A Dopamine Detection Sensor Based on Au-Decorated NiS2 and Its Medical Application
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Characterization
2.2. Structure and Morphology Characterization
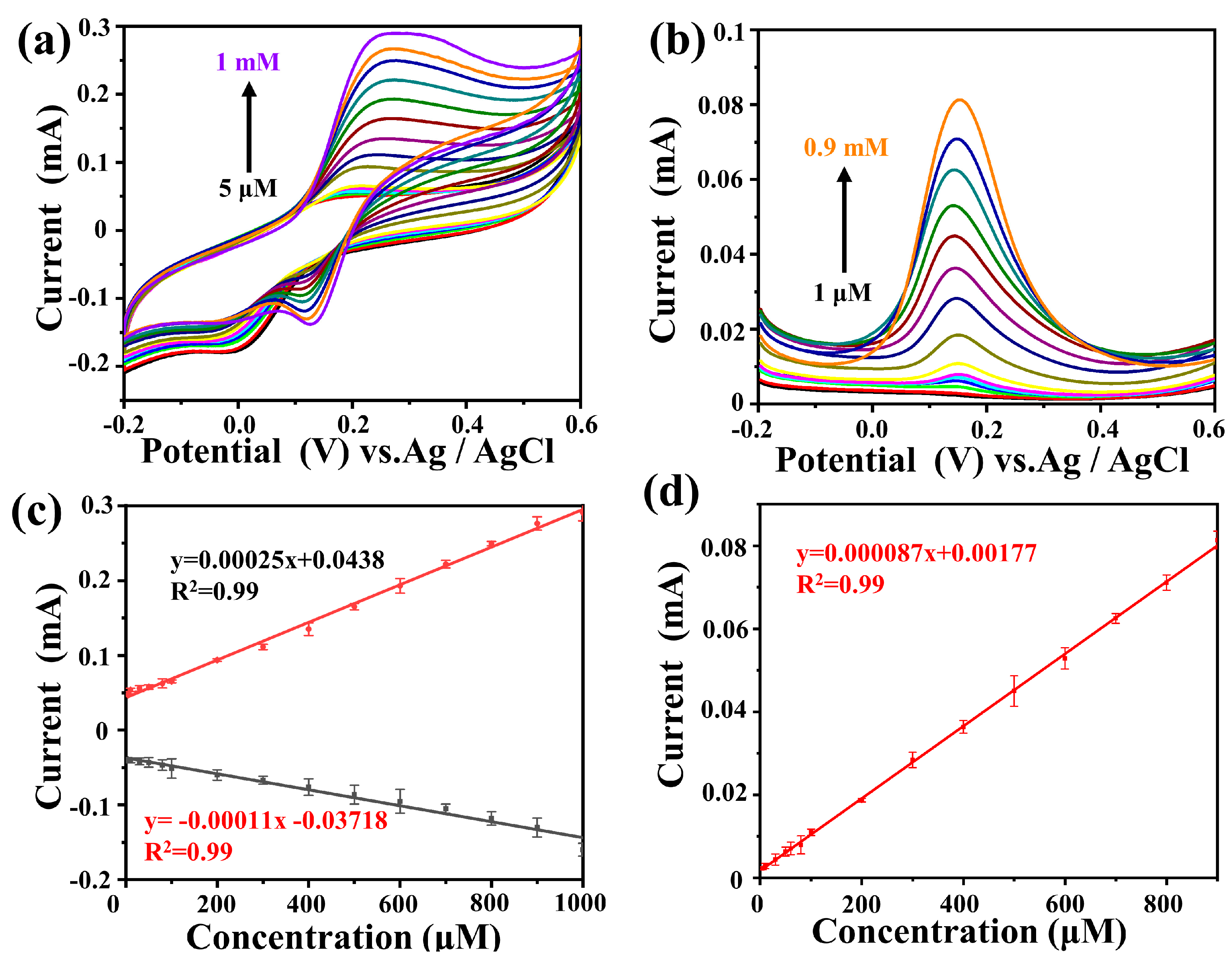
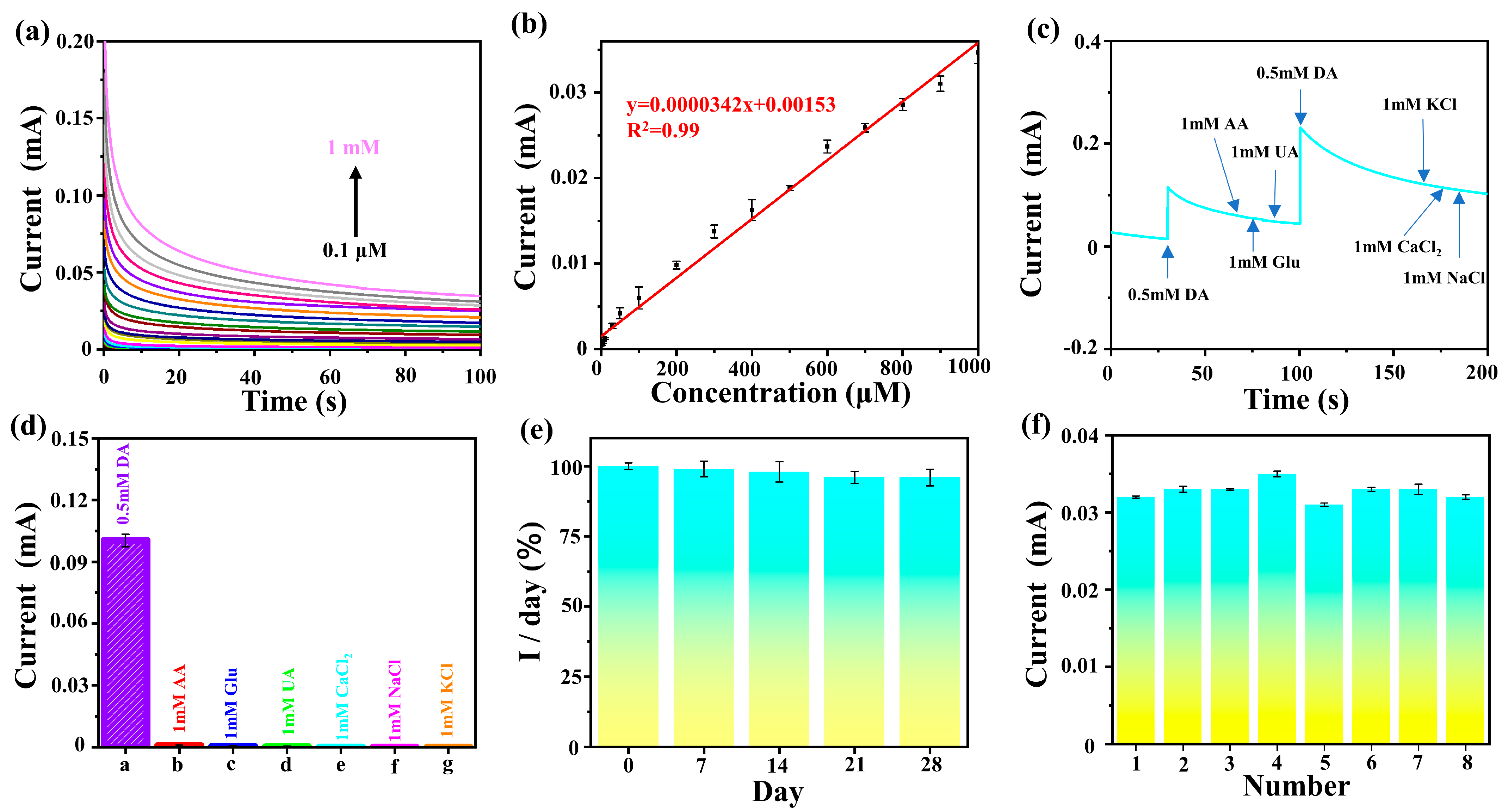
2.3. Sensor Application
3. Materials and Methods
3.1. Material Preparations
3.2. Preparation of Au@NiS2-FTO
3.3. Electrochemical Performance Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhang, X.; Chen, Y.; Xu, H.; Tan, Y.; Wang, S. Detection of Dopamine Based on Tyrosinase-Fe3O4 Nanoparticles-chitosan Nanocomposite Biosensor. Am. J. Biomed. Sci. 2010, 2, 209–216. [Google Scholar] [CrossRef]
- Luo, Q.; Su, Y.; Zhang, H. Sensitive dopamine sensor based on electrodeposited gold nanoparticles and electro-modulated MoS2 nanoflakes. J. Iran. Chem. Soc. 2022, 20, 731–738. [Google Scholar] [CrossRef]
- Jing, W.-J.; Li, F.-F.; Liu, Y.; Ma, R.-N.; Zhang, W.; Shang, L.; Li, X.-J.; Xue, Q.-W.; Wang, H.-S.; Jia, L.-P. An electrochemical ratiometric biosensor for the detection of dopamine based on an MXene-Au nanocomposite. Chem. Commun. 2023, 59, 12911–12914. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, F.; Zhang, Y.; Lin, H.; Guo, W.; Yu, K.; Qu, F. Heterophase-Structured Cobalt Hydroxide on Partly Reduced Graphene Oxide for Enhanced Dopamine Biosensing. ACS Appl. Eng. Mater. 2023, 1, 1963–1972. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Chu, Z.; Yang, D.; Subhan, S.; Li, J.; Huang, M.; Zhang, H.; Zhao, Z. A low-cost high-entropy porous CrO/CrN/C biosensor for highly sensitive simultaneous detection of dopamine and uric acid. Microchem. J. 2022, 175, 107188. [Google Scholar] [CrossRef]
