Optimization of Phenolic-Enriched Extracts from Olive Leaves via Ball Milling-Assisted Extraction Using Response Surface Methodology
Abstract
1. Introduction
2. Results and Discussion
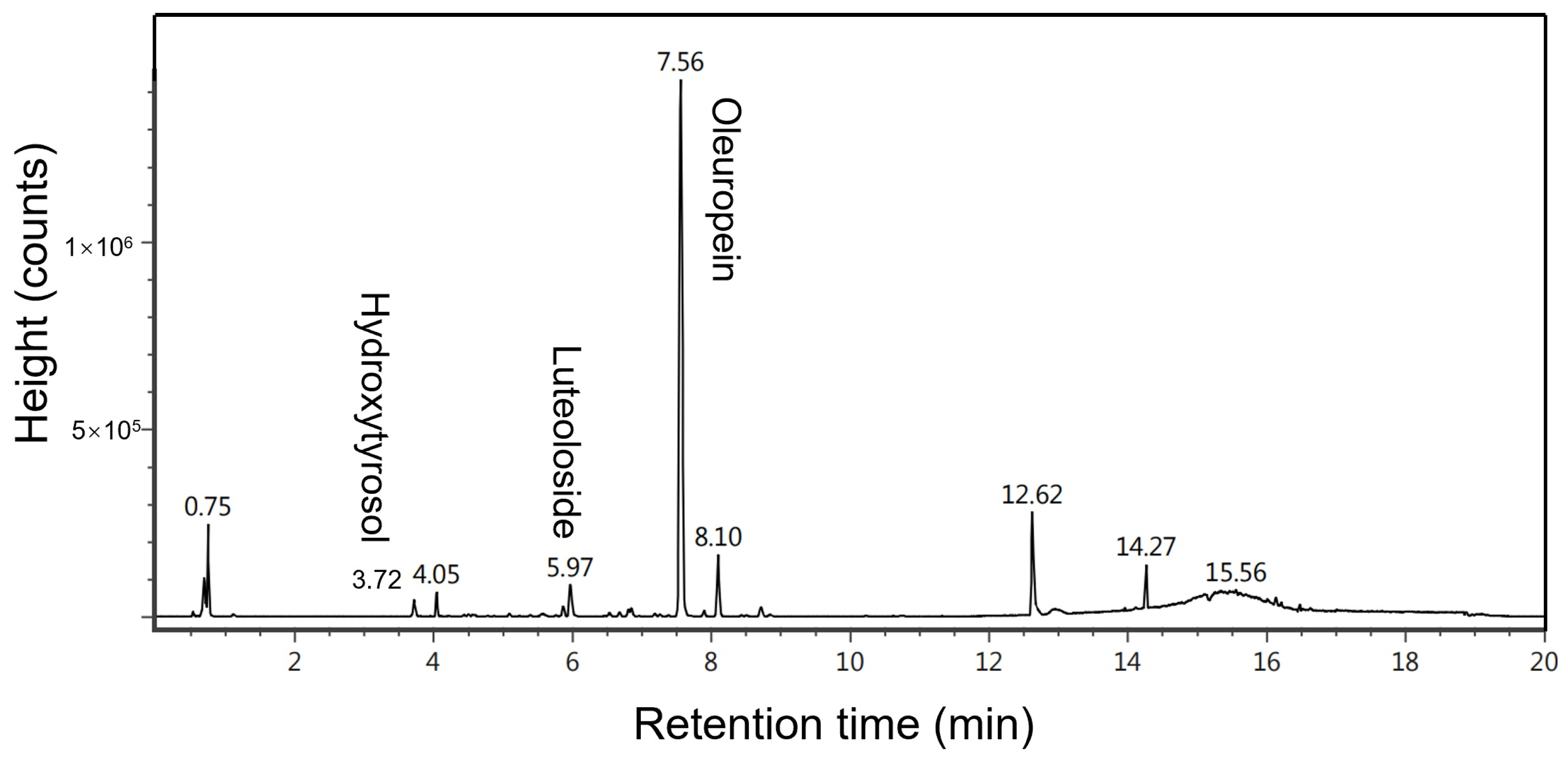
2.1. Characterization of Compounds Extracted from Raw Material via Ultrasonic Extraction Using UPLC-MS
