Susceptibility of Staphylococcus aureus to Anti-Inflammatory Drugs with a Focus on the Combinatory Effect of Celecoxib with Oxacillin In Vitro
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Staphylococcal Strains and Growth Media
4.3. Minimum Inhibitory Concentration (MIC) Determination
4.4. Evaluation of the Combined Antistaphylococcal Effect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakr, A.; Bregeon, F.; Mege, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus nasal colonisation: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef]
- Brouillette, E.; Goetz, C.; Droppa-Almeida, D.; Chamberland, S.; Jacques, M.; Malouin, F. Secondary Staphylococcus aureus intramammary colonisation is reduced by non-aureus staphylococci exoproducts. Microbes Infect. 2022, 24, 104879. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, N.; Ryan, J.E.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 2018, 31, e00084-17. [Google Scholar] [CrossRef] [PubMed]
- Kock, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.E.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J.; et al. Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in Europe. Euro Surveill. 2010, 15, pii-19688. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S. Management of bone and joint infections due to Staphylococcus aureus. Intern. Med. J. 2005, 35, S79–S96. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.D.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity, and clinical management. Nat. Rev. Microbial. 2022, 20, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Minion, J.; Skinner, S.; Wong, A. Disseminated Exophiala dermatitidis causing septic arthritis and osteomyelitis. BMC Infect. Dis. 2018, 18, 255. [Google Scholar] [CrossRef]
- Sommer, T.; Karsy, M.; Driscoll, M.J.; Jensen, R.L. Varicella-zoster virus infection and osteomyelitis of the skull. World Neurosurg. 2018, 115, 297–300. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbial. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Ferrand, J.; El Samad, Y.; Brunschweiler, B.; Grados, F.; Dehamchia-Rehailia, N.; Sejourne, A.; Schmit, J.L.; Gabrion, A.; Fardellone, P.; Paccou, J. Morbimortality in adult patients with septic arthritis: A three-year hospital-based study. BMC. Infect. Dis. 2016, 16, 239. [Google Scholar] [CrossRef]
- Huang, J.F.; Wu, Q.N.; Zheng, X.Q.; Sun, X.L.; Wu, C.Y.; Wang, X.B.; Wu, C.W.; Wang, B.; Wang, X.Y.; Bergman, M.; et al. The characteristics and mortality of osteoporosis, osteomyelitis, or rheumatoid arthritis in the diabetes population: A retrospective study. Int. J. Endocrinol. 2020, 2020, 8821978. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.; Baertl, S.; Alt, V.; Rupp, M. What is the burden of osteomyelitis in Germany? An analysis of inpatient data from 2008 through 2018. BMC Infect. Dis. 2021, 21, 550. [Google Scholar] [CrossRef] [PubMed]
- Minguez, S.; Molinos, S.; Mateo, L.; Gimenez, M.; Mateu, L.; Cabello, J.; Olive, A. Septic arthritis due to methicillin-resistant Staphylococcus aureus in adults. Reumatol. Clin. 2015, 11, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Abram, S.G.F.; Alvand, A.; Judge, A.; Beard, D.J.; Price, A.J. Mortality and adverse joint outcomes following septic arthritis of the native knee: A longitudinal cohort study of patients receiving arthroscopic washout. Lancet Infect. Dis. 2020, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Stake, S.; Scully, R.; Swenson, S.; Lee, D.; Lee, R.; Sparks, A.; Pandarinath, R. Repeat irrigation and debridement for patients with acute septic knee arthritis: Incidence and risk factors. J. Clin. Orthop. Trauma 2020, 11, S177–S183. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K.R.; Vowden, P. Wound debridement part 2: Sharp techniques. J. Wound Care. 1999, 8, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Urish, K.L.; Cassat, J.E. Staphylococcus aureus osteomyelitis: Bone, bugs, and surgery. Infect. Immun. 2020, 88, e00932-19. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Roberts, M.; Al-Kassas, R. Implantable drug delivery systems for the treatment of osteomyelitis. Drug Dev. Ind. Pharm. 2022, 48, 511–527. [Google Scholar] [CrossRef]
- Dombrowski, J.C.; Winston, L.G. Clinical failures of appropriately treated methicillin-resistant Staphylococcus aureus infections. J. Infect. 2008, 57, 110–115. [Google Scholar] [CrossRef]
