The Impact of Drying Methods on the Quality of Blanched Yellow Mealworm (Tenebrio molitor L.) Larvae
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of Blanching and Diverse Drying Methods on Drying Process, Water Activity, and Color of Yellow Mealworm Larvae
2.2. The Effects of Blanching and Diverse Drying Methods on Chemical Composition of Yellow Mealworm Larvae
2.3. The Effects of Blanching and Diverse Drying Methods on Amino Acid Profile of Yellow Mealworm Larvae
2.4. The Effects of Blanching and Diverse Drying Methods on Oil Properties of Yellow Mealworm Larvae
2.4.1. Fatty Acid Composition and Positional Distribution of Triacylglycerols
2.4.2. Oxidative Stability, Acid Value, and Peroxide Value
2.5. The Effects of Blanching and Diverse Drying Methods on the Bioactive Properties of Yellow Mealworm Larvae
| Tm_70CD | Tm_90CD | Tm_FD | |
|---|---|---|---|
| Total polyphenol content (mg chlorogenic acid/100 g d.m.) | 77.83 ± 2.03 b 1 | 76.06 ± 0.83 b | 73.00 ± 1.14 a |
| ABTS (mg TE/g d.m.) | 4.34 ± 0.15 b | 3.34 ± 0.03 a | 8.10 ± 0.44 c |
| DPPH (mg TE/g d.m.) | 5.38 ± 0.26 b | 4.21 ± 0.10 a | 5.98 ± 0.01 c |
2.6. The Effects of Blanching and Diverse Drying Methods on the Mineral Composition of Yellow Mealworm Larvae
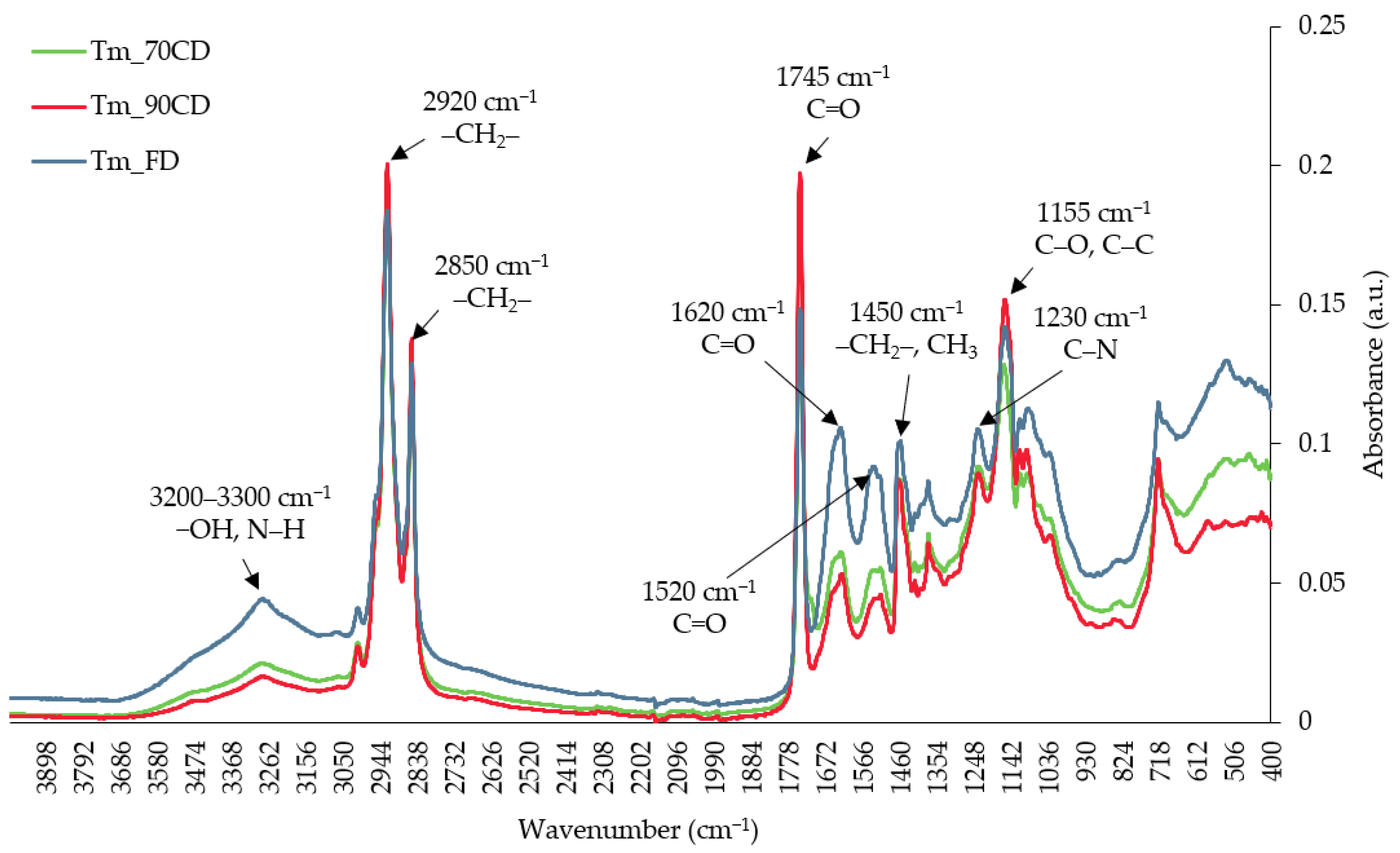
2.7. The Effects of Blanching and Diverse Drying Methods on FTIR Spectra of Yellow Mealworm Larvae
2.8. The Effects of Blanching and Diverse Drying Methods on the Allergen Content of Yellow Mealworm Larvae
2.9. The Effects of Blanching and Diverse Drying Methods on the Microbiological Quality of Yellow Mealworm Larvae
3. Materials and Methods
3.1. Material
3.2. Technological Treatment
3.2.1. Blanching
3.2.2. Drying
3.3. Water Activity
3.4. Color Measurement
3.5. Chemical Composition
3.6. Amino Acid Profile
3.7. Oil Properties
3.7.1. Extraction Procedure
3.7.2. Fatty Acid Composition
3.7.3. Fatty Acids Distribution in Triacylglycerols
3.7.4. Oxidative Stability
3.7.5. Acid Value
3.7.6. Peroxide Value
3.7.7. Health Indices
3.8. Bioactive Properties
3.8.1. Extract Preparation
3.8.2. Total Polyphenol Content
3.8.3. ABTS Assay Antioxidant Activity
3.8.4. DPPH Assay Antioxidant Activity
3.9. Mineral Composition
3.10. FTIR Measurement
3.11. Allergen Content
3.12. Microorganism Determination
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szulc, K. Edible Insects: A Study of the Availability of Insect-Based Food in Poland. Sustainability 2023, 15, 14964. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the Development of Edible Insect-Based Foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Langston, K.; Selaledi, L.; Tanga, C.; Yusuf, A. The Nutritional Profile of the Yellow Mealworm Larvae (Tenebrio molitor) Reared on Four Different Substrates. Future Foods 2024, 9, 100388. [Google Scholar] [CrossRef]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of Microwave Drying for Mealworms (Tenebrio molitor) as Alternative to Freeze Drying: Impact on Nutritional Quality and Colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Adámková, A. Nutritional and Sensory Quality of Edible Insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Vasilopoulos, S.; Giannenas, I.; Athanassiou, G.C.; Rumbos, C.; Papadopoulos, E.; Fortomaris, P. Black Soldier Fly, Mealworm and Superworm: Chemical Composition and Comparative Effect on Broiler Growth. World’s Poult. Sci. J. 2024, 1–30. [Google Scholar] [CrossRef]
- Li, A.; Dewettinck, K.; Verheust, Y.; Van de Walle, D.; Raes, K.; Diehl, B.; Tzompa-Sosa, D.A. Edible Insects as a Novel Source of Lecithin: Extraction and Lipid Characterization of Black Soldier Fly Larvae and Yellow Mealworm. Food Chem. 2024, 452, 139391. [Google Scholar] [CrossRef] [PubMed]
- Knežić, T.; Avramov, M.; Tatić, V.; Petrović, M.; Gadjanski, I.; Popović, Ž.D. Insects as a Prospective Source of Biologically Active Molecules and Pharmaceuticals—Biochemical Properties and Cell Toxicity of Tenebrio molitor and Zophobas morio Cell-Free Larval Hemolymph. Int. J. Mol. Sci. 2024, 25, 7491. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Marques, J.P.; Fernandes, T.R.; Pintado, M.E.; Carvalho, S.M.P.; Cunha, L.M. Effect of Blanching, Storage and Drying Conditions on the Macro-Composition, Color and Safety of Mealworm Tenebrio molitor Larvae. LWT 2024, 191, 115646. [Google Scholar] [CrossRef]
