From Classical to Alternative Pathways of 2-Arachidonoylglycerol Synthesis: AlterAGs at the Crossroad of Endocannabinoid and Lysophospholipid Signaling
Abstract
1. Introduction

| Enzymes | Cells or Tissues | Subcellular Localization Following Activation | Conditions of Activation | (Patho)physiological Involvement | Ref | |
|---|---|---|---|---|---|---|
| PLC | PLCβ1 | Hippocampal neurons | Plasma membrane | Gq/11-coupled receptors (mGluR1/mGluR5 or M1/M3) plus depolarization | Complex picture describing KO mice and human pathologies reviewed in detail by Katan and Cockroft [34] | [25] |
| PLCβ4 | Cerebellum (Purkinje cells) | Plasma membrane | Gq/11-coupled receptor (mGluR1) plus depolarization | [35] | ||
| PLCδ | Cultured hippocampal neurons | Plasma membrane | Depolarization (DSI)No effect of δ1, δ3, δ4 KO | [36] | ||
| PLCε | Ventral tegmental area (VTA) dopamine neurons | Plasma membrane | Depolarization (DSI) facilitated by cAMP-Epac2-Rap-PLCε cascade | Contribution to cocaine-induced disinhibition of VTA dopamine neurons | [37] | |
| PLCγ1 | Hippocampus (mossy fiber synapses onto stratum lucidum interneurons Calyx of Held (giant glutamatergic synapse) | Plasma membrane | High-frequency stimulation leading to long-term depression via endogenous BDNF release BDNF application during depolarization | [38] [39] | ||
| PLCγ2 | Macrophages, microglia | Plasma membrane | FcγR cross-linking generating a DAG–MAG–eicosanoid network | Hyperactive variants in autoimmune and inflammatory diseases or protecting from Alzheimer disease | [40] | |
| DAGL | DAGLα | Hippocampus, cerebellum, striatum slices or cultured neurons Striatonigral direct-projecting pathway medium spiny neurons | Plasma membraneRapid turnover upon membrane trafficking | Gq/11-coupled receptors or depolarization Depolarization | Production of 2-AG and AAAxon growth/guidance, neurogenesisAnxiety, fear, extinction, impairmentMetabolic phenotype similar to CB1-KO miceNeuro-ocular DAGLA related syndrome Ethanol effects | [41,42,43,44] [45,46,47,48] [49,50] [51] [52] [53] |
| DAGLα | Astrocytes Tanycytes | Plasma membrane Plasma membrane | Affective disorders, hedonic feeding Inhibition TRH release | [54,55] [56] | ||
| DAGLβ | Brain, liver, macrophages, microglia, S. nigra dopaminergic neuronsCargo protein of AP-4 vesicles | Plasma membrane AP-4 vesicles during axonal anterograde transport | Altered neurogenesis2-AG, AA, and eicosanoid production Parkinson disease2-AG-dependent axon growth (altered in AP4-deficiency) | [42] [57,58] [59] [60] | ||
| ABHD6 | Neuro-2a cells | ND | Retinoic acid-induced differentiation | [61] | ||
| ABHD11 | Ubiquitous expression | Mitochondria | No change in tissue 2-AGKO mice resistant to obesity | [62] | ||
| DDHD2 | Brain | Cytosol | In vitro determination | DAGL in vitro, TAGL in vivoPlastic paraplegia | [63,64,65,66] | |
| HSL | Neurons and astrocytes | Pre- and post-synaptic membranes | Short- and long-term memory in aged mice | [67,68] | ||
2. Variations in the Use of PLC and DAGL Isoforms Involved in 2-AG Synthesis
2.1. Phosphoinositide-Specific PLCs
2.2. Duality between DAGLα and DAGLβ
2.3. A Nuclear PLCβ-DAGLα Cascade
2.4. Other Lipases Possibly Involved in 2-AG Synthesis
2.4.1. ABHD6
2.4.2. ABHD11
2.4.3. DDHD2

2.4.4. HSL
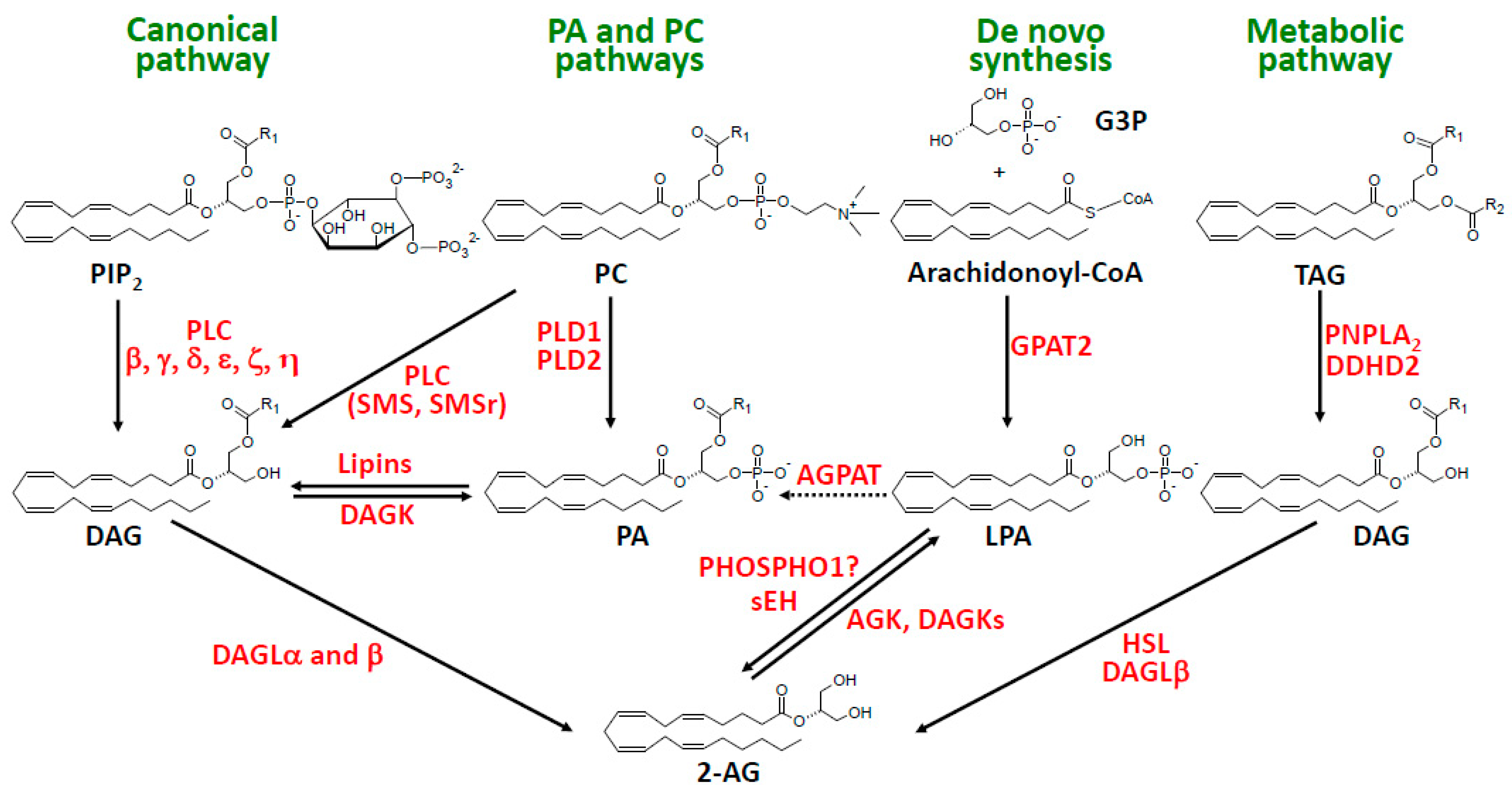
3. The PA and PC Pathways
4. The Metabolic Pathway of 2-AG Synthesis
5. The De Novo Synthetic Pathway
6. Importance of sn-2 Position of AA in 2-AG and LPLs
6.1. Regioselectivity of Various Receptors
6.1.1. CB1 Receptor
6.1.2. CB2 Receptor
6.1.3. TRPV1 Receptor
6.1.4. GPR55 Receptor
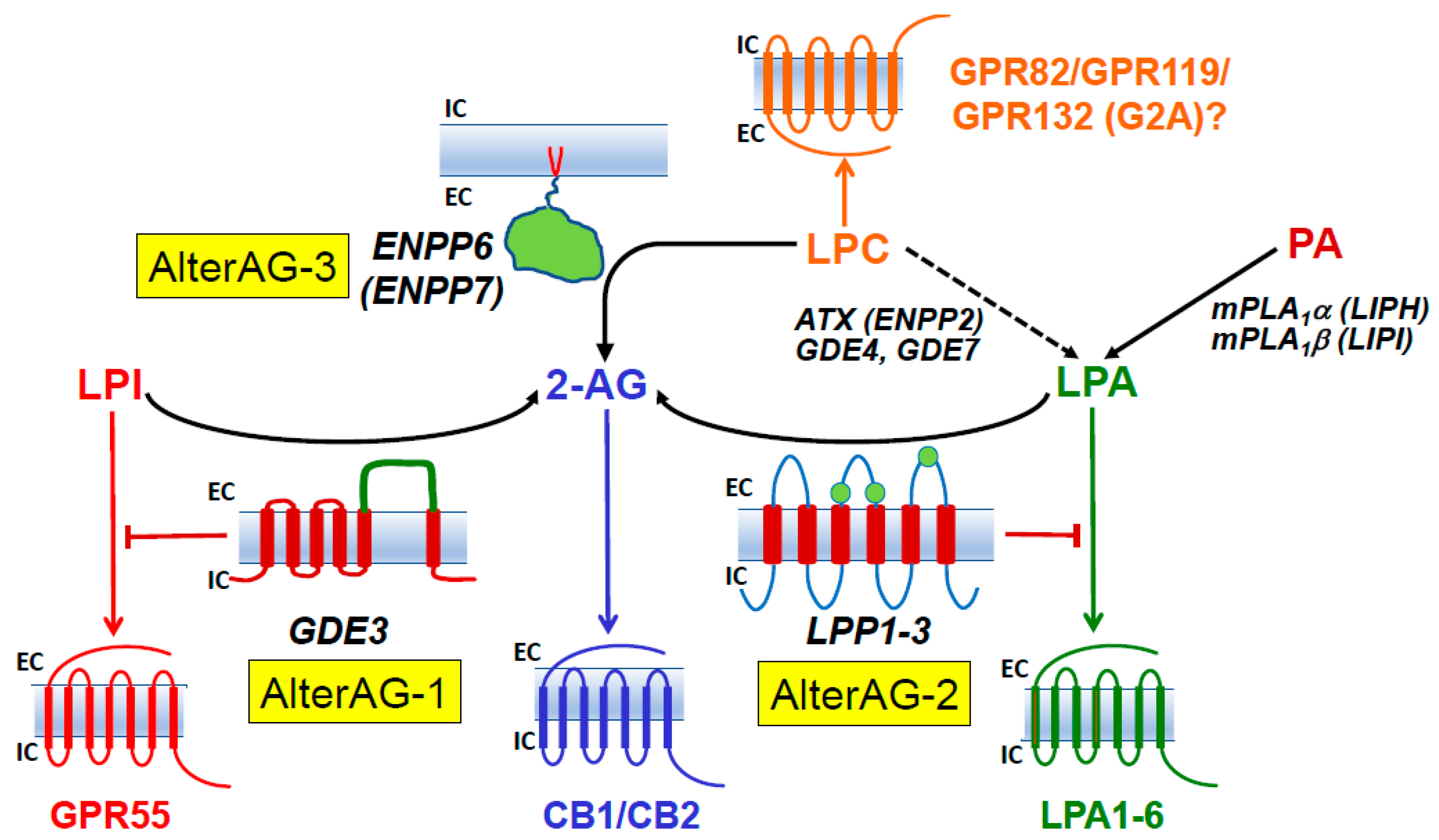
6.1.5. LPA Receptors
6.1.6. LPS Receptors
6.2. Regioselectivity of Various Lipid Acyl Hydrolases
6.2.1. MAGL, ABHD6, and ABHD12
6.2.2. FAAH
6.2.3. PLRP2
6.2.4. LYPLA1 and LYPLA2
6.2.5. ABHD16A
6.3. Regioselectivity of Various Lipid Phosphatases and Phosphodiesterases
6.3.1. LPPs
6.3.2. GDE3
6.3.3. ENPP6 and ENPP7
6.3.4. ATX
7. AlterAG Pathways
7.1. AlterAG-1
7.1.1. In Vitro Identification of GDE3 and DDHD1 as Main Actors of AlterAG-1
7.1.2. Signaling Switch between GPR55 and Classical Cannabinoid Receptors
7.1.3. Evidence That GDE3 and DDHD1 Are Functional In Vivo
7.1.4. Possible (Patho)physiological Role(s) of GDE3 and DDHD1
7.1.5. Other Activities of GDE3
7.2. AlterAG-2
7.2.1. Discovery of AlterAG-2 Pathway
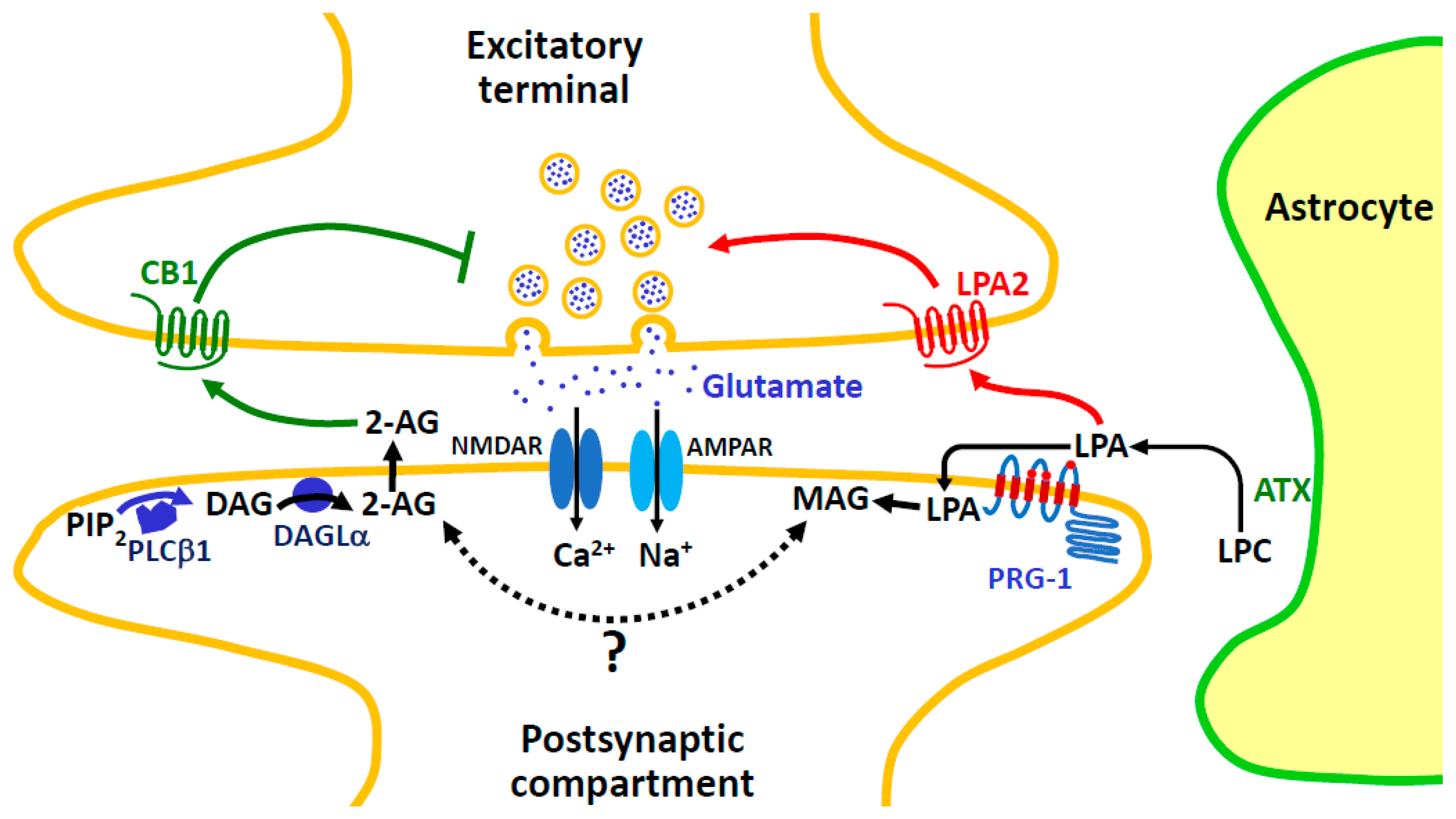
7.2.2. Signaling Switch between LPA and Classical Cannabinoid Receptors
7.2.3. Properties of LPPs
7.2.4. (Patho)physiological Roles of LPPs
7.2.5. PLA1 (LIPH and LIPI) as a Major Pathway of 2-Arachidonoyl-LPA Production
7.2.6. The Enigmatic and Fascinating Case of LPR-4/PRG-1

7.3. AlterAG-3
7.3.1. Availability of Arachidonoyl-LPC as Substrate of ENPP6 and ENPP7
7.3.2. Present Status of GPCRs Recognizing LPC as Specific Ligand
7.3.3. Properties of ENPP6 and ENPP7
8. Concluding Remarks and Potential Future Research Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-AG, 2-arachidonoylglycerol | GABA, γ-amino butyric acid |
| AA, arachidonic acid | GDE, glycerophosphodiesterase (types 1 to 7) |
| ABHD, α/β-Hydrolase Domain-Containing | GPAT, sn-glycerol-3-phosphate acyltransferase |
| AGK, acylglycerol kinase | GPC, glypican |
| AGPAT, 1-acylglycerol-3-phosphate acyltransferase | GPCR, G-protein-coupled receptor |
| A-LPA, arachidonoyl-LPA | GPR55, G-protein-coupled receptor 55 (other types with different numbers) |
| AlterAG, alternative pathway of 2-AG synthesis | HSL, hormone-sensitive lipase |
| AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor | I-LTD, long-term depression at inhibitory synapses |
| AP4, adaptator protein complex 4 | LBPA, lysobisphosphatidic acid |
| ATGL, adipose triglyceride lipase | LIPH and LIPI, lipases H and I |
| ATX, autotaxin | LPA1 to 6, LPA receptors 1 to 6 |
| BDNF, Brain-Derived Neurotrophic Factor | LPC, lysophosphatidylcholine |
| BMP, bis(monoacylglycero)phosphate (see also LBPA) | LPE, lysophosphatidylethanolamine |
| CaM, calmodulin | LPG, lysophosphatidylglycerol |
| CB1, cannabinoid receptor (types 1 or 2) | LPI, lysophosphatidylinositol |
| CNTFRα, ciliary neurotrophic factor receptor α | LPL, lysophospholipid |
| cPA, cyclic phosphatidic acid | LPLAT, lysophospholipid acyltransferase |
| D609, tricyclodecan-9-yl-xanthogenate | LPP, lipid phosphate phosphatase (types 1 to 3) |
| DAG, diacylglycerol | LPR1 to 5, LPP related proteins (see also PRG) |
| DAGK, diacylglycerol kinase | LPS, lysophosphatidylserine |
| DAGL, diacylglycerol lipase | LPS1 to 3, LPS receptors 1 to 3 |
| DDHD, DDHD containing | LYPLA1 or 2, lysophospholipase A1 or 2 |
| DHA, docosahexaenoic acid | Lyso-PtdGlc, lysophosphatidyl-β-D-glucose |
| DSE, depolarization-induced suppression of excitation | MAFP, methylarachidonoylfluorophosphonate |
| DSI, depolarization-induced suppression of inhibition | MAG, monoacylglycerol |
| EC, endocannabinoid | MAGL, monoacylglycerol lipase |
| ENPP, ecto-nucleotide pyrophosphatase/phosphodiesterase | mGluR1/5, metabotropic glutamate receptor (1 or 5) |
| Epac, exchange protein directly activated by cAMP | mPA-PLA1 (membrane-associated PA-selective PLA1 (α or β) |
| EVs, extracellular vesicles | MPSIIIB, mucopolysaccharosidosis type IIIB |
| FAAH, fatty acid amide hydrolase | NAGLU, N-acetyl-β-D-glucosaminidase gene |
| G3P, sn-glycerol-3-phosphate | NAPE, N-acyl-phosphatidylethanolamine |
| NMDAR, N-methyl-D-aspartate receptor | PNPLA2, patatin-like PLA2 |
| NODRS, neuro-ocular DAGLA-related syndrome | PP2A, protein phosphatase 2A |
| OGDHc, oxoglutarate dehydrogenase complex | PPAR, peroxisome proliferator-activated receptor |
| OX-A, orexin-A; OX-1R, orexin receptor 1 | PRG1 to 5, plasticity-related genes |
| PA, phosphatidic acid | PS, phosphatidylserine |
| PAF, platelet-activating factor or 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine | RECK, reversion-inducing cysteine-rich protein with Kazal motifs |
| PC, phosphatidylcholine | S. nigra, substantia nigra |
| PE, phosphatidylethanolamine | sAC, soluble adenylate cyclase |
| PG, phosphatidylglycerol | sEH, soluble epoxide hydrolase |
| PHOSPHO1, phosphocholine and phosphoethanolamine phosphatase | SMS, sphingomyelin synthase |
| PI, phosphatidylinositol | SMSr, sphingomyelin synthase-related protein |
| PI3P, phosphatidylinositol 3-monophosphate | TAG, triacylglycerol |
| PIP2, phosphatidylinositol 4,5-bisphosphate | TAGL, triacylglycerol lipase |
| PIP3, phosphatidylinositol 3,4,5-trisphosphate | THC, Δ-9-tetrahydocannabinol |
| PKA or PKC, protein kinase A or C | THL, tetrahydrolipstatin (lipase inhibitor) |
| PLA2, phospholipase A2 | TPA, 12-O-tetradecanoylphorbol-13-acetate |
| PLC, phospholipase C | TRPV, transient receptor potential cation channel subfamily V (various types with different numbers) |
| PLD, phospholipase D | uPAR, urokinase-type plasminogen activator receptor |
| PLRP2, pancreatic lipase related protein 2 | VTA, ventral tegmental area |
References
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Kouchaeknejad, A.; Van Der Walt, G.; De Donato, M.H.; Puighermanal, E. Imaging and Genetic Tools for the Investigation of the Endocannabinoid System in the CNS. Int. J. Mol. Sci. 2023, 24, 15829. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 2013, 280, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Nicolussi, S.; Gertsch, J. Endocannabinoid transport revisited. Vitam. Horm. 2015, 98, 441–485. [Google Scholar] [CrossRef] [PubMed]
- Kaczocha, M.; Haj-Dahmane, S. Mechanisms of endocannabinoid transport in the brain. Br. J. Pharmacol. 2022, 179, 4300–4310. [Google Scholar] [CrossRef]
- Piomelli, D.; Mabou Tagne, A. Endocannabinoid-Based Therapies. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 483–507. [Google Scholar] [CrossRef]
- Stella, N.; Schweitzer, P.; Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 1997, 388, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kishimoto, S.; Oka, S.; Gokoh, M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 2006, 45, 405–446. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998, 21, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov. Today 2010, 15, 474–483. [Google Scholar] [CrossRef]
- Cao, J.K.; Kaplan, J.; Stella, N. ABHD6: Its Place in Endocannabinoid Signaling and Beyond. Trends Pharmacol. Sci. 2019, 40, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.L.; Vaughan, C.W. Mechanisms of endocannabinoid control of synaptic plasticity. Neuropharmacology 2021, 197, 108736. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sengupta, N.; Sohn, M.; Mandal, A.; Pemberton, J.G.; Choi, U.; Balla, T. Metabolic routing maintains the unique fatty acid composition of phosphoinositides. EMBO Rep. 2022, 23, e54532. [Google Scholar] [CrossRef]
- Barneda, D.; Janardan, V.; Niewczas, I.; Collins, D.M.; Cosulich, S.; Clark, J.; Stephens, L.R.; Hawkins, P.T. Acyl chain selection couples the consumption and synthesis of phosphoinositides. EMBO J. 2022, 41, e110038. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.S.; Colangeli, R.; Dong, A.; George, A.G.; Addo-Osafo, K.; Kingsley, P.J.; Morena, M.; Wolff, M.D.; Dudok, B.; He, K.; et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron 2021, 109, 2398–2403.e4. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; He, K.; Dudok, B.; Farrell, J.S.; Guan, W.; Liput, D.J.; Puhl, H.L.; Cai, R.; Wang, H.; Duan, J.; et al. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nat. Biotechnol. 2022, 40, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Islam, A.; Chen, B.; Zhang, H.; Chi, D.H.; Mamun, M.A.; Takahashi, Y.; Sato, N.; Yamasue, H.; Nakajima, Y.; et al. Endocannabinoid 2-Arachidonoylglycerol Levels in the Anterior Cingulate Cortex, Caudate Putamen, Nucleus Accumbens, and Piriform Cortex Were Upregulated by Chronic Restraint Stress. Cells 2023, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Hashimotodani, Y.; Ohno-Shosaku, T.; Tsubokawa, H.; Ogata, H.; Emoto, K.; Maejima, T.; Araishi, K.; Shin, H.S.; Kano, M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 2005, 45, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.M.; Astarita, G.; Zhu, C.; Wallace, M.; Mackie, K.; Piomelli, D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol. Pharmacol. 2007, 72, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.J.; Puhl, H.L.; Ikeda, S.R. Molecular reconstruction of mGluR5a-mediated endocannabinoid signaling cascade in single rat sympathetic neurons. J. Neurosci. 2009, 29, 13603–13612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Howell, F.V.; Glebov, O.O.; Albrecht, D.; Williams, G.; Doherty, P. Regulated endosomal trafficking of Diacylglycerol lipase alpha (DAGLα) generates distinct cellular pools; implications for endocannabinoid signaling. Mol. Cell. Neurosci. 2016, 76, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Reisenberg, M.; Singh, P.K.; Williams, G.; Doherty, P. The diacylglycerol lipases: Structure, regulation and roles in and beyond endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3264–3275. [Google Scholar] [CrossRef]
- Alger, B.E. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): What we still do not know. J. Physiol. 2012, 590, 2203–2212. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Matsuda, R.; Morito, K.; Kawabata, K.; Yokota, M.; Nikawadori, M.; Inoue-Fujiwara, M.; Kawashima, S.; Hidaka, M.; Yamamoto, T.; et al. Identification of human glycerophosphodiesterase 3 as an ecto phospholipase C that converts the G protein-coupled receptor 55 agonist lysophosphatidylinositol to bioactive monoacylglycerols in cultured mammalian cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158761. [Google Scholar] [CrossRef] [PubMed]
- Briand-Mésange, F.; Pons, V.; Allart, S.; Masquelier, J.; Chicanne, G.; Beton, N.; Payrastre, B.; Muccioli, G.G.; Ausseil, J.; Davignon, J.L.; et al. Glycerophosphodiesterase 3 (GDE3) is a lysophosphatidylinositol-specific ectophospholipase C acting as an endocannabinoid signaling switch. J. Biol. Chem. 2020, 295, 15767–15781. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, I.; Gaspar, R.C.; Luukkonen, P.K.; Kahn, M.; Zhang, D.; Zhang, X.; Murray, S.; Golla, J.P.; Vatner, D.F.; Samuel, V.T.; et al. Lysophosphatidic acid triggers inflammation in the liver and white adipose tissue in rat models of 1-acyl-sn-glycerol-3-phosphate acyltransferase 2 deficiency and overnutrition. Proc. Natl. Acad. Sci. USA 2023, 120, e2312666120. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Cockcroft, S. Phospholipase C families: Common themes and versatility in physiology and pathology. Prog. Lipid Res. 2020, 80, 101065. [Google Scholar] [CrossRef] [PubMed]
- Maejima, T.; Oka, S.; Hashimotodani, Y.; Ohno-Shosaku, T.; Aiba, A.; Wu, D.; Waku, K.; Sugiura, T.; Kano, M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J. Neurosci. 2005, 25, 6826–6835. [Google Scholar] [CrossRef] [PubMed]
- Hashimotodani, Y.; Ohno-Shosaku, T.; Maejima, T.; Fukami, K.; Kano, M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology 2008, 54, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Liu, X.; Vickstrom, C.; Li, Y.; Yu, L.; Lu, Y.; Smrcka, A.V.; Liu, Q.S. The Epac-Phospholipase Cε Pathway Regulates Endocannabinoid Signaling and Cocaine-Induced Disinhibition of Ventral Tegmental Area Dopamine Neurons. J. Neurosci. 2017, 37, 3030–3044. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Q.; Guo, B.; Ye, F.; Ge, J.; Xue, L. BDNF Activates Postsynaptic TrkB Receptors to Induce Endocannabinoid Release and Inhibit Presynaptic Calcium Influx at a Calyx-Type Synapse. J. Neurosci. 2020, 40, 8070–8087. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Zhao, Z.; McNamara, J.O. LTD at mossy fiber synapses onto stratum lucidum interneurons requires TrkB and retrograde endocannabinoid signaling. J. Neurophysiol. 2019, 121, 609–619. [Google Scholar] [CrossRef]
- Jing, H.; Reed, A.; Ulanovskaya, O.A.; Grigoleit, J.S.; Herbst, D.M.; Henry, C.L.; Li, H.; Barbas, S.; Germain, J.; Masuda, K.; et al. Phospholipase Cγ2 regulates endocannabinoid and eicosanoid networks in innate immune cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2112971118. [Google Scholar] [CrossRef]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Gao, Y.; Vasilyev, D.V.; Goncalves, M.B.; Howell, F.V.; Hobbs, C.; Reisenberg, M.; Shen, R.; Zhang, M.Y.; Strassle, B.W.; Lu, P.; et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 2010, 30, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, A.; Yamazaki, M.; Hashimotodani, Y.; Uchigashima, M.; Kawata, S.; Abe, M.; Kita, Y.; Hashimoto, K.; Shimizu, T.; Watanabe, M.; et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 2010, 65, 320–327. [Google Scholar] [CrossRef]
- Ogasawara, D.; Deng, H.; Viader, A.; Baggelaar, M.P.; Breman, A.; den Dulk, H.; van den Nieuwendijk, A.M.; Soethoudt, M.; van der Wel, T.; Zhou, J.; et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc. Natl. Acad. Sci. USA 2016, 113, 26–33. [Google Scholar] [CrossRef]
- Oudin, M.J.; Hobbs, C.; Doherty, P. DAGL-dependent endocannabinoid signalling: Roles in axonal pathfinding, synaptic plasticity and adult neurogenesis. Eur. J. Neurosci. 2011, 34, 1634–1646. [Google Scholar] [CrossRef]
- Jung, K.M.; Astarita, G.; Thongkham, D.; Piomelli, D. Diacylglycerol lipase-alpha and -beta control neurite outgrowth in neuro-2a cells through distinct molecular mechanisms. Mol. Pharmacol. 2011, 80, 60–67. [Google Scholar] [CrossRef]
