Comparative Analysis of Exosomes and Extracellular Microvesicles in Healing Pathways: Insights for Advancing Regenerative Therapies
Abstract
1. Introduction
2. Biogenesis and Composition of Exosomes and Microvesicles
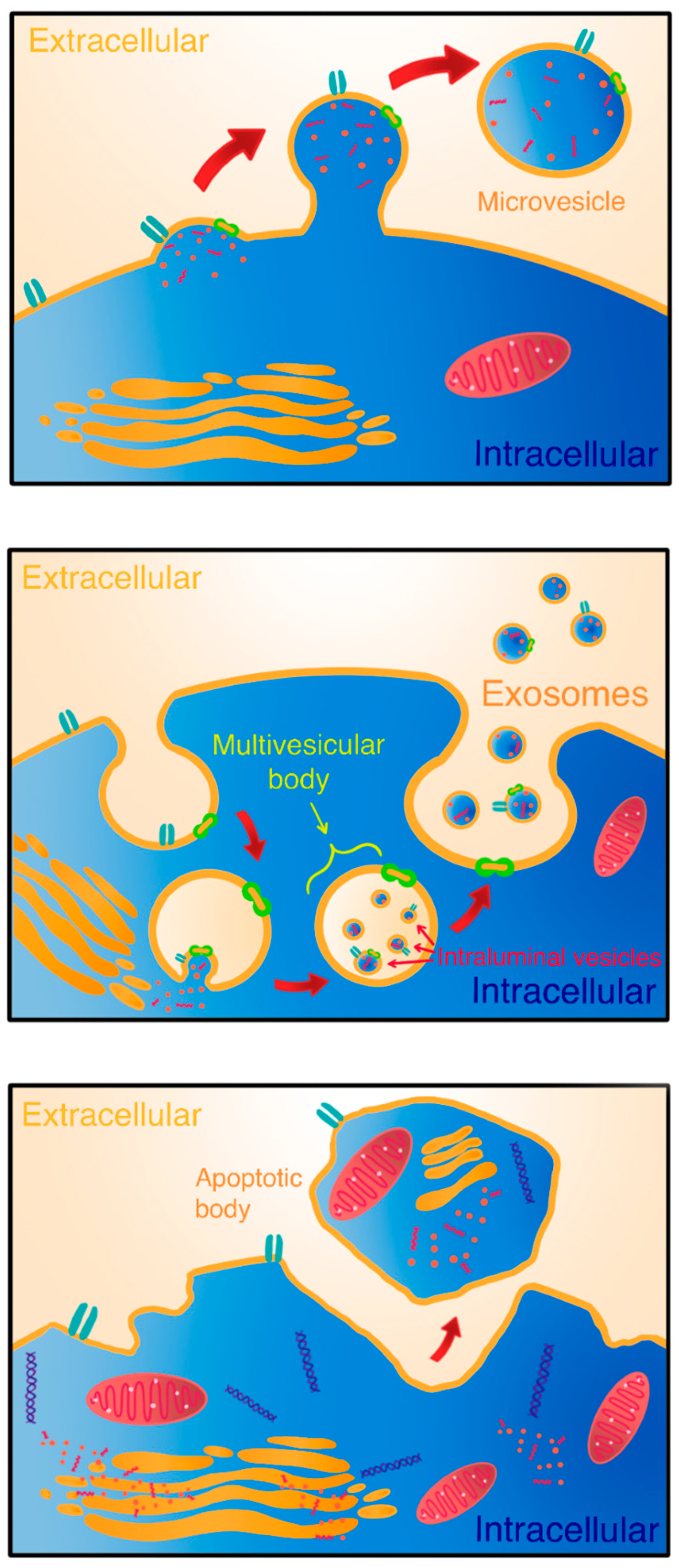
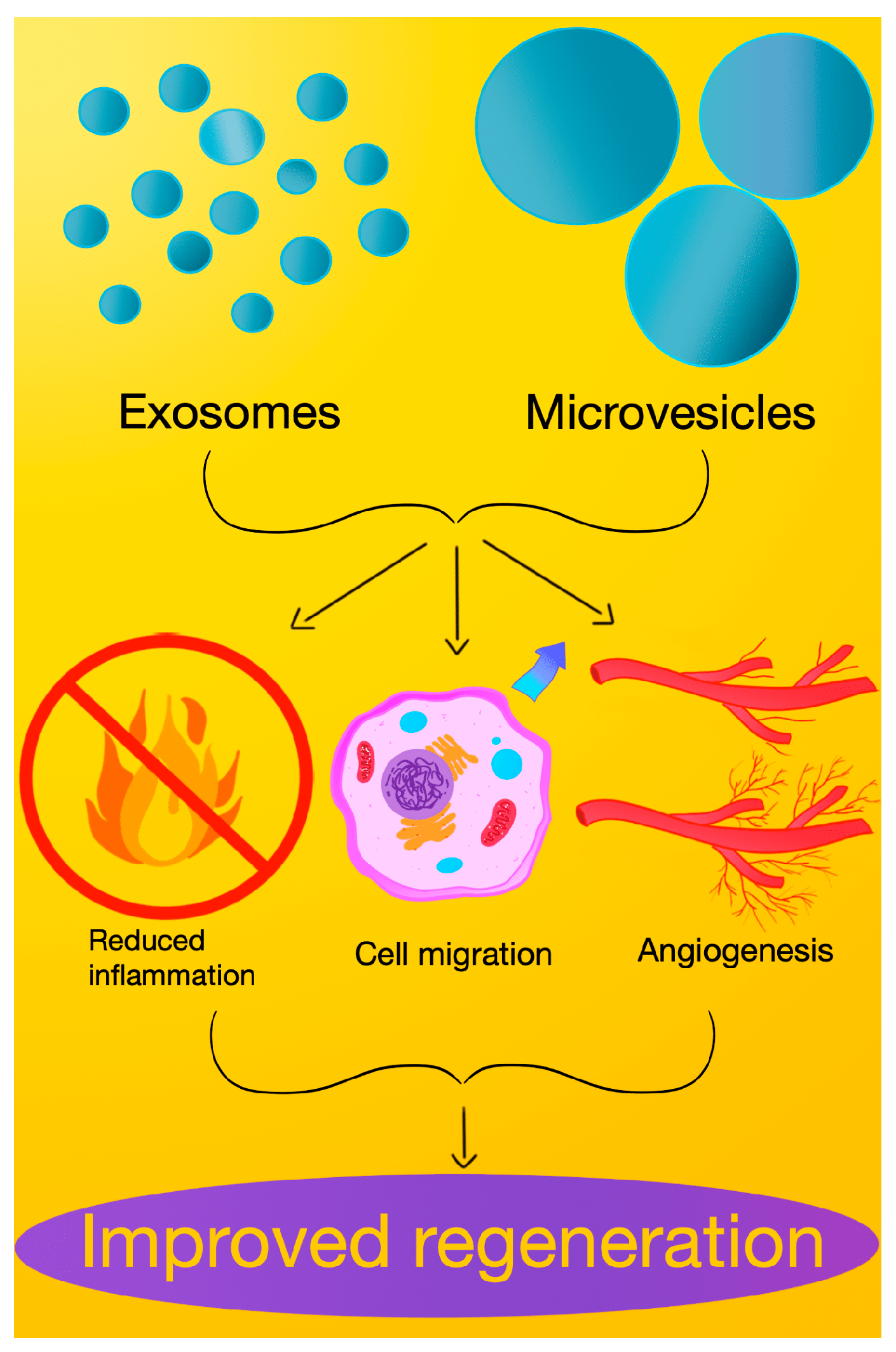
3. Role of Exosomes in Healing Pathways
4. Functions and Contributions of Extracellular Microvesicles in Healing
5. Comparative Analysis: Exosomes vs. Extracellular Microvesicles in Healing Pathways
6. Clinical Implications and Future Perspectives
| Year | Source of EVs | Experimental Model | Result |
|---|---|---|---|
| 2023 | MVs derived from mesenchymal stem cells and platelet-rich plasma | In vivo on animals | MVs derived from mesenchymal stem cells and PRP may improve burn wound healing via regulating scar formation and antioxidant mechanism [23] |
| 2023 | EXOs derived from endothelial progenitor cells | In vitro | The data showed that EPCs-EXOs promoted the proliferation and migration, while inhibited apoptosis of HaCaTs challenged by HG via elevating miR-182-5p expression level in vitro [98] |
| 2023 | EXOs derived from fibroblast cells | In vivo on animals | The results showed that the utilization of fibroblast-EXOs significantly promoted cutaneous wound healing in a rat full-thickness skin ulcer model [106] |
| 2023 | PRP-EXOs | In vivo and in vitro | PRP-EXOs can stimulate fibroblast functions and accelerate diabetic wound healing [23] |
| 2021 | dermal fibroblast-EXOs | In vitro and in vivo on animals | This research discovered that subcutaneous injections of DF-Ex could significantly promote re-epithelialization, collagen deposition, skin cell proliferation, and angiogenesis and inhibit inflammation to accelerate diabetic cutaneous wound healing [88] |
| 2021 | platelets exosome product | In vivo and in vitro | In vitro, PEP significantly promoted cell proliferation, migration, and tube formation, as well as skin organoid formation [103] |
| 2020 | PRMVs | In vitro | The research found that PRP pro-healing effects were fully replicable by PLT-MVs, suggesting a key role of MVs in the healing process and a possible clinical use as an alternative to PRP [95] |
| 2018 | BMSC-EVs | In vivo and in vitro on animals | The study concludes that murine ADSC and BMSC are equally effective in enhancing diabetic wound healing, and human diabetic ADSC is as effective as non-diabetic ADSC [99] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [PubMed]
- Elsner, C.; Ergün, S.; Wagner, N. Biogenesis and release of endothelial extracellular vesicles: Morphological aspects. Ann. Anat. 2023, 245, 152006. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2015; Volume 40, pp. 41–51. [Google Scholar] [CrossRef]