2.2. Experimental Design and BMAE Results
2.3. Model Fitting and Optimization of Recovery of Oleuropein
2.4. Model Fitting and Optimization of Recovery of Luteoloside
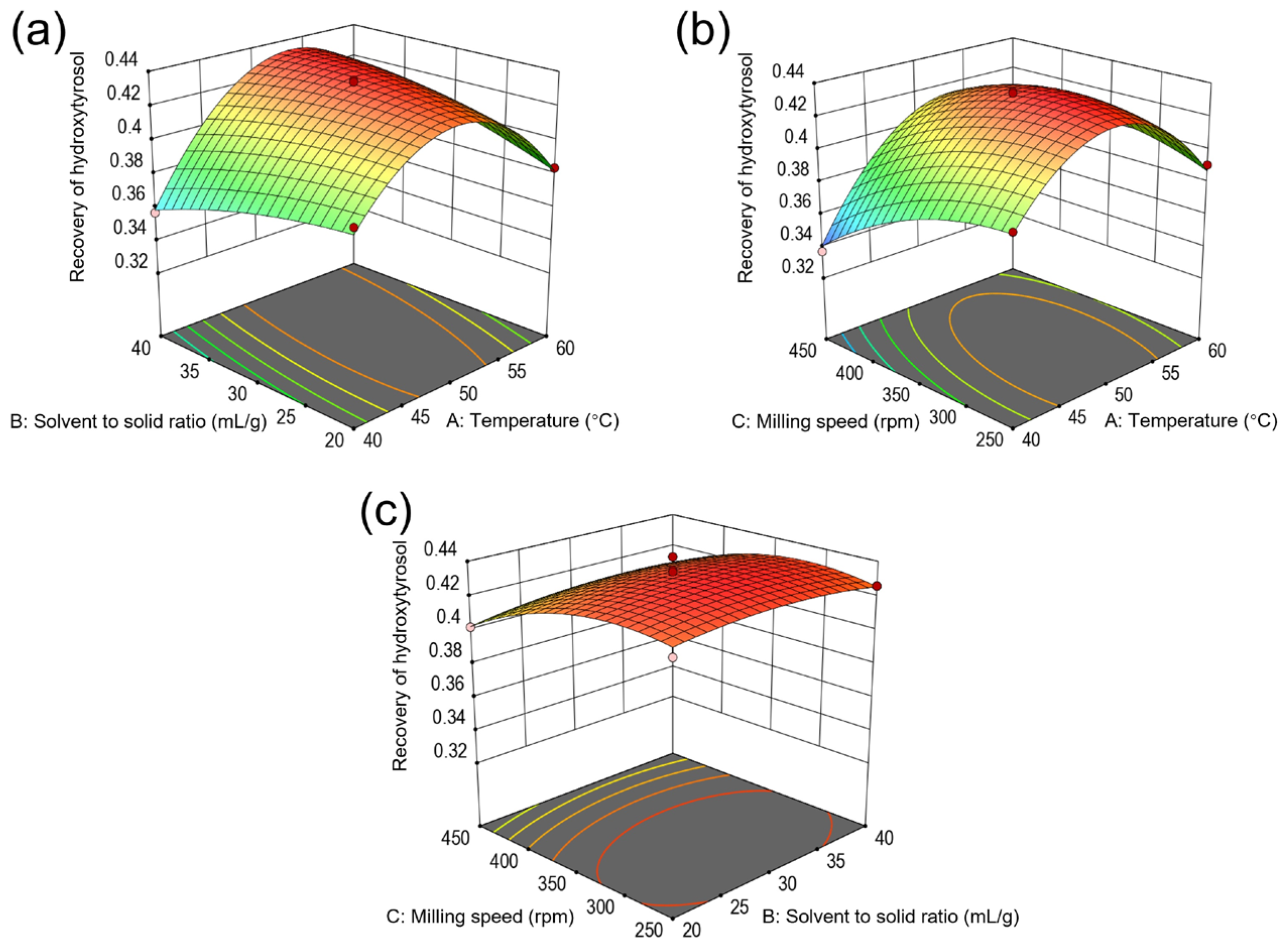
2.5. Model Fitting and Optimization of Recovery of Hydroxytyrosol