- Schuele, L.L.; Schuermann, B.; Bilkei-Gorzo, A.; Gorgzadeh, S.; Zimmer, A.; Leidmaa, E. Regulation of adult neurogenesis by the endocannabinoid-producing enzyme diacylglycerol lipase alpha (DAGLa). Sci. Rep. 2022, 12, 633. [Google Scholar] [CrossRef]
- Itami, C.; Uesaka, N.; Huang, J.Y.; Lu, H.C.; Sakimura, K.; Kano, M.; Kimura, F. Endocannabinoid-dependent formation of columnar axonal projection in the mouse cerebral cortex. Proc. Natl. Acad. Sci. USA 2022, 119, e2122700119. [Google Scholar] [CrossRef] [PubMed]
- Shonesy, B.C.; Bluett, R.J.; Ramikie, T.S.; Báldi, R.; Hermanson, D.J.; Kingsley, P.J.; Marnett, L.J.; Winder, D.G.; Colbran, R.J.; Patel, S. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 2014, 9, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Cavener, V.S.; Gaulden, A.; Pennipede, D.; Jagasia, P.; Uddin, J.; Marnett, L.J.; Patel, S. Inhibition of Diacylglycerol Lipase Impairs Fear Extinction in Mice. Front. Neurosci. 2018, 12, 479. [Google Scholar] [CrossRef]
- Powell, D.R.; Gay, J.P.; Wilganowski, N.; Doree, D.; Savelieva, K.V.; Lanthorn, T.H.; Read, R.; Vogel, P.; Hansen, G.M.; Brommage, R.; et al. Diacylglycerol Lipase α Knockout Mice Demonstrate Metabolic and Behavioral Phenotypes Similar to Those of Cannabinoid Receptor 1 Knockout Mice. Front. Endocrinol. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, M.N.; Mazumder, A.; Ogasawara, D.; Abou Jamra, R.; Bernard, G.; Bertini, E.; Burglen, L.; Cope, H.; Crawford, A.; Derksen, A.; et al. Endocannabinoid dysfunction in neurological disease: Neuro-ocular DAGLA-related syndrome. Brain 2022, 145, 3383–3390. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.M.; Gracias, A.L.; Luo, G.; Anumola, R.C.; Lovinger, D.M. Striatonigral direct pathway 2-arachidonoylglycerol contributes to ethanol effects on synaptic transmission and behavior. Neuropsychopharmacology 2023, 48, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Schuele, L.L.; Glasmacher, S.; Gertsch, J.; Roggan, M.D.; Transfeld, J.L.; Bindila, L.; Lutz, B.; Kolbe, C.C.; Bilkei-Gorzo, A.; Zimmer, A.; et al. Diacylglycerol lipase alpha in astrocytes is involved in maternal care and affective behaviors. Glia 2021, 69, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Leidmaa, E.; Prodan, A.M.; Depner, L.L.; Komorowska-Müller, J.A.; Beins, E.C.; Schuermann, B.; Kolbe, C.C.; Zimmer, A. Astrocytic Dagla Deletion Decreases Hedonic Feeding in Female Mice. Cannabis Cannabinoid Res. 2024, 9, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Kővári, D.; Penksza, V.; Szilvásy-Szabó, A.; Sinkó, R.; Gereben, B.; Mackie, K.; Fekete, C. Tanycyte specific ablation of diacylglycerol lipase alpha stimulates the hypothalamic-pituitary-thyroid axis by decreasing the endocannabinoid mediated inhibition of TRH release. J. Neuroendocrinol. 2022, 34, e13079. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.L.; Tsuboi, K.; Adibekian, A.; Pugh, H.; Masuda, K.; Cravatt, B.F. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 2012, 8, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Blankman, J.L.; Zhong, P.; Liu, X.; Schlosburg, J.E.; Joslyn, C.M.; Liu, Q.S.; Tomarchio, A.J.; Lichtman, A.H.; Selley, D.E.; et al. Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Rep. 2015, 12, 798–808. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, N.; Dong, J.; Tian, W.; Chang, L.; Ma, J.; Guo, J.; Tan, J.; Dong, A.; He, K.; et al. Deficiency in endocannabinoid synthase DAGLB contributes to early onset Parkinsonism and murine nigral dopaminergic neuron dysfunction. Nat. Commun. 2022, 13, 3490. [Google Scholar] [CrossRef]
- Davies, A.K.; Alecu, J.E.; Ziegler, M.; Vasilopoulou, C.G.; Merciai, F.; Jumo, H.; Afshar-Saber, W.; Sahin, M.; Ebrahimi-Fakhari, D.; Borner, G.H.H. AP-4-mediated axonal transport controls endocannabinoid production in neurons. Nat. Commun. 2022, 13, 1058. [Google Scholar] [CrossRef]
- van Esbroeck, A.C.M.; Kantae, V.; Di, X.; van der Wel, T.; den Dulk, H.; Stevens, A.F.; Singh, S.; Bakker, A.T.; Florea, B.I.; Stella, N.; et al. Identification of α,β-Hydrolase Domain Containing Protein 6 as a Diacylglycerol Lipase in Neuro-2a Cells. Front. Mol. Neurosci. 2019, 12, 286. [Google Scholar] [CrossRef] [PubMed]
- Escoubet, J.; Kenigsberg, M.; Derock, M.; Yaligara, V.; Bock, M.D.; Roche, S.; Massey, F.; de Foucauld, H.; Bettembourg, C.; Olivier, A.; et al. ABHD11, a new diacylglycerol lipase involved in weight gain regulation. PLoS ONE 2020, 15, e0234780. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Ohshima, N.; Aso, C.; Konishi, A.; Obinata, H.; Tatei, K.; Izumi, T. Enzymatic characterization of recombinant rat DDHD2: A soluble diacylglycerol lipase. J. Biochem. 2016, 160, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Aso, C.; Araki, M.; Ohshima, N.; Tatei, K.; Hirano, T.; Obinata, H.; Kishi, M.; Kishimoto, K.; Konishi, A.; Goto, F.; et al. Protein purification and cloning of diacylglycerol lipase from rat brain. J. Biochem. 2016, 159, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Inloes, J.M.; Hsu, K.L.; Dix, M.M.; Viader, A.; Masuda, K.; Takei, T.; Wood, M.R.; Cravatt, B.F. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc. Natl. Acad. Sci. USA 2014, 111, 14924–14929. [Google Scholar] [CrossRef] [PubMed]
- Inloes, J.M.; Kiosses, W.B.; Wang, H.; Walther, T.C.; Farese, R.V.; Cravatt, B.F. Functional Contribution of the Spastic Paraplegia-Related Triglyceride Hydrolase DDHD2 to the Formation and Content of Lipid Droplets. Biochemistry 2018, 57, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Skoug, C.; Holm, C.; Duarte, J.M.N. Hormone-sensitive lipase is localized at synapses and is necessary for normal memory functioning in mice. J. Lipid Res. 2022, 63, 100195. [Google Scholar] [CrossRef]
- Skoug, C.; Rogova, O.; Spégel, P.; Holm, C.; Duarte, J.M.N. Genetic deletion of hormone-sensitive lipase in mice reduces cerebral blood flow but does not aggravate the impact of diet-induced obesity on memory. J. Neurochem. 2024, 168, 781–800. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; van der Stelt, M.; Tsubokawa, H.; Krestel, H.; Moers, A.; Petrosino, S.; Schütz, G.; Di Marzo, V.; Offermanns, S. Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol. Cell. Biol. 2006, 26, 5888–5894. [Google Scholar] [CrossRef]
- Nakahara, M.; Shimozawa, M.; Nakamura, Y.; Irino, Y.; Morita, M.; Kudo, Y.; Fukami, K. A novel phospholipase C, PLC(eta)2, is a neuron-specific isozyme. J. Biol. Chem. 2005, 280, 29128–29134. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, J.W.; Lim, S.; Kwon, O.; Seo, J.K.; Ryu, S.H.; Suh, P.G. Phospholipase C-η1 is activated by intracellular Ca(2+) mobilization and enhances GPCRs/PLC/Ca(2+) signaling. Cell. Signal. 2011, 23, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, T.J.; Lee, Y.H.; Baek, K.J.; Suh, P.G.; Ryu, S.H.; Kim, K.T. Phospholipase C-delta1 is activated by capacitative calcium entry that follows phospholipase C-beta activation upon bradykinin stimulation. J. Biol. Chem. 1999, 274, 26127–26134. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.S.; Zhou, H.; Huang, J.; Pentyala, S.N. Activation of PLC-delta1 by Gi/o-coupled receptor agonists. Am. J. Physiol. Cell Physiol. 2004, 287, C1679–C1687. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz, S.D.; Best, A.R.; Regehr, W.G. Sustained elevation of dendritic calcium evokes widespread endocannabinoid release and suppression of synapses onto cerebellar Purkinje cells. J. Neurosci. 2006, 26, 6841–6850. [Google Scholar] [CrossRef] [PubMed]
- Chap, H. Forty five years with membrane phospholipids, phospholipases and lipid mediators: A historical perspective. Biochimie 2016, 125, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Mauco, G.; Chap, H.; Simon, M.F.; Douste-Blazy, L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie 1978, 60, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Mauco, G.; Fauvel, J.; Chap, H.; Douste-Blazy, L. Studies on enzymes related to diacylglycerol production in activated platelets. II. Subcellular distribution, enzymatic properties and positional specificity of diacylglycerol- and monoacylglycerol-lipases. Biochim. Biophys. Acta 1984, 796, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Kennerly, D.A.; Stanford, N.; Majerus, P.W. Diglyceride lipase: A pathway for arachidonate release from human platelets. Proc. Natl. Acad. Sci. USA 1979, 76, 3238–3241. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.M.; Majerus, P.W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J. Biol. Chem. 1983, 258, 764–769. [Google Scholar] [CrossRef]
- Gratacap, M.P.; Payrastre, B.; Viala, C.; Mauco, G.; Plantavid, M.; Chap, H. Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-gamma2 is an early key event in FcgammaRIIA-mediated activation of human platelets. J. Biol. Chem. 1998, 273, 24314–24321. [Google Scholar] [CrossRef]
- Gratacap, M.P.; Hérault, J.P.; Viala, C.; Ragab, A.; Savi, P.; Herbert, J.M.; Chap, H.; Plantavid, M.; Payrastre, B. FcgammaRIIA requires a Gi-dependent pathway for an efficient stimulation of phosphoinositide 3-kinase, calcium mobilization, and platelet aggregation. Blood 2000, 96, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Deciphering Complex Interactions in Bioactive Lipid Signaling. Molecules 2023, 28, 2622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M.; Bisogno, T.; Di Marzo, V.; Alger, B.E. Endocannabinoids generated by Ca2+ or by metabotropic glutamate receptors appear to arise from different pools of diacylglycerol lipase. PLoS ONE 2011, 6, e16305. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Ogasawara, D.; Joslyn, C.M.; Sanchez-Alavez, M.; Mori, S.; Nguyen, W.; Conti, B.; Cravatt, B.F. A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. Elife 2016, 5, e12345. [Google Scholar] [CrossRef] [PubMed]
- García del Caño, G.; Aretxabala, X.; González-Burguera, I.; Montaña, M.; López de Jesús, M.; Barrondo, S.; Barrio, R.J.; Sampedro, C.; Goicolea, M.A.; Sallés, J. Nuclear diacylglycerol lipase-α in rat brain cortical neurons: Evidence of 2-arachidonoylglycerol production in concert with phospholipase C-β activity. J. Neurochem. 2015, 132, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Aretxabala, X.; García Del Caño, G.; Barrondo, S.; López de Jesús, M.; González-Burguera, I.; Saumell-Esnaola, M.; Goicolea, M.A.; Sallés, J. Endocannabinoid 2-Arachidonoylglycerol Synthesis and Metabolism at Neuronal Nuclear Matrix Fractions Derived from Adult Rat Brain Cortex. Int. J. Mol. Sci. 2023, 24, 3165. [Google Scholar] [CrossRef] [PubMed]
- Payrastre, B.; Nievers, M.; Boonstra, J.; Breton, M.; Verkleij, A.J.; Van Bergen en Henegouwen, P.M. A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J. Biol. Chem. 1992, 267, 5078–5084. [Google Scholar] [CrossRef] [PubMed]
- Pusch, L.M.; Riegler-Berket, L.; Oberer, M.; Zimmermann, R.; Taschler, U. α/β-Hydrolase Domain-Containing 6 (ABHD6)- A Multifunctional Lipid Hydrolase. Metabolites 2022, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.S.J.; Ortmann, B.M.; Martinelli, A.W.; Houghton, J.W.; Costa, A.S.H.; Burr, S.P.; Antrobus, R.; Frezza, C.; Nathan, J.A. ABHD11 maintains 2-oxoglutarate metabolism by preserving functional lipoylation of the 2-oxoglutarate dehydrogenase complex. Nat. Commun. 2020, 11, 4046. [Google Scholar] [CrossRef]
- Karottki, K.J.C.; Hefzi, H.; Li, S.; Pedersen, L.E.; Spahn, P.N.; Joshi, C.; Ruckerbauer, D.; Bort, J.A.H.; Thomas, A.; Lee, J.S.; et al. A metabolic CRISPR-Cas9 screen in Chinese hamster ovary cells identifies glutamine-sensitive genes. Metab. Eng. 2021, 66, 114–122. [Google Scholar] [CrossRef]
- Soria-Gomez, E.; Pagano Zottola, A.C.; Mariani, Y.; Desprez, T.; Barresi, M.; Bonilla-Del Río, I.; Muguruza, C.; Le Bon-Jego, M.; Julio-Kalajzić, F.; Flynn, R.; et al. Subcellular specificity of cannabinoid effects in striatonigral circuits. Neuron 2021, 109, 1513–1526.e11. [Google Scholar] [CrossRef] [PubMed]
- Pagano Zottola, A.C.; Severi, I.; Cannich, A.; Ciofi, P.; Cota, D.; Marsicano, G.; Giordano, A.; Bellocchio, L. Expression of Functional Cannabinoid Type-1 (CB1) Receptor in Mitochondria of White Adipocytes. Cells 2022, 11, 2582. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ruan, Y.; Zhang, J.; Wang, X.; Wu, W.; He, P.; Wang, J.; Xiong, J.; Cheng, Y.; Liu, L.; et al. ABHD11 Is Critical for Embryonic Stem Cell Expansion, Differentiation and Lipid Metabolic Homeostasis. Front. Cell Dev. Biol. 2020, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Puy, V.; Darwiche, W.; Trudel, S.; Gomila, C.; Lony, C.; Puy, L.; Lefebvre, T.; Vitry, S.; Boullier, A.; Karim, Z.; et al. Predominant role of microglia in brain iron retention in Sanfilippo syndrome, a pediatric neurodegenerative disease. Glia 2018, 66, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Deiva, K.; Ausseil, J.; de Bournonville, S.; Zérah, M.; Husson, B.; Gougeon, M.L.; Poirier-Beaudouin, B.; Zafeiriou, D.; Parenti, G.; Heard, J.M.; et al. Intracerebral Gene Therapy in Four Children with Sanfilippo B Syndrome: 5.5-Year Follow-Up Results. Hum. Gene Ther. 2021, 32, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Gun Bilgic, D.; Gerik Celebi, H.B.; Aydin Gumus, A.; Bilgic, A.; Yazici, H.; Ceylaner, S.; Yilmaz, C.; Polat, M.; Akbal Sahin, M.; Dereli, F.; et al. Coinheritance of novel mutations in NAGLU causing mucopolysaccharidosis type IIIB and in DDHD2 causing spastic paraplegia54 in a Turkish family. J. Clin. Neurosci. 2020, 82, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Harkany, T.; Horvath, T.L. (S)Pot on Mitochondria: Cannabinoids Disrupt Cellular Respiration to Limit Neuronal Activity. Cell Metab. 2017, 25, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Jong, Y.I.; Harmon, S.K.; O’Malley, K.L. Intracellular GPCRs Play Key Roles in Synaptic Plasticity. ACS Chem. Neurosci. 2018, 9, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Recazens, E.; Mouisel, E.; Langin, D. Hormone-sensitive lipase: Sixty years later. Prog. Lipid Res. 2021, 82, 101084. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, N.; Riera Ribas, C.; Lehtonen, M.; Savinainen, J.R.; Laitinen, J.T. Brain regional cannabinoid CB(1) receptor signalling and alternative enzymatic pathways for 2-arachidonoylglycerol generation in brain sections of diacylglycerol lipase deficient mice. Eur. J. Pharm. Sci. 2014, 51, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hoover, H.S.; Blankman, J.L.; Niessen, S.; Cravatt, B.F. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg Med. Chem. Lett. 2008, 18, 5838–5841. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Yoshinaga, N.; Waku, K. Rapid generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in rat brain after decapitation. Neurosci. Lett. 2001, 297, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Brose, S.A.; Golovko, S.A.; Golovko, M.Y. Brain 2-Arachidonoylglycerol Levels Are Dramatically and Rapidly Increased Under Acute Ischemia-Injury Which Is Prevented by Microwave Irradiation. Lipids 2016, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Goracci, G.; Francescangeli, E.; Horrocks, L.A.; Porcellati, G. The reverse reaction of cholinephosphotransferase in rat brain microsomes. A new pathway for degradation of phosphatidylcholine. Biochim. Biophys. Acta 1981, 664, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kogure, K.; Yamamoto, H.; Imazawa, M.; Miyamoto, K. Mechanism of arachidonic acid liberation during ischemia in gerbil cerebral cortex. J. Neurochem. 1987, 48, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Martial, C.; Cassol, H.; Laureys, S.; Gosseries, O. Near-Death Experience as a Probe to Explore (Disconnected) Consciousness. Trends Cogn. Sci. 2020, 24, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Melck, D.; De Petrocellis, L.; Di Marzo, V. Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J. Neurochem. 1999, 72, 2113–2119. [Google Scholar] [CrossRef]
- Reue, K.; Wang, H. Mammalian lipin phosphatidic acid phosphatases in lipid synthesis and beyond: Metabolic and inflammatory disorders. J. Lipid Res. 2019, 60, 728–733. [Google Scholar] [CrossRef]
- Balboa, M.A.; de Pablo, N.; Meana, C.; Balsinde, J. The role of lipins in innate immunity and inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Lutkewitte, A.J.; Finck, B.N. Regulation of Signaling and Metabolism by Lipin-mediated Phosphatidic Acid Phosphohydrolase Activity. Biomolecules 2020, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Song, H.; Wang, F. Role of lipins in cardiovascular diseases. Lipids Health Dis. 2023, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Carrier, E.J.; Kearn, C.S.; Barkmeier, A.J.; Breese, N.M.; Yang, W.; Nithipatikom, K.; Pfister, S.L.; Campbell, W.B.; Hillard, C.J. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol. Pharmacol. 2004, 65, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kolaj, M.; Renaud, L.P. Endocannabinoid 2-AG and intracellular cannabinoid receptors modulate a low-threshold calcium spike-induced slow depolarizing afterpotential in rat thalamic paraventricular nucleus neurons. Neuroscience 2016, 322, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Eurtivong, C.; Leung, E.; Sharma, N.; Leung, I.K.H.; Reynisson, J. Phosphatidylcholine-Specific Phospholipase C as a Promising Drug Target. Molecules 2023, 28, 5637. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Yanagimoto, S.; Ikeda, S.; Gokoh, M.; Kishimoto, S.; Waku, K.; Ishima, Y.; Sugiura, T. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J. Biol. Chem. 2005, 280, 18488–18497. [Google Scholar] [CrossRef] [PubMed]
