Unveiling the Catalytic Roles of DsBBS1 and DsBBS2 in the Bibenzyl Biosynthesis of Dendrobium sinense
Abstract
1. Introduction
2. Results
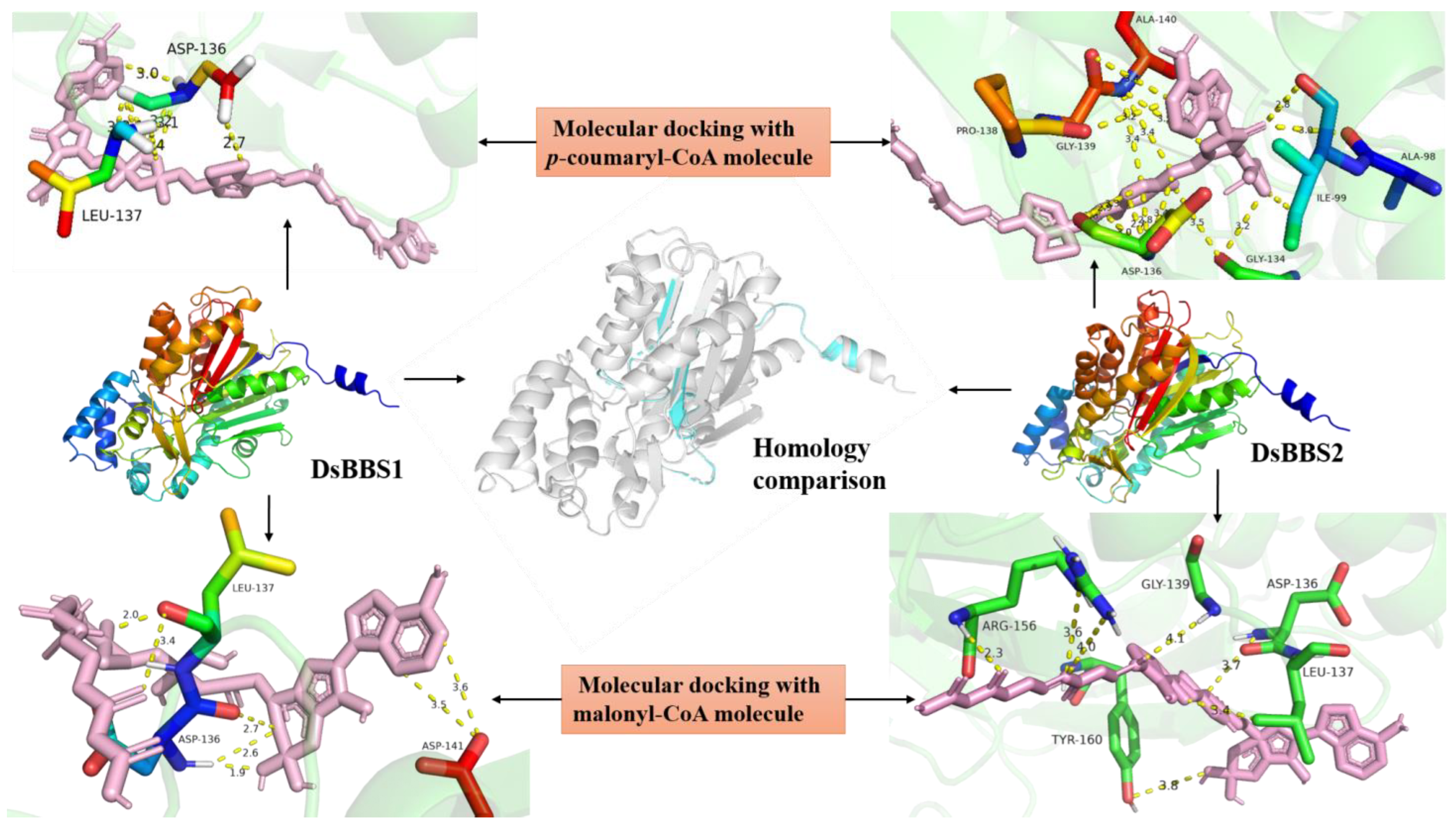
2.1. Molecular Docking Simulation of DsBBS1 and DsBBS2
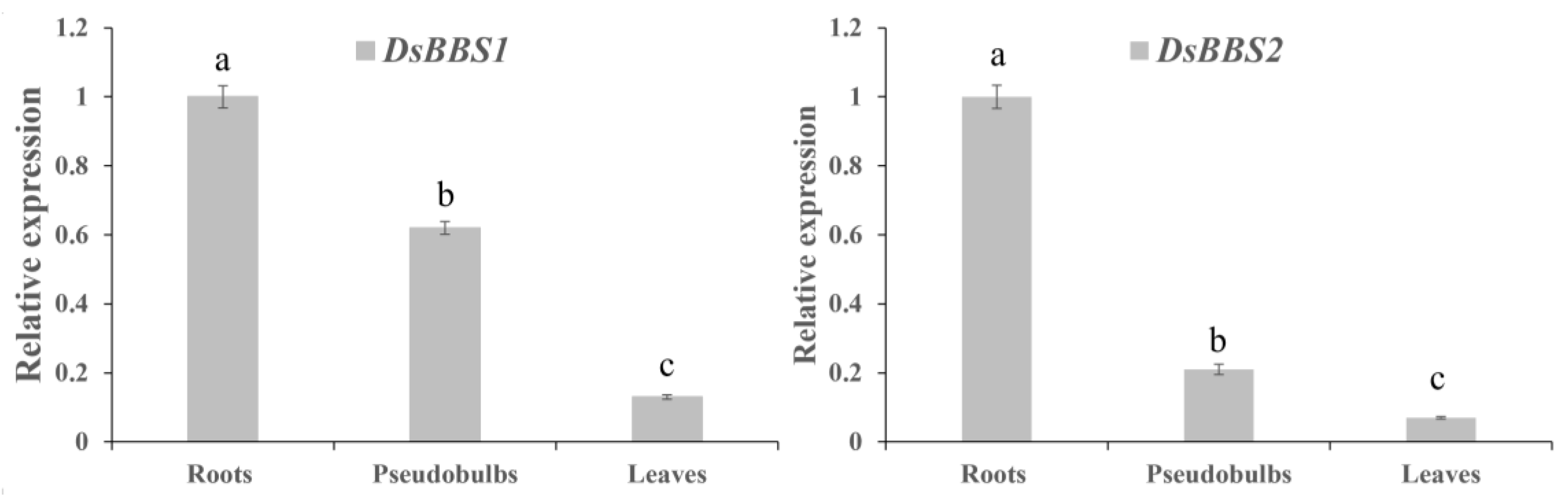
2.2. Expression Analysis of DsBBS1 and DsBBS2 in Different Tissues
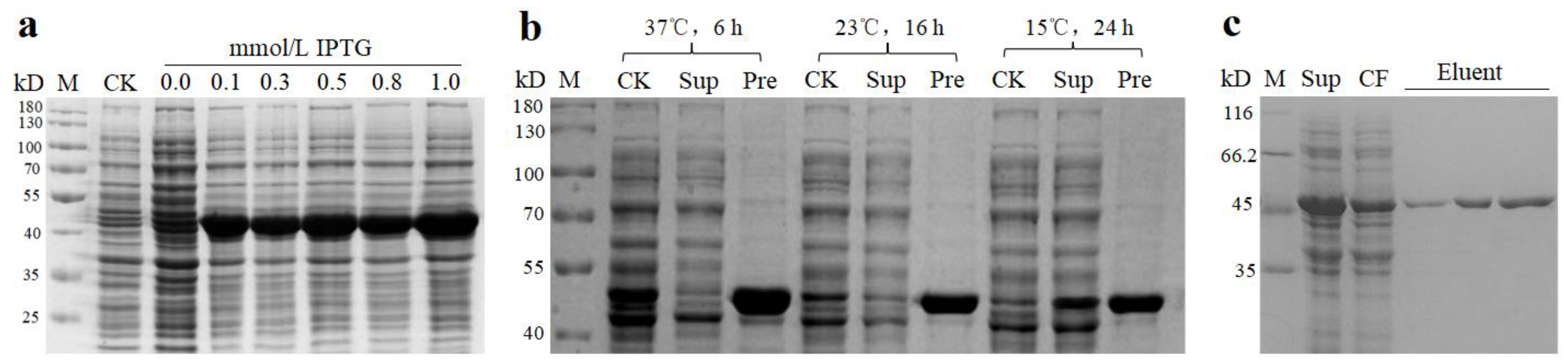
2.3. Protein Expression of DsBBS2
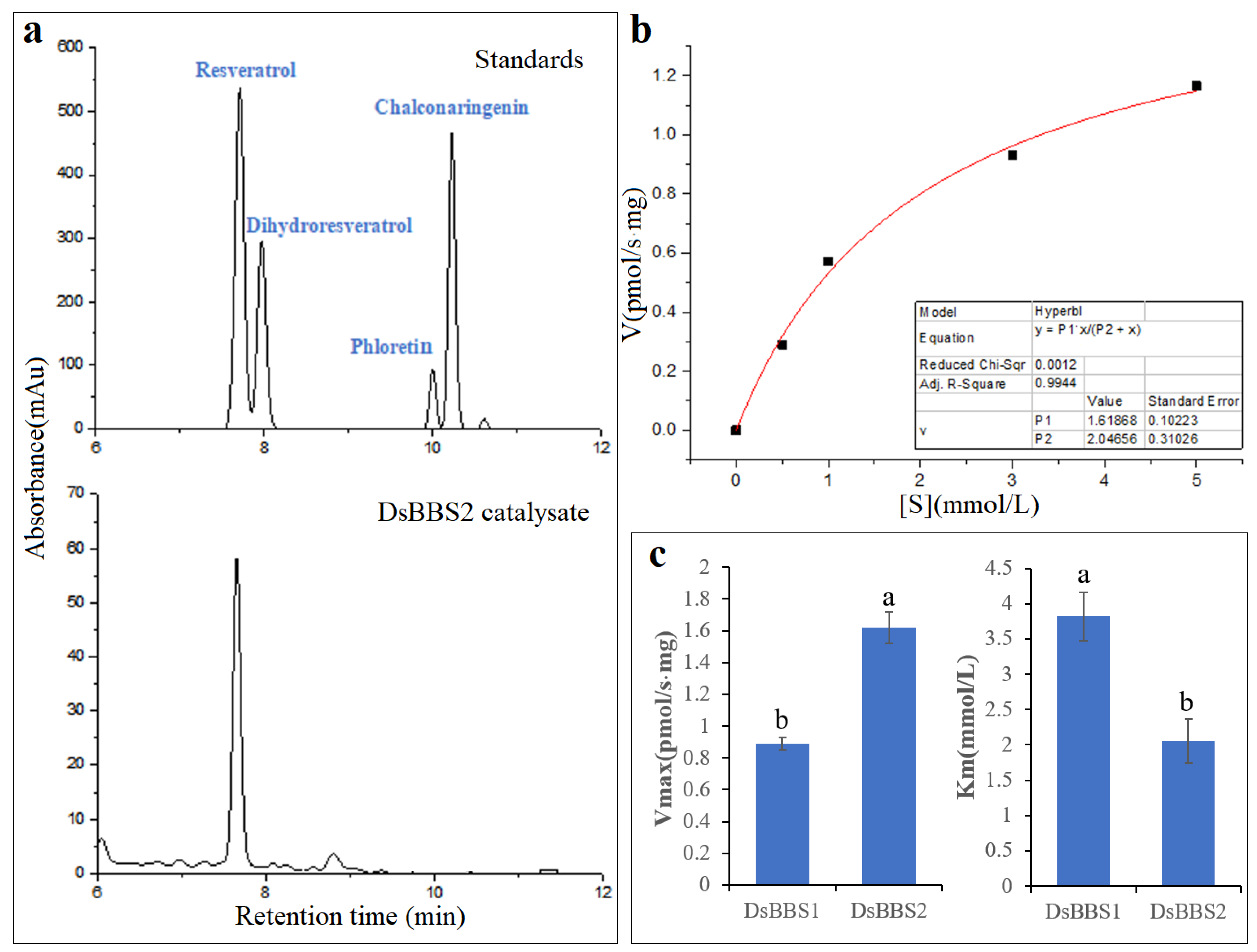
2.4. Enzyme Activity Analysis of DsBBS2
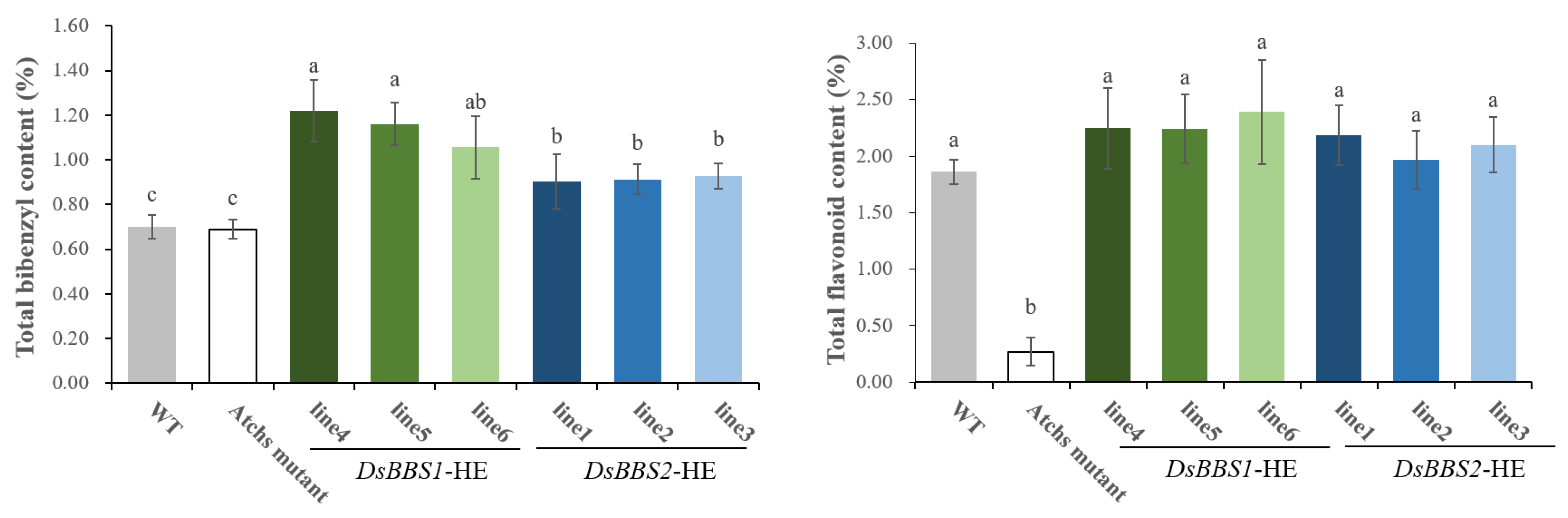
2.5. Functional Analysis of DsBBS1 and DsBBS2 in Transgenic Arabidopsis
3. Discussion
4. Materials and Methods
4.1. DsBBS Homology Modeling and Molecular Docking
4.2. RT-qPCR Analysis
4.3. DsBBS2 Cloning and Protein Purification
4.4. Enzymatic Assay
4.5. HPLC Analysis
4.6. Genetic Transformation in Arabidopsis
4.7. Determination of Total Bibenzyl and Flavonoid Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van Den Berg, C.; Schuiteman, A. An Updated Classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Li, P.-Y.; Li, L.; Wang, Y.-Z. Traditional Uses, Chemical Compositions and Pharmacological Activities of Dendrobium: A Review. J. Ethnopharmacol. 2023, 310, 116382. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Dang, P.-P.; Zhao, Z.; Yuan, L.-C.; Zhou, Z.-H.; Wolf, D.; Luo, Y.-B. An Assessment of the Chinese Medicinal Dendrobium Industry: Supply, Demand and Sustainability. J. Ethnopharmacol. 