- Wieland, B.W.; Marcantoni, J.R.; Bommarito, K.M.; Warren, D.K.; Marschall, J. A retrospective comparison of ceftriaxone versus oxacillin for osteoarticular infections due to methicillin-susceptible Staphylococcus aureus. Clin. Infect. Dis. 2012, 54, 585–590. [Google Scholar] [CrossRef]
- Woods, C.R.; Bradley, J.S.; Chatterjee, A.; Copley, L.A.; Robinson, J.; Kronman, M.P.; Arrieta, A.; Fowler, S.L.; Harrison, C.; Carrillo-Marquez, M.A.; et al. Clinical practice guideline by the pediatric infectious diseases society and the infectious diseases society of America: 2021 guideline on diagnosis and management of acute hematogenous osteomyelitis in pediatrics. J. Pediatric. Infect. Dis. Soc. 2021, 10, 801–844. [Google Scholar] [CrossRef]
- Stevens, D.L. The role of vancomycin in the treatment paradigm. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S51–S57. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chambers, H.F. Staphylococcus aureus with heterogeneous resistance to vancomycin: Epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 2003, 47, 3040–3045. [Google Scholar] [CrossRef] [PubMed]
- Marinho, D.S.; Huff, G.; Ferreira, B.L.; Castro, H.; Rodrigues, C.R.; de Sousa, V.P.; Cabral, L.M. The study of vancomycin use, and its adverse reactions associated to patients of a Brazilian university hospital. BMC Res. Notes 2011, 4, 236. [Google Scholar] [CrossRef]
- Thomas, C.; Stevenson, M.; Riley, T.V. Antibiotics, and hospital-acquired Clostridium difficile-associated diarrhoea: A systematic review. J. Antimicrob. Chemother. 2003, 51, 1339–1350. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Clinical practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 Update by IDSA. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.M.; Turnidge, J.D.; Sentry, A. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalised patients in Asia-Pacific and South Africa: Results from sentry antimicrobial surveillance programme, 1998–1999. Antimicrob. Agents Chemother. 2002, 46, 879–881. [Google Scholar] [CrossRef]
- Helito, C.P.; Zanon, B.B.; Miyahara, H.D.E.S.; Pecora, J.R.; Lima, A.L.; Oliveira, P.R.; Vicente, J.R.; Demange, M.K.; Camanho, G.L. Clinical and epidemiological differences between septic arthritis of the knee and hip caused by oxacillin-sensitive and-resistant Staphylococcus aureus. Clinics 2015, 70, 30–33. [Google Scholar] [CrossRef]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Fischbach, M.A. Combination therapies for combating antimicrobial resistance. Curr. Opin. Microbiol. 2011, 14, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on antibacterial to combat resistance: From the ancient era of plants and metals to present and future perspectives of green nanotechnological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Toews, M.L.; Bylund, D.B. Pharmacologic principles for combination therapy. Proc. Am. Thorac. Soc. 2005, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Domingos, O.D.S.; Alcantara, B.G.V.; Santos, M.F.C.; Maiolini, T.C.S.; Dias, D.F.; Baldim, J.L.; Lago, J.H.G.; Soares, M.G.; Chagas-Paula, D.A. Anti-inflammatory derivatives with dual mechanism of action from the metabolomic screening of Poincianella pluviosa. Molecules 2019, 24, 4375. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef]
- Williams, D.M. Clinical pharmacology of corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Otlu, S.; Celebi, O.; Aksu, P.; Saglam, A.G.; Dogan, A.N.C.; Mutlu, N. An investigation of antibacterial effects of steroids. Turkish J. Vet. Anim. Sci. 2017, 41, 22. [Google Scholar] [CrossRef]
- Chiu, H.C.; Lee, S.L.; Kapuriya, N.; Wang, D.; Chen, Y.R.; Yu, S.L.; Kulp, S.K.; Teng, L.J.; Chen, C.S. Development of novel antibacterial agents against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2012, 20, 4653–4660. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing celecoxib as a topical antimicrobial agent. Front. Microbiol. 2015, 6, 750. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, X.; Tang, H.; Wang, Y.; Yang, H.; Yuan, W.; Yue, B. Diclofenac resensitises methicillin-resistant Staphylococcus aureus to β-lactams and prevents implant infections. Adv. Sci. 2021, 8, 2100681. [Google Scholar] [CrossRef]
