Au Nanoshell-Based Lateral Flow Immunoassay for Colorimetric and Photothermal Dual-Mode Detection of Interleukin-6
Abstract
1. Introduction
2. Results and Discussion
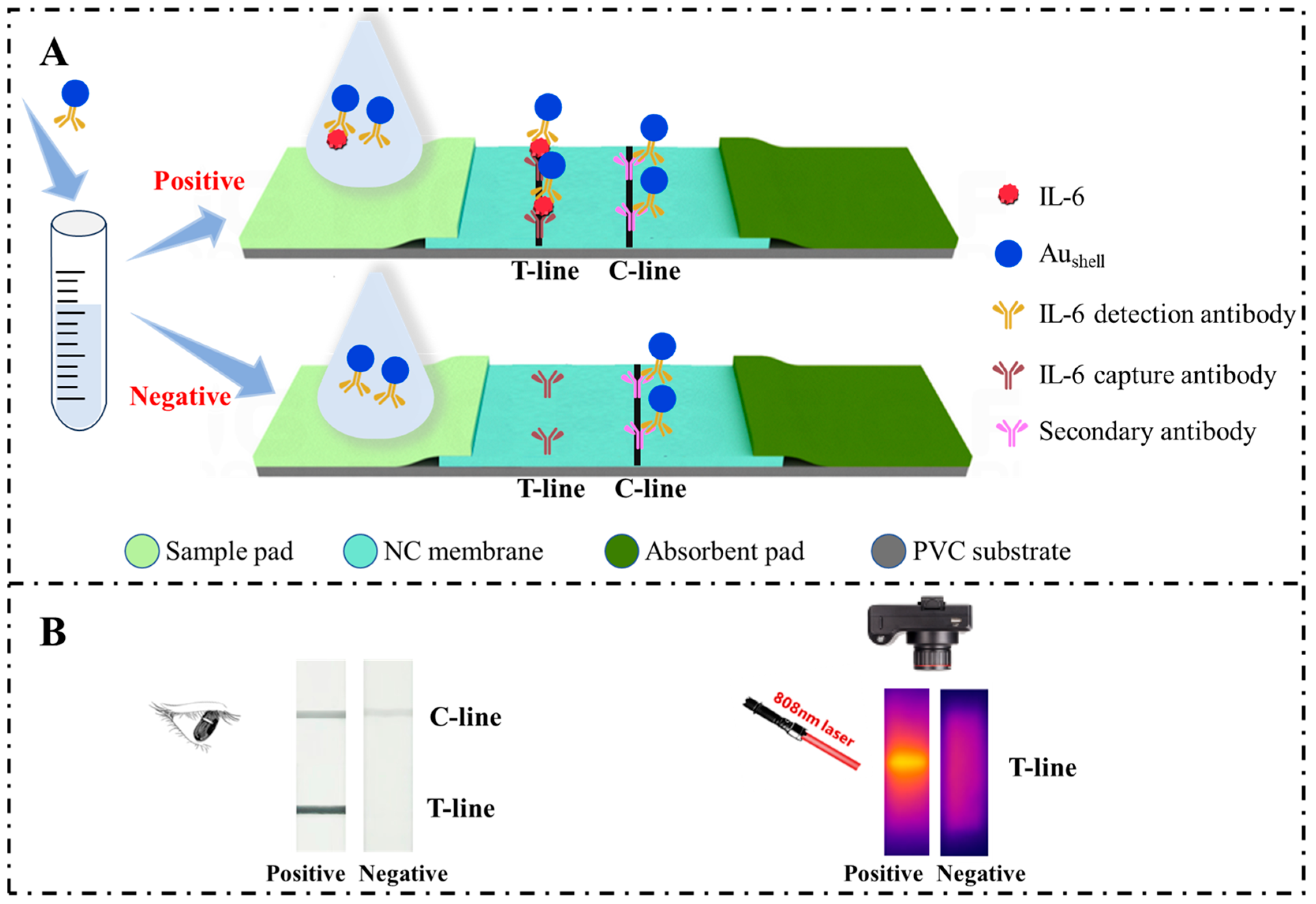
2.1. Principle of the Aushell-Based LFIA for Detecting IL-6
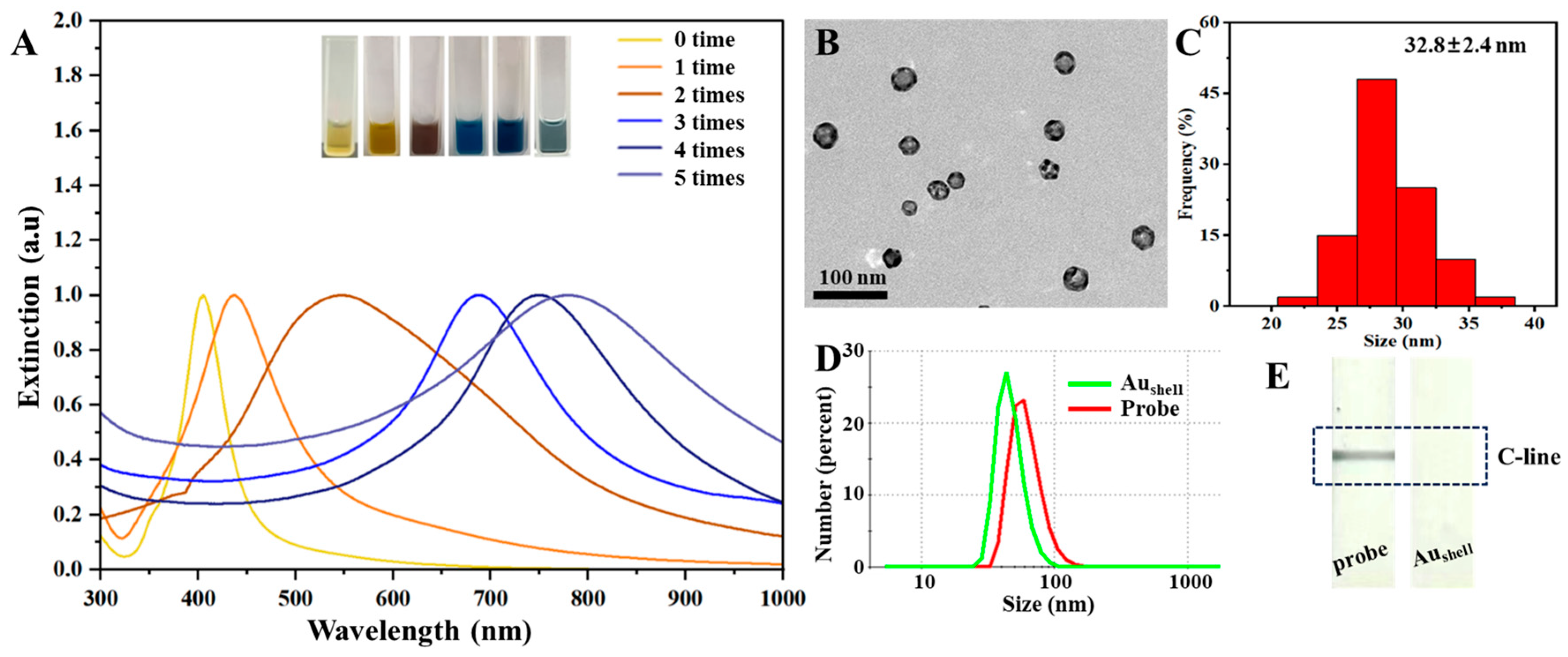
2.2. Characterization of Aushell and Aushell Probes
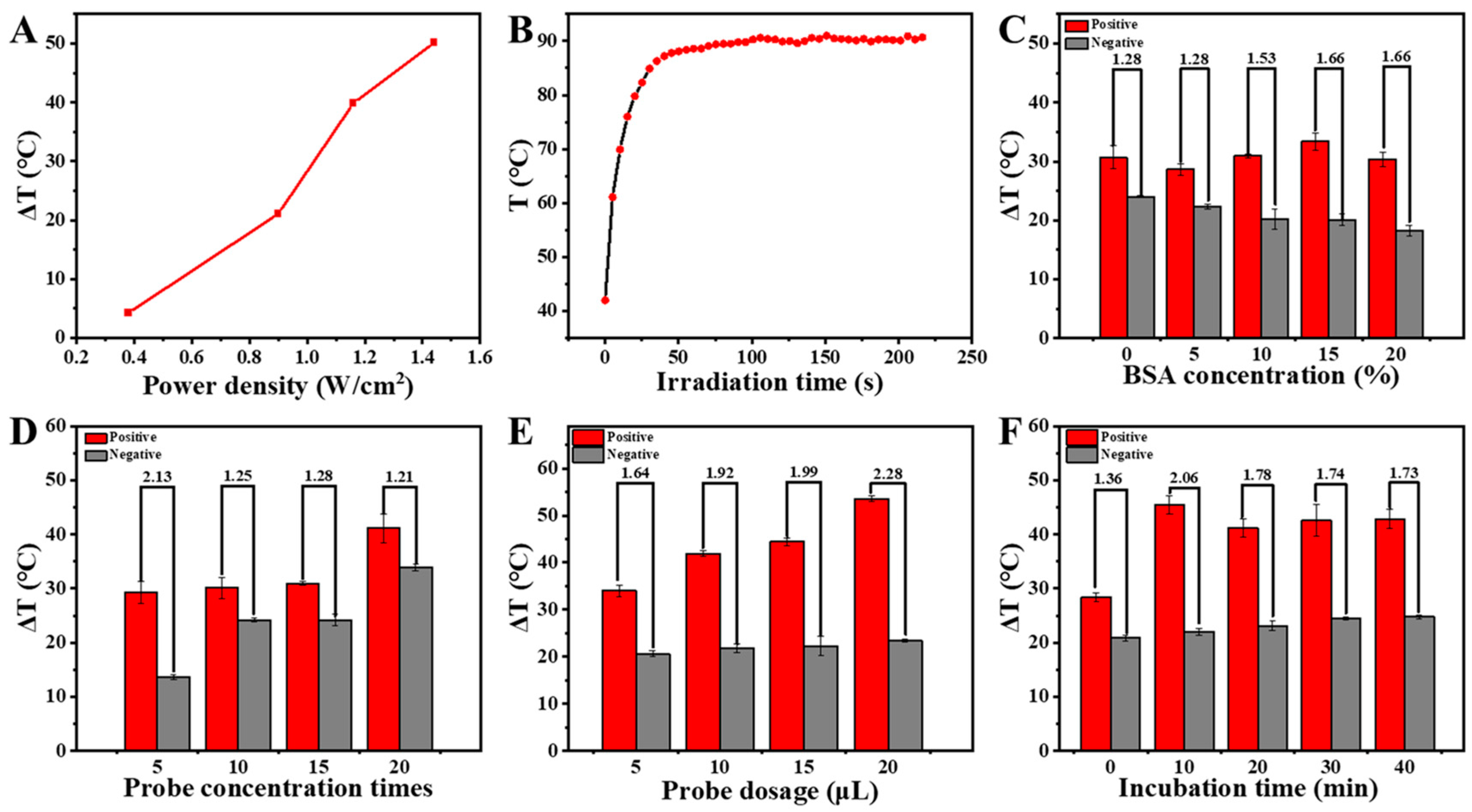
2.3. Investigation of the Optimal Detection Conditions
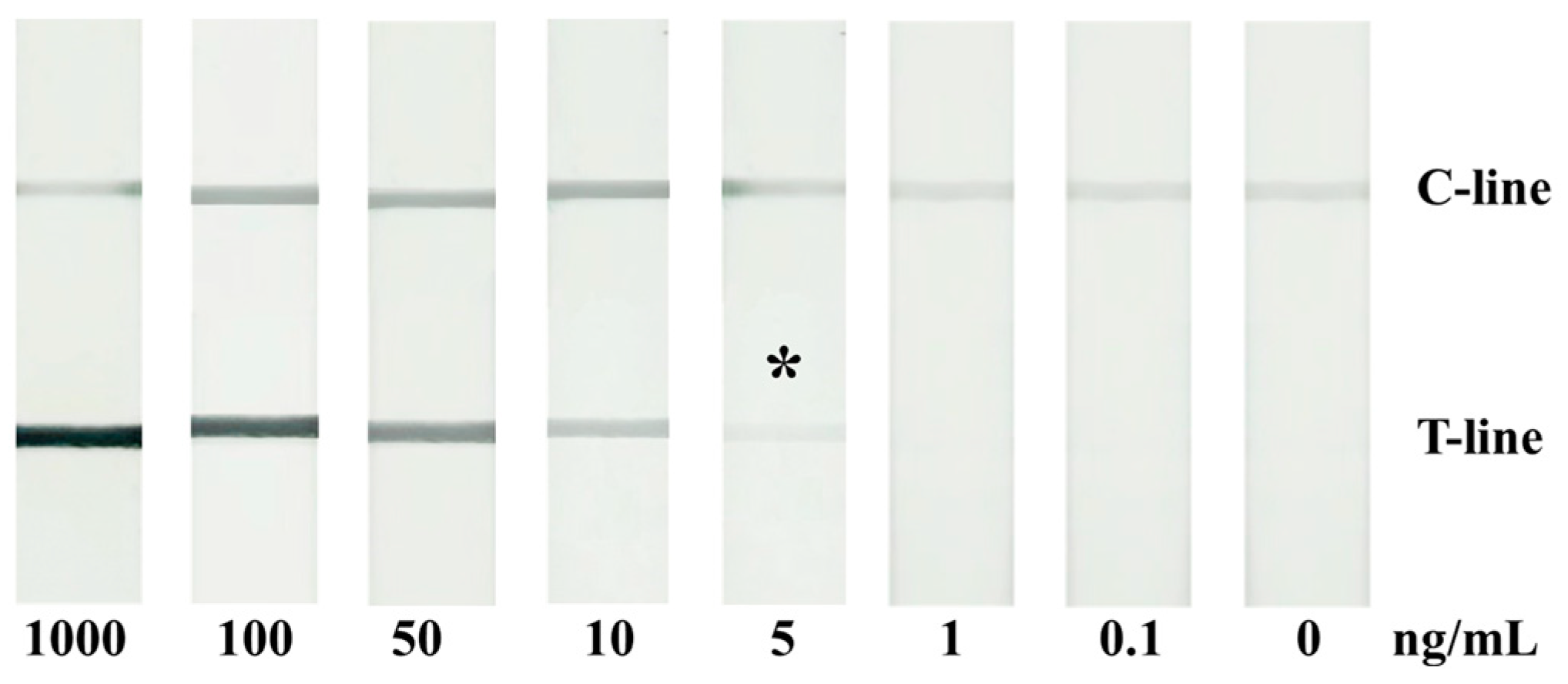
2.4. Colorimetric Qualitative Detection of IL-6 with the Aushell-Based LFIA
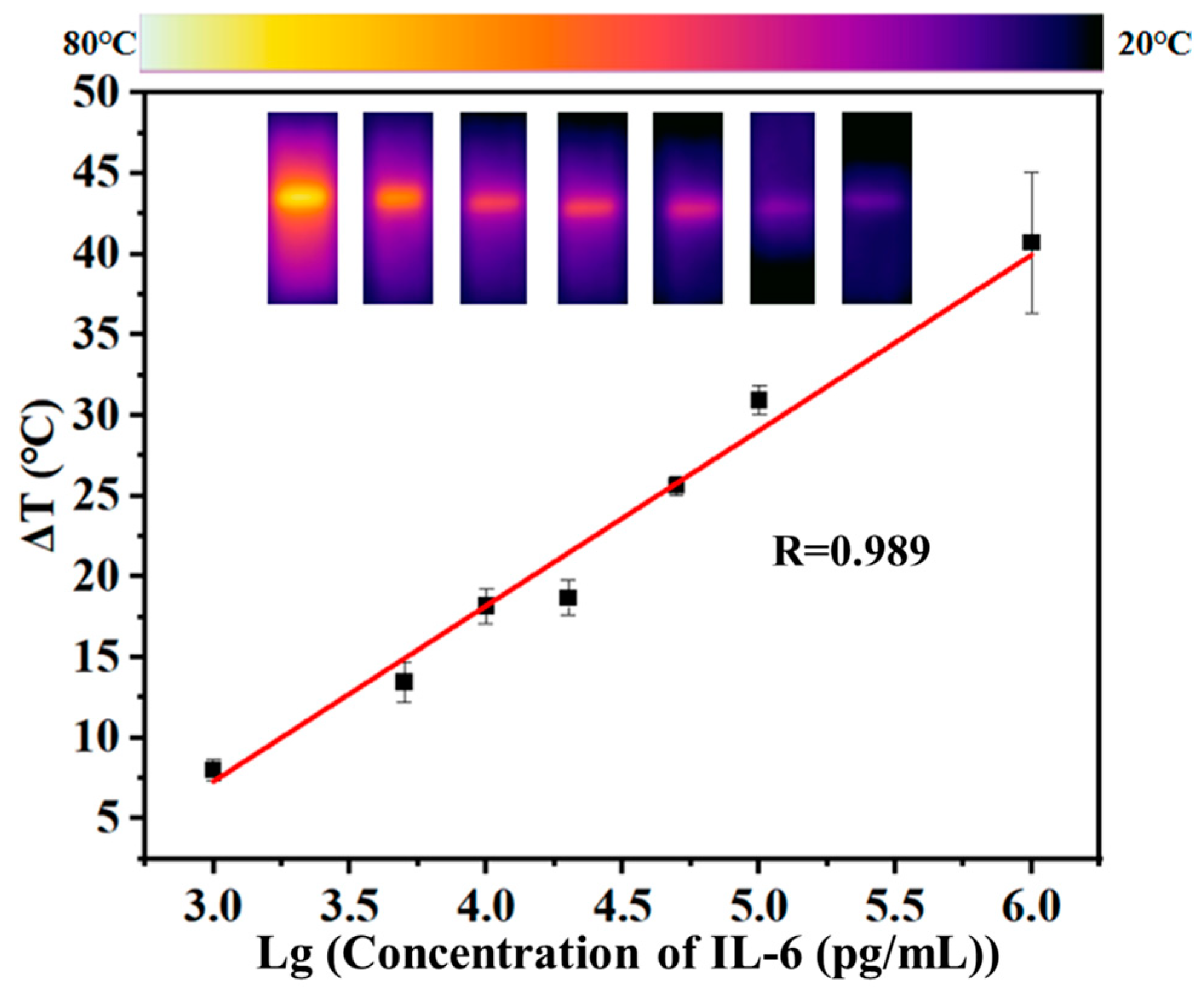
2.5. Photothermal Quantitative Detection of IL-6 with the Aushell-Based LFIA
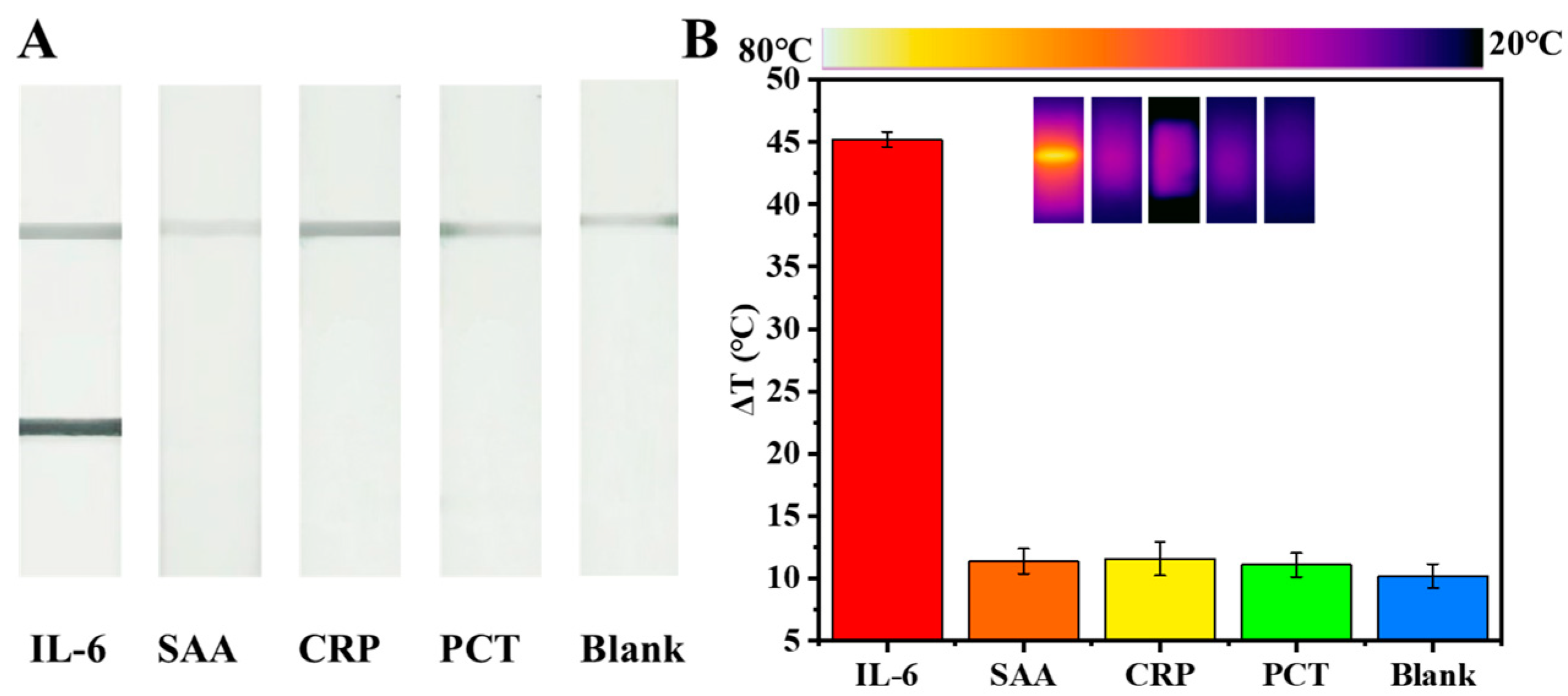
2.6. Specificity Investigation
3. Experimental Section
3.1. Reagents and Instruments
3.2. Preparation of Au Nanoshells
3.3. Construction of Aushell Probes Targeting IL-6
3.4. Fabrication of IL-6 LFIA Strips
3.5. Detection of IL-6 with the Aushell-Based LFIA
3.6. Optimization of the Detection Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Contini, G.; Micheli, L.; Signori, E. Cost-effective and disposable label-free voltammetric immunosensor for sensitive detection of interleukin-6. Biosens. Bioelectron. 2022, 213, 114467. [Google Scholar] [CrossRef] [PubMed]
- Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [CrossRef] [PubMed]