- Szwedowicz, U.; Łapińska, Z.; Gajewska-Naryniecka, A.; Choromańska, A. Exosomes and Other Extracellular Vesicles with High Therapeutic Potential: Their Applications in Oncology, Neurology, and Dermatology. Molecules 2022, 27, 1303. [Google Scholar] [CrossRef]
- Han, Q.-F.; Li, W.-J.; Hu, K.-S.; Gao, J.; Zhai, W.-L.; Yang, J.-H.; Zhang, S.-J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Khan, K.; Kim, J.-H. Biogenesis, Membrane Trafficking, Functions, and Next Generation Nanotherapeutics Medicine of Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 3357–3383. [Google Scholar] [CrossRef] [PubMed]
- Moeinzadeh, L.; Razeghian-Jahromi, I.; Zarei-Behjani, Z.; Bagheri, Z.; Razmkhah, M. Composition, Biogenesis, and Role of Exosomes in Tumor Development. Stem Cells Int. 2022, 2022, 8392509. [Google Scholar] [CrossRef] [PubMed]
- Vítková, V.; Živný, J.; Janota, J. Endothelial cell-derived microvesicles: Potential mediators and biomarkers of pathologic processes. Biomark. Med. 2018, 12, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.-J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327–11341. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Cowin, A.J.; Bayat, A.; Murray, R.Z.; Kopecki, Z. Editorial: Inflammation in Healing and Regeneration of Cutaneous Wounds. Front. Immunol. 2021, 12, 806687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef]
- Thakur, A.; Ke, X.; Chen, Y.-W.; Motallebnejad, P.; Zhang, K.; Lian, Q.; Chen, H.J. The mini player with diverse functions: Extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell 2021, 13, 631–654. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Meng, X.; Cao, F.; Wang, J.; Yang, M. Exosomes derived from platelet-rich plasma promote diabetic wound healing via the JAK2/STAT3 pathway. iScience 2023, 26, 108236. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; Ahmed, A.A.Q.; Lamboni, L.; Shi, Z.; Mukole, B.M.; Zheng, R.; Mbang, M.P.; Zhang, B.; Gauthier, M.; Yang, G. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact. Mater. 2023, 28, 74–94. [Google Scholar] [CrossRef]
- Wang, P.; Theocharidis, G.; Vlachos, I.S.; Kounas, K.; Lobao, A.; Shu, B.; Wu, B.; Xie, J.; Hu, Z.; Qi, S.; et al. Exosomes Derived from Epidermal Stem Cells Improve Diabetic Wound Healing. J. Investig. Dermatol. 2022, 142, 2508–2517.e13. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liu, S.; Wu, K.; Zhao, R.; Cao, L.; Wang, H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J. Cosmet. Dermatol. 2019, 19, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yang, W.; Cui, W.; Li, C.; Ma, C.; Ji, X.; Hong, J.; Qu, Z.; Chen, J.; Liu, A.; et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon–bone healing. J. Nanobiotechnol. 2023, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef] [PubMed]