- Kivitz, A.J.; Espinoza, L.R.; Sherrer, Y.R.; Liu-Dumaw, M.; West, C.R.A. Comparison of the efficacy and safety of celecoxib 200 mg and celecoxib 400 mg once daily in treating the signs and symptoms of psoriatic arthritis. Semin. Arthritis Rheum. 2007, 37, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Fitz Gerald, G.A. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003, 2, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Howes, L.G. Selective COX-2 inhibitors, NSAIDs, and cardiovascular events—Is celecoxib the safest choice? Ther. Clin. Risk Manag. 2007, 3, 831–845. [Google Scholar] [PubMed]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The class study: A randomised controlled trial. Celecoxib long-term arthritis safety study. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Krasselt, M.; Baerwald, C.; Petros, S.; Seifert, O. Mortality of sepsis in patients with rheumatoid arthritis: A single-center retrospective analysis and comparison with a control group. J. Intensive Care Med. 2021, 36, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.C.; Barbulescu, A.L.; Firulescu, S.C.; Chisalau, A.B.; Parvanescu, C.D.; Ciurea, P.L.; Sandu, R.E.; Turcu-Stiolica, A.; Boldeanu, M.V.; Vintila, E.M.; et al. Staphylococcus aureus-induced septic arthritis of the ankle related to malum perforans in a diabetes patient. Rom. J. Morphol. Embryol. 2021, 62, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Herawati, D.; Wibowo, M.S. The benefits and challenges of antibiotics-non-steroidal anti-inflammatory drugs non-covalent reaction. Molecules 2023, 28, 3672. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.; Kamath, A.T.; Dhara, V.; Ravindranath, V. Chronic recurrent osteomyelitis: A surgeon’s enigma. J. Oral. Pathol. Med. 2019, 48, 180–184. [Google Scholar] [CrossRef]
- Chan, E.W.L.; Yee, Z.Y.; Raja, I.; Yap, J.K.Y. Synergistic effect of non-steroidal anti-inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2017, 10, 70–74. [Google Scholar] [CrossRef]
- Wald-Dickler, N.; Holtom, P.; Spellberg, B. Busting the myth of “static vs cidal”: A systemic literature review. Clin. Infect. Dis. 2018, 66, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in treating gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Bonnaire, A.; Vernet-Garnier, V.; Lebrun, D.; Bajolet, O.; Bonnet, M.; Hentzien, M.; Ohl, X.; Diallo, S.; Bani-Sadr, F. Clindamycin combination treatment for the treatment of bone and joint infections caused by clindamycin-susceptible, erythromycin-resistant Staphylococcus spp. Diagn. Microbiol. Infect. Dis. 2021, 99, 115225. [Google Scholar] [CrossRef]
- Fidelix, T.S.; Macedo, C.R.; Maxwell, L.J.; Fernandes Moca Trevisani, V. Diacerein for osteoarthritis. Cochrane Database Syst. Rev. 2014, 10, CD005117. [Google Scholar] [CrossRef]
- Pavelka, K.; Bruyere, O.; Cooper, C. Diacerein: Benefits, risks, and place in the management of osteoarthritis. An opinion-based report from the ESCEO. Drugs Aging 2016, 33, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Nguon, S.; Novy, P.; Kokoska, L. Potentiation of the in vitro antistaphylococcal effect of oxacillin and tetracycline by the anti-inflammatory drug diacetyl rhein. Chemotherapy 2013, 59, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Yue, J.; Sun, S.; Lv, Q.; Jian, S.; Xie, Y.; Han, L.; Zhang, F.; Dai, Y.; et al. In vitro antimicrobial activity of diacerein on 76 isolates of gram-positive cocci from bacterial keratitis patients and in vivo study of diacerein eye drops on Staphylococcus aureus keratitis in mice. Antimicrob. Agents Chemother. 2019, 63, e01874-18. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Alhashimi, M.; Mayhoub, A.; Mohammad, H.; Seleem, M.N. Repurposing fenamic acid drugs to combat multidrug resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2020, 64, e02206-19. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Whittell, L.R.; Jergic, S.; Liu, M.; Harry, E.; Dixon, N.E.; Kelso, M.J.; Beck, J.L.; Oakley, A.J. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem. Biol. 2014, 21, 481–487. [Google Scholar] [CrossRef]
- Etienne, F.; Resnick, L.; Sagher, D.; Brot, N.; Weissbach, H. Reduction of sulindac to its active metabolite, sulindac sulfide: Assay and role of the methionine sulfoxide reductase system. Biochem. Biophys. Res. Commun. 2003, 312, 1005–1010. [Google Scholar] [CrossRef]
- Shirin, H.; Moss, S.F.; Kancherla, S.; Kancherla, K.; Holt, P.R.; Weinstein, I.B.; Sordillo, E.M. Nonsteroidal anti-inflammatory drugs have bacteriostatic and bactericidal activity against Helicobacter pylori. J. Gastroenterol. Hepatol. 2006, 21, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Annamanedi, M.; Kalle, A.M. Celecoxib sensitises Staphylococcus aureus to antibiotics in macrophages by modulating SIRT1. PLoS ONE 2014, 9, e99285. [Google Scholar] [CrossRef] [PubMed]
