Discrimination and Quantification of Cotton and Polyester Textile Samples Using Near-Infrared and Mid-Infrared Spectroscopies
Abstract
1. Introduction
2. Results
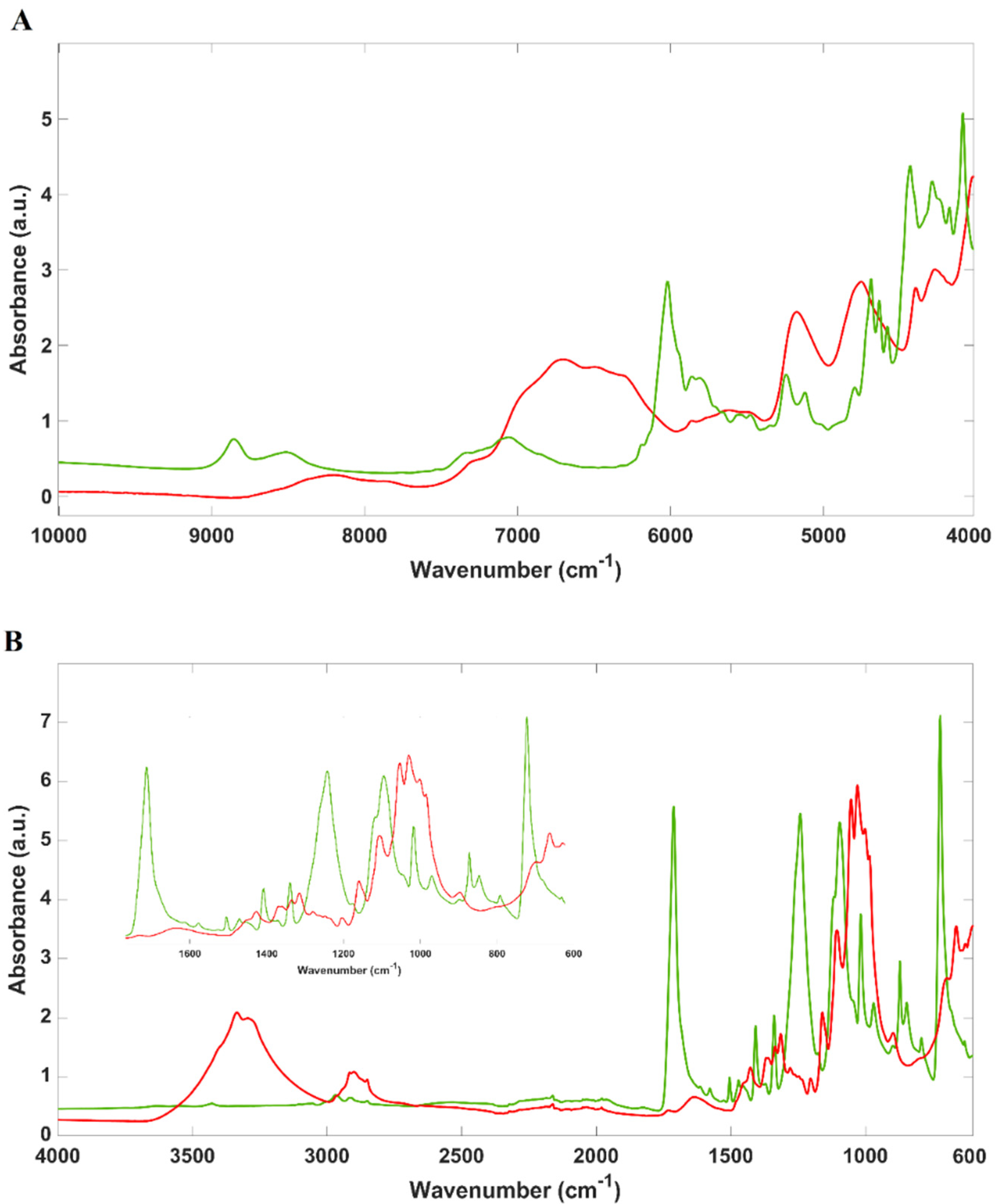
2.1. Spectral Overview
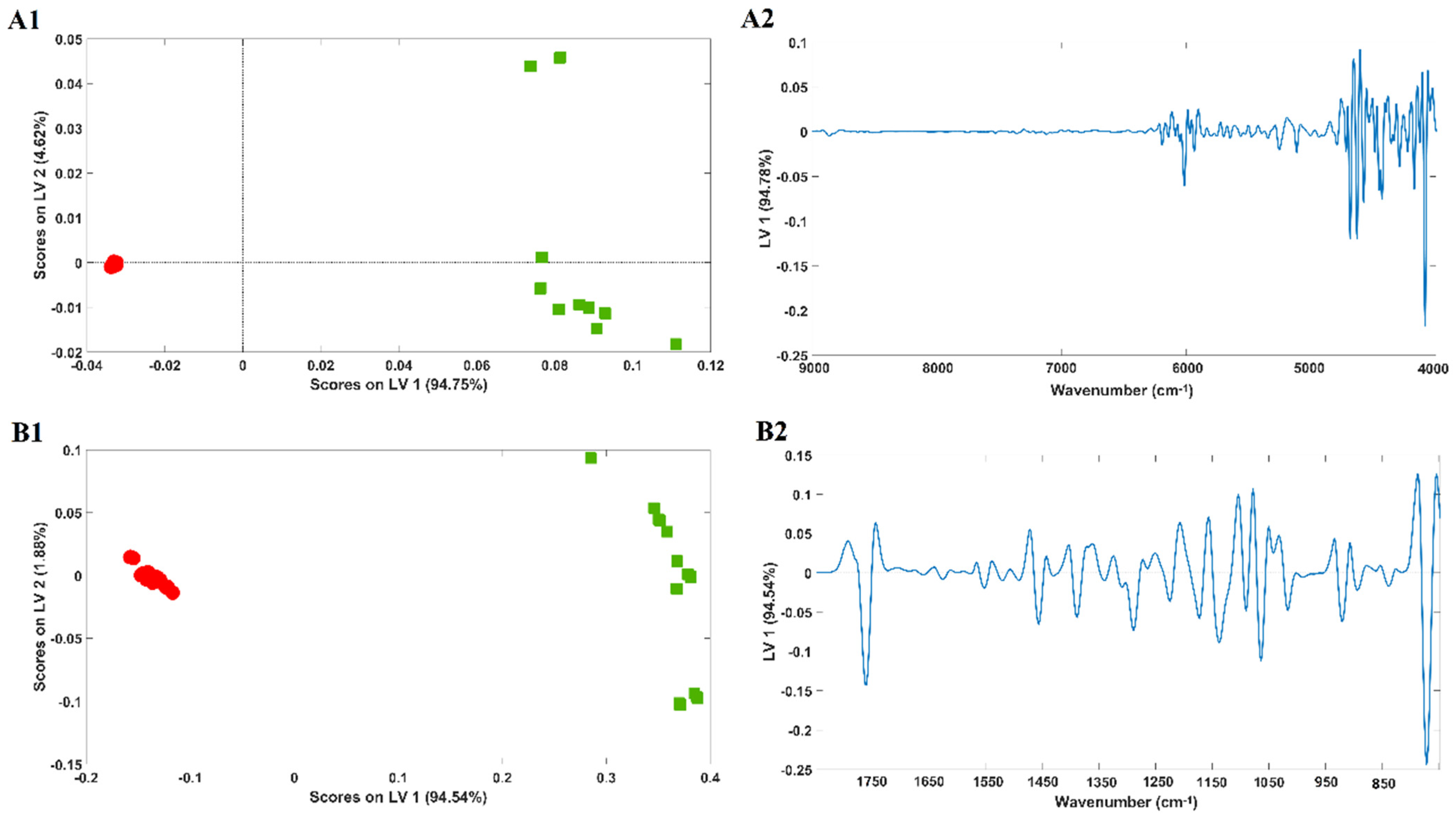
2.2. Cotton/Polyester Sample Discrimination
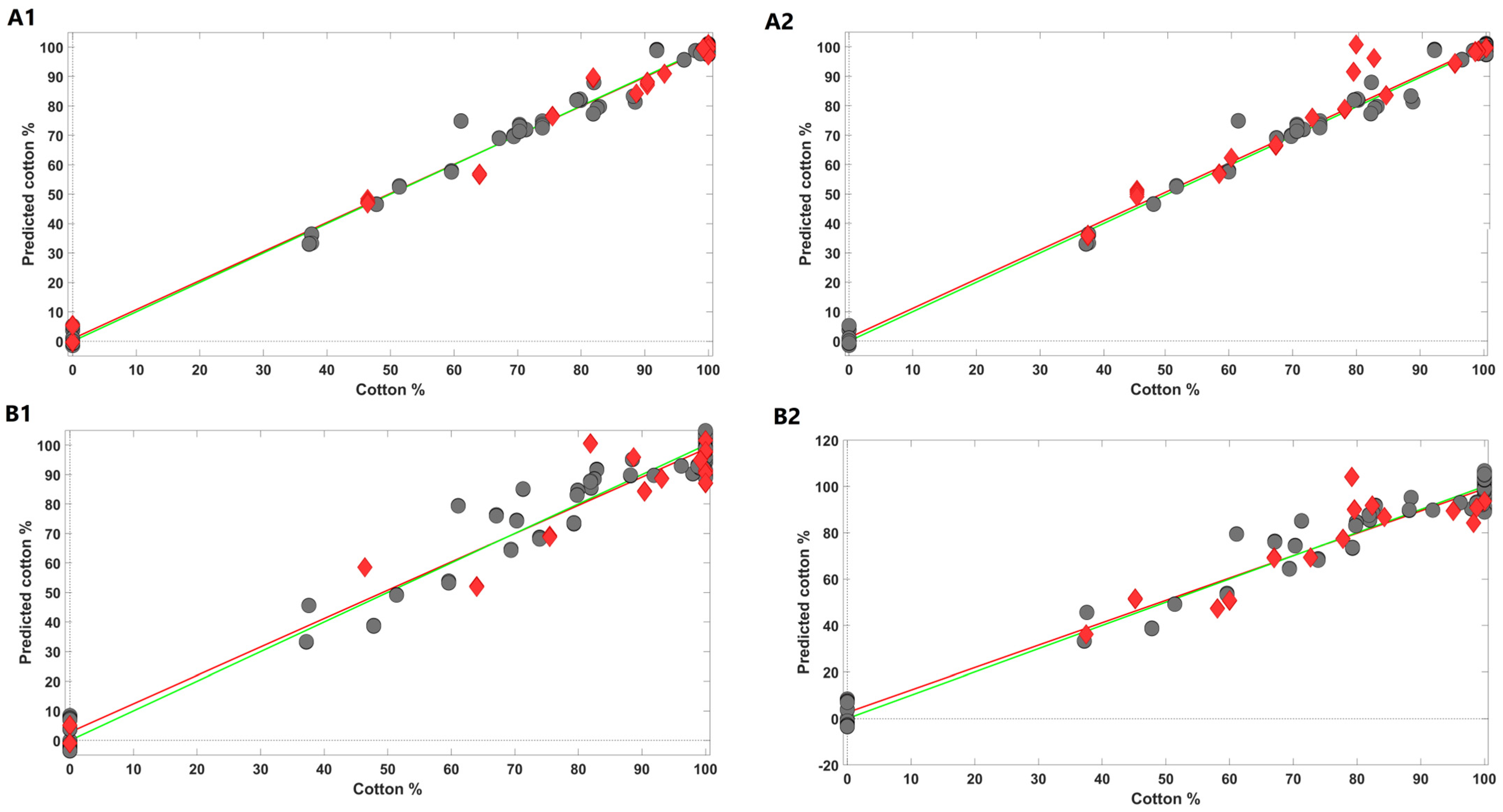
2.3. Cotton Sample Content Estimates
3. Discussion
4. Materials and Methods
4.1. Textile Samples
4.2. Near-Infrared Spectrum Acquisition
4.3. Mid-Infrared Spectrum Acquisition
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downey, N.W.; Myles, J. Near-infrared spectroscopy in the food industry. Food Technol. 1989, 43, 69–76. [Google Scholar]
- Páscoa, R.N.M.J.; Silvana, M.; Lopes, J.A.; Sousa, C. Citrus species and hybrids depicted by near- and mid-infrared spectroscopy. J. Sci. Food Agric. 2018, 98, 3953–3961. [Google Scholar] [CrossRef] [PubMed]
- Burggraeve, P.; Monteux, L.; Saeys, M. Visible and near infrared spectroscopy in archaeometry. Anal. Bioanal. Chem. 2007, 387, 1489–1508. [Google Scholar]
- Ciobica, I.; Len, A.S.; Pavel, I.E. Characterization of Flax Fibers by ATR-FTIR Spectroscopy. Cellul. Chem. Technol. 2018, 52, 683–693. [Google Scholar]
- Sun, D.; Sun, L.; Mao, X.; Liu, H. Near-Infrared Spectroscopy Prediction of Cotton Fiber Quality Parameters. J. Nat. Fibers 2015, 12, 321–330. [Google Scholar]
- Wang, H.; Siddiqui, M.Q.; Memon, H. Physical Structure, Properties and Quality of Cotton. In Cotton Science and Processing Technology; Textile Science and Clothing Technology; Wang, H., Memon, H., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Park, S.H.; Kim, H.J.; Kim, J.H. Moisture Management Properties of Cotton Fabrics Treated with Chitosan Oligosaccharide. Pigment Resin Technol. 2007, 36, 202–206. [Google Scholar]