- Xu, C.; Gu, C.; Xiao, Q.; Chen, J.; Yin, Z.-Z.; Liu, H.; Fan, K.; Li, L. A highly selective and sensitive biosensor for dopamine based on a surface molecularly imprinted layer to coordinate nano-interface functionalized acupuncture needle. Chem. Eng. J. 2022, 436, 135203. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, S.; Jiang, X.; Ai, Y.; Xu, W.; Xie, L.; Sun, H.-B.; Liang, Q. Oligo-layer graphene stabilized fully exposed Fe-sites for ultra-sensitivity electrochemical detection of dopamine. Biosens. Bioelectron. 2022, 211, 114367. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.Y.; Zhang, H.J.; Huang, S.; Lu, X.X.; Gao, X.; Song, S.S.; Wang, Z.; Wang, W.Q.; Guan, E.H. Highly sensitive and selective dopamine biosensor using Au nanoparticles-ZnO nanocone arrays/graphene foam electrode. Mater. Sci. Eng. C 2020, 108, 110490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, B.; Meng, F.; Cheng, Y.; Zhu, C. Microwave-assisted preparation of N-doped carbon dots as a biosensor for electrochemical dopamine detection. J. Colloid Interface Sci. 2015, 452, 199–202. [Google Scholar] [CrossRef]
- Liu, X.; Hou, Y.; Chen, S.; Liu, J. Controlling dopamine binding by the new aptamer for a FRET-based biosensor. Biosens. Bioelectron. 2021, 173, 112798. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Akbar, F.; Kolahdouz, M.; Larimian, S.; Radfar, B.; Radamson, H.H. Graphene synthesis, characterization and its applications in nanophotonics, nanoelectronics, and nanosensing. J. Mater. Sci. Mater. Electron. 2015, 26, 4347–4379. [Google Scholar] [CrossRef]
- Wang, K.; Liu, P.; Ye, Y.; Li, J.; Zhao, W.; Huang, X. Fabrication of a novel laccase biosensor based on silica nanoparticles modified with phytic acid for sensitive detection of dopamine. Sens. Actuators B Chem. 2014, 197, 292–299. [Google Scholar] [CrossRef]
- Park, S.J.; Song, H.S.; Kwon, O.S.; Chung, J.H.; Lee, S.H.; An, J.H.; Ahn, S.R.; Lee, J.E.; Yoon, H.; Park, T.H.; et al. Human dopamine receptor nanovesicles for gate-potential modulators in high-performance field-effect transistor biosensors. Sci. Rep. 2014, 4, 4342. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Bonavita, A.; Neri, G. Yttria-zirconia electrochemical sensor for the detection of tyrosine. Mater. Today Commun. 