- Lou, R.; Chen, J.; Zhou, F.; Zhang, T.; Chen, X.; Wang, C.; Guo, B.; Lin, L. Exosomal miRNA-155-5p from M1-polarized macrophages suppresses angiogenesis by targeting GDF6 to interrupt diabetic wound healing. Mol. Ther.—Nucleic Acids 2023, 34, 102074. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Chen, J.; Ma, Y.; Song, Y.; Cen, Y.; You, M.; Yang, G. The Notch signaling pathway regulates macrophage polarization in liver diseases. Int. Immunopharmacol. 2021, 99, 107938. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, B.; Gu, G.; Zhang, W.; Li, J.; Wang, H.; Wang, M.; Song, X.; Wei, Z.; Feng, S. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023, 14, 70. [Google Scholar] [CrossRef]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, Z.; Yang, L.; Song, S.; Fu, Z.; Tang, K.; Chen, L.; Song, Y. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J. Nanobiotechnol. 2022, 20, 110. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.; Zhang, H.; Wang, S.; Cui, J.; Hu, Y.; Liu, J.; Li, M.; Zhang, K.; Zhou, F.; et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact. Mater. 2023, 28, 273–283. [Google Scholar] [CrossRef]
- Gao, S.; Mao, F.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, M.; Yan, Y.; Yang, T.; Zhang, J.; Zhu, W.; et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp. Biol. Med. 2014, 239, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Ahuja, A.; Kim, E.; Sung, G.-H.; Cho, J.Y. STAT3 Differentially Regulates TLR4-Mediated Inflammatory Responses in Early or Late Phases. Int. J. Mol. Sci. 2020, 21, 7675. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, H.; Wang, Z.; Li, X.; Wang, P.; Cao, X.; Xu, Z.; Lv, D.; Rong, Y.; Chen, M.; et al. Analysis of miR-203a-3p/SOCS3-mediated induction of M2 macrophage polarization to promote diabetic wound healing based on epidermal stem cell-derived exosomes. Diabetes Res. Clin. Pract. 2023, 197, 110573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, C.; Jiang, L.; Zhu, X.; Zhou, F.; Xia, M.; Chen, Y. The bone mesenchymal stem cell-derived exosomal miR-146a-5p promotes diabetic wound healing in mice via macrophage M1/M2 polarization. Mol. Cell. Endocrinol. 2024, 579, 112089. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. eBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shi, Y.; Li, M.; Wang, T.; Zhao, L. Plasma Exosomes Loaded pH-Responsive Carboxymethylcellulose Hydrogel Promotes Wound Repair by Activating the Vascular Endothelial Growth Factor Signaling Pathway in Type 1 Diabetic Mice. J. Biomed. Nanotechnol. 2021, 17, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ke, Q.-F.; Tao, S.-C.; Guo, S.-C.; Rui, B.-Y.; Guo, Y.-P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Z.; Wang, Y.; Shen, H. Investigation of miR-126-3p loaded on adipose stem cell-derived exosomes for wound healing of full-thickness skin defects. Exp. Dermatol. 