- Smolen, J.S.; Beaulieu, A.; Rubbert-Roth, A.; Ramos-Remus, C.; Rovensky, J.; Alecock, E.; Woodworth, T.; Alten, R. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): A double-blind, placebo-controlled, randomised trial. Lancet 2008, 371, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Wu, Z.; Chen, W.; Yan, H.; Li, D.; You, X.-Q.; Chen, Y.-S.; Zhou, C.; Chen, S.; Zhuang, P.; et al. Au-functionalized wrinkle graphene biosensor for ultrasensitive detection of Interleukin-6. Carbon 2024, 216, 118556. [Google Scholar] [CrossRef]
- Russell, C.; Ward, A.C.; Vezza, V.; Hoskisson, P.; Alcorn, D.; Steenson, D.P.; Corrigan, D.K. Development of a needle shaped microelectrode for electrochemical detection of the sepsis biomarker interleukin-6 (IL-6) in real time. Biosens. Bioelectron. 2019, 126, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Lin, S.-W.; Wang, I.J.; Yang, C.-Y.; Hong, C.; Sun, J.-R.; Feng, P.-H.; Lee, M.-H.; Shen, C.-F.; Lee, Y.-T.; et al. Interleukin-6 Test Strip Combined With a Spectrum-Based Optical Reader for Early Recognition of COVID-19 Patients With Risk of Respiratory Failure. Front. Bioeng. Biotechnol. 2022, 10, 796996. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Lamy de la Chapelle, M.; Marty, J.L. Recent Progresses in Optical Biosensors for Interleukin 6 Detection. Biosensors 2023, 13, 898. [Google Scholar] [CrossRef]
- Fan, G.-C.; Ren, X.-L.; Zhu, C.; Zhang, J.-R.; Zhu, J.-J. A new signal amplification strategy of photoelectrochemical immunoassay for highly sensitive interleukin-6 detection based on TiO2/CdS/CdSe dual co-sensitized structure. Biosens. Bioelectron. 2014, 59, 45–53. [Google Scholar] [CrossRef]