2019, 229, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yuan, Y.; Zhang, J. How Climate Change Will Alter the Distribution of Suitable Dendrobium Habitats. Front. Ecol. Evol. 2020, 8, 536339. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Ng, T.B. The Medicinal and Pharmaceutical Importance of Dendrobium Species. Appl. Microbiol. Biot. 2017, 101, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Huang, W.; Song, X.; Niu, J. Transcriptomics and Metabolomics Reveal Purine and Phenylpropanoid Metabolism Response to Drought Stress in Dendrobium sinense, an Endemic Orchid Species in Hainan Island. Front. Genet. 2021, 12, 692702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Mei, W.-L.; Zuo, W.-J.; Zeng, Y.-B.; Guo, Z.-K.; Song, X.-Q.; Dai, H.-F. A New Antibacterial Phenanthrenequinone from Dendrobium sinense. J. Asian Nat. Prod. Res. 2013, 15, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Mei, W.-L.; Cai, C.-H.; Guo, Z.-K.; Song, X.-Q.; Dai, H.-F. Four New Bibenzyl Derivatives from Dendrobium sinense. Phytochem. Lett. 2014, 9, 107–112. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Liang, C.; Liu, L.; Song, X.; Zhao, Y.; Wang, J.; Niu, J. Characterization of the Key Bibenzyl Synthase in Dendrobium sinense. Int. J. Mol. Sci. 2022, 23, 6780. [Google Scholar] [CrossRef]