- Murakami, C.; Sakane, F. Sphingomyelin synthase-related protein generates diacylglycerol via the hydrolysis of glycerophospholipids in the absence of ceramide. J. Biol. Chem. 2021, 296, 100454. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.P.; Li, Z.; Chen, Y.; Cao, Y.; Jiang, X.C. Sphingomyelin synthase related protein is a mammalian phosphatidylethanolamine phospholipase C. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159017. [Google Scholar] [CrossRef]
- Chiang, Y.P.; Li, Z.; Chen, Y.; Cao, Y.; Jiang, X.C. Sphingomyelin synthases 1 and 2 exhibit phosphatidylcholine phospholipase C activity. J. Biol. Chem. 2021, 297, 101398. [Google Scholar] [CrossRef]
- Chiang, Y.P.; Li, Z.; He, M.; Jones, Q.; Pan, M.; Han, X.; Jiang, X.C. Sphingomyelin synthase-related protein SMSr is a phosphatidylethanolamine phospholipase C that promotes nonalcoholic fatty liver disease. J. Biol. Chem. 2023, 299, 105162. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhang, Q.; Chen, Y.; Yang, J.; Xia, Y.; Rao, B.; Li, S.; Shen, Y.; Cao, M.; Lu, H.; et al. Cryo-EM structure of human sphingomyelin synthase and its mechanistic implications for sphingomyelin synthesis. Nat. Struct. Mol. Biol. 2024, 31, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Witting, A.; Walter, L.; Wacker, J.; Möller, T.; Stella, N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl. Acad. Sci. USA 2004, 101, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Murakami, C.; Hoshino, F.; Sakai, H.; Hayashi, Y.; Yamashita, A.; Sakane, F. Diacylglycerol kinase δ and sphingomyelin synthase-related protein functionally interact via their sterile α motif domains. J. Biol. Chem. 2020, 295, 2932–2947. [Google Scholar] [CrossRef] [PubMed]
- Furuta, M.; Murakami, C.; Numagami, Y.; Suzuki, R.; Sakane, F. Diacylglycerol kinase ζ interacts with sphingomyelin synthase 1 and sphingomyelin synthase-related protein via different regions. FEBS Open Bio 2023, 13, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V.; Walther, T.C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Ralhan, I.; Chang, C.L.; Lippincott-Schwartz, J.; Ioannou, M.S. Lipid droplets in the nervous system. J. Cell Biol. 2021, 220, e202102136. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Pierce, K.A.; Roix, J.J.; Tyler, A.; Chen, H.; Teixeira, S.R. The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes 2008, 57, 1262–1268. [Google Scholar] [CrossRef]
- Matias, I.; Gonthier, M.P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Petrosino, S.; Hoareau, L.; Festy, F.; Pasquali, R.; et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Attané, C.; Estève, D.; Chaoui, K.; Iacovoni, J.S.; Corre, J.; Moutahir, M.; Valet, P.; Schiltz, O.; Reina, N.; Muller, C. Human Bone Marrow Is Comprised of Adipocytes with Specific Lipid Metabolism. Cell Rep. 2020, 30, 949–958.e6. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Uyama, T.; Tsuboi, K.; Ueda, N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1546–1561. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Ware, T.B.; Lee, H.C.; Hsu, K.L. Lipid-metabolizing serine hydrolases in the mammalian central nervous system: Endocannabinoids and beyond. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.K.; Uyama, T.; Hussain, Z.; Ueda, N. Roles of Endocannabinoids and Endocannabinoid-Like Molecules in Energy Homeostasis and Metabolic Regulation: A Nutritional Perspective. Annu. Rev. Nutr. 2021, 41, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Mock, E.D.; Gagestein, B.; van der Stelt, M. Anandamide and other N-acylethanolamines: A class of signaling lipids with therapeutic opportunities. Prog. Lipid Res. 2023, 89, 101194. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Wang, J.; Wang, X.; Chen, W.; Lu, N.; Siniossoglou, S.; Yao, Z.; Liu, K. Rewiring Neuronal Glycerolipid Metabolism Determines the Extent of Axon Regeneration. Neuron 2020, 105, 276–292.e5. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Archambault, A.S.; Dumais, É.; Martin, C.; Blanchet, M.R.; Bissonnette, E.; Ohashi, N.; Yamamoto, K.; Itoh, T.; Laviolette, M.; et al. Endocannabinoid hydrolysis inhibition unmasks that unsaturated fatty acids induce a robust biosynthesis of 2-arachidonoyl-glycerol and its congeners in human myeloid leukocytes. FASEB J. 2020, 34, 4253–4265. [Google Scholar] [CrossRef] [PubMed]
- Valentine, W.J.; Yanagida, K.; Kawana, H.; Kono, N.; Noda, N.N.; Aoki, J.; Shindou, H. Update and nomenclature proposal for mammalian lysophospholipid acyltransferases, which create membrane phospholipid diversity. J. Biol. Chem. 2022, 298, 101470. [Google Scholar] [CrossRef]
- Valentine, W.J.; Shimizu, T.; Shindou, H. Lysophospholipid acyltransferases orchestrate the compositional diversity of phospholipids. Biochimie 2023, 215, 24–33. [Google Scholar] [CrossRef]
- Wang, S.; Lee, D.P.; Gong, N.; Schwerbrock, N.M.; Mashek, D.G.; Gonzalez-Baró, M.R.; Stapleton, C.; Li, L.O.; Lewin, T.M.; Coleman, R.A. Cloning and functional characterization of a novel mitochondrial N-ethylmaleimide-sensitive glycerol-3-phosphate acyltransferase (GPAT2). Arch. Biochem. Biophys. 2007, 465, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, E.R.; Pellon-Maison, M.; Rabassa, M.E.; Lacunza, E.; Coleman, R.A.; Gonzalez-Baro, M.R. Glycerol-3-phosphate acyltransferase-2 is expressed in spermatic germ cells and incorporates arachidonic acid into triacylglycerols. PLoS ONE 2012, 7, e42986. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xin, J.; Li, X.; Yang, T.; Liu, Y.; Zhao, Y.; Xie, W.; Jiang, M. Repurposing lansoprazole to alleviate metabolic syndrome via PHOSPHO1 inhibition. Acta Pharm. Sin. B 2024, 14, 1711–1725. [Google Scholar] [CrossRef] [PubMed]
- Simic, P.; Kim, W.; Zhou, W.; Pierce, K.A.; Chang, W.; Sykes, D.B.; Aziz, N.B.; Elmariah, S.; Ngo, D.; Pajevic, P.D.; et al. Glycerol-3-phosphate is an FGF23 regulator derived from the injured kidney. J. Clin. Investig. 2020, 130, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Gaits, F.; Fourcade, O.; Le Balle, F.; Gueguen, G.; Gaigé, B.; Gassama-Diagne, A.; Fauvel, J.; Salles, J.P.; Mauco, G.; Simon, M.F.; et al. Lysophosphatidic acid as a phospholipid mediator: Pathways of synthesis. FEBS Lett. 1997, 410, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, H.; Iwata, T.; Ono, T.; Suzuki, T. Immunological characterization of sn-1,2-diacylglycerol and sn-2-monoacylglycerol kinase from pig brain. J. Biol. Chem. 1986, 261, 5597–5602. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.H.; Lin, C.H.; Strickland, K.P. The purification and properties of monoacylglycerol kinase from bovine brain. Biochem. Cell Biol. 1989, 67, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Nakane, S.; Oka, S.; Arai, S.; Waku, K.; Ishima, Y.; Tokumura, A.; Sugiura, T. 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: Occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Arch. Biochem. Biophys. 2002, 402, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Murakami, C.; Yamaki, A.; Mizuno, S.; Sakai, H.; Sakane, F. Distinct 1-monoacylglycerol and 2-monoacylglycerol kinase activities of diacylglycerol kinase isozymes. Biochim. Biophys. Acta 2016, 1864, 1170–1176. [Google Scholar] [CrossRef]
- Sakane, F.; Mizuno, S.; Takahashi, D.; Sakai, H. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv. Biol. Regul. 2018, 67, 101–108. [Google Scholar] [CrossRef]
- Waggoner, D.W.; Johnson, L.B.; Mann, P.C.; Morris, V.; Guastella, J.; Bajjalieh, S.M. MuLK, a eukaryotic multi-substrate lipid kinase. J. Biol. Chem. 2004, 279, 38228–38235. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Payne, S.G.; Liu, H.; Goparaju, S.; Milstien, S.; Spiegel, S. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J. Cell Biol. 2005, 169, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Hong, Z.; Zheng, X. Acylglycerol Kinase-Targeted Therapies in Oncology. Front. Cell Dev. Biol. 2021, 9, 659158. [Google Scholar] [CrossRef] [PubMed]
- Vukotic, M.; Nolte, H.; König, T.; Saita, S.; Ananjew, M.; Krüger, M.; Tatsuta, T.; Langer, T. Acylglycerol Kinase Mutated in Sengers Syndrome Is a Subunit of the TIM22 Protein Translocase in Mitochondria. Mol. Cell 2017, 67, 471–483.e7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, Z.; Ding, N.; Yang, M.; Zhang, L.; Fan, X.; Zhou, Y.; Zou, Q.; Hou, J.; Zheng, J.; et al. The role of AGK in thrombocytopoiesis and possible therapeutic strategies. Blood 2020, 136, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jiang, H.; Yang, M.; Bi, C.; Zhang, K.; Liu, D.; Wei, M.; Jiang, Z.; Lv, K.; Fang, C.; et al. AGK Potentiates Arterial Thrombosis by Affecting Talin-1 and αIIbβ3-Mediated Bidirectional Signaling Pathway. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qu, G.; Yu, X.; Jiang, H.; Teng, X.L.; Ding, L.; Hu, Q.; Guo, X.; Zhou, Y.; Wang, F.; et al. Acylglycerol Kinase Maintains Metabolic State and Immune Responses of CD8+ T Cells. Cell Metab. 2019, 30, 290–302.e5. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Caha, M.; Smoot, L.; Harris, D.J.; Roberts, A.E.; Sacharow, S.; Bodamer, O. Sengers syndrome and AGK-related disorders-Minireview of phenotypic variability and clinical outcomes in molecularly confirmed cases. Mol. Genet. Metab. 2023, 139, 107626. [Google Scholar] [CrossRef] [PubMed]
- Marumo, M.; Nakano, T.; Takeda, Y.; Goto, K.; Wakabayashi, I. Inhibition of thrombin-induced Ca2+ influx in platelets by R59949, an inhibitor of diacylglycerol kinase. J. Pharm. Pharmacol. 2012, 64, 855–861. [Google Scholar] [CrossRef]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Marfella, B.; Piscitelli, F.; De Girolamo, P.; Di Costanzo, A.; Di Marzo, V.; Cristino, L. Positive association between plasmatic levels of orexin A and the endocannabinoid-derived 2-arachidonoyl lysophosphatidic acid in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1004002. [Google Scholar] [CrossRef]
- B Szabo, A.; Cretin, B.; Gérard, F.; Curot, J.; J Barbeau, E.; Pariente, J.; Dahan, L.; Valton, L. Sleep: The Tip of the Iceberg in the Bidirectional Link Between Alzheimer’s Disease and Epilepsy. Front. Neurol. 2022, 13, 836292. [Google Scholar] [CrossRef] [PubMed]
- Liblau, R.S.; Latorre, D.; Kornum, B.R.; Dauvilliers, Y.; Mignot, E.J. The immunopathogenesis of narcolepsy type 1. Nat. Rev. Immunol. 2024, 24, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rilo, A.C.; Forte, N.; Palomba, L.; Tunisi, L.; Piscitelli, F.; Imperatore, R.; Di Costanzo, A.; Di Marzo, V.; Cristino, L. Orexin induces the production of an endocannabinoid-derived lysophosphatidic acid eliciting hypothalamic synaptic loss in obesity. Mol. Metab. 2023, 72, 101713. [Google Scholar] [CrossRef] [PubMed]
- Haj-Dahmane, S.; Shen, R.Y.; Elmes, M.W.; Studholme, K.; Kanjiya, M.P.; Bogdan, D.; Thanos, P.K.; Miyauchi, J.T.; Tsirka, S.E.; Deutsch, D.G.; et al. Fatty-acid-binding protein 5 controls retrograde endocannabinoid signaling at central glutamate synapses. Proc. Natl. Acad. Sci. USA 2018, 115, 3482–3487. [Google Scholar] [CrossRef] [PubMed]
- Fauzan, M.; Oubraim, S.; Yu, M.; Glaser, S.T.; Kaczocha, M.; Haj-Dahmane, S. Fatty Acid-Binding Protein 5 Modulates Brain Endocannabinoid Tone and Retrograde Signaling in the Striatum. Front. Cell. Neurosci. 2022, 16, 936939. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, M.; Piomelli, D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuroreport 2000, 11, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Marazzi, J.; Nicolussi, S.; Gertsch, J. Evidence for bidirectional endocannabinoid transport across cell membranes. J. Biol. Chem. 2012, 287, 34660–34682. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Battista, N.; Riganti, L.; Prada, I.; Antonucci, F.; Cantone, L.; Matteoli, M.; Maccarrone, M.; Verderio, C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015, 16, 213–220. [Google Scholar] [CrossRef]
- Nakamura, Y.; Dryanovski, D.I.; Kimura, Y.; Jackson, S.N.; Woods, A.S.; Yasui, Y.; Tsai, S.Y.; Patel, S.; Covey, D.P.; Su, T.P.; et al. Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. Elife 2019, 8, e47209. [Google Scholar] [CrossRef]
- Brandes, F.; Keiler, A.M.; Kirchner, B.; Borrmann, M.; Billaud, J.N.; Reithmair, M.; Klein, M.; Campolongo, P.; Thieme, D.; Pfaffl, M.W.; et al. Extracellular Vesicles and Endocannabinoid Signaling in Patients with COVID-19. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef]
- Lombardi, M.; Scaroni, F.; Gabrielli, M.; Raffaele, S.; Bonfanti, E.; Filipello, F.; Giussani, P.; Picciolini, S.; de Rosbo, N.K.; Uccelli, A.; et al. Extracellular vesicles released by microglia and macrophages carry endocannabinoids which foster oligodendrocyte differentiation. Front. Immunol. 2024, 15, 1331210. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E. Wanderung von Acyl bei den Glyceriden. Ber. Dtsch. Chem. Ges. B 1920, 53B, 1621–1633. [Google Scholar] [CrossRef]
- Moreland, D.W.; Jones, P.R. Emil Fischer’s sample collection. Bull. Hist. Chem. 2016, 41, 12–18. [Google Scholar]
- Martin, J.B. The equilibrium between symmetrical and unsymmetrical monoglycerides and determination of total monoglycerides. J. Am. Chem. Soc. 1953, 75, 5483–5486. [Google Scholar] [CrossRef]
- Mattson, F.H.; Volpenhein, R.A. Synthesis and properties of glycerides. J. Lipid Res. 1962, 3, 281–296. [Google Scholar] [CrossRef]
- Slotboom, A.J.; de Haas, G.H.; Burbach-Westerhuis, G.J.; van Deenen, L.L.M. Hydrolysis of phosphoglycerides by purified lipase preparations II. Preparation of unsaturated 2-monoacyl choline phosphoglycerides. Chem. Phys. Lipids 1970, 4, 30–36. [Google Scholar] [CrossRef]
- Plückthun, A.; Dennis, E.A. Acyl and phosphoryl migration in lysophospholipids: Importance in phospholipid synthesis and phospholipase specificity. Biochemistry 1982, 21, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Seltzman, H.H.; Fleming, D.N.; Hawkins, G.D.; Carroll, F.I. Facile synthesis and stabilization of 2-arachidonylglycerol via its 1,3-phenylboronate ester. Tetrahedron Lett. 2000, 41, 3589–3592. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Ghebreselasie, K.; Marnett, L.J. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem. Phys. Lipids 2002, 119, 69–82. [Google Scholar] [CrossRef]
- Ottria, R.; Casati, S.; Rota, P.; Ciuffreda, P. 2-Arachidonoylglycerol Synthesis: Facile and Handy Enzymatic Method That Allows to Avoid Isomerization. Molecules 2022, 27, 5190. [Google Scholar] [CrossRef]
- Sugasini, D.; Subbaiah, P.V. Rate of acyl migration in lysophosphatidylcholine (LPC) is dependent upon the nature of the acyl group. Greater stability of sn-2 docosahexaenoyl LPC compared to the more saturated LPC species. PLoS ONE 2017, 12, e0187826. [Google Scholar] [CrossRef] [PubMed]
- Okudaira, M.; Inoue, A.; Shuto, A.; Nakanaga, K.; Kano, K.; Makide, K.; Saigusa, D.; Tomioka, Y.; Aoki, J. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res. 2014, 55, 2178–2192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Gao, Y.; Btesh, J.; Kagan, N.; Kerns, E.; Samad, T.A.; Chanda, P.K. Simultaneous determination of 2-arachidonoylglycerol, 1-arachidonoylglycerol and arachidonic acid in mouse brain tissue using liquid chromatography/tandem mass spectrometry. J. Mass. Spectrom. 2010, 45, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.A.; Gutzki, F.M.; Batkai, S.; May, M.; Rakers, C.; Engeli, S.; Jordan, J.; Tsikas, D. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: A comprehensive review from an analytical and biological perspective. Biochim. Biophys. Acta 2011, 1811, 706–723. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.A.; Batkai, S.; Suchy, M.T.; Gutzki, F.M.; Engeli, S.; Jordan, J.; Tsikas, D. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: Minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2012, 883–884, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kodaka, T.; Nakane, S.; Miyashita, T.; Kondo, S.; Suhara, Y.; Takayama, H.; Waku, K.; Seki, C.; Baba, N.; et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 1999, 274, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Järvinen, T.; Laine, K.; Laitinen, J.T. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 2001, 134, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.I.; Hilston, S.; Tran, N.; Zvonok, N.; Makriyannis, A. 1-, 2- and 3-AG as substrates of the endocannabinoid enzymes and endogenous ligands of the cannabinoid receptor 1. Biochem. Biophys. Res. Commun. 2022, 591, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Dvorakova, M.; Bosquez-Berger, T.; Blahos, J.; Mackie, K. A collection of cannabinoid-related negative findings from autaptic hippocampal neurons. Sci. Rep. 2023, 13, 9610. [Google Scholar] [CrossRef]
- Dócs, K.; Mészár, Z.; Gonda, S.; Kiss-Szikszai, A.; Holló, K.; Antal, M.; Hegyi, Z. The Ratio of 2-AG to Its Isomer 1-AG as an Intrinsic Fine Tuning Mechanism of CB1 Receptor Activation. Front. Cell. Neurosci. 2017, 11, 39. [Google Scholar] [CrossRef]