2022, 31, 362–374. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, C.; Jia, J.; Hao, X.-Y.; Yu, X.-Y.; Liu, X.-Y.; Shu, M.-G. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci. Rep. 2020, 40, BSR20192549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, Y.; Gong, A.; Pan, Z.; Shi, H.; Yang, H.; Fu, H.; Yan, Y.; Zhang, X.; Wang, M.; et al. HucMSC Exosome-Delivered 14-3-3ζ Orchestrates Self-Control of the Wnt Response via Modulation of YAP during Cutaneous Regeneration. Stem Cells 2016, 34, 2485–2500. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, S.; Mallas, B.; Impola, U.; Valkeajärvi, A.; Eronen, J.; Javela, K.; Siljander, P.R.-M.; Laitinen, S. Assessment of Time-Dependent Platelet Activation Using Extracellular Vesicles, CD62P Exposure, and Soluble Glycoprotein V Content of Platelet Concentrates with Two Different Platelet Additive Solutions. Transfus. Med. Hemother. 2019, 46, 267–275. [Google Scholar] [CrossRef]
- Luan, B.; Yoon, Y.-S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef]
- Boilard, E. Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of their Biogenesis and Functions Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018, 59, 2037–2046. [Google Scholar] [CrossRef]
- Ma, R.; Ji, T.; Chen, D.; Dong, W.; Zhang, H.; Yin, X.; Ma, J.; Liang, X.; Zhang, Y.; Shen, G.; et al. Tumor cell-derived microparticles polarize M2 tumor-associated macrophages for tumor progression. OncoImmunology 2016, 5, e1118599. [Google Scholar] [CrossRef]
- Imam, R.A.; Amer, M.M. Potential therapeutic role of microvesicles derived from mesenchymal stem cells and platelet-rich plasma in murine burn wound healing: Scar regulation and antioxidant mechanism. Folia Morphol. 2023, 82, 656–667. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell–derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Liu, Y.-Y.; He, S.-S.; Bao, D.-Q.; Wang, H.-C.; Zhang, J.; Peng, X.-Y.; Zang, J.-T.; Zhu, Y.; Wu, Y.; et al. Pericytes protect rats and mice from sepsis-induced injuries by maintaining vascular reactivity and barrier function: Implication of miRNAs and microvesicles. Mil. Med. Res. 2023, 10, 13. [Google Scholar] [CrossRef]
- Katare, R.; Riu, F.; Mitchell, K.; Gubernator, M.; Campagnolo, P.; Cui, Y.; Fortunato, O.; Avolio, E.; Cesselli, D.; Beltrami, A.P.; et al. Transplantation of Human Pericyte Progenitor Cells Improves the Repair of Infarcted Heart through Activation of an Angiogenic Program Involving Micro-RNA-132. Circ. Res. 2011, 109, 894–906. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Zingarelli, B.; Fan, H. miR-145a Regulation of Pericyte Dysfunction in a Murine Model of Sepsis. J. Infect. Dis. 2020, 222, 1037–1045. [Google Scholar] [CrossRef]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef]