- Shao, Z.-H.; Mo, H.-L.; Zhao, X.; Xie, F.; Zhao, G. Atomic-precise Pt2Cu4 cluster-based fluorescent sensor for rapid interleukin-6 detection. Anal. Methods 2023, 15, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.C.; Beni, V.; Turner, A.P.F. Lateral-Flow Technology: From Visual to Instrumental. TrAC-Trend. Anal. Chem. 2016, 79, 297–305. [Google Scholar] [CrossRef]
- Boehringer, H.R.; O’Farrell, B.J. Lateral Flow Assays in Infectious Disease Diagnosis. Clin. Chem. 2022, 68, 52–58. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Khaki, P.; Jahanban-Esfahlan, A.; de la Guardia, M.; Mokhtarzadeh, A. State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1868–1912. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.L.; Wen, C.Y.; Tang, M.; Wu, L.L.; Liu, C.; Zhu, L.; Pang, D.W. Sensitive and Quantitative Detection of C-Reaction Protein Based on Immunofluorescent Nanospheres Coupled with Lateral Flow Test Strip. Anal. Chem. 2016, 88, 6577–6584. [Google Scholar] [CrossRef] [PubMed]
- Omidfar, K.; Riahi, F.; Kashanian, S. Lateral Flow Assay: A Summary of Recent Progress for Improving Assay Performance. Biosensors 2023, 13, 837. [Google Scholar] [CrossRef]