- Hernández-Álvarez, A.-J.; Mondor, M.; Piña-Domínguez, I.-A.; Sánchez-Velázquez, O.-A.; Melgar Lalanne, G. Drying Technologies for Edible Insects and Their Derived Ingredients. Dry. Technol. 2021, 39, 1991–2009. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Falacińska, J.; Kowalska, H.; Kowalska, J.; Galus, S.; Marzec, A.; Domian, E. The Effect of Pre-Treatment (Blanching, Ultrasound and Freezing) on Quality of Freeze-Dried Red Beets. Foods 2021, 10, 132. [Google Scholar] [CrossRef]
- Cacchiarelli, C.; Fratini, F.; Puccini, M.; Vitolo, S.; Paci, G.; Mancini, S. Effects of Different Blanching Treatments on Colour and Microbiological Profile of Tenebrio molitor and Zophobas morio Larvae. LWT 2022, 157, 113112. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Trusińska, M.; Rybak, K.; Wiktor, A.; Nowacka, M. The Influence of Pulsed Electric Field and Air Temperature on the Course of Hot-Air Drying and the Bioactive Compounds of Apple Tissue. Molecules 2023, 28, 2970. [Google Scholar] [CrossRef]
- Roknul Azam, S.M.; Zhang, M.; Law, C.L.; Mujumdar, A.S. Effects of Drying Methods on Quality Attributes of Peach (Prunus persica) Leather. Dry. Technol. 2019, 37, 341–351. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Yang, X.-H.; Mujumdar, A.S.; Zhao, J.-H.; Wang, D.; Zhang, Q.; Wang, J.; Gao, Z.-J.; Xiao, H.-W. Red Pepper (Capsicum annuum L.) Drying: Effects of Different Drying Methods on Drying Kinetics, Physicochemical Properties, Antioxidant Capacity, and Microstructure. Dry. Technol. 2018, 36, 893–907. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Janowicz, M.; Karwacka, M.; Galus, S.; Kowalska, J.; Gańko, K. The Effect of Hybrid Drying Methods on the Quality of Dried Carrot. Appl. Sci. 2022, 12, 10588. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Mujumdar, A.S. Recent Developments in High Efficient Freeze-Drying of Fruits and Vegetables Assisted by Microwave: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1357–1366. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Galus, S.; Karwacka, M.; Janowicz, M. The Sorption Properties, Structure and Shrinkage of Freeze-Dried Multi-Vegetable Snack Bars in the Aspect of the Environmental Water Activity. LWT 2022, 171, 114090. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.; Benning, R.; Haase, H. Effect of Different Drying Methods on Nutrient Quality of the Yellow Mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef]
- Kröncke, N.; Böschen, V.; Woyzichovski, J.; Demtröder, S.; Benning, R. Comparison of Suitable Drying Processes for Mealworms (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2018, 50, 20–25. [Google Scholar] [CrossRef]
- Selaledi, L.; Mabelebele, M. The Influence of Drying Methods on the Chemical Composition and Body Color of Yellow Mealworm (Tenebrio molitor L.). Insects 2021, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Vlahova-Vangelova, D.; Balev, D.; Kolev, N.; Dragoev, S.; Petkov, E.; Popova, T. Comparison of the Effect of Drying Treatments on the Physicochemical Parameters, Oxidative Stability, and Microbiological Status of Yellow Mealworm (Tenebrio molitor L.) Flours as an Alternative Protein Source. Agriculture 2024, 14, 436. [Google Scholar] [CrossRef]
- Cao, X.; Xu, W.; Islam, M.N. Impact of Different Drying Methods on Physicochemical Characteristics and Nutritional Compositions of Bee Larvae. Dry. Technol. 2024, 42, 1037–1050. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Severini, C. Understanding the Drying Kinetic and Hygroscopic Behaviour of Larvae of Yellow Mealworm (Tenebrio molitor) and the Effects on Their Quality. J. Insects Food Feed 2016, 2, 233–244. [Google Scholar] [CrossRef]
- Vanqa, N.; Mshayisa, V.V.; Basitere, M. Proximate, Physicochemical, Techno-Functional and Antioxidant Properties of Three Edible Insect (Gonimbrasia belina, Hermetia illucens and Macrotermes subhylanus) Flours. Foods 2022, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Krzyżaniak, M.; Aljewicz, M.; Bordiean, A.; Stolarski, M.J. Yellow Mealworm Composition after Convective and Freeze Drying—Preliminary Results. Agriculture 2022, 12, 149. [Google Scholar] [CrossRef]