- Annamanedi, M.; Varma, G.Y.N.; Anuradha, K.; Kalle, A.M. Celecoxib enhances the efficacy of low-dose antibiotic treatment against polymicrobial sepsis in mice and clinical Isolates of ESKAPE pathogens. Front. Microbiol. 2017, 8, 805. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.W.; Dokla, E.M.E.; Serya, R.A.T.; Abouzid, K.A.M. Penicillin binding protein 2a: An overview and a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 199, 112312. [Google Scholar] [CrossRef]
- Santiago, C.; Pang, E.L.; Lim, K.H.; Loh, H.S.; Ting, K.N. Inhibition of penicillin-binding protein 2a (PBP2a) in methicillin-resistant Staphylococcus aureus (MRSA) by combination of ampicillin and a bioactive fraction from Duabanga grandiflora. MC Complement. Altern. Med. 2015, 15, 178. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Santiago, C.; Pang, E.L.; Lim, K.H.; Loh, H.S.; Ting, K.N. Reversal of ampicillin resistance in MRSA via inhibition of penicillin-binding protein 2a by Acalypha wilkesiana. BioMed. Res. Int. 2014, 2014, 9653482014. [Google Scholar] [CrossRef]
- Zhou, T.; Li, Z.; Kang, O.H.; Mun, S.H.; Seo, Y.S.; Kong, R.; Shin, D.W.; Liu, X.Q.; Kwon, D.Y. Antimicrobial activity, and synergism of ursolic acid 3-O-α-L-Arabinopyranoside with oxacillin against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Med. 2017, 40, 1285–1293. [Google Scholar] [CrossRef][Green Version]
- Pinho, M.G.; Filipe, S.R.; de Lencastre, H.; Tomasz, A. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 2001, 183, 6525–6531. [Google Scholar] [CrossRef]
- Fuda, C.; Suvorov, M.; Vakulenko, S.B.; Mobashery, S. The basis for resistance to beta-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2004, 279, 40802–40806. [Google Scholar] [CrossRef]
- Sadeghian, H.; Sadeghian, A.; Pordel, M.; Rahimizadeh, M.; Jahandari, P.; Orafaie, A.; Bakavoli, M. Design, synthesis, and structure–activity relationship study of 5-amido-1-(2,4-dinitrophenyl)-1H-4-pyrazolecarbonitrils as DD-carboxypeptidase/penicillin-binding protein inhibitors with Gram-positive antibacterial activity. Med. Chem. Res. 2010, 19, 103–119. [Google Scholar] [CrossRef]
- Li, Z.; Francisco, G.D.; Hu, W.; Labthavikul, P.; Petersen, P.J.; Severin, A.; Singh, G.; Yang, Y.; Rasmussen, B.A.; Lin, Y.; et al. 2-Phenyl-5,6-dihydro-2H-thieno[3,2-c]pyrazol-3-ol derivatives as new inhibitors of bacterial cell wall biosynthesis. Bioorg. Med. Chem. Lett. 2003, 13, 2591–2594. [Google Scholar] [CrossRef] [PubMed]
- Preuer, K.; Lewis, R.P.I.; Hochreiter, S.; Bender, A.; Bulusu, K.C.; Klambauer, G. DeepSynergy: Predicting anti-cancer drug synergy with deep Learning. Bioinformatics 2018, 34, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Kok, E.Y.; Vallejo, J.G.; Sommer, L.M.; Rosas, L.; Kaplan, S.L.; Hulten, K.G.; McNeil, J.C. Association of vancomycin MIC and molecular characteristics with clinical outcomes in methicillin-susceptible Staphylococcus aureus acute hematogenous osteoarticular infections in children. Antimicrob. Agents Chemother. 2018, 62, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.; Anderson, T.L.; Roberts, S.A.; Gao, W.; Christiansen, K.J.; et al. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J. Infect. Dis. 2011, 204, 340–347. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Zheng, C.; Zheng, C.; Wang, B.; Shen, P.; Xie, Z.; Qu, Y. Efficacy of pre-emptive use of cyclooxygenase-2 inhibitors for total knee arthroplasty: A mini-review. Arthroplasty 2019, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- FDA. Centre for Drug Evaluation and Research: Application Number NDA 20-998; FDA: Silver Spring, MD, USA, 1998. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20998AP_clinphrmr_P1.pdf (accessed on 3 August 2023).
- Sidney, L.E.; Heathman, T.R.; Britchford, E.R.; Abed, A.; Rahman, C.V.; Buttery, L.D. Investigation of localized delivery of diclofenac sodium from poly (D, L-lactic acid-co-glycolic acid)/poly (ethylene glycol) scaffolds using an in vitro osteoblast inflammation model. Tissue Eng. Part A 2015, 21, 362–373. [Google Scholar] [CrossRef]
- Heraeus Medical. Palacos R+G: High-Viscosity, Bone Cement With Gentamicin; Heraeus Medical GmbH, Germany. Available online: https://www.heraeus-medical.com/en/healthcare-professionals/products/palacos-rg/ (accessed on 15 August 2023).