- Ochiai, K.; Hirooka, R.; Sakaino, M.; Takeuchi, S.; Hira, T. 2-Arachidonoyl glycerol potently induces cholecystokinin secretion in murine enteroendocrine STC-1 cells via cannabinoid receptor CB1. Lipids 2021, 56, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Kishimoto, S.; Miyashita, T.; Nakane, S.; Kodaka, T.; Suhara, Y.; Takayama, H.; Waku, K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 2000, 275, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Saito, O.; Tanabe, M.; Inayoshi, K.; Kobata, K.; Uno, S.; Morita, A.; Watanabe, T. Monoacylglycerols activate capsaicin receptor, TRPV1. Lipids 2008, 43, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jönsson, B.A.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols activate TRPV1--a link between phospholipase C and TRPV1. PLoS ONE 2013, 8, e81618. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Nakajima, K.; Yamashita, A.; Kishimoto, S.; Sugiura, T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007, 362, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Toshida, T.; Maruyama, K.; Nakajima, K.; Yamashita, A.; Sugiura, T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: A possible natural ligand for GPR55. J. Biochem. 2009, 145, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Guy, A.T.; Nagatsuka, Y.; Ooashi, N.; Inoue, M.; Nakata, A.; Greimel, P.; Inoue, A.; Nabetani, T.; Murayama, A.; Ohta, K.; et al. Glycerophospholipid regulation of modality-specific sensory axon guidance in the spinal cord. Science 2015, 349, 974–977. [Google Scholar] [CrossRef]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef]
- Yanagida, K.; Shimizu, T. Lysophosphatidic acid, a simple phospholipid with myriad functions. Pharmacol. Ther. 2023, 246, 108421. [Google Scholar] [CrossRef]
- Bandoh, K.; Aoki, J.; Taira, A.; Tsujimoto, M.; Arai, H.; Inoue, K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000, 478, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Aoki, J.; Hiramatsu, T.; Ishida, M.; Bandoh, K.; Nagai, Y.; Taguchi, R.; Inoue, K.; Arai, H. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J. Biol. Chem. 2002, 277, 34254–34263. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Masago, K.; Nakanishi, H.; Kihara, Y.; Hamano, F.; Tajima, Y.; Taguchi, R.; Shimizu, T.; Ishii, S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J. Biol. Chem. 2009, 284, 17731–17741. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Arima, N.; Ishiguro, J.; Prestwich, G.D.; Arai, H.; Aoki, J. LPA-producing enzyme PA-PLA₁α regulates hair follicle development by modulating EGFR signalling. EMBO J. 2011, 30, 4248–4260. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Nishihara, M.; Tokumura, A. Three lysophosphatidic acids with a distinct long chain moiety differently affect cell differentiation of human colon epithelial cells to goblet cells. Life Sci. 2018, 197, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, R.; Inoue, A.; Sayama, M.; Uwamizu, A.; Yamashita, K.; Hirata, K.; Yoshida, M.; Tanaka, Y.; Kato, H.E.; Nakada-Nakura, Y.; et al. Structural insights into ligand recognition by the lysophosphatidic acid receptor LPA6. Nature 2017, 548, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, R.; Takemoto, M.; Inoue, A.; Ishitani, R.; Nureki, O. Lateral access mechanism of LPA receptor probed by molecular dynamics simulation. PLoS ONE 2022, 17, e0263296. [Google Scholar] [CrossRef] [PubMed]
- Omi, J.; Kano, K.; Aoki, J. Current Knowledge on the Biology of Lysophosphatidylserine as an Emerging Bioactive Lipid. Cell Biochem. Biophys. 2021, 79, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Makide, K.; Shuto, A.; Ikubo, M.; Inoue, A.; Suzuki, K.; Sato, Y.; Nakamura, S.; Otani, Y.; Ohwada, T.; et al. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J. Biochem. 2012, 151, 511–518. [Google Scholar] [CrossRef]
- Uwamizu, A.; Inoue, A.; Suzuki, K.; Okudaira, M.; Shuto, A.; Shinjo, Y.; Ishiguro, J.; Makide, K.; Ikubo, M.; Nakamura, S.; et al. Lysophosphatidylserine analogues differentially activate three LysoPS receptors. J. Biochem. 2015, 157, 151–160. [Google Scholar] [CrossRef]
- Navia-Paldanius, D.; Savinainen, J.R.; Laitinen, J.T. Biochemical and pharmacological characterization of human α/β-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12). J. Lipid Res. 2012, 53, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Shaikh, M.; Singh, S.; Rajendran, A.; Mhetre, A.; Kamat, S.S. Biochemical characterization of the PHARC-associated serine hydrolase ABHD12 reveals its preference for very-long-chain lipids. J. Biol. Chem. 2018, 293, 16953–16963. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, J.; Chap, H.; Roques, V.; Sarda, L.; Douste-Blazy, L. Substrate specificity of two cationic lipases with high phospholipase A1 activity purified from guinea pig pancreas. I. Studies on neutral glycerides. Biochim. Biophys. Acta 1984, 792, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, J.; Chap, H.; Roques, V.; Douste-Blazy, L. Substrate specificity of two cationic lipases with high phospholipase A1 activity purified from guinea pig pancreas. II. Studies on glycerophospholipids. Biochim. Biophys. Acta 1984, 792, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wepy, J.A.; Galligan, J.J.; Kingsley, P.J.; Xu, S.; Goodman, M.C.; Tallman, K.A.; Rouzer, C.A.; Marnett, L.J. Lysophospholipases cooperate to mediate lipid homeostasis and lysophospholipid signaling. J. Lipid Res. 2019, 60, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Manna, J.D.; Wepy, J.A.; Hsu, K.L.; Chang, J.W.; Cravatt, B.F.; Marnett, L.J. Identification of the major prostaglandin glycerol ester hydrolase in human cancer cells. J. Biol. Chem. 2014, 289, 33741–33753. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Patel, J.Z.; Parkkari, T.; Navia-Paldanius, D.; Marjamaa, J.J.; Laitinen, T.; Nevalainen, T.; Laitinen, J.T. Biochemical and pharmacological characterization of the human lymphocyte antigen B-associated transcript 5 (BAT5/ABHD16A). PLoS ONE 2014, 9, e109869. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Baker, D.L.; Virag, T.; Liliom, K.; Byun, H.S.; Tigyi, G.; Bittman, R. Stereochemical properties of lysophosphatidic acid receptor activation and metabolism. Biochim. Biophys. Acta 2002, 1582, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, G.; Granci, V.; Rogalle, P.; Briand-Mésange, F.; Wilson, M.; Klaébé, A.; Tercé, F.; Chap, H.; Salles, J.P.; Simon, M.F.; et al. A lysophosphatidic acid analogue is revealed as a potent inhibitor of phosphatidylcholine synthesis, inducing apoptosis. Biochem. J. 2002, 368, 447–459. [Google Scholar] [CrossRef][Green Version]
- Bolen, A.L.; Naren, A.P.; Yarlagadda, S.; Beranova-Giorgianni, S.; Chen, L.; Norman, D.; Baker, D.L.; Rowland, M.M.; Best, M.D.; Sano, T.; et al. The phospholipase A1 activity of lysophospholipase A-I links platelet activation to LPA production during blood coagulation. J. Lipid Res. 2011, 52, 958–970. [Google Scholar] [CrossRef]
- Qian, L.; Xu, Y.; Hasegawa, Y.; Aoki, J.; Mills, G.B.; Prestwich, G.D. Enantioselective responses to a phosphorothioate analogue of lysophosphatidic acid with LPA3 receptor-selective agonist activity. J. Med. Chem. 2003, 46, 5575–5578. [Google Scholar] [CrossRef] [PubMed]
- Chrencik, J.E.; Roth, C.B.; Terakado, M.; Kurata, H.; Omi, R.; Kihara, Y.; Warshaviak, D.; Nakade, S.; Asmar-Rovira, G.; Mileni, M.; et al. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell 2015, 161, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Yaginuma, S.; Kawana, H.; Aoki, J. Current Knowledge on Mammalian Phospholipase A1, Brief History, Structures, Biochemical and Pathophysiological Roles. Molecules 2022, 27, 2487. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Yong, X.; Zhang, C.; Lin, G.; Jia, G.; Zhao, C.; Wang, X.; Hao, Y.; Wang, Y.; Zhou, P.; et al. Cryo-EM structures of human GPR34 enable the identification of selective antagonists. Proc. Natl. Acad. Sci. USA 2023, 120, e2308435120. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. 2012, 204, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Betters, J.L.; Lord, C.C.; Brown, A.L.; Marshall, S.; Ferguson, D.; Sawyer, J.; Davis, M.A.; Melchior, J.T.; Blume, L.C.; et al. The serine hydrolase ABHD6 Is a critical regulator of the metabolic syndrome. Cell Rep. 2013, 5, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Pribasnig, M.A.; Mrak, I.; Grabner, G.F.; Taschler, U.; Knittelfelder, O.; Scherz, B.; Eichmann, T.O.; Heier, C.; Grumet, L.; Kowaliuk, J.; et al. α/β Hydrolase Domain-containing 6 (ABHD6) Degrades the Late Endosomal/Lysosomal Lipid Bis(monoacylglycero)phosphate. J. Biol. Chem. 2015, 290, 29869–29881. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Fawzy, N.; Pribasnig, M.A.; Trieb, M.; Taschler, U.; Holzer, M.; Schweiger, M.; Wolinski, H.; Kolb, D.; Horvath, A.; et al. Metabolic disease and ABHD6 alter the circulating bis(monoacylglycerol)phosphate profile in mice and humans. J. Lipid Res. 2019, 60, 1020–1031. [Google Scholar] [CrossRef]
- Masquelier, J.; Alhouayek, M.; Terrasi, R.; Bottemanne, P.; Paquot, A.; Muccioli, G.G. Lysophosphatidylinositols in inflammation and macrophage activation: Altered levels and anti-inflammatory effects. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1458–1468. [Google Scholar] [CrossRef]
- Blankman, J.L.; Long, J.Z.; Trauger, S.A.; Siuzdak, G.; Cravatt, B.F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci. USA 2013, 110, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Ichu, T.A.; Reed, A.; Ogasawara, D.; Ulanovskaya, O.; Roberts, A.; Aguirre, C.A.; Bar-Peled, L.; Gao, J.; Germain, J.; Barbas, S.; et al. ABHD12 and LPCAT3 Interplay Regulates a Lyso-phosphatidylserine-C20:4 Phosphatidylserine Lipid Network Implicated in Neurological Disease. Biochemistry 2020, 59, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, D.; Ichu, T.A.; Vartabedian, V.F.; Benthuysen, J.; Jing, H.; Reed, A.; Ulanovskaya, O.A.; Hulce, J.J.; Roberts, A.; Brown, S.; et al. Selective blockade of the lyso-PS lipase ABHD12 stimulates immune responses in vivo. Nat. Chem. Biol. 2018, 14, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, D.; Ichu, T.A.; Jing, H.; Hulce, J.J.; Reed, A.; Ulanovskaya, O.A.; Cravatt, B.F. Discovery and Optimization of Selective and in Vivo Active Inhibitors of the Lysophosphatidylserine Lipase α/β-Hydrolase Domain-Containing 12 (ABHD12). J. Med. Chem. 2019, 62, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, J.; Bonnefis, M.J.; Sarda, L.; Chap, H.; Thouvenot, J.P.; Douste-Blazy, L. Purification of two lipases with high phospholipase A1 activity from guinea-pig pancreas. Biochim. Biophys. Acta 1981, 663, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.E. Properties and function of pancreatic lipase related protein 2. Biochimie 2000, 82, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Inoue, A.; Makide, K.; Saiki, N.; Arai, H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 2007, 89, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Fang, Q.; Zhu, F.; Huang, D.; Yang, C. Structure and Function of Pancreatic Lipase-Related Protein 2 and Its Relationship With Pathological States. Front. Genet. 2021, 12, 693538. [Google Scholar] [CrossRef] [PubMed]
- Alves, B.N.; Leong, J.; Tamang, D.L.; Elliott, V.; Edelnant, J.; Redelman, D.; Singer, C.A.; Kuhn, A.R.; Miller, R.; Lowe, M.E.; et al. Pancreatic lipase-related protein 2 (PLRP2) induction by IL-4 in cytotoxic T lymphocytes (CTLs) and reevaluation of the negative effects of its gene ablation on cytotoxicity. J. Leukoc. Biol. 2009, 86, 701–712. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, W.; Sun, Q.; Yang, X.; Liu, J.; Ge, W.; Yang, Y.; Zhao, Y.; Xu, X.; Zhang, J. Pancreatic lipase-related protein 2 is responsible for the increased hepatic retinyl ester hydrolase activity in vitamin A-deficient mice. FEBS J. 2019, 286, 4232–4244. [Google Scholar] [CrossRef]
- Ge, W.; Gao, Y.; Zhao, Y.; Yang, Y.; Sun, Q.; Yang, X.; Xu, X.; Zhang, J. Decreased T-cell mediated hepatic injury in concanavalin A-treated PLRP2-deficient mice. Int. Immunopharmacol. 2020, 85, 106604. [Google Scholar] [CrossRef] [PubMed]
- Kuge, H.; Miyamoto, I.; Yagyu, K.I.; Honke, K. PLRP2 selectively localizes synaptic membrane proteins via acyl-chain remodeling of phospholipids. J. Lipid Res. 2020, 61, 1747–1763. [Google Scholar] [CrossRef]
- Ding, Z.; Cheng, R.; Liu, J.; Zhao, Y.; Ge, W.; Yang, Y.; Xu, X.; Wang, S.; Zhang, J. The suppression of pancreatic lipase-related protein 2 ameliorates experimental hepatic fibrosis in mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159102. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.P.; Lu, T.F.; Li, S.; Jia, G.X.; Zhang, X.N.; Yang, Q.E.; Hou, Y.P. Pancreatic lipase-related protein 2 is selectively expressed by peritubular myoid cells in the murine testis and sustains long-term spermatogenesis. Cell. Mol. Life Sci. 2023, 80, 217. [Google Scholar] [CrossRef] [PubMed]
- Sahaka, M.; Mateos-Diaz, E.; Amara, S.; Wattanakul, J.; Gray, D.; Lafont, D.; Gontero, B.; Launay, H.; Carrière, F. In situ monitoring of galactolipid digestion by infrared spectroscopy in both model micelles and spinach chloroplasts. Chem. Phys. Lipids 2023, 252, 105291. [Google Scholar] [CrossRef]
- Record, M.; Amara, S.; Subra, C.; Jiang, G.; Prestwich, G.D.; Ferrato, F.; Carrière, F. Bis (monoacylglycero) phosphate interfacial properties and lipolysis by pancreatic lipase-related protein 2, an enzyme present in THP-1 human monocytes. Biochim. Biophys. Acta 2011, 1811, 419–430. [Google Scholar] [CrossRef]
- Gilleron, M.; Lepore, M.; Layre, E.; Cala-De Paepe, D.; Mebarek, N.; Shayman, J.A.; Canaan, S.; Mori, L.; Carrière, F.; Puzo, G.; et al. Lysosomal Lipases PLRP2 and LPLA2 Process Mycobacterial Multi-acylated Lipids and Generate T Cell Stimulatory Antigens. Cell Chem. Biol. 2016, 23, 1147–1156. [Google Scholar] [CrossRef]
- Hirano, T.; Kishi, M.; Sugimoto, H.; Taguchi, R.; Obinata, H.; Ohshima, N.; Tatei, K.; Izumi, T. Thioesterase activity and subcellular localization of acylprotein thioesterase 1/lysophospholipase 1. Biochim. Biophys. Acta 2009, 1791, 797–805. [Google Scholar] [CrossRef]
- Kamat, S.S.; Camara, K.; Parsons, W.H.; Chen, D.H.; Dix, M.M.; Bird, T.D.; Howell, A.R.; Cravatt, B.F. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat. Chem. Biol. 2015, 11, 164–171. [Google Scholar] [CrossRef]
- Kim, H.Y. Phospholipids: A neuroinflammation emerging target. Nat. Chem. Biol. 2015, 11, 99–100. [Google Scholar] [CrossRef]
- Singh, S.; Joshi, A.; Kamat, S.S. Mapping the Neuroanatomy of ABHD16A, ABHD12, and Lysophosphatidylserines Provides New Insights into the Pathophysiology of the Human Neurological Disorder PHARC. Biochemistry 2020, 59, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Voeltz, G.K. An ER phospholipid hydrolase drives ER-associated mitochondrial constriction for fission and fusion. Elife 2022, 11, e84279. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, X.; Xu, Z.; Shen, L.; Ding, Y.; Chen, S.; Mao, L.; Liu, W.; Xu, J. ABHD16A Negatively Regulates the Palmitoylation and Antiviral Function of IFITM Proteins. mBio 2022, 13, e0228922. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, R.; Rajasekharan, R.; Usharani, D. Molecular insights on PS-PLA1 lipase activity of human ABHD16B. Biophys. Chem. 2023, 296, 106976. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.C.; Thomas, G.; Brown, J.M. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim. Biophys. Acta 2013, 1831, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Brindley, D.N. Lipid phosphate phosphatases and related proteins: Signaling functions in development, cell division, and cancer. J. Cell Biochem. 2004, 92, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Sigal, Y.J.; McDermott, M.I.; Morris, A.J. Integral membrane lipid phosphatases/phosphotransferases: Common structure and diverse functions. Biochem. J. 2005, 387, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Benesch, M.G.; Brindley, D.N. Lipid phosphate phosphatases and their roles in mammalian physiology and pathology. J. Lipid Res. 2015, 56, 2048–2060. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Brindley, D.N. Lipid Phosphate Phosphatases and Cancer. Biomolecules 2020, 10, 1263. [Google Scholar] [CrossRef]
- Stefan, C.; Jansen, S.; Bollen, M. NPP-type ectophosphodiesterases: Unity in diversity. Trends Biochem. Sci. 2005, 30, 542–550. [Google Scholar] [CrossRef]
- Borza, R.; Salgado-Polo, F.; Moolenaar, W.H.; Perrakis, A. Structure and function of the ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP) family: Tidying up diversity. J. Biol. Chem. 2022, 298, 101526. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Aoki, J.; Natori, Y.; Nishikawa, K.; Kakehi, Y.; Natori, Y.; Arai, H. Biochemical and molecular characterization of a novel choline-specific glycerophosphodiester phosphodiesterase belonging to the nucleotide pyrophosphatase/phosphodiesterase family. J. Biol. Chem. 2005, 280, 23084–23093. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.M.; Cravatt, B.F. Endocannabinoid metabolism in the absence of fatty acid amide hydrolase (FAAH): Discovery of phosphorylcholine derivatives of N-acyl ethanolamines. Biochemistry 2006, 45, 11267–11277. [Google Scholar] [CrossRef] [PubMed]
- Greiner-Tollersrud, L.; Berg, T.; Stensland, H.M.; Evjen, G.; Greiner-Tollersrud, O.K. Bovine brain myelin glycerophosphocholine choline phosphodiesterase is an alkaline lysosphingomyelinase of the eNPP-family, regulated by lysosomal sorting. Neurochem. Res. 2013, 38, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Morita, J.; Kano, K.; Kato, K.; Takita, H.; Sakagami, H.; Yamamoto, Y.; Mihara, E.; Ueda, H.; Sato, T.; Tokuyama, H.; et al. Structure and biological function of ENPP6, a choline-specific glycerophosphodiester-phosphodiesterase. Sci. Rep. 2016, 6, 20995. [Google Scholar] [CrossRef] [PubMed]