- He, L.; Su, Q.; Bai, L.; Li, M.; Liu, J.; Liu, X.; Zhang, C.; Jiang, Z.; He, J.; Shi, J.; et al. Recent Research Progress on Natural Small Molecule Bibenzyls and Its Derivatives in Dendrobium Species. Eur. J. Med. Chem. 2020, 204, 112530. [Google Scholar] [CrossRef]
- Chanvorachote, P.; Kowitdamrong, A.; Ruanghirun, T.; Sritularak, B.; Mungmee, C.; Likhitwitayawuid, K. Anti-Metastatic Activities of Bibenzyls from Dendrobium pulchellum. Nat. Prod. Commun. 2013, 8, 1934578X1300800127. [Google Scholar] [CrossRef]
- Zhu, Q.; Sheng, Y.; Li, W.; Wang, J.; Ma, Y.; Du, B.; Tang, Y. Erianin, a Novel Dibenzyl Compound in Dendrobium Extract, Inhibits Bladder Cancer Cell Growth via the Mitochondrial Apoptosis and JNK Pathways. Toxicol. Appl. Pharm. 2019, 371, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Deng, B.W.; Zhang, C.Y.; Cui, Y.D.; Bi, J.Y.; Zhang, G.G. New Phenanthrene and 9, 10-Dihydrophenanthrene Derivatives from the Stems of Dendrobium officinale with Their Cytotoxic Activities. J. Nat. Med. 2018, 72, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Sarakulwattana, C.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. New Bisbibenzyl and Phenanthrene Derivatives from Dendrobium scabrilingue and Their α-Glucosidase Inhibitory Activity. Nat. Prod. Res. 2020, 34, 1694–1701. [Google Scholar] [CrossRef]
- Huang, J.-M.; Huang, F.-I.; Yang, C.-R. Moscatilin Ameliorates Tau Phosphorylation and Cognitive Deficits in Alzheimer’s Disease Models. J. Nat. Prod. 2019, 82, 1979–1988. [Google Scholar] [CrossRef]