- Humez, M.; Domann, E.; Thormann, K.M.; Folsch, C.; Strathausen, R.; Vogt, S.; Alt, V.; Kuhn, K.D. Daptomycin-impregnated PMMA cement against vancomycin-resistant germs: Dosage, handling, elution, mechanical stability, and effectiveness. Antibiotics 2023, 12, 1567. [Google Scholar] [CrossRef]
- PRO-IMPLANT Foundation. Pocket Guide to Diagnosing and Treating the Periprosthetic Joint Infection (PJI); PRO-IMPLANT Foundation: Berlin, Germany, 2018; Available online: https://pro-implant.org/tools/pocket-guide/1 (accessed on 22 May 2024).
- Gogia, J.S.; Meehan, J.P.; Di Cesare, P.E.; Jamali, A.A. Local antibiotic therapy in osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef]
- Gunay, H.; Bakan, O.M.; Mirzazade, J.; Sozbilen, M.C. A new perspective on the diagnosis of septic arthritis: High-resolution thermal imaging. J. Clin. Med. 2023, 12, 1573. [Google Scholar] [CrossRef]
- Cunha, B.A. Methicillin-resistant Staphylococcus aureus: Clinical manifestations and antimicrobial therapy. Microbiol. Infect. 2005, 11 (Suppl. S4), 33–42. [Google Scholar] [CrossRef]
- Missiakas, D.M.; Schneewind, O. Growth and laboratory maintenance of Staphylococcus aureus. Curr. Protoc. Microbiol. 2013, 28, 9C.1.1–9C.1.9. [Google Scholar] [CrossRef]
- Dolenc, A.; Kristl, J.; Baumgartner, S.; Planinsek, O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int. J. Pharm. 2009, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Yet, B.; Nemutlu, E.; Akdag, Y.C.; Eroglu, H.; Oner, L. Celecoxib Nanoformulations with Enhanced solubility, dissolution rate, and oral bioavailability: Experimental approaches over in vitro/in vivo evaluation. Pharmaceutics 2023, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- John Hopkins Arthritis Centre. Rheumatoid Arthritis Treatment; John Hopkins Arthritis Centre: Baltimore, MD, USA, 2024; Available online: https://www.hopkinsarthritis.org/arthritis-info/rheumatoid-arthritis/ra-treatment/#NSAID (accessed on 24 April 2024).
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Derry, S.; McQuay, H.J. Single dose oral acemetacin for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2009, 2009, CD007589. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sheraz, M.A.; Ahmad, I. Tolfenamic Acid. Profiles Drug Subst. Excip. Relat. Methodol. 2018, 43, 255–319. [Google Scholar] [CrossRef]