2023, 35, 106036. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Algethami, J.S.; Alsaiari, M.; Harraz, F.A. A novel In2O3-doped ZnO decorated mesoporous carbon nanocomposite as a sensitive and selective dopamine electrochemical sensor. J. Mater. Res. Technol. 2024, 29, 540–549. [Google Scholar] [CrossRef]
- Wang, S.; Ning, P.; Huang, S.; Wang, W.; Fei, S.; He, Q.; Zai, J.; Jiang, Y.; Hu, Z.; Qian, X.; et al. Multi-functional NiS2/FeS2/N-doped carbon nanorods derived from metal-organic frameworks with fast reaction kinetics for high performance overall water splitting and lithium-ion batteries. J. Power Sources 2019, 436, 226857. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Z.; Duan, H.; Zhang, F.; Zhai, B.; Zhao, J.; Wang, X. Controlled synthesis of rod-like three-dimensional NiS2/graphene nanostructures from metal complexes and their application in supercapacitor electrodes. J. Phys. Chem. Solids 2022, 167, 110716. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Paulmony, T. Evaluation of a New Biosensor Based on in Situ Synthesized PPy-Ag-PVP Nanohybrid for Selective Detection of Dopamine. J. Phys. Chem. B 2017, 121, 1118–1127. [Google Scholar] [CrossRef]
- Kajisa, T.; Li, W.; Michinobu, T.; Sakata, T. Well-designed dopamine-imprinted polymer interface for selective and quantitative dopamine detection among catecholamines using a potentiometric biosensor. Biosens. Bioelectron. 2018, 117, 810–817. [Google Scholar] [CrossRef]
- Shin, J.-W.; Yoon, J.; Shin, M.; Choi, J.-W. Electrochemical Dopamine Biosensor Composed of Silver Encapsulated MoS2 Hybrid Nanoparticle. Biotechnol. Bioprocess Eng. 2019, 24, 135–144. [Google Scholar] [CrossRef]
- Dong, X.; Lu, X.; Zhang, K.; Zhang, Y. Chronocoulometric DNA biosensor based on a glassy carbon electrode modified with gold nanoparticles, poly(dopamine) and carbon nanotubes. Microchim. Acta 2012, 180, 101–108. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Zhao, H.; An, H.; Cao, H.; Zhang, Y.; Fan, Z. Tuning sulfur doping in graphene for highly sensitive dopamine biosensors. Carbon 2015, 86, 197–206. [Google Scholar] [CrossRef]