- Tobolková, B.; Takáč, P.; Mangová, B.; Kozánek, M. A Comparative Study of Colour Characteristics of Thermally/Non-Thermally Treated Mealworm Larvae (Tenebrio molitor) by Means of UV/Vis Spectroscopy and Multivariate Analysis. J. Food Meas. Charact. 2021, 15, 3791–3799. [Google Scholar] [CrossRef]
- Ruszkowska, M.; Tańska, M.; Kowalczewski, P.Ł. Extruded Corn Snacks with Cricket Powder: Impact on Physical Parameters and Consumer Acceptance. Sustainability 2022, 14, 16578. [Google Scholar] [CrossRef]
- Seo, H.; Kim, H.R.; Cho, I.H. Aroma Characteristics of Raw and Cooked Tenebrio molitor Larvae (Mealworms). Food Sci. Anim. Resour. 2020, 40, 649–658. [Google Scholar] [CrossRef]
- Qiu, Y.; Bi, J.; Jin, X.; Wu, X.; Hu, L.; Chen, L. Investigation on the Rehydration Mechanism of Freeze-Dried and Hot-Air Dried Shiitake Mushrooms from Pores and Cell Wall Fibrous Material. Food Chem. 2022, 383, 132360. [Google Scholar] [CrossRef]
- Melis, R.; Braca, A.; Mulas, G.; Sanna, R.; Spada, S.; Serra, G.; Fadda, M.L.; Roggio, T.; Uzzau, S.; Anedda, R. Effect of Freezing and Drying Processes on the Molecular Traits of Edible Yellow Mealworm. Innov. Food Sci. Emerg. Technol. 2018, 48, 138–149. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Wang, X.; Chen, Z. Effect of Freezing on Soybean Protein Solution. Foods 2023, 12, 2650. [Google Scholar] [CrossRef] [PubMed]
- Perez-Santaescolastica, C.; de Pril, I.; van de Voorde, I.; Fraeye, I. Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods 2023, 12, 4090. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the Nutritional Value of Mysore Thorn Borer (Anoplophora chinensis) and Mealworm Larva (Tenebrio molitor): Amino Acid, Fatty Acid, and Element Profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef] [PubMed]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Duda, A.; Adamczak, J.; Chełmińska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and Nutritional/Textural Properties of Durum Wheat Pasta Enriched with Cricket Powder. Foods 2019, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Labuza, T.P. Water Activity and Food Preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007; p. 463. ISBN 978-0-429-19108-4. [Google Scholar]
- Son, Y.-J.; Choi, S.Y.; Hwang, I.-K.; Nho, C.W.; Kim, S.H. Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients? Foods 2020, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, T.-K.; Cha, J.Y.; Yong, H.I.; Kang, M.-C.; Jang, H.W.; Choi, Y.-S. Physicochemical Characteristics and Aroma Patterns of Oils Prepared from Edible Insects. LWT 2022, 167, 113888. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Tuccinardi, T.; Turchi, B.; Nuvoloni, R.; Paci, G. Effects of Different Blanching Treatments on Microbiological Profile and Quality of the Mealworm (Tenebrio molitor). J. Insects Food Feed 2019, 5, 225–234. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological Profile and Bioactive Properties of Insect Powders Used in Food and Feed Formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef]
- Kilar, J.; Kasprzyk, A. Fatty Acids and Nutraceutical Properties of Lipids in Fallow Deer (Dama Dama) Meat Produced in Organic and Conventional Farming Systems. Foods 2021, 10, 2290. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Kwiatkowska, K.; Baranowska-Wójcik, E.; Szwajgier, D.; Zaricka, E. Fatty Acid Profile, Antioxidative Status and Dietary Value of the Breast Muscle of Broiler Chickens Receiving Glycine-Zn Chelates. Anim. Prod. Sci. 2020, 60, 1095. [Google Scholar] [CrossRef]
- Ghafari, H.; Rezaeian, M.; Sharifi, S.D.; Khadem, A.A.; Afzalzadeh, A. Effects of Dietary Sesame Oil on Growth Performance and Fatty Acid Composition of Muscle and Tail Fat in Fattening Chaal Lambs. Anim. Feed Sci. Technol. 2016, 220, 216–225. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M. Mercury, Fatty Acids Content and Lipid Quality Indexes in Muscles of Freshwater and Marine Fish on the Polish Market. Risk Assessment of Fish Consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Nicula, C.; Peter, A.; Mihaly Cozmuta, L.; Nartea, A.; Kuhalskaya, A.; Pacetti, D.; Silvi, S.; Fiorini, D.; Pruteanu, L. Cricket and Yellow Mealworm Powders Promote Higher Bioaccessible Fractions of Mineral Elements in Functional Bread. J. Funct. Foods 2022, 99, 105310. [Google Scholar] [CrossRef]