- Corda, D.; Kudo, T.; Zizza, P.; Iurisci, C.; Kawai, E.; Kato, N.; Yanaka, N.; Mariggiò, S. The developmentally regulated osteoblast phosphodiesterase GDE3 is glycerophosphoinositol-specific and modulates cell growth. J. Biol. Chem. 2009, 284, 24848–24856. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, A.; Liu, F.; Illes, K.; Nagar, B. Crystal structure of the human alkaline sphingomyelinase provides insights into substrate recognition. J. Biol. Chem. 2017, 292, 7087–7094. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.D.; Bergman, T.; Xu, N.; Wu, J.; Cheng, Y.; Duan, J.; Nelander, S.; Palmberg, C.; Nilsson, A. Identification of human intestinal alkaline sphingomyelinase as a novel ecto-enzyme related to the nucleotide phosphodiesterase family. J. Biol. Chem. 2003, 278, 38528–38536. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Nilsson, A.; Jönsson, B.A.; Stenstad, H.; Agace, W.; Cheng, Y.; Duan, R.D. Intestinal alkaline sphingomyelinase hydrolyses and inactivates platelet-activating factor by a phospholipase C activity. Biochem. J. 2006, 394, 299–308. [Google Scholar] [CrossRef]
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002, 277, 39436–39442. [Google Scholar] [CrossRef]
- Umezu-Goto, M.; Kishi, Y.; Taira, A.; Hama, K.; Dohmae, N.; Takio, K.; Yamori, T.; Mills, G.B.; Inoue, K.; Aoki, J.; et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002, 158, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nakanaga, K.; Hama, K.; Aoki, J. Autotaxin--an LPA producing enzyme with diverse functions. J. Biochem. 2010, 148, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Inoue, A.; Okudaira, S. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 2008, 1781, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, W.H.; Perrakis, A. Insights into autotaxin: How to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 2011, 12, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-function and signaling. J. Lipid Res. 2014, 55, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Dusaulcy, R.; Tréguer, K.; Grès, S.; Attané, C.; Saulnier-Blache, J.S. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie 2014, 96, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Barbayianni, E.; Kaffe, E.; Aidinis, V.; Kokotos, G. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog. Lipid Res. 2015, 58, 76–96. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic Acid signaling in the nervous system. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, R.; Houssin, A.; Peyruchaud, O. Platelets, autotaxin and lysophosphatidic acid signalling: Win-win factors for cancer metastasis. Br. J. Pharmacol. 2018, 175, 3100–3110. [Google Scholar] [CrossRef]
- Tang, X.; Benesch, M.G.K.; Brindley, D.N. Role of the autotaxin-lysophosphatidate axis in the development of resistance to cancer therapy. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158716. [Google Scholar] [CrossRef]
- Jose, A.; Kienesberger, P.C. Autotaxin-LPA-LPP3 Axis in Energy Metabolism and Metabolic Disease. Int. J. Mol. Sci. 2021, 22, 9575. [Google Scholar] [CrossRef] [PubMed]
- Alioli, C.; Demesmay, L.; Peyruchaud, O.; Machuca-Gayet, I. Autotaxin/Lysophosphatidic Acid Axis: From Bone Biology to Bone Disorders. Int. J. Mol. Sci. 2022, 23, 3427. [Google Scholar] [CrossRef] [PubMed]
- Karalis, T.; Poulogiannis, G. The Emerging Role of LPA as an Oncometabolite. Cells 2024, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Tang, X.; Brindley, D.N.; Takabe, K. Autotaxin and Lysophosphatidate Signaling: Prime Targets for Mitigating Therapy Resistance in Breast Cancer. World J. Oncol. 2024, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Taira, A.; Takanezawa, Y.; Kishi, Y.; Hama, K.; Kishimoto, T.; Mizuno, K.; Saku, K.; Taguchi, R.; Arai, H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 2002, 277, 48737–48744. [Google Scholar] [CrossRef] [PubMed]
- Clair, T.; Aoki, J.; Koh, E.; Bandle, R.W.; Nam, S.W.; Ptaszynska, M.M.; Mills, G.B.; Schiffmann, E.; Liotta, L.A.; Stracke, M.L. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 2003, 63, 5446–5453. [Google Scholar] [PubMed]
- Hausmann, J.; Kamtekar, S.; Christodoulou, E.; Day, J.E.; Wu, T.; Fulkerson, Z.; Albers, H.M.; van Meeteren, L.A.; Houben, A.J.; van Zeijl, L.; et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 2011, 18, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Okudaira, S.; Hama, K.; Mihara, E.; Dohmae, N.; Inoue, A.; Ishitani, R.; Takagi, J.; Aoki, J.; Nureki, O. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat. Struct. Mol. Biol. 2011, 18, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tabchy, A.; Tigyi, G.; Mills, G.B. Location, location, location: A crystal-clear view of autotaxin saturating LPA receptors. Nat. Struct. Mol. Biol. 2011, 18, 117–118. [Google Scholar] [CrossRef][Green Version]
- Sano, T.; Baker, D.; Virag, T.; Wada, A.; Yatomi, Y.; Kobayashi, T.; Igarashi, Y.; Tigyi, G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J. Biol. Chem. 2002, 277, 21197–21206. [Google Scholar] [CrossRef]
- Salgado-Polo, F.; Borza, R.; Matsoukas, M.T.; Marsais, F.; Jagerschmidt, C.; Waeckel, L.; Moolenaar, W.H.; Ford, P.; Heckmann, B.; Perrakis, A. Autotaxin facilitates selective LPA receptor signaling. Cell Chem. Biol. 2023, 30, 69–84.e14. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.; Yun, M.K.; Frank, M.M.; Rock, C.O. Lysophosphatidylglycerol (LPG) phospholipase D maintains membrane homeostasis in Staphylococcus aureus by converting LPG to lysophosphatidic acid. J. Biol. Chem. 2023, 299, 104863. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Okudaira, S.; Moriya-Ito, K.; Shimamoto, C.; Tanaka, M.; Aoki, J.; Arai, H.; Murakami-Murofushi, K.; Kobayashi, T. Cyclic phosphatidic acid is produced by autotaxin in blood. J. Biol. Chem. 2006, 397, 26081–26088. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.; Gotoh, M.; Uwamizu, A.; Hirokawa, T.; Ishikawa, M.; Shimizu, Y.; Yamamoto, S.; Iwasa, K.; Yoshikawa, K.; Aoki, J.; et al. 2-Carba-lysophosphatidic acid is a novel β-lysophosphatidic acid analogue with high potential for lysophosphatidic acid receptor activation and autotaxin inhibition. Sci. Rep. 2021, 11, 17360. [Google Scholar] [CrossRef] [PubMed]
- Tserendavga, B.; Ohshima, N.; Fujita, C.; Yuzawa, K.; Ohshima, M.; Yanaka, N.; Minamishima, Y.A.; Izumi, T. Characterization of recombinant murine GDE4 and GDE7, enzymes producing lysophosphatidic acid and/or cyclic phosphatidic acid. J. Biochem. 2022, 170, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, K.; Ali, H.; Kimoto, R.; Takenouchi, Y.; Ishimaru, H.; Yamashita, A.; Ueda, N.; Tanaka, T.; Okamoto, Y.; Tsuboi, K. GDE7 produces cyclic phosphatidic acid in the ER lumen functioning as a lysophospholipid mediator. Commun. Biol. 2023, 6, 524. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kobayashi, T.; Kishimoto, M.; Tsutsumi, T.; Okuyama, H. A possible pathway of phosphoinositide metabolism through EDTA-insensitive phospholipase A1 followed by lysophosphoinositide-specific phospholipase C in rat brain. J. Neurochem. 1993, 61, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Murase, S.; Okuyama, H. A membrane-bound phospholipase C with an apparent specificity for lysophosphatidylinositol in porcine platelets. J. Biol. Chem. 1985, 260, 262–265. [Google Scholar] [CrossRef]
- Volwerk, J.J.; Birrell, G.B.; Hedberg, K.K.; Griffith, O.H. A high level of cell surface phosphatidylinositol-specific phospholipase C activity is characteristic of growth-arrested 3T3 fibroblasts but not of transformed variants. J. Cell. Physiol. 1992, 151, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Birrell, G.B.; Hedberg, K.K.; Volwerk, J.J.; Griffith, O.H. Differential expression of phospholipase C specific for inositol phospholipids at the cell surface of rat glial cells and REF52 rat embryo fibroblasts. J. Neurochem. 1993, 60, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Kobayashi, T.; Ueda, H.; Yamauchi, E.; Watanabe, S.; Okuyama, H. Lysophosphoinositide-specific phospholipase C in rat brain synaptic plasma membranes. Neurochem. Res. 1994, 19, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Kobayashi, T.; Miyashita, M.; Watanabe, S.; Homma, Y.; Okuyama, H. A lysophosphoinositide-specific phospholipase C distinct from other phospholipase C families in rat brain. Arch. Biochem. Biophys. 1995, 317, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, N. Mammalian glycerophosphodiester phosphodiesterases. Biosci. Biotechnol. Biochem. 2007, 71, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Corda, D.; Mosca, M.G.; Ohshima, N.; Grauso, L.; Yanaka, N.; Mariggiò, S. The emerging physiological roles of the glycerophosphodiesterase family. FEBS J. 2014, 281, 998–1016. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, S.M.; Pirmoradi, S.; Zununi Vahed, S.; Kumar Roy, R.; Hosseiniyan Khatibi, S.M. An update on Glycerophosphodiester Phosphodiesterases; From Bacteria to Human. Protein J. 2024, 43, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.M.; Cravatt, B.F. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J. Biol. Chem. 2008, 283, 9341–9349. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, N.; Imai, Y.; Kawai, E.; Akatsuka, H.; Wakimoto, K.; Nogusa, Y.; Kato, N.; Chiba, H.; Kotani, E.; Omori, K.; et al. Novel membrane protein containing glycerophosphodiester phosphodiesterase motif is transiently expressed during osteoblast differentiation. J. Biol. Chem. 2003, 278, 43595–43602. [Google Scholar] [CrossRef]
- van Veen, M.; Matas-Rico, E.; van de Wetering, K.; Leyton-Puig, D.; Kedziora, K.M.; De Lorenzi, V.; Stijf-Bultsma, Y.; van den Broek, B.; Jalink, K.; Sidenius, N.; et al. Negative regulation of urokinase receptor activity by a GPI-specific phospholipase C in breast cancer cells. Elife 2017, 6, e23649. [Google Scholar] [CrossRef]
- Dobrowolski, M.; Cave, C.; Levy-Myers, R.; Lee, C.; Park, S.; Choi, B.R.; Xiao, B.; Yang, W.; Sockanathan, S. GDE3 regulates oligodendrocyte precursor proliferation via release of soluble CNTFRα. Development 2020, 147, dev180695. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.E.; Pagano, R.E. Detection of a phosphatidylinositol-specific phospholipase C at the surface of Swiss 3T3 cells and its potential role in the regulation of cell growth. J. Biol. Chem. 1990, 265, 5337–5340. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.E.; Pagano, R.E. Density-dependent inhibition of cell growth is correlated with the activity of a cell surface phosphatidylinositol-specific phospholipase C. Eur. J. Cell Biol. 1991, 56, 401–406. [Google Scholar] [PubMed]
- Higgs, H.N.; Han, M.H.; Johnson, G.E.; Glomset, J.A. Cloning of a phosphatidic acid-preferring phospholipase A1 from bovine testis. J. Biol. Chem. 1998, 273, 5468–5477. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Kumazawa, T.; Koga, H.; Suzuki, N.; Oka, S.; Sugiura, T. Generation of lysophosphatidylinositol by DDHD domain containing 1 (DDHD1): Possible involvement of phospholipase D/phosphatidic acid in the activation of DDHD1. Biochim. Biophys. Acta 2010, 1801, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Nemoto-Sasaki, Y.; Oka, S.; Arai, S.; Wada, I.; Yamashita, A. Phosphorylation of human phospholipase A1 DDHD1 at newly identified phosphosites affects its subcellular localization. J. Biol. Chem. 2021, 297, 100851. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, R.; Maffucci, T.; Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 2011, 30, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Emmanouilidi, A.; Casari, I.; Gokcen Akkaya, B.; Maffucci, T.; Furic, L.; Guffanti, F.; Broggini, M.; Chen, X.; Maxuitenko, Y.Y.; Keeton, A.B.; et al. Inhibition of the Lysophosphatidylinositol Transporter ABCC1 Reduces Prostate Cancer Cell Growth and Sensitizes to Chemotherapy. Cancers 2020, 12, 2022. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A. L-α-lysophosphatidylinositol meets GPR55: A deadly relationship. Trends Pharmacol. Sci. 2011, 32, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Masquelier, J.; Muccioli, G.G. Lysophosphatidylinositols, from Cell Membrane Constituents to GPR55 Ligands. Trends Pharmacol. Sci. 2018, 39, 586–604. [Google Scholar] [CrossRef]
- Zachowski, A. Phospholipids in animal eukaryotic membranes: Transverse asymmetry and movement. Biochem. J. 1993, 294 Pt 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lhermusier, T.; Chap, H.; Payrastre, B. Platelet membrane phospholipid asymmetry: From the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J. Thromb. Haemost. 2011, 9, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Menon, A.K. Transbilayer lipid asymmetry. Curr. Biol. 2018, 28, R386–R391. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.D.; Gu, B.; Capelluto, D.G.; Dou, D.; Feldman, E.; Rumore, A.; Arredondo, F.D.; Hanlon, R.; Fudal, I.; Rouxel, T.; et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell 2010, 142, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Kanemaru, K.; Matsubara, A.; Takai, E.; Shimozawa, M.; Satow, R.; Yamaguchi, H.; Nakamura, Y.; Fukami, K. Phosphatidylinositol 4,5-bisphosphate is localized in the plasma membrane outer leaflet and regulates cell adhesion and motility. Biochem. Biophys. Res. Commun. 2020, 527, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Mujalli, A.; Viaud, J.; Severin, S.; Gratacap, M.P.; Chicanne, G.; Hnia, K.; Payrastre, B.; Terrisse, A.D. Exploring the Role of PI3P in Platelets: Insights from a Novel External PI3P Pool. Biomolecules 2023, 13, 583. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.H.; Kang, G.H.; Hur, J.; Lee, J.; Jung, Y.; Hong, I.S.; Lee, H.; Seo, S.Y.; Lee, D.H.; Lee, C.S.; et al. Externalized phosphatidylinositides on apoptotic cells are eat-me signals recognized by CD14. Cell Death Differ. 2022, 29, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kim, H.; Chan, R.B.; Agarwal, S.; Williamson, R.; Cho, W.; Paolo, G.D.; Satchell, K.J. Autophagy and endosomal trafficking inhibition by Vibrio cholerae MARTX toxin phosphatidylinositol-3-phosphate-specific phospholipase A1 activity. Nat. Commun. 2015, 6, 8745. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Balenga, N.; Parzmair, G.P.; Brown, A.J.; Heinemann, A.; Waldhoer, M. The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. J. Biol. Chem. 2012, 287, 44234–44248. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Reyes-Resina, I.; Oñatibia-Astibia, A.; Zamarbide, M.; Ricobaraza, A.; Navarro, G.; Moreno, E.; Dopeso-Reyes, I.G.; Sierra, S.; Rico, A.J.; et al. CB1 and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum. Exp. Neurol. 2014, 261, 44–52. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Rico, A.J.; Rivas-Santisteban, R.; Lillo, J.; Roda, E.; Navarro, G.; Franco, R.; Lanciego, J.L. Expression of cannabinoid CB1 R-GPR55 heteromers in neuronal subtypes of the Macaca fascicularis striatum. Ann. N. Y. Acad. Sci. 2020, 1475, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Aguinaga, D.; Navarro, G.; Rico, A.J.; Oyarzábal, J.; Sánchez-Arias, J.A.; Lanciego, J.L.; Franco, R. Targeting CB1 and GPR55 Endocannabinoid Receptors as a Potential Neuroprotective Approach for Parkinson’s Disease. Mol. Neurobiol. 2019, 56, 5900–5910. [Google Scholar] [CrossRef] [PubMed]
- Balenga, N.A.; Aflaki, E.; Kargl, J.; Platzer, W.; Schröder, R.; Blättermann, S.; Kostenis, E.; Brown, A.J.; Heinemann, A.; Waldhoer, M. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 2011, 21, 1452–1469. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Andradas, C.; Medrano, M.; Caffarel, M.M.; Pérez-Gómez, E.; Blasco-Benito, S.; Gómez-Cañas, M.; Pazos, M.R.; Irving, A.J.; Lluís, C.; et al. Targeting CB2-GPR55 receptor heteromers modulates cancer cell signaling. J. Biol. Chem. 2014, 289, 21960–21972. [Google Scholar] [CrossRef] [PubMed]
- Balenga, N.A.; Martínez-Pinilla, E.; Kargl, J.; Schröder, R.; Peinhaupt, M.; Platzer, W.; Bálint, Z.; Zamarbide, M.; Dopeso-Reyes, I.G.; Ricobaraza, A.; et al. Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. Br. J. Pharmacol. 2014, 171, 5387–5406. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Rabal, O.; Reyes-Resina, I.; Zamarbide, M.; Navarro, G.; Sánchez-Arias, J.A.; de Miguel, I.; Lanciego, J.L.; Oyarzabal, J.; Franco, R. Two Affinity Sites of the Cannabinoid Subtype 2 Receptor Identified by a Novel Homogeneous Binding Assay. J. Pharmacol. Exp. Ther. 2016, 358, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Akimov, M.G.; Gretskaya, N.M.; Dudina, P.V.; Sherstyanykh, G.D.; Zinchenko, G.N.; Serova, O.V.; Degtyaryova, K.O.; Deyev, I.E.; Bezuglov, V.V. The Mechanisms of GPR55 Receptor Functional Selectivity during Apoptosis and Proliferation Regulation in Cancer Cells. Int. J. Mol. Sci. 2023, 24, 5524. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.L.; Sánchez-Miranda, E.; Castillo-Arellano, J.I.; Cervantes-Villagrana, R.D.; Ibarra-Sánchez, A.; González-Espinosa, C. Anandamide inhibits FcεRI-dependent degranulation and cytokine synthesis in mast cells through CB2 and GPR55 receptor activation. Possible involvement of CB2-GPR55 heteromers. Int. Immunopharmacol. 2018, 64, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Pérez, C.; Rivas-Santisteban, R.; Del Valle, E.; Tolivia, J.; Navarro, A.; Franco, R.; Martínez-Pinilla, E. Heteromers Formed by GPR55 and Either Cannabinoid CB1 or CB2 Receptors Are Upregulated in the Prefrontal Cortex of Multiple Sclerosis Patients. Int. J. Mol. Sci. 2024, 25, 4176. [Google Scholar] [CrossRef]
- BioGPS. Available online: http://biogps.org/#goto=genereport&id=71584 (accessed on 29 December 2023).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000130055-GDPD2 (accessed on 29 December 2023).