- Kongkatitham, V.; Muangnoi, C.; Kyokong, N.; Thaweesest, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. Anti-Oxidant and Anti-Inflammatory Effects of New Bibenzyl Derivatives from Dendrobium parishii in Hydrogen Peroxide and Lipopolysaccharide Treated RAW264. 7 Cells. Phytochem. Lett. 2018, 24, 31–38. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.L.; Wang, Y.J.; Wang, F.F.; Guo, S.X.; Yang, J.S.; Xiao, P.G. Four New Bibenzyl Derivatives from Dendrobium candidum. Chem. Pharm. Bull. 2009, 57, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, H.; Zhang, S.; Lan, T. The Type III Polyketide Synthase Supergene Family in Plants: Complex Evolutionary History and Functional Divergence. Plant J. 2022, 112, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xu, F.; Zeng, J.; Zhan, J. Type III Polyketide Synthases in Natural Product Biosynthesis. IUBMB Life 2012, 64, 285–295. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ou, Q.; You, H.; Wang, J.; Niu, J. Alternative First Exons Drive Enzymatic Activity Variation in Chalcone synthase 3 of Dendrobium sinense. Forests 2023, 14, 1702. [Google Scholar] [CrossRef]
- Morita, H.; Yamashita, M.; Shi, S.-P.; Wakimoto, T.; Kondo, S.; Kato, R.; Sugio, S.; Kohno, T.; Abe, I. Synthesis of Unnatural Alkaloid Scaffolds by Exploiting Plant Polyketide Synthase. Proc. Natl. Acad. Sci. USA 2011, 108, 13504–13509. [Google Scholar] [CrossRef]