- Prokopi, M.; Pula, G.; Mayr, U.; Devue, C.; Gallagher, J.; Xiao, Q.; Boulanger, C.M.; Westwood, N.; Urbich, C.; Willeit, J.; et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood 2009, 114, 723–732. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, R.; Bo, Y.; Zhang, M.; Chen, Y.; Wang, X.; Huang, M.; Liu, B.; Zhang, L. Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration. Theranostics 2020, 10, 9970–9983. [Google Scholar] [CrossRef]
- Arif, S.; Larochelle, S.; Moulin, V.J. PLGF-1 contained in normal wound myofibroblast-derived microvesicles stimulated collagen production by dermal fibroblasts. J. Cell Commun. Signal. 2020, 14, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair. Regen 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, Y.; Huang, Y.; Zhu, N.; Shen, Y.-Q. Extracellular vesicles modulate key signalling pathways in refractory wound healing. Burn. Trauma 2023, 11, tkad039. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef]
- Deng, F.; Peng, L.; Li, Z.; Tan, G.; Liang, E.; Chen, S.; Zhao, X.; Zhi, F. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 2018, 9, 153. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Wang, X.; Wang, J.; Duan, X.; Li, R.; Peng, Y.; Ye, Q.; He, Y. Characteristics of culture-condition stimulated exosomes or their loaded hydrogels in comparison with other extracellular vesicles or MSC lysates. Front. Bioeng. Biotechnol. 2022, 10, 1016833. [Google Scholar] [CrossRef]
- Guan, F.; Xiang, X.; Xie, Y.; Li, H.; Zhang, W.; Shu, Y.; Wang, J.-H.; Qin, W. Simultaneous metabolomics and proteomics analysis of plasma-derived extracellular vesicles. Anal. Methods 2021, 13, 1930–1938. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef]
- Stachurska, A.; Dorman, M.; Korsak, J.; Gaweł, D.; Grzanka, M.; Trybus, W.; Fabijanska-Mitek, J. Selected CD molecules and the phagocytosis of microvesicles released from erythrocytes ex vivo. Vox Sang. 2019, 114, 576–587. [Google Scholar] [CrossRef]
- Wen, J.; Creaven, D.; Luan, X.; Wang, J. Comparison of immunotherapy mediated by apoptotic bodies, microvesicles and exosomes: Apoptotic bodies’ unique anti-inflammatory potential. J. Transl. Med. 2023, 21, 478. [Google Scholar] [CrossRef]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Control. Release 2021, 338, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-dependent cellular uptake of exosomes. Nanomedicine 2017, 13, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Huang, X.; Jabeen, A.; Ahsan, A.; Seidu, T.A.; Kutoka, P.T.; Wang, B. Enhanced cellular uptake and cytotoxicity of vorinostat through encapsulation in TPGS-modified liposomes. Colloids Surf. B Biointerfaces 2020, 199, 111523. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.R.; Riazifar, M.; Pone, E.J.; Yeri, A.; Van Keuren-Jensen, K.; Lässer, C.; Lotvall, J.; Zhao, W. Isolation and characterization of microvesicles from mesenchymal stem cells. Methods 2020, 177, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.R.; Oropallo, A.R.; Grande, D.A.; Kirsner, R.S.; Badiavas, E.V. Extracellular Vesicles as Therapeutic Tools for the Treatment of Chronic Wounds. Pharmaceutics 2021, 13, 1543. [Google Scholar] [CrossRef]
- Fu, L.; Wu, S.S. Advances in studies on exosomes and microvesicles as markers of cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2622–2629. [Google Scholar] [CrossRef]
- Pedersen, S.; Jensen, K.P.; Honoré, B.; Kristensen, S.R.; Pedersen, C.H.; Szejniuk, W.M.; Maltesen, R.G.; Falkmer, U. Circulating microvesicles and exosomes in small cell lung cancer by quantitative proteomics. Clin. Proteom. 2022, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Hornung, S.; Taha, H.B.; Bitan, G. Biomarkers for parkinsonian disorders in CNS-originating EVs: Promise and challenges. Acta Neuropathol. 2023, 145, 515–540. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Hertel, F.C.; da Silva, A.S.; Sabino, A.d.P.; Valente, F.L.; Reis, E.C.C. Preconditioning Methods to Improve Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Bone Regeneration—A Systematic Review. Biology 2022, 11, 733. [Google Scholar] [CrossRef]
- Didamoony, M.A.; Atwa, A.M.; Ahmed, L.A. Modulatory effect of rupatadine on mesenchymal stem cell-derived exosomes in hepatic fibrosis in rats: A potential role for miR-200a. Life Sci. 2023, 324, 121710. [Google Scholar] [CrossRef] [PubMed]
- Al-Masawa, M.E.; Alshawsh, M.A.; Ng, C.Y.; Ng, A.M.H.; Foo, J.B.; Vijakumaran, U.; Subramaniam, R.; Ghani, N.A.A.; Witwer, K.W.; Law, J.X. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: A systematic review and meta-analysis of animal studies. Theranostics 2022, 12, 6455–6508. [Google Scholar] [CrossRef]
- Chen, S.; Sun, F.; Qian, H.; Xu, W.; Jiang, J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022, 2022, 1779346. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Hussain, A.; Behfar, A.; Moran, S.L.; Zhao, C. The Therapeutic Potential of Exosomes in Soft Tissue Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 3869. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Han, X.; Wu, P.; Li, L.; Sahal, H.M.; Ji, C.; Zhang, J.; Wang, Y.; Wang, Q.; Qian, H.; Shi, H.; et al. Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the Akt/β-catenin pathway. Cell Cycle 2021, 20, 616–629. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Yan, C. Recent advances in the use of extracellular vesicles from adipose-derived stem cells for regenerative medical therapeutics. J. Nanobiotechnol. 2024, 22, 316. [Google Scholar] [CrossRef]