- Piasecka, I.; Brzezińska, R.; Ostrowska-Ligęza, E.; Wiktor, A.; Górska, A. Ultrasound-Assisted Extraction of Cranberry Seed Oil: Food Waste Valorization Approach. Eur. Food Res. Technol. 2023, 249, 2763–2775. [Google Scholar] [CrossRef]
- Hurtado-Ribeira, R.; Hernández, D.M.; Villanueva-Bermejo, D.; García-Risco, M.R.; Hernández, M.D.; Vázquez, L.; Fornari, T.; Martin, D. The Interaction of Slaughtering, Drying, and Defatting Methods Differently Affects Oxidative Quality of the Fat from Black Soldier Fly (Hermetia illucens) Larvae. Insects 2023, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.; Barbosa, L.; Júnior, I.; Nascimento, T.; Dornelas, C.; Grillo, L. Lipase Activity in the Larval Midgut of Rhynchophorus Palmarum: Biochemical Characterization and the Effects of Reducing Agents. Insects 2017, 8, 100. [Google Scholar] [CrossRef]
- Mirzaei-Baktash, H.; Hamdami, N.; Torabi, P.; Fallah-Joshaqani, S.; Dalvi-Isfahan, M. Impact of Different Pretreatments on Drying Kinetics and Quality of Button Mushroom Slices Dried by Hot-Air or Electrohydrodynamic Drying. LWT 2022, 155, 112894. [Google Scholar] [CrossRef]
- Li, M.; Wang, B.; Wang, Y.; Liu, J.; Zhang, M. Evaluation of the Uniformity, Quality and Energy Cost of Four Types of Vegetables and Fruits after Pilot-Scale Pulse-Spouted Bed Microwave (915 MHz) Freeze-Drying. Dry. Technol. 2023, 41, 290–307. [Google Scholar] [CrossRef]
- Baek, M.; Kim, M.; Kwon, Y.; Hwang, J.; Goo, T.; Jun, M.; Yun, E. Effects of Processing Methods on Nutritional Composition and Antioxidant Activity of Mealworm (Tenebrio molitor) Larvae. Entomol. Res. 2019, 49, 284–293. [Google Scholar] [CrossRef]
- Gumul, D.; Oracz, J.; Kowalski, S.; Mikulec, A.; Skotnicka, M.; Karwowska, K.; Areczuk, A. Bioactive Compounds and Antioxidant Composition of Nut Bars with Addition of Various Edible Insect Flours. Molecules 2023, 28, 3556. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Effect of Drying Processes in the Chemical, Physico-Chemical, Techno-Functional and Antioxidant Properties of Flours Obtained from House Cricket (Acheta domesticus). Eur. Food Res. Technol. 2019, 245, 1451–1458. [Google Scholar] [CrossRef]
- Zambelli, A. Current Status of High Oleic Seed Oils in Food Processing. J. Am. Oil Chem. Soc. 2021, 98, 129–137. [Google Scholar] [CrossRef]
- Bogusz, R.; Pobiega, K.; Kowalczewski, P.Ł.; Onopiuk, A.; Szulc, K.; Wiktor, A.; Rybak, K.; Nowacka, M. Nutritional Value and Microbiological Aspects of Dried Yellow Mealworm (Tenebrio molitor L.) Larvae Pretreated with a Pulsed Electric Field. Appl. Sci. 2024, 14, 968. [Google Scholar] [CrossRef]
- Oz, F.; Aksu, M.I.; Turan, M. The Effects of Different Cooking Methods on Some Quality Criteria and Mineral Composition of Beef Steaks. J. Food Process. Preserv. 2017, 41, e13008. [Google Scholar] [CrossRef]
- Orkusz, A. Edible Insects versus Meat—Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef]
- Pistón, M.; Suárez, A.; Bühl, V.; Tissot, F.; Silva, J.; Panizzolo, L. Influence of Cooking Processes on Cu, Fe, Mn, Ni, and Zn Levels in Beef Cuts. J. Food Compos. Anal. 2020, 94, 103624. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Regnard, M.; Jessen, F.; Mohammadifar, M.A.; Sloth, J.J.; Petersen, H.O.; Ajalloueian, F.; Brouzes, C.M.C.; Fraihi, W.; Fallquist, H.; et al. Physico-Chemical and Colloidal Properties of Protein Extracted from Black Soldier Fly (Hermetia illucens) Larvae. Int. J. Biol. Macromol. 2021, 186, 714–723. [Google Scholar] [CrossRef]