- Ghadimi, H.; Mahmoudian, M.R.; Basirun, W.J. A sensitive dopamine biosensor based on ultra-thin polypyrrole nanosheets decorated with Pt nanoparticles. RSC Adv. 2015, 5, 39366–39374. [Google Scholar] [CrossRef]
- Arya Nair, J.S.; Saisree, S.; Aswathi, R.; Sandhya, K.Y. Ultra-selective and real-time detection of dopamine using molybdenum disulphide decorated graphene-based electrochemical biosensor. Sens. Actuators B Chem. 2022, 354, 131254. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, S.; Wu, P.; Yuan, T.; Wang, X. Lignosulfonate in situ-modified reduced graphene oxide biosensors for the electrochemical detection of dopamine. RSC Adv. 2022, 12, 31083–31090. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Tang, T.W.; Pan, B.; Liu, H.; Zhang, K.; Luo, Z. Strategies for Controlled Growth of Transition Metal Dichalcogenides by Chemical Vapor Deposition for Integrated Electronics. ACS Mater. Au 2022, 2, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Vomiero, A. 2D Transition Metal Dichalcogenides-Based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2208994. [Google Scholar] [CrossRef]
- Lu, T.; Wang, Y.; Cai, G.; Jia, H.; Liu, X.; Zhang, C.; Meng, S.; Liu, M. Synthesizability of transition-metal dichalcogenides: A systematic first-principles evaluation. Mater. Futures 2023, 2, 015001. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Y. Enhancing catalytic properties of noble metal@MoS2/WS2 heterojunction for the hydrogen evolution reaction. Appl. Surf. Sci. 2022, 591, 153168. [Google Scholar] [CrossRef]
- Wei, C.; Cheng, C.; Cheng, Y.; Wang, Y.; Xu, Y.; Du, W.; Pang, H. Comparison of NiS2 and α-NiS hollow spheres for supercapacitors, non-enzymatic glucose sensors and water treatment. Dalton Trans. 2015, 44, 17278–17285. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Liu, T.; Wang, G.; Sun, M.; Jiang, Y.; He, H.; Wang, Y.; Zou, P.; Wang, X.; et al. A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chem. Eng. J. 2020, 389, 124417. [Google Scholar] [CrossRef]