- Rondevaldova, J.; Hummelova, J.; Tauchen, J.; Kokoska, L. In vitro antistaphylococcal synergistic effect of isoflavone metabolite demethyltexasin with amoxicillin and oxacillin. Microb. Drug Resist. 2018, 24, 24–29. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Hamed, M.I.; Panitch, A.; Seleem, M.N. Targeting methicillin-resistant Staphylococcus aureus with short salt-resistant synthetic peptides. Antimicrob. Agents Chemother. 2014, 58, 4113–4122. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro proof-of-concept. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D.; Washington, J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In Manual of Clinical Microbiology, 7th ed Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 1526–1543. [Google Scholar]
- Frankova, A.; Vistejnova, L.; Merinas-Amo, T.; Leheckova, Z.; Doskocil, I.; Wong Soon, J.; Kudera, T.; Laupua, F.; Alonso-Moraga, A.; Kokoska, L. In vitro antibacterial activity of extracts from Samoan medicinal plants and their effect on proliferation and migration of human fibroblasts. J. Ethnopharmacol. 2021, 264, 113220. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Hollanders, M.; Benkendorff, K. Out of control: The need for standardised solvent approaches and data reporting in antibiofilm assays incorporating dimethyl-sulfoxide (DMSO). Biofilm 2022, 4, 100081. [Google Scholar] [CrossRef]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E-test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the assessment of in vitro and in vivo antifungal combinations. J. Fungi 2022, 7, 113. [Google Scholar] [CrossRef] [PubMed]
| Compound | Minimum Inhibitory Concentration in (mg/L) and (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standard ATCC a Strains | Clinical Isolates | |||||||||
| 25923 | 29213 | 33591 b | 33592 b | 43300 b | BAA 976 b | MRSA1 b | MRSA2 b | MRSA3 b | MRSA4 b | |
| Acetylsalicylic acid | - | - | - | - | - | - | - | - | - | - |
| Acemetacin | - | - | - | - | - | - | - | - | - | - |
| Ampyrone | - | - | - | - | - | - | - | - | - | - |