- Alagappan, S.; Ma, S.; Nastasi, J.R.; Hoffman, L.C.; Cozzolino, D. Evaluating the Use of Vibrational Spectroscopy to Detect the Level of Adulteration of Cricket Powder in Plant Flours: The Effect of the Matrix. Sensors 2024, 24, 924. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Gómez, C.; Avendaño, C.; Harmsen, I.; Ortiz, D.; Ceballos, R.; Villamizar-Sarmiento, M.G.; Oyarzun-Ampuero, F.; Wacyk, J.; Valenzuela, C. House Fly (Musca domestica) Larvae Meal as an Ingredient with High Nutritional Value: Microencapsulation and Improvement of Organoleptic Characteristics. Food Res. Int. 2021, 145, 110423. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Kaveh, M.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods. Molecules 2022, 27, 2164. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Bakuła, T.; Bralewska-Piotrowicz, B.; Karczmarczyk, K.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Szyryńska, N.; Lewczuk, B. The Use of Chitin from the Molts of Mealworm (Tenebrio molitor) for the Removal of Anionic and Cationic Dyes from Aqueous Solutions. Materials 2023, 16, 545. [Google Scholar] [CrossRef]
- García-Gutiérrez, N.; Mellado-Carretero, J.; Bengoa, C.; Salvador, A.; Sanz, T.; Wang, J.; Ferrando, M.; Güell, C.; de Lamo-Castellví, S. ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder. Foods 2021, 10, 1806. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. A Comparative Study of Mid-Infrared, UV–Visible and Fluorescence Spectroscopy in Combination with Chemometrics for the Detection of Adulteration of Fresh Olive Oils with Old Olive Oils. Food Control 2019, 105, 209–218. [Google Scholar] [CrossRef]
- Przybył, K.; Koszela, K.; Adamski, F.; Samborska, K.; Walkowiak, K.; Polarczyk, M. Deep and Machine Learning Using SEM, FTIR, and Texture Analysis to Detect Polysaccharide in Raspberry Powders. Sensors 2021, 21, 5823. [Google Scholar] [CrossRef] [PubMed]
- Mshayisa, V.V.; Van Wyk, J.; Zozo, B. Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates. Foods 2022, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Emara, A.M.; Gan, X.; Li, H. Effects of Malondialdehyde as a Byproduct of Lipid Oxidation on Protein Oxidation in Rabbit Meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef]
- Broekman, H.; Verhoeckx, K.C.; den Hartog Jager, C.F.; Kruizinga, A.G.; Pronk-Kleinjan, M.; Remington, B.C.; Bruijnzeel-Koomen, C.A.; Houben, G.F.; Knulst, A.C. Majority of Shrimp-Allergic Patients Are Allergic to Mealworm. J. Allergy Clin. Immunol. 2016, 137, 1261–1263. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Frozen and Dried Formulations from Whole Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06778. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Moura, M.B.M.V.; Teixeira, C.S.S.; Costa, J.; Mafra, I. Monitoring Yellow Mealworm (Tenebrio molitor) as a Potential Novel Allergenic Food: Effect of Food Processing and Matrix. Nutrients 2023, 15, 482. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.J.R.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. An Overview on Fish and Shellfish Allergens and Current Methods of Detection. Food Agric. Immunol. 2015, 26, 848–869. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Kuang, H.; Tang, C.; Song, J. Edible Insects as Ingredients in Food Products: Nutrition, Functional Properties, Allergenicity of Insect Proteins, and Processing Modifications. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, R.; Pobiega, K.; Rybak, K.; Wiktor, A.; Parniakov, O.; Smetana, S.; Nowacka, M. The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) Larvae. Appl. Sci. 2023, 13, 10251. [Google Scholar] [CrossRef]
- Nyangena, D.N.; Mutungi, C.; Imathiu, S.; Kinyuru, J.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Effects of Traditional Processing Techniques on the Nutritional and Microbiological Quality of Four Edible Insect Species Used for Food and Feed in East Africa. Foods 2020, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2021/882 of 1 June 2021 Authorising the Placing on the Market of Dried Tenebrio molitor Larva as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Im. Off. J. Eur. Union 2021, 50, 16–20. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2022, 30, 10–16. [Google Scholar]
- Vandeweyer, D.; Lenaerts, S.; Callens, A.; Van Campenhout, L. Effect of Blanching Followed by Refrigerated Storage or Industrial Microwave Drying on the Microbial Load of Yellow Mealworm Larvae (Tenebrio molitor). Food Control 2017, 71, 311–314. [Google Scholar] [CrossRef]
- AOAC Official Method 994.12 Amino Acids in Feeds. In Official Methods of Analysis of AOAC International; Oxford University Press: New York, NY, USA, 2023.