- Inloes, J.M.; Jing, H.; Cravatt, B.F. The Spastic Paraplegia-Associated Phospholipase DDHD1 Is a Primary Brain Phosphatidylinositol Lipase. Biochemistry 2018, 57, 5759–5767. [Google Scholar] [CrossRef]
- Morikawa, T.; Ohishi, H.; Kosaka, K.; Shimojo, T.; Nagano, A.; Taniguchi, I.; Fujioka, R.; Moriyama, K.; Unoki, M.; Takahashi, M.; et al. Ddhd1 knockout mouse as a model of locomotive and physiological abnormality in familial spastic paraplegia. Biosci. Rep. 2021, 41, BSR20204171. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Takahashi, M.; Izumi, Y.; Bamba, T.; Moriyama, K.; Hattori, G.; Fujioka, R.; Miura, S.; Shibata, H. Oleic Acid-Containing Phosphatidylinositol Is a Blood Biomarker Candidate for SPG28. Biomedicines 2023, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Kawabata, K.; Yamazaki, N.; Tsukigawa, K.; Nishi, H.; Tokumura, A. Extracellular and intracellular productions of lysophosphatidic acids and cyclic phosphatidic acids by lysophospholipase D from exogenously added lysophosphatidylcholines to cultured NRK52E cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159349. [Google Scholar] [CrossRef] [PubMed]
- Greiner-Tollersrud, O.K. The non-classical N-glycan processing pathway of bovine brain ecto-nucleotide phosphodiesterase/pyrophosphatase 6 (eNPP6) is brain specific and not due to mannose-6-phosphorylation. Neurochem. Res. 2014, 39, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, F.; Nilsson, A.; Duan, R.D. Pancreatic trypsin cleaves intestinal alkaline sphingomyelinase from mucosa and enhances the sphingomyelinase activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G967–G973. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.; Leal Rato, M.; Silva, C.S.; Soares, M.P.; Carvalho, V.; Guedes, L.C. A novel frameshift DDHD1 mutation in a patient with hereditary spastic paraplegia type 28: Case report and review of the literature. Park. Relat. Disord. 2024, 118, 105931. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Kashiwagi, Y.; Arimitsu, N.; Kogure, T.; Edo, A.; Maruyama, T.; Nakao, K.; Nakanishi, H.; Kinoshita, M.; Frohman, M.A.; et al. Phosphatidic acid (PA)-preferring phospholipase A1 regulates mitochondrial dynamics. J. Biol. Chem. 2014, 289, 11497–11511. [Google Scholar] [CrossRef]
- Liguori, R.; Giannoccaro, M.P.; Arnoldi, A.; Citterio, A.; Tonon, C.; Lodi, R.; Bresolin, N.; Bassi, M.T. Impairment of brain and muscle energy metabolism detected by magnetic resonance spectroscopy in hereditary spastic paraparesis type 28 patients with DDHD1 mutations. J. Neurol. 2014, 261, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Mignarri, A.; Rubegni, A.; Tessa, A.; Stefanucci, S.; Malandrini, A.; Cardaioli, E.; Meschini, M.C.; Stromillo, M.L.; Doccini, S.; Federico, A.; et al. Mitochondrial dysfunction in hereditary spastic paraparesis with mutations in DDHD1/SPG28. J. Neurol. Sci. 2016, 362, 287–291. [Google Scholar] [CrossRef]
- Yadav, P.K.; Rajasekharan, R. Misregulation of a DDHD Domain-containing Lipase Causes Mitochondrial Dysfunction in Yeast. J. Biol. Chem. 2016, 291, 18562–18581. [Google Scholar] [CrossRef]
- Maemoto, Y.; Maruyama, T.; Nemoto, K.; Baba, T.; Motohashi, M.; Ito, A.; Tagaya, M.; Tani, K. DDHD1, but Not DDHD2, Suppresses Neurite Outgrowth in SH-SY5Y and PC12 Cells by Regulating Protein Transport From Recycling Endosomes. Front. Cell Dev. Biol. 2020, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Doan, R.N.; Lim, E.T.; De Rubeis, S.; Betancur, C.; Cutler, D.J.; Chiocchetti, A.G.; Overman, L.M.; Soucy, A.; Goetze, S.; Freitag, C.M.; et al. Recessive gene disruptions in autism spectrum disorder. Nat. Genet. 2019, 51, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Cristaldi, M.; Fontana, S.; Saieva, L.; Monteleone, F.; Calabrese, G.; Giavaresi, G.; Parenti, R.; Alessandro, R. The phospholipase DDHD1 as a new target in colorectal cancer therapy. J. Exp. Clin. Cancer Res. 2018, 37, 82. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, W.; Pan, Y.; Zhou, Q.; Xu, J.; Han, S. Immune-related genes in tumor-specific CD4+ and CD8+ T cells in colon cancer. BMC Cancer 2020, 20, 585. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, C.; Sonoda, H.; Anzai, H.; Nagai, Y.; Abe, S.; Yokoyama, Y.; Ishii, H.; Kishikawa, J.; Emoto, S.; Murono, K.; et al. Expression of Lysophosphatidylinositol Signaling-relevant Molecules in Colorectal Cancer. Anticancer Res. 2021, 41, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.Y.; Yang, P.J.; Zhang, W.S.; Chen, J.B.; Tian, Q.Y.; Zhang, Y.; Han, B. Identification of a Prognostic Gene Signature Based on Lipid Metabolism-Related Genes in Esophageal Squamous Cell Carcinoma. Pharmgenomics Pers. Med. 2023, 16, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.; Sabharwal, P.; Zhang, M.; Meyers, C.L.; Sockanathan, S. GDE2 promotes neurogenesis by glycosylphosphatidylinositol-anchor cleavage of RECK. Science 2013, 339, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Li, Y.; Choi, B.R.; Matas-Rico, E.; Troncoso, J.; Takahashi, C.; Sockanathan, S. GDE2-RECK controls ADAM10 α-secretase-mediated cleavage of amyloid precursor protein. Sci. Transl. Med. 2021, 13, eabe6178. [Google Scholar] [CrossRef] [PubMed]
- Matas-Rico, E.; van Veen, M.; Leyton-Puig, D.; van den Berg, J.; Koster, J.; Kedziora, K.M.; Molenaar, B.; Weerts, M.J.; de Rink, I.; Medema, R.H.; et al. Glycerophosphodiesterase GDE2 Promotes Neuroblastoma Differentiation through Glypican Release and Is a Marker of Clinical Outcome. Cancer Cell 2016, 30, 548–562. [Google Scholar] [CrossRef]
- Cave, C.; Park, S.; Rodriguez, M.; Nakamura, M.; Hoke, A.; Pletnikov, M.; Sockanathan, S. GDE2 is essential for neuronal survival in the postnatal mammalian spinal cord. Mol. Neurodegener. 2017, 12, 8. [Google Scholar] [CrossRef]
- Westerhaus, A.; Joseph, T.; Meyers, A.J.; Jang, Y.; Na, C.H.; Cave, C.; Sockanathan, S. The distribution and function of GDE2, a regulator of spinal motor neuron survival, are disrupted in Amyotrophic Lateral Sclerosis. Acta Neuropathol. Commun. 2022, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- van Veen, M.; Mans, L.A.; Matas-Rico, E.; van Pelt, J.; Perrakis, A.; Moolenaar, W.H.; Haramis, A.G. Glycerophosphodiesterase GDE2/GDPD5 affects pancreas differentiation in zebrafish. Int. J. Biochem. Cell Biol. 2018, 94, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Daudelin, D.; Westerhaus, A.; Zhang, N.; Leyder, E.; Savonenko, A.; Sockanathan, S. Loss of GDE2 leads to complex behavioral changes including memory impairment. Behav. Brain Funct. 2024, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Cave, C.; Na, C.H.; Sockanathan, S. GDE2-Dependent Activation of Canonical Wnt Signaling in Neurons Regulates Oligodendrocyte Maturation. Cell Rep. 2020, 31, 107540. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Dobrowolski, M.; Sockanathan, S. GDE2 expression in oligodendroglia regulates the pace of oligodendrocyte maturation. Dev. Dyn. 2021, 250, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zheng, J.; He, X.; Zhang, X. Tiki proteins are glycosylphosphatidylinositol-anchored proteases. FEBS Lett. 2022, 596, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Nogusa, Y.; Fujioka, Y.; Komatsu, R.; Kato, N.; Yanaka, N. Isolation and characterization of two serpentine membrane proteins containing glycerophosphodiester phosphodiesterase, GDE2 and GDE6. Gene 2004, 337, 173–179. [Google Scholar] [CrossRef] [PubMed]
- McKean, M.; Napoli, F.R.; Hasan, T.; Joseph, T.; Wheeler, A.; Beebe, K.; Soriano-Cruz, S.; Kawano, M.; Cave, C. GDE6 promotes progenitor identity in the vertebrate neural tube. Front. Neurosci. 2023, 17, 1047767. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Polo, F.; van Veen, M.; van den Broek, B.; Jalink, K.; Leyton-Puig, D.; Perrakis, A.; Moolenaar, W.H.; Matas-Rico, E. Sequence-dependent trafficking and activity of GDE2, a GPI-specific phospholipase promoting neuronal differentiation. J. Cell Sci. 2020, 133, jcs235044. [Google Scholar] [CrossRef]
- Levy-Myers, R.; Daudelin, D.; Na, C.H.; Sockanathan, S. An independent regulator of global release pathways in astrocytes generates a subtype of extracellular vesicles required for postsynaptic function. Sci. Adv. 2023, 9, eadg2067. [Google Scholar] [CrossRef]
- Brindley, D.N.; Waggoner, D.W. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 1998, 273, 24281–24284. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Ghil, S. Crosstalk between cannabinoid receptor 2 and lysophosphatidic acid receptor 5. Biochem. Biophys. Res. Commun. 2023, 666, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Fa, H.; Xiao, D.; Wang, J. MicroRNA-184 alleviates insulin resistance in cardiac myocytes and high fat diet-induced cardiac dysfunction in mice through the LPP3/DAG pathway. Mol. Cell. Endocrinol. 2020, 508, 110793. [Google Scholar] [CrossRef] [PubMed]
- Hooks, S.B.; Santos, W.L.; Im, D.S.; Heise, C.E.; Macdonald, T.L.; Lynch, K.R. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 2001, 276, 4611–4621. [Google Scholar] [CrossRef] [PubMed]
- Smyth, S.S.; Sciorra, V.A.; Sigal, Y.J.; Pamuklar, Z.; Wang, Z.; Xu, Y.; Prestwich, G.D.; Morris, A.J. Lipid phosphate phosphatases regulate lysophosphatidic acid production and signaling in platelets: Studies using chemical inhibitors of lipid phosphate phosphatase activity. J. Biol. Chem. 2003, 278, 43214–43223. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R.; Khandoga, A.L.; Goyal, P.; Fells, J.I.; Perygin, D.H.; Siess, W.; Parrill, A.L.; Tigyi, G.; Fujiwara, Y. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J. Biol. Chem. 2009, 284, 17304–17319. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, R.; Zhang, Q.X.; Pilquil, C.; Singh, I.; Xu, J.; Dewald, J.; Dillon, D.A.; Berthiaume, L.G.; Carman, G.M.; Waggoner, D.W.; et al. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 1999, 340 Pt 3, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Pilquil, C.; Dewald, J.; Cherney, A.; Gorshkova, I.; Tigyi, G.; English, D.; Natarajan, V.; Brindley, D.N. Lipid phosphate phosphatase-1 regulates lysophosphatidate-induced fibroblast migration by controlling phospholipase D2-dependent phosphatidate generation. J. Biol. Chem. 2006, 281, 38418–38429. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Yokoyama, K.; Tigyi, G.; Pyne, N.J.; Pyne, S. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid- and platelet-derived-growth-factor-induced cell migration. Biochem. J. 2006, 394, 495–500. [Google Scholar] [CrossRef]
- Yukiura, H.; Kano, K.; Kise, R.; Inoue, A.; Aoki, J. LPP3 localizes LPA6 signalling to non-contact sites in endothelial cells. J. Cell Sci. 2015, 128, 3871–3877. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Snyder, A.H.; Berdyshev, E.V.; Skobeleva, A.; Mataya, C.; Natarajan, V.; Brindley, D.N.; Lynch, K.R. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 2009, 419, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.A.; Yang, L.; Ubele, M.; Mao, G.; Brandon, J.; Vandra, J.; Nichols, T.C.; Escalante-Alcalde, D.; Morris, A.J.; Smyth, S.S. Coronary Artery Disease Risk-Associated Plpp3 Gene and Its Product Lipid Phosphate Phosphatase 3 Regulate Experimental Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Manzini, S.; Hilvo, M.; Parolini, C.; Ganzetti, G.S.; Dellera, F.; Ekroos, K.; Jänis, M.; Escalante-Alcalde, D.; Sirtori, C.R.; et al. Liver-specific deletion of the Plpp3 gene alters plasma lipid composition and worsens atherosclerosis in apoE-/- mice. Sci. Rep. 2017, 7, 44503. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Escalante-Alcalde, D.; Bhuiyan, M.S.; Orr, A.W.; Kevil, C.; Morris, A.J.; Nam, H.; Dominic, P.; McCarthy, K.J.; Miriyala, S.; et al. Cardiac-specific inactivation of LPP3 in mice leads to myocardial dysfunction and heart failure. Redox Biol. 2018, 14, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Yokoyama, K.; Balazs, L.; Baker, D.L.; Smalley, D.; Pilquil, C.; Brindley, D.N.; Tigyi, G. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell. Signal. 2004, 16, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sundberg, J.P.; Gridley, T. Mice mutant for Ppap2c, a homolog of the germ cell migration regulator wunen, are viable and fertile. Genesis 2000, 27, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Alcalde, D.; Hernandez, L.; Le Stunff, H.; Ryu Maeda, R.; Lee, H.S.; Cheng, J.G.; Sciorra, V.A.; Daar, I.; Spiegel, S.; Morris, A.J.; et al. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development 2003, 130, 4623–4637. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Kai, M.; Wada, I.; Sakane, F.; Kanoh, H. Differential localization of lipid phosphate phosphatases 1 and 3 to cell surface subdomains in polarized MDCK cells. FEBS Lett. 2003, 552, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Sakane, F.; Jia, Y.J.; Imai, S.; Yasuda, S.; Kanoh, H. Lipid phosphate phosphatases 1 and 3 are localized in distinct lipid rafts. J. Biochem. 2006, 140, 677–686. [Google Scholar] [CrossRef]
- Tang, X.; Cromwell, C.R.; Liu, R.; Godbout, R.; Hubbard, B.P.; McMullen, T.P.W.; Brindley, D.N. Lipid phosphate phosphatase-2 promotes tumor growth through increased c-Myc expression. Theranostics 2022, 12, 5675–5690. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Wu, R.; Tang, X.; Brindley, D.N.; Ishikawa, T.; Takabe, K. Decreased Lipid Phosphate Phosphatase 1/3 and Increased Lipid Phosphate Phosphatase 2 Expression in the Human Breast Cancer Tumor Microenvironment Promotes Tumor Progression and Immune System Evasion. Cancers 2023, 15, 2299. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Manzini, S.; Parolini, C.; Escalante-Alcalde, D.; Chiesa, G. Lipid phosphate phosphatase 3 in vascular pathophysiology. Atherosclerosis 2018, 271, 156–165. [Google Scholar] [CrossRef]
- Smyth, S.S.; Kraemer, M.; Yang, L.; Van Hoose, P.; Morris, A.J. Roles for lysophosphatidic acid signaling in vascular development and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158734. [Google Scholar] [CrossRef]
- Davidson, J.; Rotondo, D. The potential role of the lipid phosphate phosphatase 3 (Plpp3) gene in cardiovascular disease. Curr. Opin. Lipidol. 2020, 31, 258–259. [Google Scholar] [CrossRef]
- Jose, A.; Fernando, J.; Kienesberger, P. Lysophosphatidic Acid Metabolism and Signaling in Heart Disease. Can. J. Physiol. Pharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Salous, A.K.; Brandon, J.; Miriyala, S.; Wheeler, J.; Patil, P.; Sunkara, M.; Morris, A.J.; Escalante-Alcalde, D.; Smyth, S.S. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, I.; Baruah, J.; Lurie, E.E.; Wary, K.K. Endothelial lipid phosphate phosphatase-3 deficiency that disrupts the endothelial barrier function is a modifier of cardiovascular development. Cardiovasc. Res. 2016, 111, 105–118. [Google Scholar] [CrossRef]
- Tripathi, H.; Shindo, K.; Donahue, R.R.; Gao, E.; Kuppa, A.; ElKammar, M.; Morris, A.J.; Smyth, S.S.; Abdel-Latif, A. Myeloid-Specific Deletion of Lipid Plpp3 (Phosphate Phosphatase 3) Increases Cardiac Inflammation After Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 379–381. [Google Scholar] [CrossRef]
- Chabowski, D.S.; Hughes, W.E.; Hockenberry, J.C.; LoGiudice, J.; Beyer, A.M.; Gutterman, D.D. Lipid phosphate phosphatase 3 maintains NO-mediated flow-mediated dilatation in human adipose resistance arterioles. J. Physiol. 2023, 601, 469–481. [Google Scholar] [CrossRef]
- Wu, C.; Huang, R.T.; Kuo, C.H.; Kumar, S.; Kim, C.W.; Lin, Y.C.; Chen, Y.J.; Birukova, A.; Birukov, K.G.; Dulin, N.O.; et al. Mechanosensitive PPAP2B Regulates Endothelial Responses to Atherorelevant Hemodynamic Forces. Circ. Res. 2015, 117, e41–e53. [Google Scholar] [CrossRef]
- Bot, M.; de Jager, S.C.; MacAleese, L.; Lagraauw, H.M.; van Berkel, T.J.; Quax, P.H.; Kuiper, J.; Heeren, R.M.; Biessen, E.A.; Bot, I. Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation. J. Lipid Res. 2013, 54, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Van Hoose, P.M.; Yang, L.; Kraemer, M.; Ubele, M.; Morris, A.J.; Smyth, S.S. Lipid phosphate phosphatase 3 in smooth muscle cells regulates angiotensin II-induced abdominal aortic aneurysm formation. Sci. Rep. 2022, 12, 5664. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Chattopadhyay, A.; Hough, G.; Meriwether, D.; Fogelman, S.I.; Wagner, A.C.; Grijalva, V.; Su, F.; Anantharamaiah, G.M.; Hwang, L.H.; et al. Source and role of intestinally derived lysophosphatidic acid in dyslipidemia and atherosclerosis. J. Lipid Res. 2015, 56, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, T.; Sonoda, H.; Takanezawa, Y.; Morikawa, R.; Ishida, M.; Kasahara, K.; Sanai, Y.; Taguchi, R.; Aoki, J.; Arai, H. Biochemical and molecular characterization of two phosphatidic acid-selective phospholipase A1s, mPA-PLA1alpha and mPA-PLA1beta. J. Biol. Chem. 2003, 278, 49438–49447. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Broedl, U.C.; Monajemi, H.; Glick, J.M.; Rader, D.J. Lipase H, a new member of the triglyceride lipase family synthesized by the intestine. Genomics 2002, 80, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Remington, S.G.; Nelson, J.D. mRNA encoding a new lipolytic enzyme expressed in rabbit lacrimal glands. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3617–3624. [Google Scholar]
- Holmes, R.S.; Cox, L.A. Review. Comparative structures and evolution of mammalian lipase I (LIPI) genes and proteins: A close relative of vertebrate phospholipase LIPH. Nat. Sci. 2012, 4, 1165–1178. [Google Scholar] [CrossRef]
- Nanjundan, M.; Possmayer, F. Pulmonary lipid phosphate phosphohydrolase in plasma membrane signalling platforms. Biochem. J. 2001, 358, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Sciorra, V.A.; Morris, A.J. Sequential actions of phospholipase D and phosphatidic acid phosphohydrolase 2b generate diglyceride in mammalian cells. Mol. Biol. Cell 1999, 10, 3863–3876. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Gordon, C.M.; Williamson, B.; Lee, S.Y.; Chen, Y.T.; Stockert, E.; Jungbluth, A.; Ritter, G.; Jäger, D.; Jäger, E.; et al. Identification of cancer/testis genes by database mining and mRNA expression analysis. Int. J. Cancer 2002, 98, 485–492. [Google Scholar] [CrossRef]