- Yin, X.; Liu, S.; Kukkar, D.; Wang, J.; Zhang, D.; Kim, K.-H. Performance enhancement of the lateral flow immunoassay by use of composite nanoparticles as signal labels. TrAC-Trend. Anal. Chem. 2024, 170, 117441. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric in Vitro Diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Luciano, K.; Xia, X. Catalytic Gold-Iridium Nanoparticles as Labels for Sensitive Colorimetric Lateral Flow Assay. ACS Nano 2022, 16, 21609–21617. [Google Scholar] [CrossRef]
- Rahbar, M.; Wu, Y.; Subramony, J.A.; Liu, G. Sensitive Colorimetric Detection of Interleukin-6 via Lateral Flow Assay Incorporated Silver Amplification Method. Front. Bioeng. Biotechnol. 2021, 9, 778269. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Varitsev, Y.A.; Drenova, N.V.; Kornev, K.P.; Zherdev, A.V.; Dzantiev, B.B. Development of the Sensitive Lateral Flow Immunoassay with Silver Enhancement for the Detection of Ralstonia solanacearum in Potato Tubers. Talanta 2016, 152, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Arain, H.; Obaid, M.; Sabhnani, N.; Mohan, C. Ultra-Sensitive and Semi-Quantitative Vertical Flow Assay for the Rapid Detection of Interleukin-6 in Inflammatory Diseases. Biosensors 2022, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Wang, D.; Arain, H.; Mohan, C. Design of Gold Nanoparticle Vertical Flow Assays for Point-of-Care Testing. Diagnostics 2022, 12, 1107. [Google Scholar] [CrossRef]