- Tomczak, A.; Zielińska-Dawidziak, M.; Piasecka-Kwiatkowska, D.; Lampart-Szczapa, E. Blue Lupine Seeds Protein Content and Amino Acids Composition. Plant Soil Environ. 2018, 64, 147–155. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty Acids and Sterols Composition, and Antioxidant Activity of Oils Extracted from Plant Seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef] [PubMed]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- Bryś, J.; Flores, L.F.V.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC Methods to Characterize Human Milk Fat Substitutes Obtained from Lard and Milk Thistle Oil Mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- Ziarno, M.; Bryś, J.; Parzyszek, M.; Veber, A. Effect of Lactic Acid Bacteria on the Lipid Profile of Bean-Based Plant Substitute of Fermented Milk. Microorganisms 2020, 8, 1348. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, W.; Kmiecik, D.; Baranowska, H.M.; Staroszczyk, H.; Sommer, A.; Kowalczewski, P.Ł. Chemical Characteristics and Thermal Oxidative Stability of Novel Cold-Pressed Oil Blends: GC, LF NMR, and DSC Studies. Foods 2023, 12, 2660. [Google Scholar] [CrossRef]
- Kurdi, P.; Chaowiwat, P.; Weston, J.; Hansawasdi, C. Studies on Microbial Quality, Protein Yield, and Antioxidant Properties of Some Frozen Edible Insects. Int. J. Food Sci. 2021, 2021, 5580976. [Google Scholar] [CrossRef]
| Tm_70CD | Tm_90CD | Tm_FD | |
|---|---|---|---|
| Drying Time to MR = 0.02 (min) | 175 ± 7 b 1 | 120 ± 7 a | 2235 ± 21 c |
| Water Activity (-) | 0.225 ± 0.005 c | 0.220 ± 0.008 b | 0.068 ± 0.002 a |
| L* | 26.5 ± 0.5 a | 34.6 ± 0.7 b | 43.7 ± 0.7 c |
| a* | 4.9 ± 0.1 a | 6.6 ± 0.1 b | 8.3 ± 0.2 c |
| b* | 11.7 ± 0.4 a | 16.9 ± 0.3 b | 23.6 ± 0.5 c |
| Browning Index (BI) | 70.5 ± 4.5 a | 79.7 ± 3.6 b | 88.8 ± 4.5 c |
| Photo |  |  |  |
| Component | Tm_70CD | Tm_90CD | Tm_FD |
|---|---|---|---|
| Moisture (%) | 3.10 ± 0.05 b 1 | 3.24 ± 0.02 b | 1.57 ± 0.31 a |
| Protein (g/100 g d.m.) | 41.14 ± 0.18 b | 42.14 ± 0.80 b | 37.57 ± 0.05 a |
| Fat (g/100 g d.m.) | 36.62 ± 1.06 a | 35.65 ± 1.14 a | 35.12 ± 0.30 a |
| Ash (g/100 g d.m.) | 3.42 ± 0.04 a | 3.39 ± 0.02 a | 3.34 ± 0.04 a |
| Amino Acid (mg/g of Protein) | Tm_70CD | Tm_90CD | Tm_FD |
|---|---|---|---|
| Essential amino acids (EAAs) | |||
| Phenylalanine (Phe) | 35.40 ± 0.83 a 1 | 41.60 ± 3.41 b | 37.10 ± 2.86 ab |
| Histidine (His) | 42.87 ± 2.50 a | 45.44 ± 2.25 a | 43.44 ± 4.26 a |
| Isoleucine (Ile) | 36.62 ± 2.02 a | 39.89 ± 0.42 a | 39.74 ± 5.34 a |
| Leucine (Leu) | 69.42 ± 2.28 ab | 75.13 ± 1.10 b | 64.40 ± 5.85 a |
| Lysine (Lys) | 54.29 ± 2.12 a | 60.73 ± 2.30 a | 59.72 ± 6.86 a |
| Valine (Val) | 52.88 ± 1.82 a | 58.72 ± 1.57 b | 54.14 ± 3.75 ab |
| Methionine (Met) | 4.74 ± 1.38 a | 5.78 ± 0.47 a | 5.13 ± 1.03 a |
| Threonine (Thr) | 38.68 ± 1.26 a | 42.43 ± 0.54 a | 41.44 ± 3.53 a |
| Total EAAs | 334.90 ± 10.14 a | 369.71 ± 9.08 b | 345.10 ± 24.23 ab |
| Non-essential amino acids (NEAAs) | |||
| Asparagine (Asp) | 81.59 ± 3.65 a | 86.51 ± 3.94 a | 83.92 ± 3.94 a |
| Serine (Ser) | 47.27 ± 0.29 a | 51.01 ± 2.24 b | 49.11 ± 2.16 ab |
| Glutamic acid (Glu) | 113.81 ± 5.02 a | 115.09 ± 5.81 a | 111.08 ± 4.07 a |
| Proline (Pro) | 78.96 ± 2.24 a | 88.71 ± 1.60 b | 80.60 ± 5.50 a |
| Glycine (Gly) | 44.95 ± 1.07 a | 49.43 ± 1.96 b | 47.74 ± 2.90 ab |
| Alanine (Ala) | 70.14 ± 2.90 ab | 76.27 ± 3.08 b | 67.69 ± 4.28 a |
| Cysteine (Cys) | 5.97 ± 1.65 a | 6.13 ± 0.62 a | 4.14 ± 0.91 a |
| Tyrosine (Tyr) | 1.93 ± 0.72 a | 5.35 ± 2.37 b | 4.80 ± 0.41 b |
| Arginine (Arg) | 49.92 ± 0.92 a | 56.44 ± 3.86 b | 55.01 ± 1.48 b |