- Kim, J.; Byun, S.; Smith, A.J.; Yu, J.; Huang, J. Enhanced Electrocatalytic Properties of Transition-Metal Dichalcogenides Sheets by Spontaneous Gold Nanoparticle Decoration. J. Phys. Chem. Lett. 2013, 4, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.R.; Alsulami, Q.A.; Asnag, G.M.; Rajeh, A. Enhanced optical, morphological, dielectric, and conductivity properties of gold nanoparticles doped with PVA/CMC blend as an application in organoelectronic devices. J. Mater. Sci. Mater. Electron. 2021, 32, 10443–10457. [Google Scholar] [CrossRef]
- Chen, J.-L.; Yan, X.-P.; Meng, K.; Wang, S.-F. Graphene Oxide Based Photoinduced Charge Transfer Label-Free Near-Infrared Fluorescent Biosensor for Dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef] [PubMed]
- Renganathan, V.; Balaji, R.; Chen, S.-M.; Singh, V. The electrochemical determination of hazardous 4-hydroxynitrobenzene using NiS2 decorated graphene oxide nanocomposite in the river water sample. Microchem. J. 2020, 153, 104502. [Google Scholar] [CrossRef]
- Xia, N.; Deng, D.; Zhang, L.; Yuan, B.; Jing, M.; Du, J.; Liu, L. Sandwich-type electrochemical biosensor for glycoproteins detection based on dual-amplification of boronic acid-gold nanoparticles and dopamine-gold nanoparticles. Biosens. Bioelectron. 2013, 43, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.R.; Vieira, I.C. A biosensor based on gold nanoparticles stabilized in poly(allylamine hydrochloride) and decorated with laccase for determination of dopamine. Analyst 2016, 141, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Baynosa, M.L.; Dhakal, G.; Shim, J.-J. Sphere-like Ni3S4/NiS2/MoOx composite modified glassy carbon electrode for the electrocatalytic determination of d-penicillamine. J. Mol. Liq. 2020, 301, 112447. [Google Scholar] [CrossRef]