- Jiang, N.; Ahmed, R.; Damayantharan, M.; Ünal, B.; Butt, H.; Yetisen, A.K. Lateral and Vertical Flow Assays for Point-of-Care Diagnostics. Adv. Healthc. Mater. 2019, 8, 1900244. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Fan, L.; Liu, X.; Tang, Y.; Wang, P.; Shu, Z.; Zhang, W.; Zhu, L. Lateral Flow Immunoassay Based on Quantum-Dot Nanobeads for Detection of Chloramphenicol in Aquatic Products. Molecules 2023, 28, 7496. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhou, T.; Zhou, P.; Li, J.; Deng, A. Ultrasensitive and Specific Detection of Anticancer Drug 5-Fluorouracil in Blood Samples by a Surface-Enhanced Raman Scattering (SERS)-Based Lateral Flow Immunochromatographic Assay. Molecules 2022, 27, 4019. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, Y.Z.; Tang, M.; Wu, L.L.; Xie, H.Y.; Zhang, Z.L.; Pang, D.W. Colorimetric-Fluorescent-Magnetic Nanosphere-Based Multimodal Assay Platform for Salmonella Detection. Anal. Chem. 2019, 91, 1178–1184. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, G.; Liu, J.; Li, X.; Yang, X.; Liu, M.; Fu, C.; Zeng, J.; Li, J. One-pot synthesized Au@Pt nanostars-based lateral flow immunoassay for colorimetric and photothermal dual-mode detection of SARS-CoV-2 nucleocapsid antibody. Anal. Chim. Acta 2024, 1292, 342241. [Google Scholar] [CrossRef]
- Bradley, Z.; Coleman, P.A.; Courtney, M.A.; Fishlock, S.; McGrath, J.; Uniacke-Lowe, T.; Bhalla, N.; McLaughlin, J.A.; Hogan, J.; Hanrahan, J.P.; et al. Effect of Selenium Nanoparticle Size on IL-6 Detection Sensitivity in a Lateral Flow Device. ACS Omega 2023, 8, 8407–8414. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, K.Q.; Kwee, S.; Tang, L.; Wang, Z.H.; Xia, J.F.; Li, X.J. Gold Nanoparticle Aggregation-Induced Quantitative Photothermal Biosensing Using a Thermometer: A Simple and Universal Biosensing Platform. Anal. Chem. 2020, 92, 2739–2747. [Google Scholar] [CrossRef]
- Zhang, D.; Du, S.; Su, S.; Wang, Y.; Zhang, H. Rapid detection method and portable device based on the photothermal effect of gold nanoparticles. Biosens. Bioelectron. 2019, 123, 19–24. [Google Scholar] [CrossRef]
- Liang, J.; Wu, L.; Wang, Y.; Liang, W.; Hao, Y.; Tan, M.; He, G.; Lv, D.; Wang, Z.; Zeng, T.; et al. SERS/photothermal-based dual-modal lateral flow immunoassays for sensitive and simultaneous antigen detection of respiratory viral infections. Sens. Actuators B Chem. 2023, 389, 133875. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Liang, X.; Jiang, Y.; Wen, C.-Y.; Zeng, J. Near-Infrared Responsive Ag@Au Nanoplates with Exceptional Stability for Highly Sensitive Colorimetric and Photothermal Dual-Mode Lateral Flow Immunoassay. Anal. Chem. 2024, 96, 3208–3216. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Wang, X.; Hao, Z.; Han, S.; Wang, T.; Zhang, H. Photothermal-based nanomaterials and photothermal-sensing: An overview. Biosens. Bioelectron. 2023, 220, 114883. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bai, X.; Wang, L. Exploration of photothermal sensors based on photothermally responsive materials: A brief review. Inorg. Chem. Front. 2018, 5, 751–759. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.-J.; Xu, Z.; Liu, X.; Zhou, J.; Qu, X.-F.; Wang, W.-L.; Feng, Y.; Peng, C. An ultra-sensitive photothermal lateral flow immunoassay for 17β-estradiol in food samples. Food Chem. 2023, 404, 134482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Qu, X.; Zhou, J.; Yang, H.; Wang, W.; Yang, C. A photothermal lateral flow immunoassay for zearalenone with high sensitivity and wide detection range. Sens. Actuators B Chem. 2023, 390, 133909. [Google Scholar] [CrossRef]
- Hao, W.; Huang, Y.; Wang, L.; Liang, J.; Yang, S.; Su, L.; Zhang, X. Smartphone-Based Photothermal Lateral Flow Immunoassay Using Rhenium Diselenide Nanosheet. ACS Appl. Mater. Interfaces 2023, 15, 9665–9674. [Google Scholar] [CrossRef]
- Wen, C.Y.; Zhao, L.J.; Wang, Y.; Wang, K.; Li, H.W.; Li, X.; Zi, M.; Zeng, J.B. Colorimetric and photothermal dual-mode lateral flow immunoassay based on Au-Fe3O4 multifunctional nanoparticles for detection of Salmonella typhimurium. Microchim. Acta 2023, 190, 57. [Google Scholar] [CrossRef]
- Guo, G.; Zhao, T.; Sun, R.; Song, M.; Liu, H.; Wang, S.; Li, J.; Zeng, J. Au-Fe3O4 dumbbell-like nanoparticles based lateral flow immunoassay for colorimetric and photothermal dual-mode detection of SARS-CoV-2 spike protein. Chin. Chem. Lett. 2024, 35, 109198. [Google Scholar] [CrossRef]