| Total NEAAs | 494.53 ± 12.69 a | 534.95 ± 8.26 b | 504.09 ± 10.61 a |
| Fatty Acid (%) | Tm_70CD | Tm_90CD | Tm_FD |
|---|---|---|---|
| Caprylic acid (C8:0) | 0.08 ± 0.00 b 1 | nd | 0.02 ± 0.00 a |
| Capric acid (C10:0) | 0.08 ± 0.00 b | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| Lauric acid (C12:0) | 0.77 ± 0.00 b | 0.59 ± 0.03 a | 0.52 ± 0.10 a |
| Tridecanoic acid (C13:0) | 0.05 ± 0.01 a | 0.09 ± 0.00 b | 0.07 ± 0.02 a |
| Myristic acid (C14:0) | 3.60 ± 0.08 a | 4.82 ± 0.14 a | 4.38 ± 1.27 a |
| Pentadecanoic acid (C15:0) | 0.07 ± 0.00 a | 0.09 ± 0.00 b | 0.08 ± 0.01 ab |
| Palmitic acid (C16:0) | 13.48 ± 0.34 a | 15.06 ± 0.08 b | 15.36 ± 1.25 b |
| Heptadecanoic acid (C17:0) | 0.25 ± 0.03 a | 0.29 ± 0.00 b | 0.23 ± 0.01 a |
| Stearic acid (C18:0) | 3.08 ± 0.00 a | 3.04 ± 0.03 a | 3.42 ± 0.16 b |
| Arachidic acid (C20:0) | 0.10 ± 0.02 a | 0.10 ± 0.00 a | 0.12 ± 0.04 a |
| Total SFAs | 21.55 ± 0.42 a | 24.10 ± 0.11 ab | 24.21 ± 2.47 b |
| Myristoleic acid (C14:1) | 0.18 ± 0.00 a | 0.25 ± 0.01 b | 0.20 ± 0.06 ab |
| Palmitoleic acid (C16:1) | 2.19 ± 0.01 a | 2.31 ± 0.03 a | 2.20 ± 0.18 a |
| cis-10-Heptadecenoic acid (C17:1) | 0.12 ± 0.01 ab | 0.12 ± 0.00 b | 0.11 ± 0.00 a |
| Oleic acid (C18:1 n-9c) | 52.27 ± 0.50 ab | 49.91 ± 0.13 a | 53.42 ± 2.38 b |
| cis-11-Eicosenoic acid (C20:1 n-9c) | 0.14 ± 0.01 ab | 0.16 ± 0.01 b | 0.13 ± 0.01 a |
| Total MUFAs | 54.89 ± 0.52 ab | 52.74 ± 0.15 a | 56.05 ± 2.14 b |
| Linoleic acid (C18:2 n-6c) | 21.15 ± 0.02 b | 20.68 ± 0.02 b | 17.52 ± 0.58 a |
| α-Linolenic acid (C18:3 n-3) | 0.83 ± 0.01 b | 0.86 ± 0.01 c | 0.63 ± 0.01 a |
| Total PUFAs | 21.97 ± 0.03 b | 21.53 ± 0.01 b | 18.15 ± 0.59 a |
| Total unidentified fatty acids | 1.57 ± 0.16 a | 1.64 ± 0.05 a | 1.60 ± 0.26 a |
| Health Indices | |||
| N-6/n-3 | 25.63 ± 0.19 b | 24.18 ± 0.22 a | 27.81 ± 0.30 c |
| Atherogenicity index (AI) | 0.37 ± 0.01 a | 0.47 ± 0.01 a | 0.45 ± 0.10 a |
| Thrombogenicity index (TI) | 0.50 ± 0.01 a | 0.58 ± 0.00 ab | 0.60 ± 0.08 b |
| Hypercholesterolemic ratio (HH) | 4.35 ± 0.14 b | 3.59 ± 0.02 a | 3.67 ± 0.62 a |
| Fatty Acid (%) | Tm_70CD | Tm_90CD | Tm_FD | |
|---|---|---|---|---|
| Myristic acid (C14:0) | sn-1,3 | 4.73 ± 0.02 a 1 | 6.73 ± 0.03 c | 6.09 ± 0.04 b |
| sn-2 | 1.35 ± 0.04 b | 1.00 ± 0.02 a | 0.96 ± 0.08 a | |
| sn-2* | 12.50 ± 0.39 b | 6.88 ± 0.44 a | 7.31 ± 0.65 a | |
| Palmitic acid (C16:0) | sn-1,3 | 17.83 ± 0.09 a | 21.02 ± 0.01 c | 20.46 ± 0.03 b |
| sn-2 | 4.79 ± 0.18 b | 3.14 ± 0.02 a | 5.15 ± 0.06 c | |
| sn-2* | 11.83 ± 0.44 c | 6.94 ± 0.05 a | 11.17 ± 0.14 b | |
| Stearic acid (C18:0) | sn-1,3 | 3.50 ± 0.03 a | 3.91 ± 0.02 b | 3.92 ± 0.02 b |
| sn-2 | 2.24 ± 0.06 b | 1.30 ± 0.04 a | 2.42 ± 0.05 c | |
| sn-2* | 24.24 ± 0.61 b | 14.25 ± 0.47 a | 23.54 ± 0.48 b | |
| Oleic acid (C18:1 n-9c) | sn-1,3 | 47.47 ± 0.07 b | 42.99 ± 0.59 a | 47.24 ± 0.76 b |
| sn-2 | 61.86 ± 0.13 a | 63.74 ± 1.18 a | 65.77 ± 1.52 b | |
| sn-2* | 39.45 ± 0.09 a | 42.57 ± 0.79 c | 41.04 ± 0.95 b | |
| Linoleic acid (C18:2 n-6c) | sn-1,3 | 18.75 ± 0.14 c | 16.56 ± 0.22 b | 14.07 ± 0.08 a |
| sn-2 | 25.95 ± 0.29 b | 28.91 ± 0.43 c | 24.42 ± 0.16 a | |
| sn-2* | 40.90 ± 0.46 a | 46.60 ± 0.70 b | 46.46 ± 0.30 b |
| Tm_70CD | Tm_90CD | Tm_FD | |
|---|---|---|---|
| Oxidative Stability (min) | 6.56 ± 0.20 c 1 | 4.76 ± 0.01 b | 1.73 ± 0.07 a |
| Acid Value (mg KOH/g) | 16.72 ± 0.94 b | 4.65 ± 0.01 a | 3.76 ± 0.10 a |
| Peroxide Value (meq O2/kg) | 6.55 ± 0.93 b | 9.51 ± 1.32 c | <0.01 a |
| Mineral (mg/100 g d.m.) | Tm_70CD | Tm_90CD | Tm_FD | PRI/AI (mg/day) | %PRI/AI |
|---|---|---|---|---|---|