- Martín, M.; Salazar, P.; Villalonga, R.; Campuzano, S.; Pingarrón, J.M.; González-Mora, J.L. Preparation of core–shell Fe3O4@poly(dopamine) magnetic nanoparticles for biosensor construction. J. Mater. Chem. B 2014, 2, 739–746. [Google Scholar] [CrossRef]
- Baloach, Q.-U.; Nafady, A.; Tahira, A.; Sirajuddin; Sherazi, S.T.H.; Shaikh, T.; Arain, M.; Willander, M.; Ibupoto, Z.H. An amperometric sensitive dopamine biosensor based on novel copper oxide nanostructures. Microsyst. Technol. 2016, 23, 1229–1235. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, C.; Zeng, X.; Lei, J.; Hou, J.; Huo, D.; Hou, C. Co Single-Atom Nanozymes for the Simultaneous Electrochemical Detection of Uric Acid and Dopamine in Biofluids. ACS Appl. Nano Mater. 2024, 7, 6273–6283. [Google Scholar] [CrossRef]
- Xie, Z.; Shao, M.; Liu, Z.; Ren, X.; Gao, M.; Ma, H.; Zhang, N.; Wei, Q. Ultrasensitive aggregation-induced electrochemiluminescence sensor for dopamine detection in polymer hydrogel system. Sens. Actuators B Chem. 2024, 398, 134781. [Google Scholar] [CrossRef]
- Kaya, H.K.; Cinar, S.; Altundal, G.; Bayramlı, Y.; Unaleroglu, C.; Kuralay, F. A novel design thia-bilane structure-based molecular imprinted electrochemical sensor for sensitive and selective dopamine determination. Sens. Actuators B Chem. 2021, 346, 130425. [Google Scholar] [CrossRef]
- Li, S.-M.; Wang, Y.-S.; Hsiao, S.-T.; Liao, W.-H.; Lin, C.-W.; Yang, S.-Y.; Tien, H.-W.; Ma, C.-C.M.; Hu, C.-C. Fabrication of a silver nanowire-reduced graphene oxide-based electrochemical biosensor and its enhanced sensitivity in the simultaneous determination of ascorbic acid, dopamine, and uric acid. J. Mater. Chem. C 2015, 3, 9444–9453. [Google Scholar] [CrossRef]
- Rahman, S.F.; Min, K.; Park, S.-H.; Park, J.-H.; Yoo, J.C.; Park, D.-H. Highly sensitive and selective dopamine detection by an amperometric biosensor based on tyrosinase/MWNT/GCE. Korean J. Chem. Eng. 2016, 33, 3442–3447. [Google Scholar] [CrossRef]