| Celecoxib | 64 (168) | 64 (168) | 64 (168) | 64 (168) | 64 (168) | 64 (168) | 32 (84) | 64 (168) | 64 (168) | 64 (168) |
| Cortisone | - | - | - | - | - | - | - | - | - | - |
| Diacerein | 128 (347) | 128 (347) | 128 (347) | 64 (174) | 128 (347) | 128 (347) | 128 (347) | 128 (347) | 64 (174) | 128 (347) |
| Diclofenac sodium | - | - | - | - | - | - | - | - | - | - |
| Diflunisal | 512 (2046) | 512 (2046) | 512 (2046) | 512 (2046) | 512 (2046) | - | 512 (2046) | 512 (2046) | - | - |
| Ethenzamide | - | - | - | - | - | - | - | - | - | - |
| Felbinac | - | - | - | - | - | - | - | - | - | - |
| Ibuprofen | - | - | - | - | - | - | - | - | - | - |
| Mefenamic acid | 512 (2121) | 512 (2121) | 512 (2121) | 512 (2121) | 512 (2121) | - | - | 512 (2121) | 512 (2121) | - |
| Nabumetone | - | - | - | - | - | - | - | - | - | - |
| Propyphenazone | - | - | - | - | - | - | - | - | - | - |
| Sulindac sulfide | 128 (376) | 128 (376) | 128 (376) | 128 (376) | 128 (376) | 128 (376) | 128 (376) | 128 (376) | 128 (376) | 128 (376) |
| Sulindac | - | - | - | - | - | - | - | - | - | - |
| Tolfenamic acid | 256 (978) | 256 (978) | 256 (978) | 256 (978) | 256 (978) | 256 (978) | 256 (978) | 512 (1957) | 256 (978) | 128 (489) |
| Oxacillin c | 0.25 (0.623) | 0.5 (1.247) | 512 (1276) | 512 (1276) | 256 (637) | 64 (159) | 512 (1276) | 256 (637) | 256 (637) | 256 (637) |
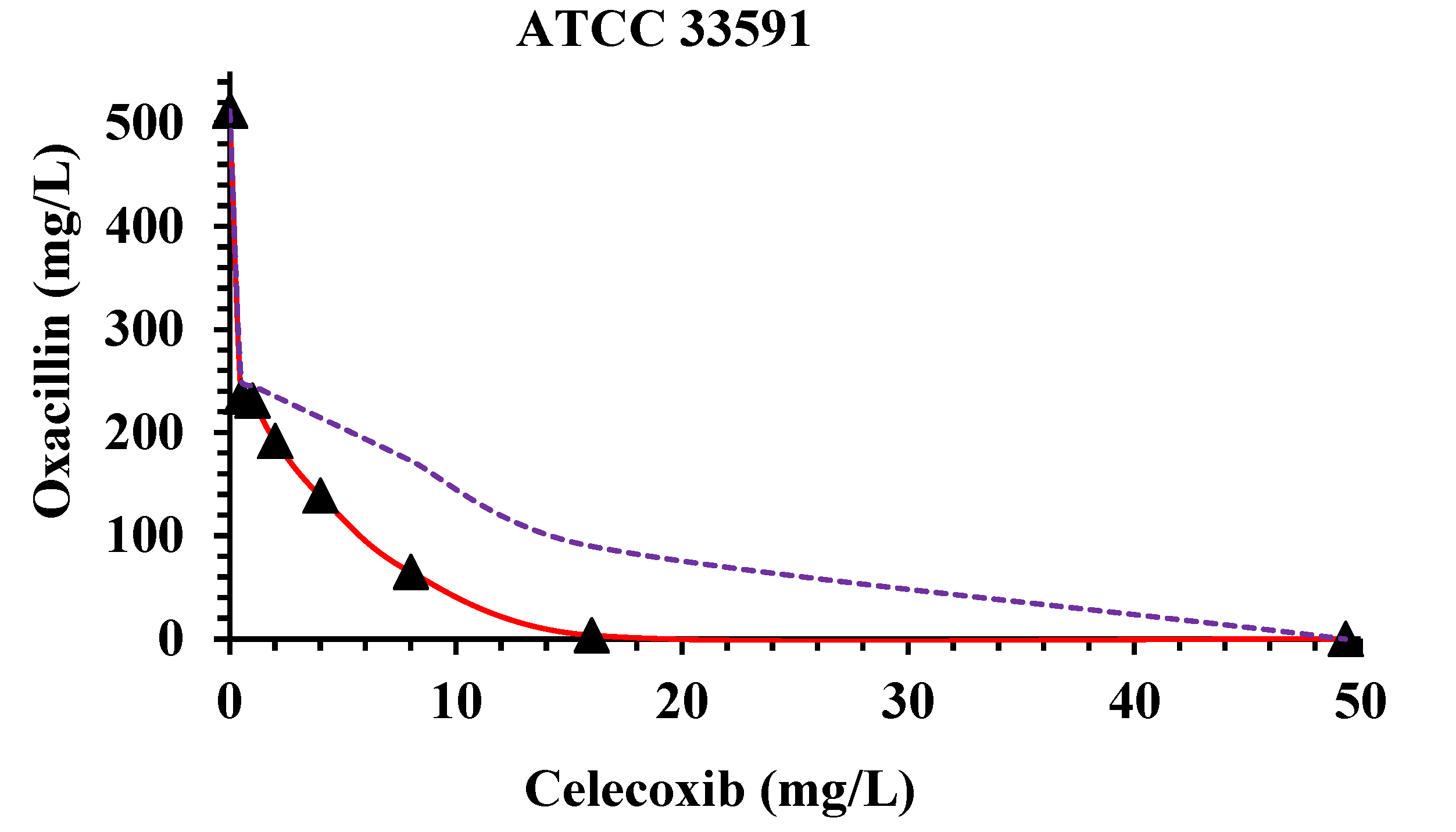
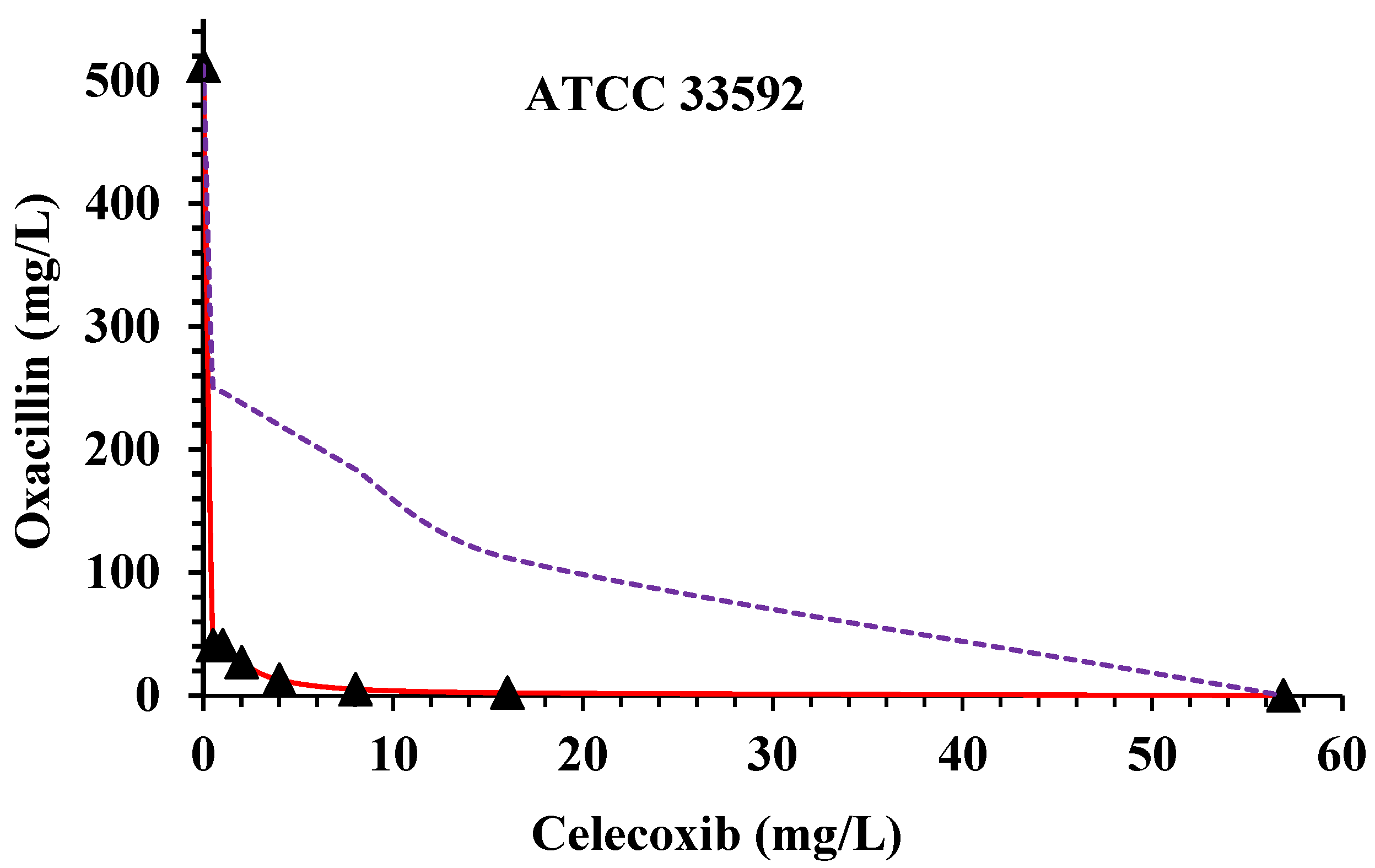
| S. aureus | MICs a Alone | MICs of CX and OX Combinations and Related FICI b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OX c | CX d | CX MIC 16 | CX MIC 8 | CX MIC 4 | CX MIC 2 | CX MIC 1 | CX MIC 0.5 | |||||||
| OX MIC | FICI | OX MIC | FICI | OX MIC | FICI | OX MIC | FICI | OX MIC | FICI | OX MIC | FICI | |||
| ATCC e 29213 | 0.5 | 60.444 | 0.225 | 0.716 | 0.189 | 0.510 | 0.166 | 0.399 | 0.194 | 0.421 | 0.180 | 0.377 | 0.180 | 0.369 |
| ATCC 25923 | 0.25 | 56.888 | 0.230 | 1.204 | 0.263 | 1.196 | 0.166 | 0.736 | 0.152 | 0.646 | 0.125 | 0.517 | 0.090 | 0.369 |
| ATCC 33591 | 512 | 49.777 | 3.555 | 0.328 | 64.888 | 0.287 | 138.890 | 0.351 | 192.000 | 0.415 | 231.110 | 0.471 | 234.670 | 0.468 |