- Zhang, B.; Bi, Y.; Wang, K.; Guo, X.; Liu, Z.; Li, J.; Wu, M. Stem Cell-Derived Extracellular Vesicles: Promising Therapeutic Opportunities for Diabetic Wound Healing. Int. J. Nanomed. 2024, 19, 4357–4375. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, F.; Rasouli, H.R.; Talebi, S.; Golchin, D.; Esmailinejad, M.R.; Razie, A. Effects of exosomes derived from fibroblast cells on skin wound healing in Wistar rats. Burns 2023, 49, 1372–1381. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Author Correction: Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2021, 11, 3245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, X.; Li, H.; Zhang, C.; Zhang, Z.; Wu, C.; Zhang, J.; Hu, J.; Zhang, J. The role of mesenchymal stem cell-derived EVs in diabetic wound healing. Front. Immunol. 2023, 14, 1136098. [Google Scholar] [CrossRef]
- Lovisolo, F.; Carton, F.; Gino, S.; Migliario, M.; Reno, F. Platelet rich plasma-derived microvesicles increased in vitro wound healing. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9658–9664. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Ren, L.; Feng, B.; Chen, Z.; Li, Z.; Cheng, B.; Xia, J. Development of an Antiswelling Hydrogel System Incorporating M2-Exosomes and Photothermal Effect for Diabetic Wound Healing. ACS Nano 2023, 17, 22106–22120. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, Z.; Zeng, F.; Gu, C.; Chen, X. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater. Today Bio. 2023, 20, 100649. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Lu, S.; Lu, L.; Liu, Y.; Li, Z.; Fang, Y.; Chen, Z.; Zhou, J. Native and engineered extracellular vesicles for wound healing. Front. Bioeng. Biotechnol. 2022, 10, 1053217. [Google Scholar] [CrossRef]
- Guo, J.; Hu, H.; Gorecka, J.; Bai, H.; He, H.; Assi, R.; Isaji, T.; Wang, T.; Setia, O.; Lopes, L.; et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am. J. Physiol. Physiol. 2018, 315, C885–C896. [Google Scholar] [CrossRef]
- Jia, Q.; Zhao, H.; Wang, Y.; Cen, Y.; Zhang, Z. Mechanisms and applications of adipose-derived stem cell-extracellular vesicles in the inflammation of wound healing. Front. Immunol. 2023, 14, 1214757. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wen, X.; Zhou, L.; Fang, X. The value of platelet-rich plasma-derived extracellular vesicles in modern medicine. Ann. Med. 2023, 55, 2287705. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Piao, Y.; Liu, Q.; Yang, X. Platelet-rich plasma-derived extracellular vesicles: A superior alternative in regenerative medicine? Cell Prolif. 2021, 54, e13123. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Li, J.; Qiu, X.; Sabbah, M.; Boroumand, S.; Huang, T.C.-T.; Zhao, C.; Terzic, A.; Behfar, A.; Moran, S.L. TGF-β loaded exosome enhances ischemic wound healing in vitro and in vivo. Theranostics 2021, 11, 6616–6631. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2020, 29, 13–31. [Google Scholar] [CrossRef]