- Ghosh, D.; Tabassum, R.; Sarkar, P.P.; Rahman, M.D.A.; Jalal, A.H.; Islam, N.; Ashraf, A. Graphene Nanocomposite Ink Coated Laser Transformed Flexible Electrodes for Selective Dopamine Detection and Immunosensing. ACS Appl. Bio Mater. 2024, 7, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Yasser, M.; Ahmad, A.; Natsir, H.; Wahid Wahab, A.; Fauziah, S.; Taba, P.; Pratama, I.; Rosalin; Rajab, A.; et al. A review: Progress and trend advantage of dopamine electrochemical sensor. J. Electroanal. Chem. 2024, 959, 118157. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Li, Y. Molecularly imprinted polymer-decorated signal on-off ratiometric electrochemical sensor for selective and robust dopamine detection. Biosens. Bioelectron. 2019, 135, 224–230. [Google Scholar] [CrossRef]
- Beatto, T.G.; Gomes, W.E.; Etchegaray, A.; Gupta, R.; Mendes, R.K. Dopamine levels determined in synthetic urine using an electrochemical tyrosinase biosensor based on ZnO@Au core–shell. RSC Adv. 2023, 13, 33424–33429. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Zhang, X.; Liu, X.; Jian, J.; Kong, D.; Zeng, D.; Yuan, H.; Feng, S. Electrochemical dopamine sensor based on superionic conducting potassium ferrite. Biosens. Bioelectron. 2020, 153, 112045. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Kang, P.; Wang, S.-Q.; Liu, Z.-G.; Li, Y.-X.; Guo, Z. Ag nanoparticles anchored onto porous CuO nanobelts for the ultrasensitive electrochemical detection of dopamine in human serum. Sens. Actuators B Chem. 2021, 327, 128878. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, T.; Zhou, L.; Offenhäusser, A.; Mayer, D. Label-Free Split Aptamer Sensor for Femtomolar Detection of Dopamine by Means of Flexible Organic Electrochemical Transistors. Materials 2020, 13, 2577. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Anithaa, A.C.; Lavanya, N.; Asokan, K.; Sekar, C. WO3 nanoparticles based direct electrochemical dopamine sensor in the presence of ascorbic acid. Electrochim. Acta 2015, 167, 294–302. [Google Scholar] [CrossRef]