| ATCC 33592 | 512 | 56.888 | 2.222 | 0.285 | 5.110 | 0.150 | 12.777 | 0.095 | 26.888 | 0.087 | 40.888 | 0.097 | 40.888 | 0.088 |
| ATCC 43300 | 227.56 | 53.333 | 2.000 | 0.308 | 44.222 | 0.344 | 88.000 | 0.461 | 156.444 | 0.725 | 156.444 | 0.706 | - | - |
| BAA 976 | 64 | 64 | 55.999 | 1.124 | 39.999 | 0.749 | 42.666 | 0.729 | 32.000 | 0.531 | 32.000 | 0.515 | 32.000 | 0.507 |
| MRSA1 | 512 | 53.333 | 199.560 | 0.689 | 216.111 | 0.572 | 231.111 | 0.526 | 202.666 | 0.433 | 181.333 | 0.372 | 181.333 | 0.363 |
| MRSA2 | 256 | 64 | 114.000 | 0.695 | 213.333 | 0.958 | 241.777 | 1.006 | 241.777 | 0.975 | 241.777 | 0.960 | 241.777 | 0.952 |
| MRSA3 | 256 | 64 | 88.666 | 0.596 | 135.111 | 0.652 | 142.222 | 0.618 | 156.444 | 0.642 | 156.444 | 0.626 | 156.444 | 0.618 |
| MRSA4 | 256 | 64 | 55.555 | 0.467 | 117.333 | 0.583 | 142.222 | 0.618 | 156.444 | 0.642 | 156.444 | 0.626 | 156.444 | 0.618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okpala, O.E.; Rondevaldova, J.; Osei-Owusu, H.; Kudera, T.; Kokoskova, T.; Kokoska, L. Susceptibility of Staphylococcus aureus to Anti-Inflammatory Drugs with a Focus on the Combinatory Effect of Celecoxib with Oxacillin In Vitro. Molecules 2024, 29, 3665. https://doi.org/10.3390/molecules29153665
Okpala OE, Rondevaldova J, Osei-Owusu H, Kudera T, Kokoskova T, Kokoska L. Susceptibility of Staphylococcus aureus to Anti-Inflammatory Drugs with a Focus on the Combinatory Effect of Celecoxib with Oxacillin In Vitro. Molecules. 2024; 29(15):3665. https://doi.org/10.3390/molecules29153665
Chicago/Turabian StyleOkpala, Onyedika Emmanuel, Johana Rondevaldova, Hayford Osei-Owusu, Tomas Kudera, Tersia Kokoskova, and Ladislav Kokoska. 2024. "Susceptibility of Staphylococcus aureus to Anti-Inflammatory Drugs with a Focus on the Combinatory Effect of Celecoxib with Oxacillin In Vitro" Molecules 29, no. 15: 3665. https://doi.org/10.3390/molecules29153665
APA StyleOkpala, O. E., Rondevaldova, J., Osei-Owusu, H., Kudera, T., Kokoskova, T., & Kokoska, L. (2024). Susceptibility of Staphylococcus aureus to Anti-Inflammatory Drugs with a Focus on the Combinatory Effect of Celecoxib with Oxacillin In Vitro. Molecules, 29(15), 3665. https://doi.org/10.3390/molecules29153665