- Hesse, M.; Willscher, E.; Schmiedel, B.J.; Posch, S.; Golbik, R.P.; Staege, M.S. Sequence and expression of the chicken membrane-associated phospholipases A1 alpha (LIPH) and beta (LIPI). Mol. Biol. Rep. 2012, 39, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Willier, S.; Butt, E.; Grunewald, T.G. Lysophosphatidic acid (LPA) signalling in cell migration and cancer invasion: A focussed review and analysis of LPA receptor gene expression on the basis of more than 1700 cancer microarrays. Biol. Cell 2013, 105, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.Y.; Hegele, R.A.; Wang, J.; Wang, D.Y.; Cheung, J.; Wilson, M.; Yahyapour, M.; Bai, Y.; Zhuang, L.; Skaug, J.; et al. Identification of a novel lipase gene mutated in lpd mice with hypertriglyceridemia and associated with dyslipidemia in humans. Hum. Mol. Genet. 2003, 12, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Yoshida, Y.; Ishimine, H.; Shinozaki-Ushiku, A.; Ito, Y.; Sumitomo, K.; Nakajima, J.; Fukayama, M.; Michiue, T.; Asashima, M.; et al. Lipase member H is a novel secreted protein selectively upregulated in human lung adenocarcinomas and bronchioloalveolar carcinomas. Biochem. Biophys. Res. Commun. 2014, 443, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Qiao, X.; Gu, X.; Xue, J.; Han, Y.; Sun, L.; Cui, M.; Liu, C. LIPH promotes metastasis by enriching stem-like cells in triple-negative breast cancer. J. Cell. Mol. Med. 2020, 24, 9125–9134. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Jiang, Y.; Shi, M.; Gan, L.; Wu, Z.; Xue, M.; Zhu, Y.; Xiong, C.; Wang, T.; Lin, X.; et al. LIPH contributes to glycolytic phenotype in pancreatic ductal adenocarcinoma by activating LPA/LPAR axis and maintaining ALDOA stability. J. Transl. Med. 2023, 21, 838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, L.; Liu, X.Y.; Liu, F.X.; Zhang, H.; Zhang, Y.J.; Tang, X.M.; Ma, Y.S.; Wu, H.Y.; Diao, X.; et al. KLK10/LIPH/PARD6B/SLC52A3 are promising molecular biomarkers for the prognosis of pancreatic cancer through a ceRNA network. Heliyon 2024, 10, e24287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yao, H.; Wang, H.; Sui, T. Development of Woolly Hair and Hairlessness in a CRISPR-Engineered Mutant Mouse Model with KRT71 Mutations. Cells 2023, 12, 1781. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004, 15, 477–489. [Google Scholar] [CrossRef]
- Fourcade, O.; Simon, M.F.; Viodé, C.; Rugani, N.; Leballe, F.; Ragab, A.; Fournié, B.; Sarda, L.; Chap, H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 1995, 80, 919–927. [Google Scholar] [CrossRef]
- Le Balle, F.; Simon, M.F.; Meijer, S.; Fourcade, O.; Chap, H. Membrane sidedness of biosynthetic pathways involved in the production of lysophosphatidic acid. Adv. Enzym. Regul. 1999, 39, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, A.U.; Nitsch, R. Plasticity-related genes (PRGs/LRPs): A brain-specific class of lysophospholipid-modifying proteins. Biochim. Biophys. Acta 2008, 1781, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Strauss, U.; Bräuer, A.U. Current views on regulation and function of plasticity-related genes (PRGs/LPPRs) in the brain. Biochim. Biophys. Acta 2013, 1831, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Trimbuch, T.; Beed, P.; Vogt, J.; Schuchmann, S.; Maier, N.; Kintscher, M.; Breustedt, J.; Schuelke, M.; Streu, N.; Kieselmann, O.; et al. Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell 2009, 138, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Tokumitsu, H.; Hatano, N.; Tsuchiya, M.; Yurimoto, S.; Fujimoto, T.; Ohara, N.; Kobayashi, R.; Sakagami, H. Identification and characterization of PRG-1 as a neuronal calmodulin-binding protein. Biochem. J. 2010, 431, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Yang, J.W.; Mobascher, A.; Cheng, J.; Li, Y.; Liu, X.; Baumgart, J.; Thalman, C.; Kirischuk, S.; Unichenko, P.; et al. Molecular cause and functional impact of altered synaptic lipid signaling due to a prg-1 gene SNP. EMBO Mol. Med. 2016, 8, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huai, J.; Endle, H.; Schlüter, L.; Fan, W.; Li, Y.; Richers, S.; Yurugi, H.; Rajalingam, K.; Ji, H.; et al. PRG-1 Regulates Synaptic Plasticity via Intracellular PP2A/β1-Integrin Signaling. Dev. Cell 2016, 38, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, G.G.; Matin, N.; Wild, A.R.; Tosefsky, K.; Flibotte, S.; Stacey, R.G.; Hollman, R.B.; Foster, L.J.; Bamji, S.X. Synaptic activity-dependent changes in the hippocampal palmitoylome. Sci. Signal. 2022, 15, eadd2519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Pilquil, C.S.; Dewald, J.; Berthiaume, L.G.; Brindley, D.N. Identification of structurally important domains of lipid phosphate phosphatase-1: Implications for its sites of action. Biochem. J. 2000, 345 Pt 2, 181–184. [Google Scholar] [CrossRef]
- Bräuer, A.U.; Savaskan, N.E.; Kühn, H.; Prehn, S.; Ninnemann, O.; Nitsch, R. A new phospholipid phosphatase, PRG-1, is involved in axon growth and regenerative sprouting. Nat. Neurosci. 2003, 6, 572–578. [Google Scholar] [CrossRef]
- McDermott, M.I.; Sigal, Y.J.; Sciorra, V.A.; Morris, A.J. Is PRG-1 a new lipid phosphatase. Nat. Neurosci. 2004, 7, 789. [Google Scholar] [CrossRef] [PubMed]
- Pagano, R.E.; Longmuir, K.J. Phosphorylation, transbilayer movement, and facilitated intracellular transport of diacylglycerol are involved in the uptake of a fluorescent analog of phosphatidic acid by cultured fibroblasts. J. Biol. Chem. 1985, 260, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.Z.; Morris, A.J. Role of phosphatidic acid phosphatase 2a in uptake of extracellular lipid phosphate mediators. Biochim. Biophys. Acta 2000, 1487, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Renault, A.D.; Sigal, Y.J.; Morris, A.J.; Lehmann, R. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science 2004, 305, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Vacratsis, P.O.; Firestein, R.; Cleary, M.L.; Dixon, J.E. Regulation of myotubularin-related (MTMR)2 phosphatidylinositol phosphatase by MTMR5, a catalytically inactive phosphatase. Proc. Natl. Acad. Sci. USA 2003, 100, 4492–4497. [Google Scholar] [CrossRef] [PubMed]
- Rohde, H.M.; Tronchère, H.; Payrastre, B.; Laporte, J. Detection of myotubularin phosphatases activity on phosphoinositides in vitro and ex vivo. Methods Mol. Biol. 2009, 462, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Sreelatha, A. Emerging functions of pseudoenzymes. Biochem. J. 2023, 480, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Bitar, L.; Uphaus, T.; Thalman, C.; Muthuraman, M.; Gyr, L.; Ji, H.; Domingues, M.; Endle, H.; Groppa, S.; Steffen, F.; et al. Inhibition of the enzyme autotaxin reduces cortical excitability and ameliorates the outcome in stroke. Sci. Transl. Med. 2022, 14, eabk0135. [Google Scholar] [CrossRef] [PubMed]
- Unichenko, P.; Kirischuk, S.; Yang, J.W.; Baumgart, J.; Roskoden, T.; Schneider, P.; Sommer, A.; Horta, G.; Radyushkin, K.; Nitsch, R.; et al. Plasticity-Related Gene 1 Affects Mouse Barrel Cortex Function via Strengthening of Glutamatergic Thalamocortical Transmission. Cereb. Cortex 2016, 26, 3260–3272. [Google Scholar] [CrossRef]
- Schneider, P.; Petzold, S.; Sommer, A.; Nitsch, R.; Schwegler, H.; Vogt, J.; Roskoden, T. Altered synaptic phospholipid signaling in PRG-1 deficient mice induces exploratory behavior and motor hyperactivity resembling psychiatric disorders. Behav. Brain Res. 2018, 336, 1–7. [Google Scholar] [CrossRef]
- Thalman, C.; Horta, G.; Qiao, L.; Endle, H.; Tegeder, I.; Cheng, H.; Laube, G.; Sigurdsson, T.; Hauser, M.J.; Tenzer, S.; et al. Synaptic phospholipids as a new target for cortical hyperexcitability and E/I balance in psychiatric disorders. Mol. Psychiatry 2018, 23, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Tüscher, O.; Muthuraman, M.; Horstmann, J.P.; Horta, G.; Radyushkin, K.; Baumgart, J.; Sigurdsson, T.; Endle, H.; Ji, H.; Kuhnhäuser, P.; et al. Altered cortical synaptic lipid signaling leads to intermediate phenotypes of mental disorders. Mol. Psychiatry 2024. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Endle, H.; Schumann, L.; Wilken-Schmitz, A.; Kaiser, J.; Gerber, S.; Vogelaar, C.F.; Schmidt, M.H.H.; Nitsch, R.; Snodgrass, I.; et al. Prevention of age-associated neuronal hyperexcitability with improved learning and attention upon knockout or antagonism of LPAR2. Cell. Mol. Life Sci. 2021, 78, 1029–1050. [Google Scholar] [CrossRef] [PubMed]
- Endle, H.; Horta, G.; Stutz, B.; Muthuraman, M.; Tegeder, I.; Schreiber, Y.; Snodgrass, I.F.; Gurke, R.; Liu, Z.W.; Sestan-Pesa, M.; et al. AgRP neurons control feeding behaviour at cortical synapses via peripherally derived lysophospholipids. Nat. Metab. 2022, 4, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Knierim, E.; Vogt, J.; Kintscher, M.; Ponomarenko, A.; Baumgart, J.; Beed, P.; Korotkova, T.; Trimbuch, T.; Panzer, A.; Steinlein, O.K.; et al. Mutations in plasticity-related-gene-1 (PRG-1) protein contribute to hippocampal seizure susceptibility and modify epileptic phenotype. Cereb. Cortex 2023, 33, 7454–7467. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.; Zhang, C.; Li, C.; Fan, Y.; Li, Z.; Hou, K.; Che, X.; Liu, Y.; Qu, X. LPPR4 promotes peritoneal metastasis via Sp1/integrin α/FAK signaling in gastric cancer. Am. J. Cancer Res. 2020, 10, 1026–1044. [Google Scholar] [PubMed]
- Gaaya, A.; Poirier, O.; Mougenot, N.; Hery, T.; Atassi, F.; Marchand, A.; Saulnier-Blache, J.S.; Amour, J.; Vogt, J.; Lompré, A.M.; et al. Plasticity-related gene-1 inhibits lysophosphatidic acid-induced vascular smooth muscle cell migration and proliferation and prevents neointima formation. Am. J. Physiol. Cell Physiol. 2012, 303, C1104–C1114. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, V.; Montero, F.; González-Forero, D.; Rodríguez-Bey, G.; Gómez-Pérez, L.; Medialdea-Wandossell, M.J.; Domínguez-Vías, G.; García-Verdugo, J.M.; Moreno-López, B. Membrane-derived phospholipids control synaptic neurotransmission and plasticity. PLoS Biol. 2015, 13, e1002153. [Google Scholar] [CrossRef] [PubMed]
- Roza, C.; Campos-Sandoval, J.A.; Gómez-García, M.C.; Peñalver, A.; Márquez, J. Lysophosphatidic Acid and Glutamatergic Transmission. Front. Mol. Neurosci. 2019, 12, 138. [Google Scholar] [CrossRef]
- Croset, M.; Brossard, N.; Polette, A.; Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000, 345 Pt 1, 61–67. [Google Scholar] [CrossRef]
- Ojala, P.J.; Hirvonen, T.E.; Hermansson, M.; Somerharju, P.; Parkkinen, J. Acyl chain-dependent effect of lysophosphatidylcholine on human neutrophils. J. Leukoc. Biol. 2007, 82, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Kano, K.; Hara, M.; Tsukamoto, K.; Aoki, J.; Yatomi, Y. Regulation of plasma glycero-lysophospholipid levels by lipoprotein metabolism. Biochem. J. 2019, 476, 3565–3581. [Google Scholar] [CrossRef] [PubMed]
- Sekas, G.; Patton, G.M.; Lincoln, E.C.; Robins, S.J. Origin of plasma lysophosphatidylcholine: Evidence for direct hepatic secretion in the rat. J. Lab. Clin. Med. 1985, 105, 190–194. [Google Scholar] [PubMed]
- Brindley, D.N. Hepatic secretion of lysophosphatidylcholine: A novel transport system for polyunsaturated fatty acids and choline. J. Nutr. Biochem. 1993, 4, 442–449. [Google Scholar] [CrossRef]
- Sugasini, D.; Yang, P.; Ng, D.; Khetarpal, S.A.; Vitali, C.; Rader, D.J.; Subbaiah, P.V. Potential role of hepatic lipase in the accretion of docosahexaenoic acid (DHA) by the brain. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159002. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; McHowat, J.; Hsu, F.F.; Brennan, M.L.; Hazen, S.L.; Ford, D.A. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation 2003, 108, 3128–3133. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Sumitani, M.; Akiyama, Y.; Yamada, M.; Fujimura, D.; Yamaki, S.; Kano, K.; Aoki, J.; Hayakawa, K.; Takahashi, T.; et al. Usefulness of lysophosphatidylcholine measurement in the cerebrospinal fluid for differential diagnosis of neuropathic pain: Possible introduction into clinical laboratory testing. Clin. Chim. Acta 2023, 541, 117249. [Google Scholar] [CrossRef] [PubMed]
- Thiés, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem. 1992, 59, 1110–1116. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Cater, R.J.; Chua, G.L.; Erramilli, S.K.; Keener, J.E.; Choy, B.C.; Tokarz, P.; Chin, C.F.; Quek, D.Q.Y.; Kloss, B.; Pepe, J.G.; et al. Structural basis of omega-3 fatty acid transport across the blood-brain barrier. Nature 2021, 595, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.A.P.; Zhang, J.; Aydin, D.; Xu, Y.; Andreone, B.J.; Langen, U.H.; Dror, R.O.; Gu, C.; Feng, L. Structure and mechanism of blood-brain-barrier lipid transporter MFSD2A. Nature 2021, 596, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Sakai, E.; Yatomi, Y. Understanding modulations of lipid mediators in cancer using a murine model of carcinomatous peritonitis. Cancer Med. 2022, 11, 3491–3507. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, D.; Hamano, F.; Masuda, K.; Dahlström, M.; Kobayashi, D.; Sato, N.; Hamakubo, T.; Shimizu, T.; Ishii, S. Inverse agonism of lysophospholipids with cationic head groups at Gi-coupled receptor GPR82. Eur. J. Pharmacol. 2023, 954, 175893. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Huang, S.; Guo, S.; Yun, Y.; Cheng, X.; He, X.; Cai, P.; Lan, Y.; Zhou, H.; Jiang, H.; et al. Structural identification of lysophosphatidylcholines as activating ligands for orphan receptor GPR119. Nat. Struct. Mol. Biol. 2022, 29, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Kristinsson, H.; Sałaga, M.; Zatorski, H.; Koziołkiewicz, M.; Gendaszewska-Darmach, E.; Bergsten, P. Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol. Cell. Endocrinol. 2018, 472, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Szustak, M.; Korkus, E.; Madaj, R.; Chworos, A.; Dąbrowski, G.; Czaplicki, S.; Tabandeh, E.; Maciejewska, G.; Koziołkiewicz, M.; Konopka, I.; et al. Lysophosphatidylcholines Enriched with cis and trans Palmitoleic Acid Regulate Insulin Secretion via GPR119 Receptor. ACS Med. Chem. Lett. 2024, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, J.; Yang, L.; Liu, Y.; Wang, L.; Liu, W.; Lin, Y.; Yang, H.; Ma, L.; Ye, S.; et al. Activation and signaling mechanism revealed by GPR119-Gs complex structures. Nat. Commun. 2022, 13, 7033. [Google Scholar] [CrossRef]
- Kabarowski, J.H. G2A and LPC: Regulatory functions in immunity. Prostaglandins Other Lipid Mediat. 2009, 89, 73–81. [Google Scholar] [CrossRef]
- Sisignano, M.; Fischer, M.J.M.; Geisslinger, G. Proton-Sensing GPCRs in Health and Disease. Cells 2021, 10, 2050. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Ngai, D.; Schilperoort, M.; Tabas, I. Efferocytosis-induced lactate enables the proliferation of pro-resolving macrophages to mediate tissue repair. Nat. Metab. 2023, 5, 2206–2219. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Huang, F.; Naikawadi, R.P.; Kim, K.S.; Said, T.; Lum, H. Lysophosphatidylcholine impairs endothelial barrier function through the G protein-coupled receptor GPR4. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L91–L101. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Yokomizo, T.; Okuno, T.; Shimizu, T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 2004, 279, 42484–42491. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.G.; Yang, L.V.; Riedinger, M.; Au, M.; Witte, O.N. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 245–250. [Google Scholar] [CrossRef]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Frasch, S.C.; Zemski-Berry, K.; Murphy, R.C.; Borregaard, N.; Henson, P.M.; Bratton, D.L. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J. Immunol. 2007, 178, 6540–6548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, W.; Liu, J.; Yang, H.; Hu, Y.; Zhang, M.; Bai, T.; Chang, F. Lysophosphatidylcholine promotes intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression in human umbilical vein endothelial cells via an orphan G protein receptor 2-mediated signaling pathway. Bioengineered 2021, 12, 4520–4535. [Google Scholar] [CrossRef]
- Le, L.Q.; Kabarowski, J.H.; Weng, Z.; Satterthwaite, A.B.; Harvill, E.T.; Jensen, E.R.; Miller, J.F.; Witte, O.N. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity 2001, 14, 561–571. [Google Scholar] [CrossRef]
- Bolick, D.T.; Skaflen, M.D.; Johnson, L.E.; Kwon, S.C.; Howatt, D.; Daugherty, A.; Ravichandran, K.S.; Hedrick, C.C. G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circ. Res. 2009, 104, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Jung, J.S.; Lee, J.E.; Lee, J.; Huh, S.O.; Kim, H.S.; Jung, K.C.; Cho, J.Y.; Nam, J.S.; Suh, H.W.; et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004, 10, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Jang, J.H.; Jung, J.S.; Shin, J.; Park, C.O.; Kim, Y.J.; Ahn, W.G.; Nam, J.S.; Hong, C.W.; Lee, J.; et al. G2A Protects Mice against Sepsis by Modulating Kupffer Cell Activation: Cooperativity with Adenosine Receptor 2b. J. Immunol. 2019, 202, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Radu, C.G.; Yang, L.V.; Bentolila, L.A.; Riedinger, M.; Witte, O.N. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol. Biol. Cell 2005, 16, 2234–2247. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Hattori, T.; Nakane, S.; Tatei, K.; Izumi, T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J. Biol. Chem. 2005, 280, 40676–40683. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.R.; Ueno, S.; Chen, M.X.; Harvey, J.; Dowell, S.J.; Irving, A.J.; Brown, A.J. N-Palmitoylglycine and other N-acylamides activate the lipid receptor G2A/GPR132. Pharmacol. Res. Perspect. 2019, 7, e00542. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.J.; Kang, H.S.; Chu, J.; Huang, Y.H.; Gordon, E.A.; Reddy, B.V.; Ternei, M.A.; Craig, J.W.; Brady, S.F. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4825–E4834. [Google Scholar] [CrossRef] [PubMed]
- Lahvic, J.L.; Ammerman, M.; Li, P.; Blair, M.C.; Stillman, E.R.; Fast, E.M.; Robertson, A.L.; Christodoulou, C.; Perlin, J.R.; Yang, S.; et al. Specific oxylipins enhance vertebrate hematopoiesis via the receptor GPR132. Proc. Natl. Acad. Sci. USA 2018, 115, 9252–9257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Dou, X.D.; Cheng, J.; Gao, M.X.; Xu, G.F.; Ding, W.; Ding, J.H.; Li, Y.; Wang, S.H.; Ji, Z.W.; et al. Functional screening and rational design of compounds targeting GPR132 to treat diabetes. Nat. Metab. 2023, 5, 1726–1746. [Google Scholar] [CrossRef]
- Carneiro, A.B.; Iaciura, B.M.; Nohara, L.L.; Lopes, C.D.; Veas, E.M.; Mariano, V.S.; Bozza, P.T.; Lopes, U.G.; Atella, G.C.; Almeida, I.C.; et al. Lysophosphatidylcholine triggers TLR2- and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-κB translocation and MAPK/ERK phosphorylation. PLoS ONE 2013, 8, e76233. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000164303-ENPP6 (accessed on 9 April 2024).