- Moreau, L.M.; Schurman, C.A.; Kewalramani, S.; Shahjamali, M.M.; Mirkin, C.A.; Bedzyk, M.J. How Ag Nanospheres Are Transformed into AgAu Nanocages. J. Am. Chem. Soc. 2017, 139, 12291–12298. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, D.; Li, H.; Sun, R.; Zhang, Z.; Zhao, T.; Guo, G.; Zeng, J.; Wen, C.-Y. High-density Au nanoshells assembled onto Fe3O4 nanoclusters for integrated enrichment and photothermal/colorimetric dual-mode detection of SARS-CoV-2 nucleocapsid protein. Biosens. Bioelectron. 2023, 241, 115688. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Dou, Y.; Liu, Y.; Jiang, X.; Tu, X.; Zhang, R. Au Nanoshell-Based Lateral Flow Immunoassay for Colorimetric and Photothermal Dual-Mode Detection of Interleukin-6. Molecules 2024, 29, 3683. https://doi.org/10.3390/molecules29153683
Wen C, Dou Y, Liu Y, Jiang X, Tu X, Zhang R. Au Nanoshell-Based Lateral Flow Immunoassay for Colorimetric and Photothermal Dual-Mode Detection of Interleukin-6. Molecules. 2024; 29(15):3683. https://doi.org/10.3390/molecules29153683
Chicago/Turabian StyleWen, Congying, Yue Dou, Yao Liu, Xuan Jiang, Xiaomei Tu, and Ruiqiao Zhang. 2024. "Au Nanoshell-Based Lateral Flow Immunoassay for Colorimetric and Photothermal Dual-Mode Detection of Interleukin-6" Molecules 29, no. 15: 3683. https://doi.org/10.3390/molecules29153683
APA StyleWen, C., Dou, Y., Liu, Y., Jiang, X., Tu, X., & Zhang, R. (2024). Au Nanoshell-Based Lateral Flow Immunoassay for Colorimetric and Photothermal Dual-Mode Detection of Interleukin-6. Molecules, 29(15), 3683. https://doi.org/10.3390/molecules29153683