- Joo, J.H.; Choe, E.K.; Kim, S.H. A Review of Recent Developments in Sustainable Polyesters. Polym. Int. 2020, 99, 2844–2853. [Google Scholar]
- Li, X.; Xin, J.H. Wrinkle Resistance of Polyester Fabrics: Chemical and Physical Aspects. Text. Res. J. 2007, 77, 1044–1051. [Google Scholar]
- McDonald, R. Polyester/Cotton Blended Fabrics–Care and Cleaning. Int. J. Cloth. Sci. Technol. 2007, 19, 391–399. [Google Scholar]
- Peets, P.; Leito, I.; Pelt, J.; Vahur, S. Identification and classification of textile fibres using ATR-FT-IR spectroscopy with chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 175–181. [Google Scholar] [CrossRef]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR spectroscopy as a viable option for textile fibre identification. Herit. Sci. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Riba, J.-R.; Cantero, R.; Puig, R. Classification of textile samples using data fusion combining near- and mid-infrared spectral information. Polymers 2022, 14, 3073. [Google Scholar] [CrossRef] [PubMed]
- Cleve, E.; Bach, E.; Schollmeyer, E. Using chemometric methods and NIR spectrophotometry in the textile industry. Anal. Chim. Acta 2000, 420, 163–167. [Google Scholar] [CrossRef]
- Zhou, C.; Han, G.; Via, B.; Song, Y.; Gao, S.; Jiang, W. Rapid identification of fibers from different waste fabrics using the near-infrared spectroscopy technique. Text. Res. J. 2019, 89, 3610–3616. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, M.; Sun, Y. Classification of textiles fabrics by use of spectroscopy-based pattern recognition methods. Spectrosc. Lett. 2016, 49, 96–102. [Google Scholar] [CrossRef]