- Boddington, K.F.; Soubeyrand, E.; Van Gelder, K.; Casaretto, J.A.; Perrin, C.; Forrester, T.J.; Parry, C.; Al-Abdul-Wahid, M.S.; Jentsch, N.G.; Magolan, J.; et al. Bibenzyl Synthesis in Cannabis sativa L. Plant J. 2022, 109, 693–707. [Google Scholar] [CrossRef]
- Nam, B.; Ryu, S.M.; Lee, D.; Jung, C.-H.; Jin, C.H.; Kim, J.-B.; Lee, I.-S.; Han, A.-R. Identification of Two New Phenanthrenes from Dendrobii Herba and Their Cytotoxicity towards Human Hypopharynx Squamous Carcinoma Cell (FaDu). Molecules 2019, 24, 2339. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.-J.; Luo, H.-R.; Cui, J.; Zhou, J.; Wang, X.-J.; Sheng, J.; Hu, J.-M. Two New Dendrocandins with Neurite Outgrowth-Promoting Activity from Dendrobium officinale. J. Asian Nat. Prod. Res. 2015, 17, 125–131. [Google Scholar] [CrossRef]
- Choonong, R.; Sermpradit, W.; Kitisripanya, T.; Sritularak, B.; Putalun, W. The Contents of Bibenzyl Derivatives, Flavonoids and a Phenanthrene in Selected Dendrobium Spp. and the Correlation with Their Antioxidant Activity. Scienceasia 2019, 45, 245–252. [Google Scholar] [CrossRef]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical Constituents of Dendrobium venustum and Their Antimalarial and Anti-Herpetic Properties. Nat. Prod. Commun. 2014, 9, 1934578X1400900625. [Google Scholar] [CrossRef]
- Abe, I. Biosynthesis of Medicinally Important Plant Metabolites by Unusual Type III Polyketide Synthases. J. Nat. Med. 2020, 74, 639–646. [Google Scholar] [CrossRef]