- Xiao, L.; Ohayon, D.; McKenzie, I.A.; Sinclair-Wilson, A.; Wright, J.L.; Fudge, A.D.; Emery, B.; Li, H.; Richardson, W.D. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 2016, 19, 1210–1217. [Google Scholar] [CrossRef]
- DeMichele-Sweet, M.A.A.; Klei, L.; Creese, B.; Harwood, J.C.; Weamer, E.A.; McClain, L.; Sims, R.; Hernandez, I.; Moreno-Grau, S.; Tárraga, L.; et al. Genome-wide association identifies the first risk loci for psychosis in Alzheimer disease. Mol. Psychiatry 2021, 26, 5797–5811. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Levey, A.I.; Lah, J.J.; Seyfried, N.T.; Mitchell, C.S. Machine Learning Selection of Most Predictive Brain Proteins Suggests Role of Sugar Metabolism in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 92, 411–424. [Google Scholar] [CrossRef]
- Stewart, A.J.; Leong, D.T.K.; Farquharson, C. PLA2 and ENPP6 may act in concert to generate phosphocholine from the matrix vesicle membrane during skeletal mineralization. FASEB J. 2018, 32, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.; Suchacki, K.; Hsu, S.N.; Stephen, L.A.; Wang, R.; Cawthorn, W.P.; Stewart, A.J.; Nudelman, F.; Morton, N.M.; Farquharson, C. Ablation of Enpp6 Results in Transient Bone Hypomineralization. JBMR Plus 2021, 5, e10439. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, P.; Xu, S.C.; Yang, L.; Voss, U.; Ekblad, E.; Wu, Y.; Min, Y.; Hertervig, E.; Nilsson, Å.; et al. Enhanced colonic tumorigenesis in alkaline sphingomyelinase (NPP7) knockout mice. Mol. Cancer Ther. 2015, 14, 259–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Hansen, G.H.; Niels-Christiansen, L.L.; Koentgen, F.; Ohlsson, L.; Nilsson, A.; Duan, R.D. Crucial role of alkaline sphingomyelinase in sphingomyelin digestion: A study on enzyme knockout mice. J. Lipid Res. 2011, 52, 771–781. [Google Scholar] [CrossRef]
- Tian, Y.; Li, X.; Wang, X.; Pei, S.T.; Pan, H.X.; Cheng, Y.Q.; Li, Y.C.; Cao, W.T.; Petersen, J.D.; Zhang, P. Alkaline sphingomyelinase deficiency impairs intestinal mucosal barrier integrity and reduces antioxidant capacity in dextran sulfate sodium-induced colitis. World J. Gastroenterol. 2024, 30, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.; Kotarsky, K.; Wu, J.; Agace, W.; Duan, R.D. Expression of alkaline sphingomyelinase in yeast cells and anti-inflammatory effects of the expressed enzyme in a rat colitis model. Dig. Dis. Sci. 2009, 54, 1440–1448. [Google Scholar] [CrossRef]
- Duan, R.D. Alkaline sphingomyelinase (NPP7) in hepatobiliary diseases: A field that needs to be closely studied. World J. Hepatol. 2018, 10, 246–253. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.; Guo, Z.; Zhang, T.; Zhang, P. Transcriptome analysis of intestine from alk-SMase knockout mice reveals the effect of alk-SMase. Cancer Cell Int. 2022, 22, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, L.; Li, X.; Lan, D.; Song, L.; Li, Y.; Cheng, Y.; Zhang, P. Transcriptome analysis alk-SMase knockout mice reveals the effect of alkaline sphingomyelinase on liver. Biochem. Biophys. Rep. 2022, 30, 101240. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, M.; Kadivar, M.; Erjefält, J.; Johansson-Lindbom, B.; Duan, R.D.; Nilsson, Å.; Marsal, J. Alkaline sphingomyelinase (NPP7) impacts the homeostasis of intestinal T lymphocyte populations. Front. Immunol. 2022, 13, 1050625. [Google Scholar] [CrossRef] [PubMed]
- Slieker, R.C.; Donnelly, L.A.; Akalestou, E.; Lopez-Noriega, L.; Melhem, R.; Güneş, A.; Abou Azar, F.; Efanov, A.; Georgiadou, E.; Muniangi-Muhitu, H.; et al. Identification of biomarkers for glycaemic deterioration in type 2 diabetes. Nat. Commun. 2023, 14, 2533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ma, M.; Zhang, L.; Wang, S.; Guo, Y.; Ling, X.; Lin, H.; Lai, N.; Lin, S.; Du, L.; et al. System Analysis Based on Lipid-Metabolism-Related Genes Identifies AGT as a Novel Therapy Target for Gastric Cancer with Neoadjuvant Chemotherapy. Pharmaceutics 2023, 15, 810. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nishikado, S.; Omi, J.; Aoki, J. The Many Roles of Lysophospholipid Mediators and Japanese Contributions to This Field. Biol. Pharm. Bull. 2022, 45, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Bottemanne, P.; Paquot, A.; Ameraoui, H.; Alhouayek, M.; Muccioli, G.G. The α/β-hydrolase domain 6 inhibitor WWL70 decreases endotoxin-induced lung inflammation in mice, potential contribution of 2-arachidonoylglycerol, and lysoglycerophospholipids. FASEB J. 2019, 33, 7635–7646. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Li, K.; Song, Z.; Wang, Q.; Wang, J.; Li, X.; Li, Y.; Zhang, Q.; Zhu, Y.; Chen, H. Nitric oxide hinders club cell proliferation through Gdpd2 during allergic airway inflammation. FEBS Open Bio 2023, 13, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.; Mowbray, S.; Dürk, H.; Homburg, S.; Fleming, I.; Fisslthaler, B.; Oesch, F.; Arand, M. The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc. Natl. Acad. Sci. USA 2003, 100, 1552–1557. [Google Scholar] [CrossRef]
- Newman, J.W.; Morisseau, C.; Harris, T.R.; Hammock, B.D. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc. Natl. Acad. Sci. USA 2003, 100, 1558–1563. [Google Scholar] [CrossRef]
- Oguro, A.; Imaoka, S. Lysophosphatidic acids are new substrates for the phosphatase domain of soluble epoxide hydrolase. J. Lipid Res. 2012, 53, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Oguro, A.; Kaga, Y.; Sato, H.; Fujiyama, T.; Fujimoto, S.; Nagai, S.; Matsuyama, M.; Miyara, M.; Ishihara, Y.; Yamazaki, T.; et al. Mice deficient in the phosphatase activity of sEH show decreased levels of the endocannabinoid 2-AG in the olfactory bulb and depressive-like behavior. FEBS Lett 2024. [Google Scholar] [CrossRef] [PubMed]
- Leuillier, M.; Duflot, T.; Ménoret, S.; Messaoudi, H.; Djerada, Z.; Groussard, D.; Denis, R.G.P.; Chevalier, L.; Karoui, A.; Panthu, B.; et al. CRISPR/Cas9-mediated inactivation of the phosphatase activity of soluble epoxide hydrolase prevents obesity and cardiac ischemic injury. J. Adv. Res. 2023, 43, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Su, W.M.; Gu, X.J.; Dou, M.; Duan, Q.Q.; Jiang, Z.; Yin, K.F.; Cai, W.C.; Cao, B.; Wang, Y.; Chen, Y.P. Systematic druggable genome-wide Mendelian randomisation identifies therapeutic targets for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2023, 94, 954–961. [Google Scholar] [CrossRef] [PubMed]
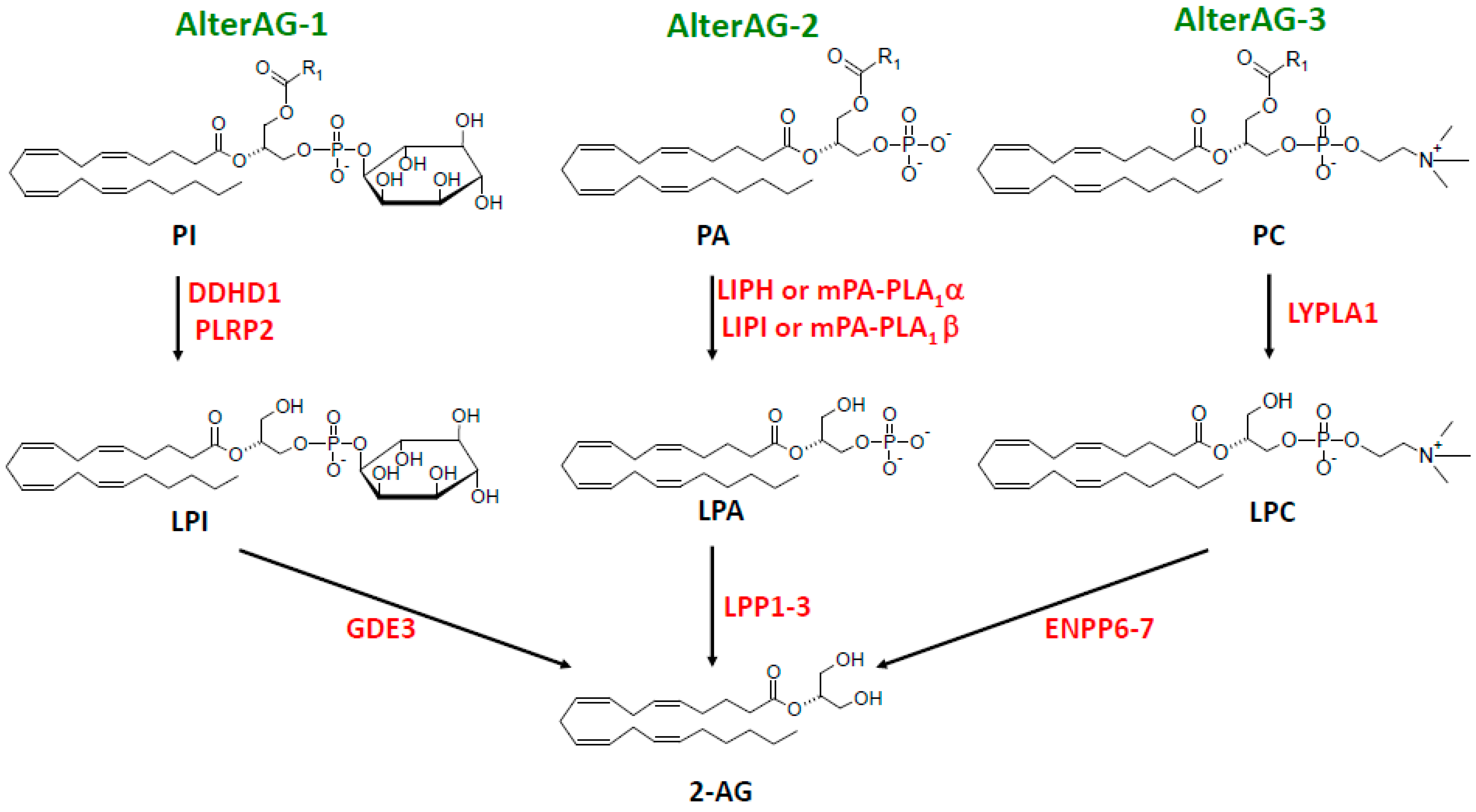
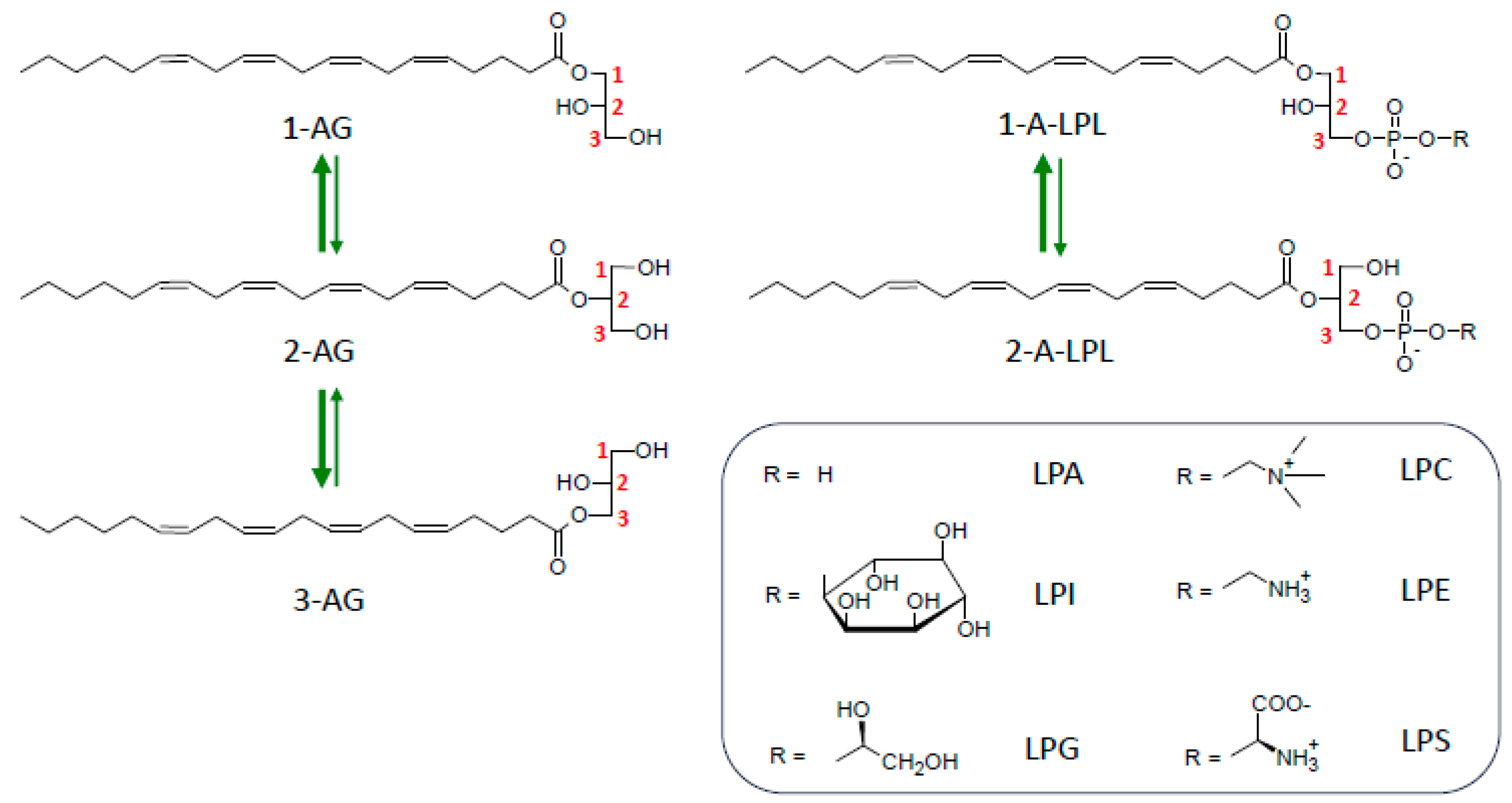
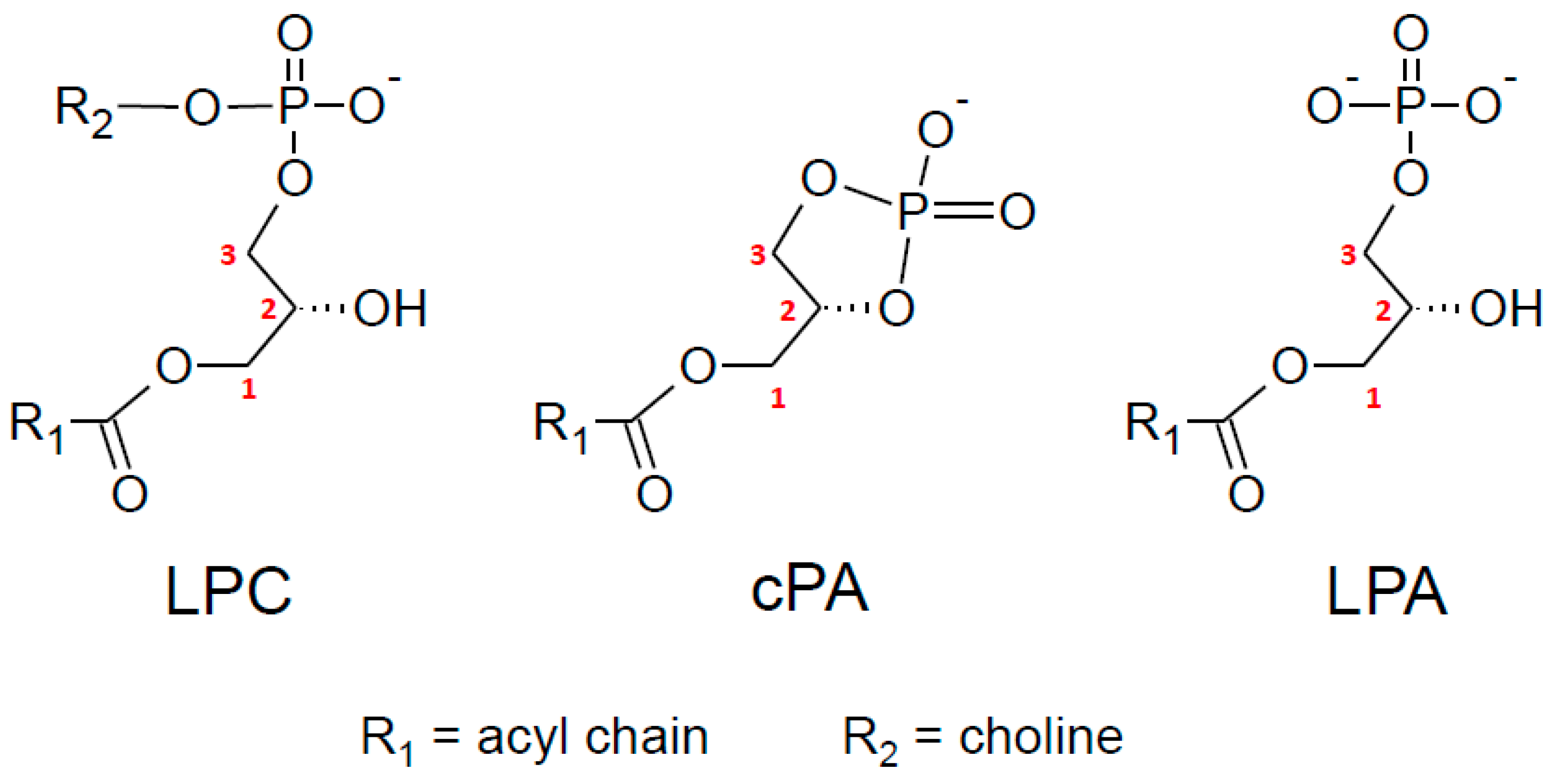
| PLC Subtype | Activation Mechanism | Main Localization Tested | References |
|---|---|---|---|
| PLCβ1 | Gαq | Hippocampus | [25] |
| PLCβ4 | Gαq | Cerebellum | [35] |
| PLCδ1,δ2,δ3 | Ca2+ (10 μM) | Brain | [36,70,72,73] |
| PLCη1,η2 | Ca2+ (1 μM) | Brain | [70,71] |
| PLCε | cAMP via Epac | Ventral tegmental area | [37] |
| PLCγ1 | Tyr phosphorylation | Brain | [38,39] |
| PLCγ2 | Tyr phosphorylation | Myeloid cells | [40] |
| Serine Hydrolases | Substrate | Effect of THL | References |
|---|---|---|---|
| DAGLα | DAG | Inhibited | [41] |
| DAGLβ | DAG | Inhibited | [41] |
| ABHD12 | MAG, LysoPS | Inhibited | [103] |
| ABHD16A | PS | Inhibited | [103] |
| TPP2 | Tripeptide | Inhibited | [103] |
| PLA2G7 | PAF | Inhibited | [103] |
| HSL | DAG > TAG > MAG | No effect | [103] |
| DDHD2 | DAG > TAG | Inhibited | [63] |
| Receptors | Ligand or Substrate Preference | References |
|---|---|---|
| CB1 | 2-AG > 3-AG > 1-AG | [11,186,187,188,189,190,191] |
| CB2 | 2-AG > 1-AG = 3-AG | [192] |
| TRPV1 | 2-AG = 1(3)-AG | [193,194,195] |
| GPR55 | 2-A-LPI most potent among LPI species | [196,197,198] |
| LPA3 | 2-acyl-LPA > 1-acyl-LPA (2-arachidonoyl-LPA < 2-oleoyl-LPA) | [199,200,201,202] |
| LPA6 | 2-acyl-LPA > 1-acyl-LPA (2-arachidonoyl-LPA < 2-oleoyl-LPA) | [199,200,203,204,205,206,207] |
| GPR34 | 2-acyl-LPS > 1-acyl-LPS | [208,209,210] |
| Enzymes | ||
| MAGL | 3-AG > 2-AG > 1-AG; 1(3)-AG = 2-AG | [188,211] |
| ABHD6 | 1-AG = 3-AG > 2-AG | [188,211] |
| ABHD12 | 1(3)-AG > 2-AG | [211,212] |
| FAAH | 1-AG = 2-AG = 3-AG | [188] |
| PLRP2 | 1(3)-acyl-sn-glycerol > 2-acyl-glycerol = 0;1-acyl-LPC >> 2-acyl-LPC = 0 | [213,214] |
| LYPLA1/LYPLA2 | 1(3)-AG >> 2-AG = 0;1-palmitoyl-LPC >> 2-palmitoyl-LPC = 0 | [215,216] |
| ABHD16A | 1(3)-linoleoyl-sn-glycerol > 2-linoleoyl-glycerol | [217] |
| LPP1–3 | LPP1 non stereospecific | [218,219] |
| GDE3 | 2-acyl-LPI = 1-acyl-LPI | [32] |
| ENPP6–7 | Not determined | |
| ATX/ENPP2 | 1-O-alkyl-LPC >> 2-O-alkyl-LPC | [220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briand-Mésange, F.; Gennero, I.; Salles, J.; Trudel, S.; Dahan, L.; Ausseil, J.; Payrastre, B.; Salles, J.-P.; Chap, H. From Classical to Alternative Pathways of 2-Arachidonoylglycerol Synthesis: AlterAGs at the Crossroad of Endocannabinoid and Lysophospholipid Signaling. Molecules 2024, 29, 3694. https://doi.org/10.3390/molecules29153694
Briand-Mésange F, Gennero I, Salles J, Trudel S, Dahan L, Ausseil J, Payrastre B, Salles J-P, Chap H. From Classical to Alternative Pathways of 2-Arachidonoylglycerol Synthesis: AlterAGs at the Crossroad of Endocannabinoid and Lysophospholipid Signaling. Molecules. 2024; 29(15):3694. https://doi.org/10.3390/molecules29153694
Chicago/Turabian StyleBriand-Mésange, Fabienne, Isabelle Gennero, Juliette Salles, Stéphanie Trudel, Lionel Dahan, Jérôme Ausseil, Bernard Payrastre, Jean-Pierre Salles, and Hugues Chap. 2024. "From Classical to Alternative Pathways of 2-Arachidonoylglycerol Synthesis: AlterAGs at the Crossroad of Endocannabinoid and Lysophospholipid Signaling" Molecules 29, no. 15: 3694. https://doi.org/10.3390/molecules29153694
APA StyleBriand-Mésange, F., Gennero, I., Salles, J., Trudel, S., Dahan, L., Ausseil, J., Payrastre, B., Salles, J.-P., & Chap, H. (2024). From Classical to Alternative Pathways of 2-Arachidonoylglycerol Synthesis: AlterAGs at the Crossroad of Endocannabinoid and Lysophospholipid Signaling. Molecules, 29(15), 3694. https://doi.org/10.3390/molecules29153694