- Ruckebusch, C.; Orhan, F.; Durand, A.; Boubellouta, T.; Huvenne, J. Quantitative Analysis of Cotton-Polyester Textile Blends from Near-Infrared Spectra. Appl. Spectrosc. 2006, 60, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, M.-X.; Sun, Y.-Z. Variables selection for quantitative determination of cotton content in textile blends by near infrared spectroscopy. Infrared Phys. Technol. 2016, 77, 65–72. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Liu, W.; Yang, X.; Luo, J. Least-squares support vector machine and successive projection algorithm for quantitative analysis of cotton-polyester textile by near infrared spectroscopy. J. Near Infrared Spectrosc. 2018, 26, 34–43. [Google Scholar] [CrossRef]
- Makela, M.; Rissanen, M.; Sixta, H. Machine vision estimates the polyester content in recyclable waste textiles. Resour. Conserv. Recycl. 2020, 161, 105007. [Google Scholar] [CrossRef]
- Dochia, M.; Roskwitalski, Z. Handbook of Natural Fibres: Types, Properties and Factors Affecting Breeding and Cultivation; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Wei, S.C.; Ding, X.; Li, W.X. Model establishment and validation of waste polyester fiber products based on near infrared quantitative analysis. Text. Res. J. 2018, 39, 63–66. [Google Scholar]
- van der Ploeg, R.J. A note on the near-infrared spectroscopic analysis of polyesters. Polym. Test. 2001, 20, 277–282. [Google Scholar]
- Yousefi, M.; Mohseni-Shahri, A.R.; Arabzadeh, H.; Razavi-Nia, S.; Karimi-Alavijeh, M. Near-infrared (NIR) spectroscopy for the determination of poly(ethylene terephthalate) (PET) content in PET/cotton blend fabrics. Fibers Polym. 2001, 12, 1424–1430. [Google Scholar]
