Substituents for Enhanced Flame Retardancy. Macromol. Chem. Phys. 2015, 216, 1447–1461. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Annamalai, P.K.; Martin, D.J. The use of cellulose nanocrystals to enhance the thermal insulation properties and sustainability of rigid polyurethane foam. Ind. Crops Prod. 2017, 107, 114–121. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid polyurethane foams with halogen-free flame retardants: Thermal insulation, mechanical, and flame retardant properties. J. Appl. Polym. Sci. 2020, 137, 47611. [Google Scholar] [CrossRef]
- Bykov, Y.; Wagner, S.; Walter, O.; Doering, M.; Fischer, O.; Pospiech, D.; Koeppl, T.; Altstaedt, V. Synthesis of new dibenzo [c.e] [1,2] oxaphosphorine 2-oxide containing diols based on diethanolamine. Heteroat. Chem. 2012, 23, 146–153. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Synthesis of a novel flame retardant based on DOPO derivatives and its application in waterborne polyurethane. RSC Adv. 2019, 9, 7411–7419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, F.; Hu, Z.; Wu, F.; Gao, M.; Chai, Z.; Wang, Y.; Gu, X.; Wang, Y. Study on the flame retardancy of rigid polyurethane foam with phytic acid-functionalized graphene oxide. Molecules 2023, 28, 6267. [Google Scholar] [CrossRef]
- Zhong, W.; Yu, Z.; Zhang, Z.X. Development of polyamide12 composite foam by supercritical CO2: The effect of flame retardants on foaming behavior and properties. Eur. Polym. J. 2022, 179, 111562. [Google Scholar] [CrossRef]

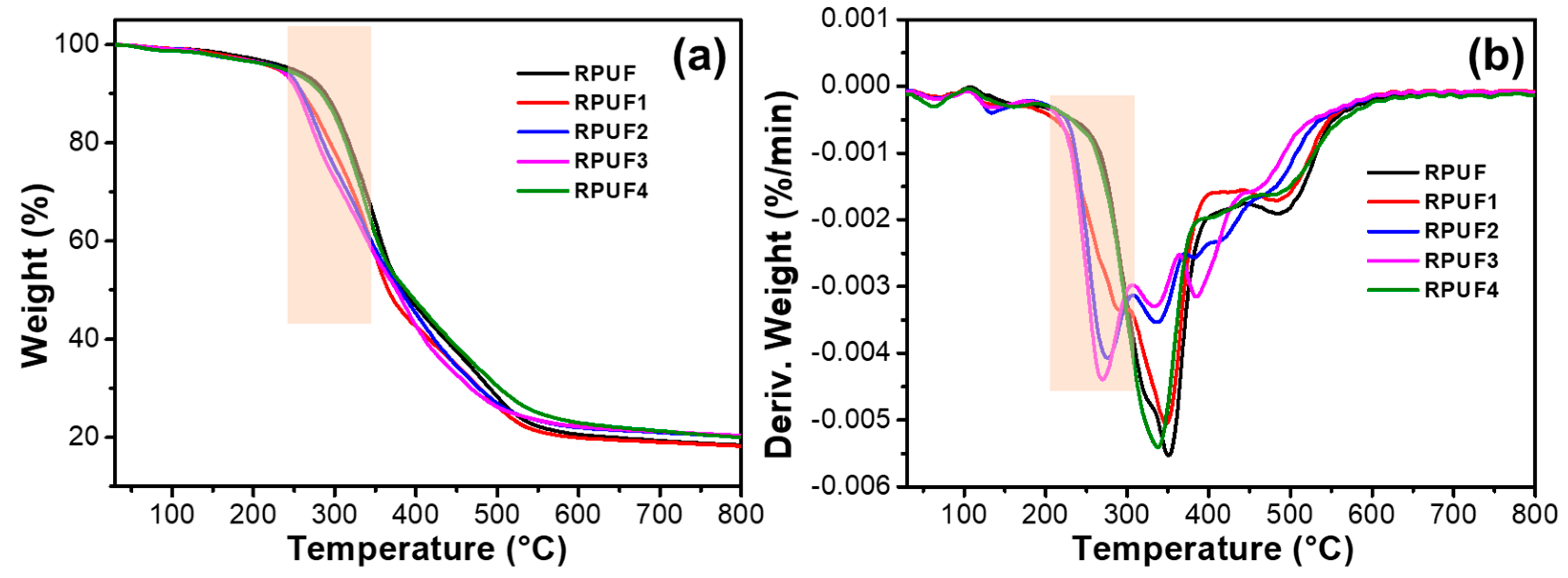


| Samples | T5% (°C) | Tmax (°C) | Residues (%) | |
|---|---|---|---|---|
| Tmax1 | Tmax2 | |||
| RPUF | 247.6 | / | 350.6 | 18.3 |
| RPUF1 | 229.3 | 292.3 | 347.3 | 18.2 |
| RPUF2 | 233.0 | 275.6 | 336.3 | 20.1 |
| RPUF3 | 231.3 | 269.3 | 333.6 | 20.4 |
| RPUF4 | 237.3 | / | 347.0 | 19.9 |
| Samples | LOI (%) | UL 94 (HB) | PHRR (kW/m2) | THR (MJ/m2) | av-CO2 (kg/kg) | FPI (m2s/kW) |
|---|---|---|---|---|---|---|
| RPUF | 19.1 | NR | 353.7 | 16.6 | 1.5 | <0.001 |
| RPUF1 | 23.6 | HF2 | 419.3 | 15.2 | 2.6 | 0.038 |
| RPUF3 | 28.3 | HF1 | 295.5 | 14.2 | 2.3 | 0.068 |
| RPUF4 | 25.4 | HF2 | 320.1 | 15.1 | 1.8 | 0.006 |
| Samples | Component A (g) | Component B (g) | Potassium Salt (g) | DFD (g) |
|---|---|---|---|---|
| RPUF | 50 | 50 | 0 | 0 |
| RPUF1 | 49 | 49 | 2 | 0 |
| RPUF2 | 46.5 | 46.5 | 2 | 5 |
| RPUF3 | 44 | 44 | 2 | 10 |
| RPUF4 | 45 | 45 | 0 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Wang, L.; Gao, T.; Zhong, Y.; Ren, K. Enhanced Fire Safety of Energy-Saving Foam by Self-Cleavage CO2 Pre-Combustion and Phosphorus Release Post-Combustion. Molecules 2024, 29, 3708. https://doi.org/10.3390/molecules29153708
Sun F, Wang L, Gao T, Zhong Y, Ren K. Enhanced Fire Safety of Energy-Saving Foam by Self-Cleavage CO2 Pre-Combustion and Phosphorus Release Post-Combustion. Molecules. 2024; 29(15):3708. https://doi.org/10.3390/molecules29153708
Chicago/Turabian StyleSun, Fengyun, Lijun Wang, Tiantian Gao, Yuanyuan Zhong, and Kefa Ren. 2024. "Enhanced Fire Safety of Energy-Saving Foam by Self-Cleavage CO2 Pre-Combustion and Phosphorus Release Post-Combustion" Molecules 29, no. 15: 3708. https://doi.org/10.3390/molecules29153708
APA StyleSun, F., Wang, L., Gao, T., Zhong, Y., & Ren, K. (2024). Enhanced Fire Safety of Energy-Saving Foam by Self-Cleavage CO2 Pre-Combustion and Phosphorus Release Post-Combustion. Molecules, 29(15), 3708. https://doi.org/10.3390/molecules29153708