| Potassium (K) | 1317.25 ± 12.40 b | 1334.22 ± 33.92 b | 1173.52 ± 14.40 a | 3500 | 33.53–38.12 |
| Magnesium (Mg) | 305.43 ± 7.36 b | 291.75 ± 7.44 b | 260.79 ± 5.12 a | 300 | 86.93–101.81 |
| Sodium (Na) | 169.72 ± 2.13 b 1 | 174.25 ± 2.08 b | 153.26 ± 3.01 a | 1500 | 10.22–11.62 |
| Calcium (Ca) | 73.16 ± 9.10 a | 69.13 ± 12.36 a | 65.45 ± 11.09 a | 950 | 6.89–7.70 |
| Zinc (Zn) | 18.99 ± 0.40 b | 20.04 ± 0.56 b | 17.16 ± 0.34 a | 9.3 | 184.56–215.51 |
| Iron (Fe) | 5.76 ± 0.13 b | 6.31 ± 0.05 c | 4.94 ± 0.03 a | 11 | 44.92–57.33 |
| Copper (Cu) | 2.60 ± 0.06 b | 2.66 ± 0.05 b | 2.32 ± 0.04 a | 1.3 | 178.58–204.54 |
| Manganese (Mn) | 1.35 ± 0.04 b | 1.35 ± 0.02 b | 1.19 ± 0.04 a | 3 | 39.58–45.13 |
| Selenium (Se) | 0.28 ± 0.00 a | 0.27 ± 0.00 a | 0.28 ± 0.00 a | 0.07 | 385.29–397.48 |
| Molybdenum (Mo) | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.09 ± 0.00 a | 0.65 | 14.45–15.62 |
| Aluminum (Al) | 0.26 ± 0.01 b | 0.21 ± 0.01 a | 0.31 ± 0.00 c | – | – |
| Barium (Ba) | 0.14 ± 0.00 b | 0.15 ± 0.00 c | 0.12 ± 0.00 a | – | – |
| Boron (B) | 0.10 ± 0.00 b | 0.09 ± 0.00 b | 0.08 ± 0.01 a | – | – |
| Chromium (Cr) | 0.04 ± 0.00 b | 0.04 ± 0.00 a | 0.05 ± 0.00 c | – | – |
| Nickel (Ni) | 0.02 ± 0.00 a | 0.03 ± 0.00 b | 0.03 ± 0.00 ab | – | – |
| Cobalt (Co) | 0.00 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a | – | – |
| Arsenic (As) | 1.08 ± 0.02 b | 1.00 ± 0.02 a | 0.99 ± 0.02 a | – | – |
| Cadmium (Cd) | 0.01 ± 0.00 b | 0.01 ± 0.00 c | 0.01 ± 0.00 a | – | – |
| Allergen (ppb) | Tm_70CD | Tm_90CD | Tm_FD |
|---|---|---|---|
| Crustaceans | 4047.24 ± 19.80 a 1 | 4818.91 ± 48.54 b | 5274.72 ± 29.83 c |
| Mollusks | 2512.49 ± 32.02 c | 2335.48 ± 42.83 b | 1952.59 ± 64.31 a |
| Microorganism (log CFU/g) | Tm_70CD | Tm_90CD | Tm_FD |
|---|---|---|---|
| Total viable count | 2.01 ± 0.12 | 2.21 ± 0.16 | 2.10 ± 0.06 |
| Total yeast and mold count | 2.29 ± 0.08 | 1.49 ± 0.16 | 1.86 ± 0.07 |
| Listeria monocytogenes | ≤1.00 | ≤1.00 | ≤1.00 |
| Enterobacteriaceae | ≤1.00 | ≤1.00 | ≤1.00 |
| Escherichia coli | ≤1.00 | ≤1.00 | ≤1.00 |
| Staphylococcus aureus | ≤1.00 | ≤1.00 | ≤1.00 |
| Aerobic spore-forming bacteria | 1.70 ± 0.16 | 1.56 ± 0.25 | 1.74 ± 0.10 |
| Anaerobic spore-forming bacteria | ≤1.00 | ≤1.00 | ≤1.00 |
| Presence of Salmonella | absence in 25 g | absence in 25 g | absence in 25 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusz, R.; Bryś, J.; Onopiuk, A.; Pobiega, K.; Tomczak, A.; Kowalczewski, P.Ł.; Rybak, K.; Nowacka, M. The Impact of Drying Methods on the Quality of Blanched Yellow Mealworm (Tenebrio molitor L.) Larvae. Molecules 2024, 29, 3679. https://doi.org/10.3390/molecules29153679
Bogusz R, Bryś J, Onopiuk A, Pobiega K, Tomczak A, Kowalczewski PŁ, Rybak K, Nowacka M. The Impact of Drying Methods on the Quality of Blanched Yellow Mealworm (Tenebrio molitor L.) Larvae. Molecules. 2024; 29(15):3679. https://doi.org/10.3390/molecules29153679
Chicago/Turabian StyleBogusz, Radosław, Joanna Bryś, Anna Onopiuk, Katarzyna Pobiega, Aneta Tomczak, Przemysław Łukasz Kowalczewski, Katarzyna Rybak, and Małgorzata Nowacka. 2024. "The Impact of Drying Methods on the Quality of Blanched Yellow Mealworm (Tenebrio molitor L.) Larvae" Molecules 29, no. 15: 3679. https://doi.org/10.3390/molecules29153679
APA StyleBogusz, R., Bryś, J., Onopiuk, A., Pobiega, K., Tomczak, A., Kowalczewski, P. Ł., Rybak, K., & Nowacka, M. (2024). The Impact of Drying Methods on the Quality of Blanched Yellow Mealworm (Tenebrio molitor L.) Larvae. Molecules, 29(15), 3679. https://doi.org/10.3390/molecules29153679