- Lin, Z.; Qu, X. Emerging Diversity in Polyketide Synthase. Tetrahedron Lett. 2022, 110, 154183. [Google Scholar] [CrossRef]
- Spies, M.A.; Reese, J.G.; Dodd, D.; Pankow, K.L.; Blanke, S.R.; Baudry, J. Determinants of Catalytic Power and Ligand Binding in Glutamate Racemase. J. Am. Chem. Soc. 2009, 131, 5274–5284. [Google Scholar] [CrossRef][Green Version]
- Halwachs, W. KM and Vmax from Only One Experiment. Biotechnol. Bioeng. 1978, 20, 281–285. [Google Scholar] [CrossRef]
- Strickland, M.; Kale, S.; Strub, M.-P.; Schwieters, C.D.; Liu, J.; Peterkofsky, A.; Tjandra, N. Potential Regulatory Role of Competitive Encounter Complexes in Paralogous Phosphotransferase Systems. J. Mol. Biol. 2019, 431, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Naake, T.; Maeda, H.A.; Proost, S.; Tohge, T.; Fernie, A.R. Kingdom-Wide Analysis of the Evolution of the Plant Type III Polyketide Synthase Superfamily. Plant Physiol. 2021, 185, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The Flavonoid Biosynthetic Pathway in Arabidopsis: Structural and Genetic Diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Serazetdinova, L.; Oldach, K.H.; Lörz, H. Expression of Transgenic Stilbene Synthases in Wheat Causes the Accumulation of Unknown Stilbene Derivatives with Antifungal Activity. J. Plant Physiol. 2005, 162, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, K.; Parray, Z.A.; Ahamad, S.; Ahmad, F.; Ahmed, A.; Freeh Alamery, S.; Hussain, T.; Hassan, M.I.; Islam, A. Interactions under Crowding Milieu: Chemical-Induced Denaturation of Myoglobin Is Determined by the Extent of Heme Dissociation on Interaction with Crowders. Biomolecules 2020, 10, 490. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, X.; Fu, Y.; Huo, K.; Liang, C.; Liu, L.; Wang, J.; Niu, J. Genome-Wide Identification of Superior Reference Genes in Dendrobium sinense. Chin. J. Trop. Crops 2023, 44, 1365. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; You, H.; Ye, L.; Ou, Q.; Zhao, Y.; Wang, J.; Niu, J. Unveiling the Catalytic Roles of DsBBS1 and DsBBS2 in the Bibenzyl Biosynthesis of Dendrobium sinense. Molecules 2024, 29, 3682. https://doi.org/10.3390/molecules29153682
Liu L, You H, Ye L, Ou Q, Zhao Y, Wang J, Niu J. Unveiling the Catalytic Roles of DsBBS1 and DsBBS2 in the Bibenzyl Biosynthesis of Dendrobium sinense. Molecules. 2024; 29(15):3682. https://doi.org/10.3390/molecules29153682
Chicago/Turabian StyleLiu, Liyan, Huiyan You, Lixuan Ye, Qiongjian Ou, Ying Zhao, Jia Wang, and Jun Niu. 2024. "Unveiling the Catalytic Roles of DsBBS1 and DsBBS2 in the Bibenzyl Biosynthesis of Dendrobium sinense" Molecules 29, no. 15: 3682. https://doi.org/10.3390/molecules29153682
APA StyleLiu, L., You, H., Ye, L., Ou, Q., Zhao, Y., Wang, J., & Niu, J. (2024). Unveiling the Catalytic Roles of DsBBS1 and DsBBS2 in the Bibenzyl Biosynthesis of Dendrobium sinense. Molecules, 29(15), 3682. https://doi.org/10.3390/molecules29153682