- Arvand, M.; Ghodsi, N. Electrospun TiO2 nanofiber/graphite oxide modified electrode for electrochemical detection of l-DOPA in human cerebrospinal fluid. Sens. Actuators B Chem. 2014, 204, 393–401. [Google Scholar] [CrossRef]
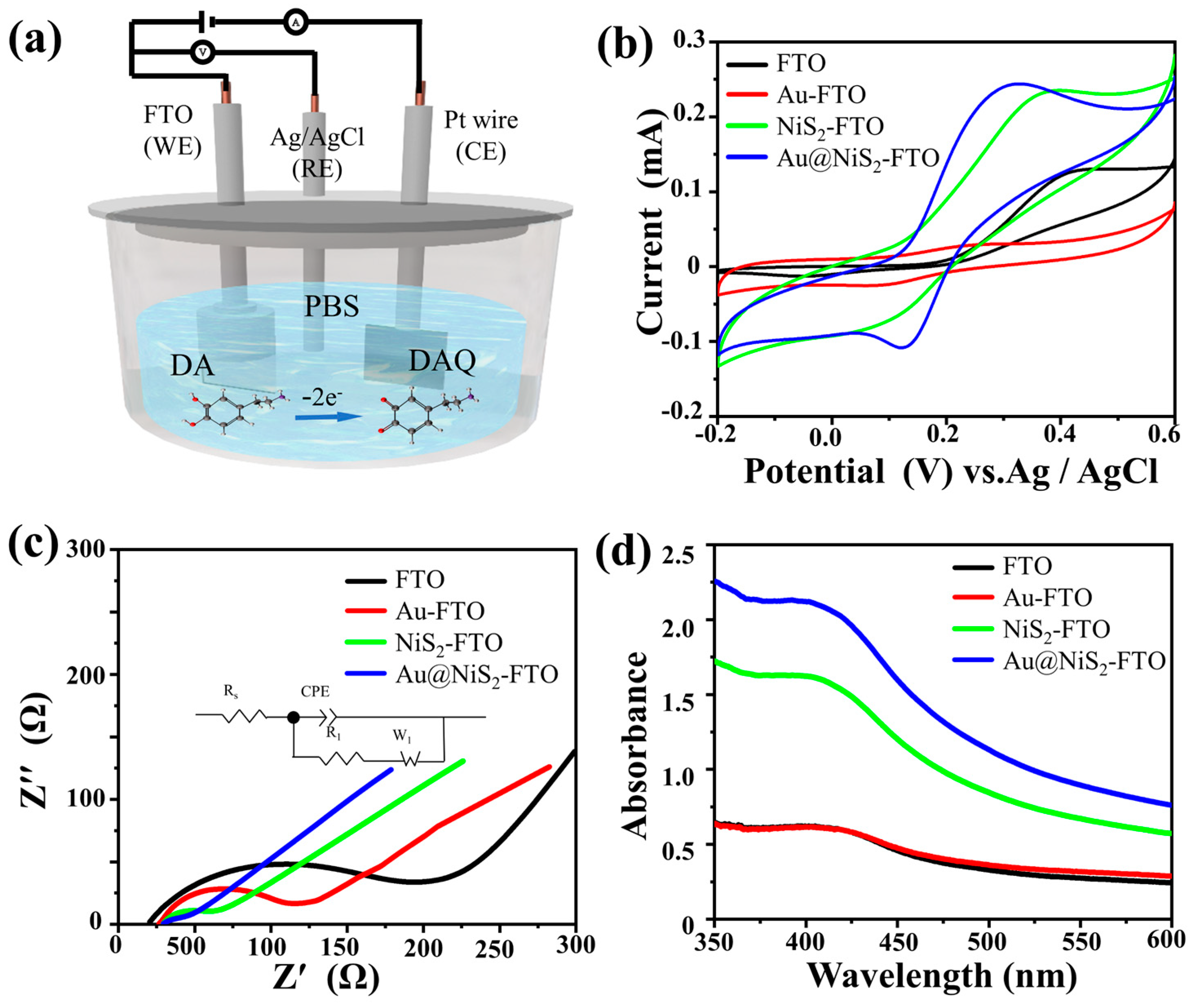
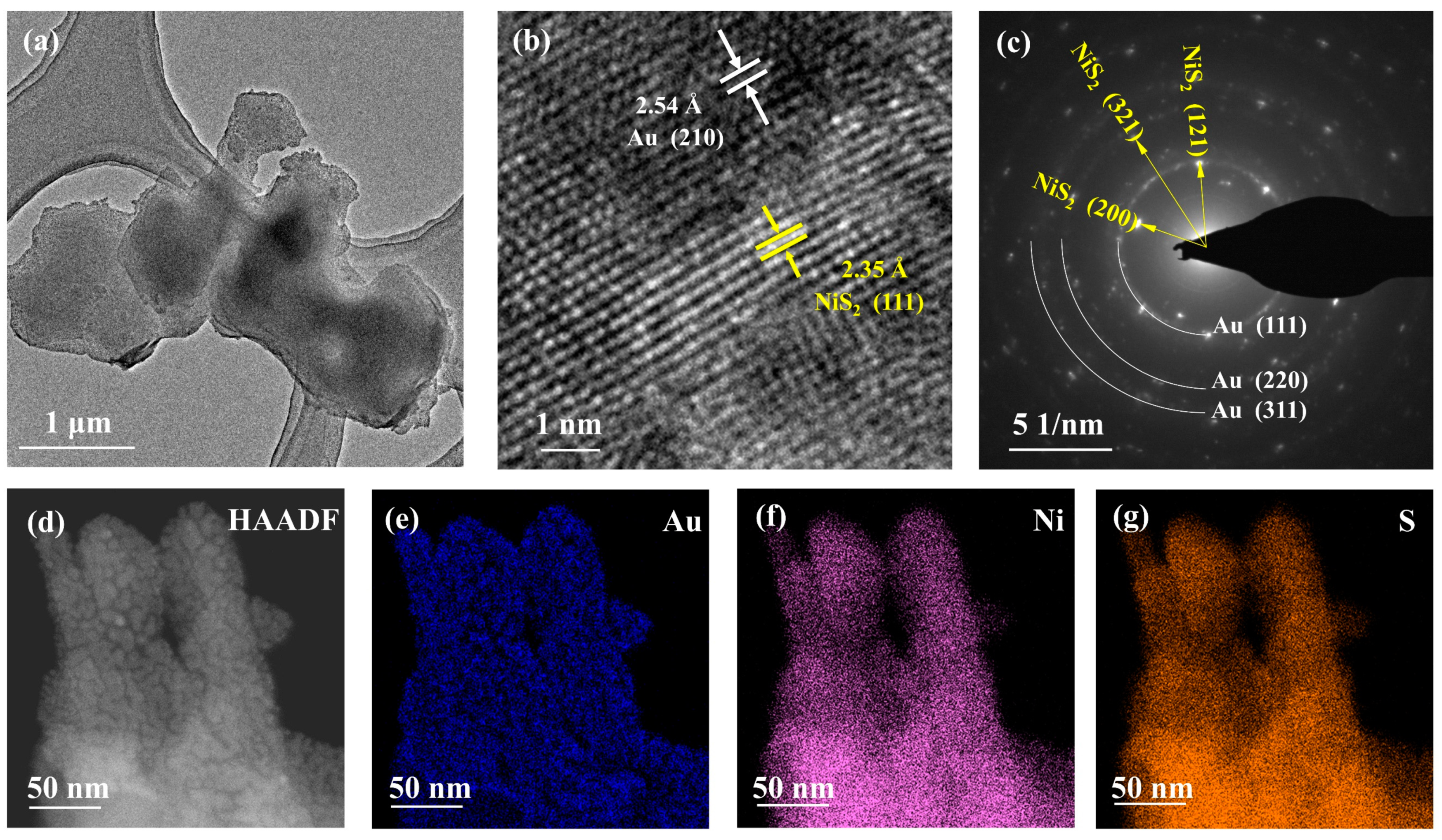
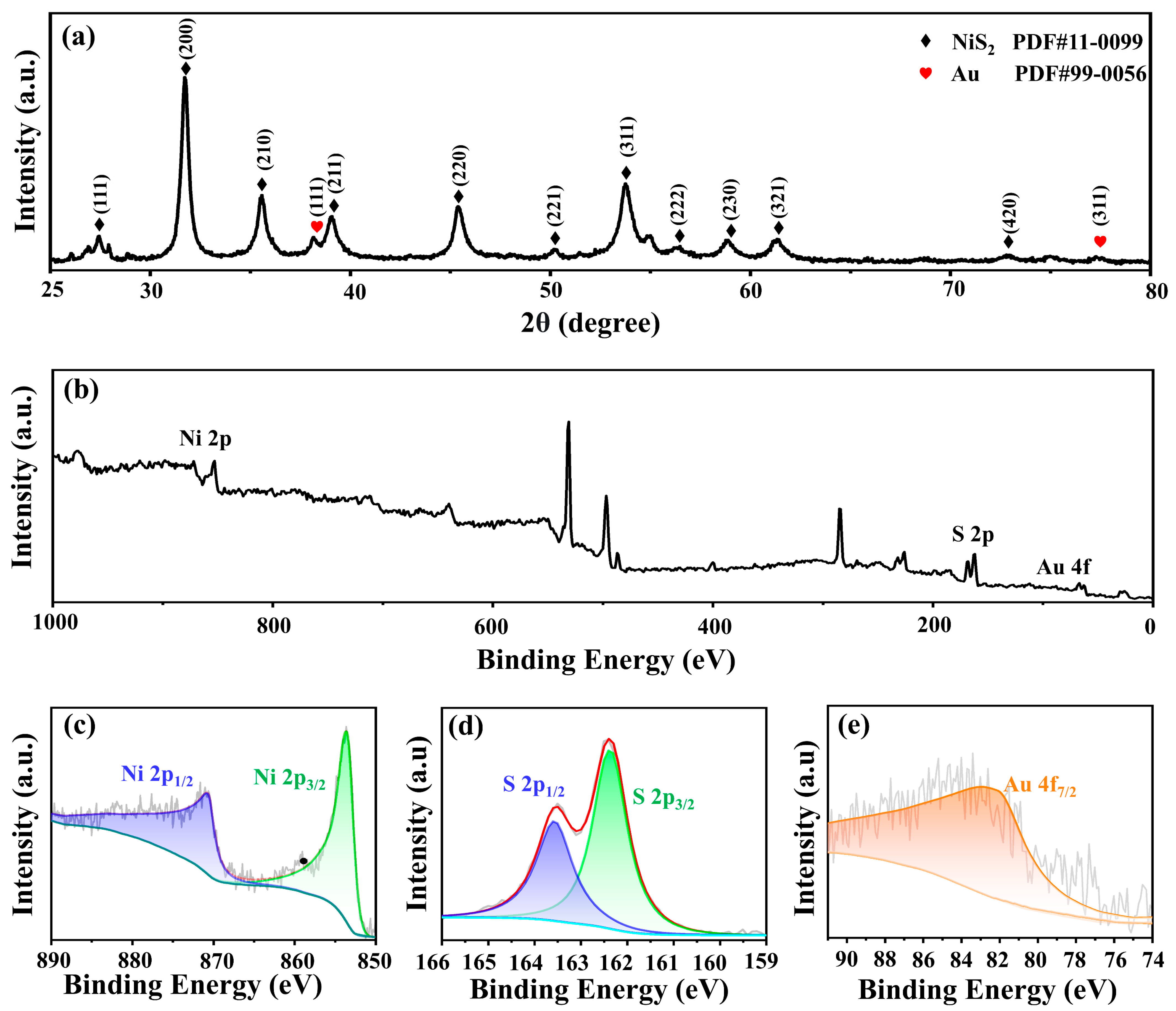
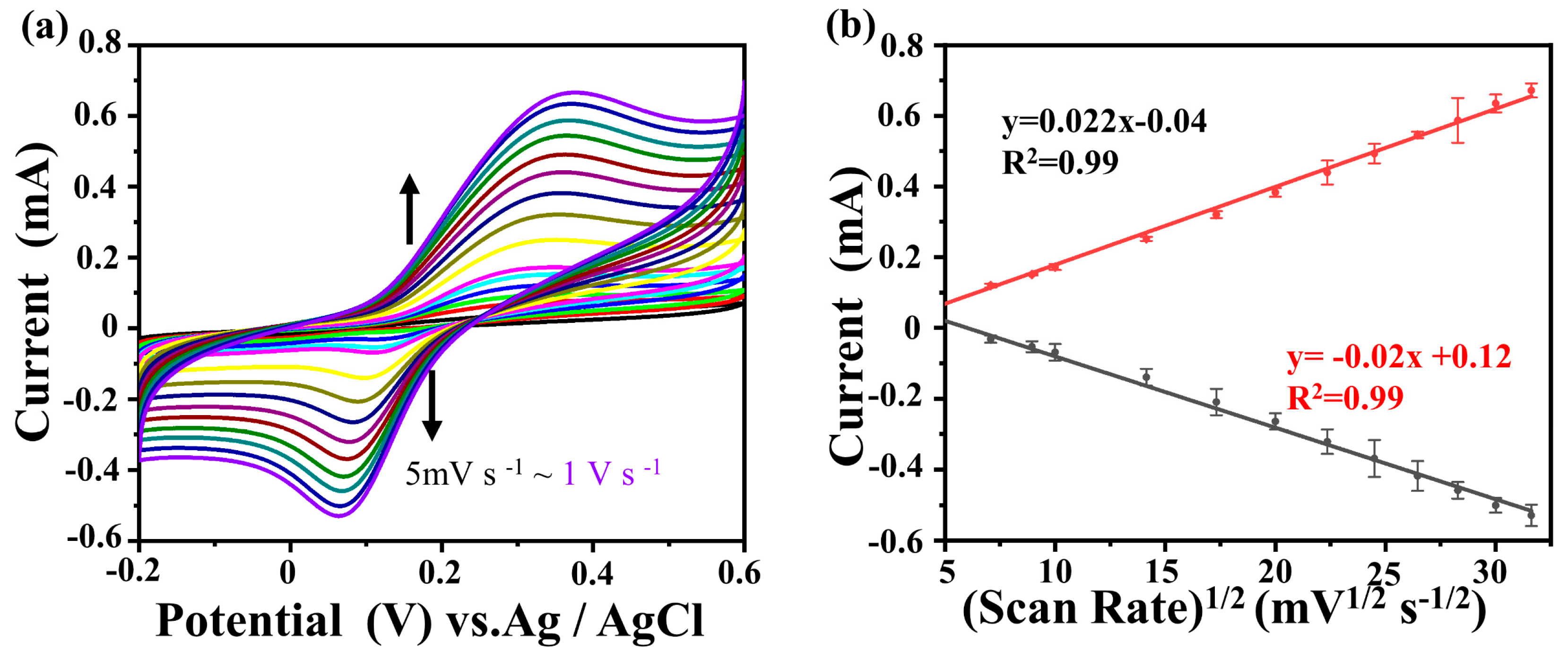
| Electrode | RCT (Ω) |
|---|---|
| FTO | 153 |
| Au-FTO | 83 |
| NiS2-FTO | 31 |
| Au@NiS2-FTO | 20 |
| Materials | Linear Range (μM) | LOD (nM) | Reference |
|---|---|---|---|
| Au@NiS2-FTO | 0.1–1000 | 1 | This work |
| SWCNTs-GCE | 0.5–100 | 190 | [49] |
| ZnO@Au | 0.1–500 | 8.5 | [50] |
| K2Fe4O7/GCE | 1–140 | 22 | [51] |
| Ag/CuO PNBs | 0.04–10 | 7 | [52] |
| Split aptamer sensor | 5–50 | 1000 | [53] |
| pS-BIL MIP PeGE | 0.05–250 | 20 | [44] |
| Gallic acid-RGO/AuNPs | 0.01–100.3 | 2.6 | [54] |
| WO3 NPs-GCE | 0.1–50, 50–600 | 24 | [55] |
| CuO | 5–40 | 110 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Wen, Y.; Qiao, Y.; Shen, K.Z.; Yuan, H. A Dopamine Detection Sensor Based on Au-Decorated NiS2 and Its Medical Application. Molecules 2024, 29, 2925. https://doi.org/10.3390/molecules29122925
Ma C, Wen Y, Qiao Y, Shen KZ, Yuan H. A Dopamine Detection Sensor Based on Au-Decorated NiS2 and Its Medical Application. Molecules. 2024; 29(12):2925. https://doi.org/10.3390/molecules29122925
Chicago/Turabian StyleMa, Chongchong, Yixuan Wen, Yuqing Qiao, Kevin Z. Shen, and Hongwen Yuan. 2024. "A Dopamine Detection Sensor Based on Au-Decorated NiS2 and Its Medical Application" Molecules 29, no. 12: 2925. https://doi.org/10.3390/molecules29122925
APA StyleMa, C., Wen, Y., Qiao, Y., Shen, K. Z., & Yuan, H. (2024). A Dopamine Detection Sensor Based on Au-Decorated NiS2 and Its Medical Application. Molecules, 29(12), 2925. https://doi.org/10.3390/molecules29122925