- Cai, Y.; Liu, J.; Feng, X.; Sun, D. Characterization of Bacterial Cellulose by Fourier Transform Infrared Spectroscopy. J. Mol. Struct. 2007, 844, 28–34. [Google Scholar] [CrossRef]
- Colom, X.; Bras, J.; Fort, R. Determination of Cellulose Crystallinity by Near Infrared Spectroscopy. Carbohydr. Res. 2003, 343, 11–19. [Google Scholar]
- Bhattacharya, S.S.; Chaudhari, S.B. Study on structural, mechanical and functional properties of polyester silica nanocomposite fabric. Int. J. Pure Appl. Sci. Technol. 2014, 21, 43–52. [Google Scholar]
- Liu, Y.; Chang, S. Comprehensive analysis of cotton fiber infrared maturity distribution and its relation to fiber HVI and AFIS properties. Fiber Polym. 2024, 25, 1127–1136. [Google Scholar] [CrossRef]
- Parida, D.; Sangtani, R.; Nogueira, R.; Bala, K. Scrutinizing the chemical and morphological alterations of microfibers released from household washing machines under varying temperature conditions. Clean Soil Air Water 2024, 2300285. [Google Scholar] [CrossRef]
- Prajapati, S.; Beal, M.; Maley, J.; Brinkmann, M. Qualitative and quantitative microplastics and microfiber contamination in effluents of the city of Saskatoon wastewater treatment plant. Environ. Sci. Pollut. Res. 2021, 28, 32545–32553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, W.; Wei, Z. Qualitative classification of waste textiles based on near infrared spectroscopy and the convolutional network. Text. Res. J. 2020, 90, 1057–1066. [Google Scholar] [CrossRef]