| Mechanism | Active Component | Signaling Pathways |
|---|---|---|
| Macrophage polarization M1 M2 | miRNA + snRNA | TGFβ signaling [25] |
| miRNA let-7b | inhibition of NF-κB pathway [30] | |
| MFG-E8 | STAT3 pathway [32] | |
| miR-203a-3p | STAT3 pathway [39] | |
| miR-146a-5p | inhibition of NF-κB pathway [40] | |
| miR-181c | inhibition of NF-κB pathway [41] | |
| The promotion of proliferation state | bFGF, PDGFBB, and TGF-β | PI3K/AKT + Erk pathway [21] |
| - | VEGF signaling pathway [42,43] | |
| miR-126-3p | PI3K/AKT and Erk pathways [44] | |
| lncRNA MALAT1 | activation of Wnt/β-catenin pathway [46] | |
| Inhibition of scar formation | lncRNA MALAT1 | Wnt/β-catenin pathway [46] |
| 14-3-3ζ | Wnt/β-catenin pathway [47] |
| Mechanism | Active Component | Signaling Pathways |
|---|---|---|
| Macrophage polarization M1 M2 | STAT signaling axis [52] | |
| The promotion of proliferation state | miRNA-145 and miRNA-132 | S1P signaling pathway [55] |
| VEGF, FGF-2, and PDGF | PI3/AKT + Erk pathway [58] | |
| miR-16-5p | p38/MARK pathway [61] | |
| Wnt4 | Wnt/β-catenin pathway [79] | |
| Inhibition of scar formation | 14–3-3ζ protein | Wnt/β-catenin pathway [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sędzik, M.; Rakoczy, K.; Sleziak, J.; Kisiel, M.; Kraska, K.; Rubin, J.; Łuniewska, W.; Choromańska, A. Comparative Analysis of Exosomes and Extracellular Microvesicles in Healing Pathways: Insights for Advancing Regenerative Therapies. Molecules 2024, 29, 3681. https://doi.org/10.3390/molecules29153681
Sędzik M, Rakoczy K, Sleziak J, Kisiel M, Kraska K, Rubin J, Łuniewska W, Choromańska A. Comparative Analysis of Exosomes and Extracellular Microvesicles in Healing Pathways: Insights for Advancing Regenerative Therapies. Molecules. 2024; 29(15):3681. https://doi.org/10.3390/molecules29153681
Chicago/Turabian StyleSędzik, Mikołaj, Katarzyna Rakoczy, Jakub Sleziak, Michał Kisiel, Karolina Kraska, Jakub Rubin, Wiktoria Łuniewska, and Anna Choromańska. 2024. "Comparative Analysis of Exosomes and Extracellular Microvesicles in Healing Pathways: Insights for Advancing Regenerative Therapies" Molecules 29, no. 15: 3681. https://doi.org/10.3390/molecules29153681
APA StyleSędzik, M., Rakoczy, K., Sleziak, J., Kisiel, M., Kraska, K., Rubin, J., Łuniewska, W., & Choromańska, A. (2024). Comparative Analysis of Exosomes and Extracellular Microvesicles in Healing Pathways: Insights for Advancing Regenerative Therapies. Molecules, 29(15), 3681. https://doi.org/10.3390/molecules29153681