- Li, W.; Wei, Z.; Liu, Z.; Du, Y.; Zheng, J.; Wang, H.; Zhang, S. Qualitative identification of waste textiles based on near-infrared spectroscopy and the back propagation artificial neural network. Text. Res. J. 2021, 91, 2459–2467. [Google Scholar] [CrossRef]
- Du, W.; Zheng, J.; Li, W.; Liu, Z.; Wang, H.; Han, X. Efficient recognition and automatic sorting technology of waste textiles based on online near infrared spectroscopy and convolutional neural network. Resour. Conserv. Recycl. 2022, 180, 106157. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis; Springer: New York, NY, USA, 1986. [Google Scholar]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Naes, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2002. [Google Scholar]
- Steinier, J.; Termonia, Y.; Deltour, J. Smoothing and differentiation of data by simplified least squares procedure. Anal. Chem. 1972, 36, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Grosso, F.; Meirinhos-Soares, L.; Peixe, L.; Lopes, J. Identification of carbapenem-resistant Acinetobacter baumannii clones using infrared spectroscopy. J. Biophotonics 2014, 7, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Rambo, M.K.D.; Amorim, E.P.; Ferreira, M.M.C. Potential of visible-near infrared spectroscopy combines with chemometrics for analysis of some constituents of coffee and banana residues. Anal. Chim. Acta 2013, 775, 41–49. [Google Scholar] [CrossRef]
- Tekin, Y.; Tumsavas, Z.; Mouazen, A.M. Effect of Moisture content on prediction of organic carbon and pH using visible and near-infrared spectroscopy. Soil Sci. Soc. Am. J. 2011, 76, 188–198. [Google Scholar] [CrossRef]


| Sample Composition | Set | Figures-of-Merit | Near-IR | Mid-IR |
|---|---|---|---|---|
| Cotton//PES | Cal | RMSEC | 3.0 | 5.8 |
| R2C | 0.99 | 0.97 | ||
| RMSECV | 3.2 | 6.5 | ||
| R2CV | 0.99 | 0.96 | ||
| Pred 1 | RMSEP | 3.6 | 6.5 | |
| R2P | 0.99 | 0.97 | ||
| RER | 27.9 | 15.4 | ||
| RPD | 9.17 | 5.08 | ||
| Cotton//PES//Other | Pred 2 | RMSEP | 7.8 | 8.0 |
| R2P | 0.90 | 0.82 |
| Nº Samples | Type of Sample (Nº) | Composition (% Range) |
|---|---|---|
| 26 | Raw (7) Yarn (12) Woven fabric (7) | 100% cotton |
| 10 | Raw (3) Yarn (3) Knitted fabric (3) Woven fabric (1) | 100% polyester |
| 40 | Raw (3) Yarn (19) Knitted fabric (11) Woven fabric (7) | 99.7–37.2% cotton 0.3–62.8% polyester |
| 8 | Raw (1) Yarn (5) Woven fabric (2) | 98.3–37.5% cotton 54.3–0.3% polyester 19.8–0.4% other fibre (linen, viscose, elastane, and polyamide) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz, M.L.; Sousa, C. Discrimination and Quantification of Cotton and Polyester Textile Samples Using Near-Infrared and Mid-Infrared Spectroscopies. Molecules 2024, 29, 3667. https://doi.org/10.3390/molecules29153667
Paz ML, Sousa C. Discrimination and Quantification of Cotton and Polyester Textile Samples Using Near-Infrared and Mid-Infrared Spectroscopies. Molecules. 2024; 29(15):3667. https://doi.org/10.3390/molecules29153667
Chicago/Turabian StylePaz, Maria Luís, and Clara Sousa. 2024. "Discrimination and Quantification of Cotton and Polyester Textile Samples Using Near-Infrared and Mid-Infrared Spectroscopies" Molecules 29, no. 15: 3667. https://doi.org/10.3390/molecules29153667
APA StylePaz, M. L., & Sousa, C. (2024). Discrimination and Quantification of Cotton and Polyester Textile Samples Using Near-Infrared and Mid-Infrared Spectroscopies. Molecules, 29(15), 3667. https://doi.org/10.3390/molecules29153667