2.6. Optimal Extraction Conditions of Oleuropein, Luteoloside, and Hydroxytyrosol
3. Materials and Methods
3.1. Materials
3.2. Ultrasonic Extraction of Olive Leaves
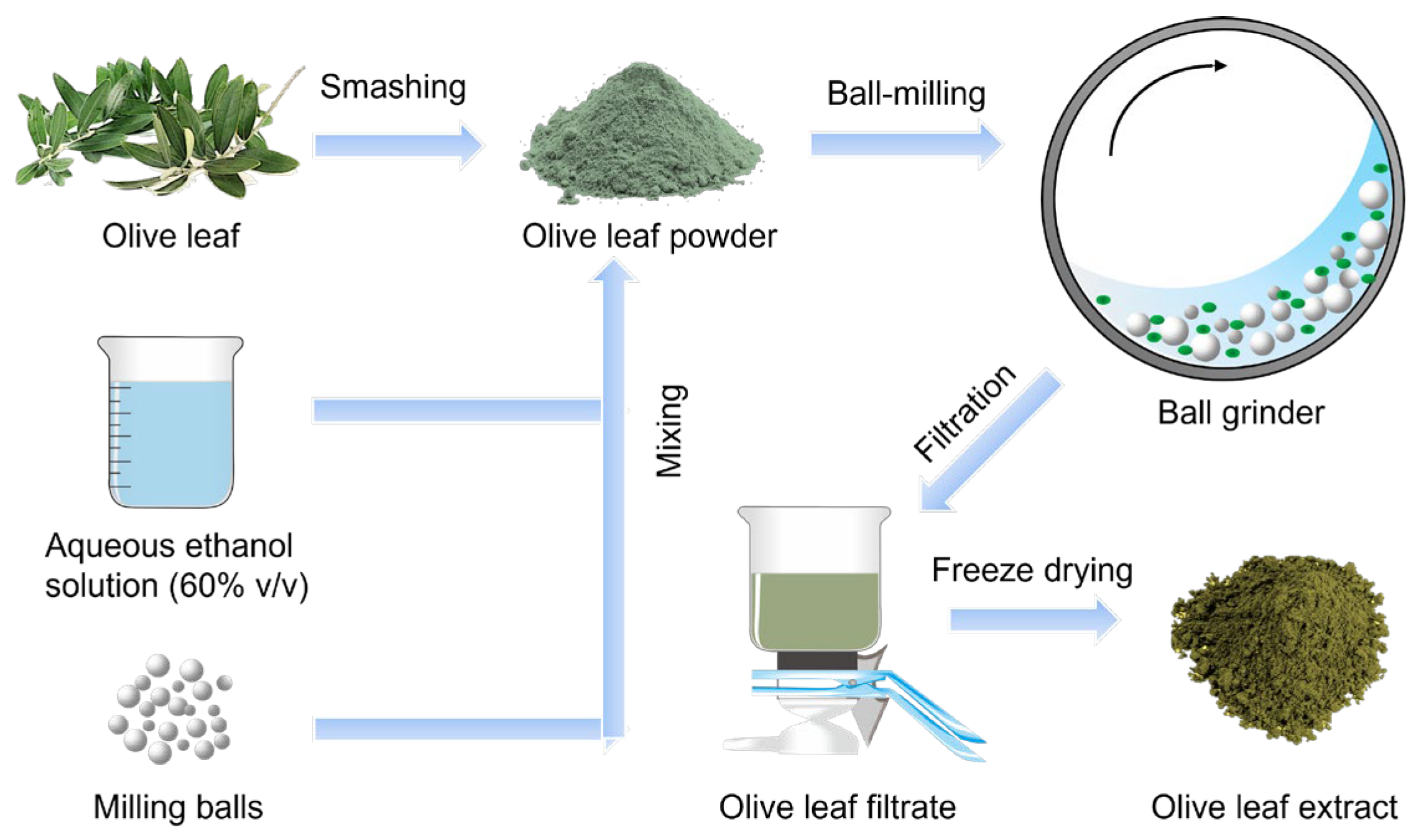
3.3. Ball Milling Extraction of Olive Leaves
3.4. Design of Experiments and Response Surface Methodology
3.5. Analysis of Active Substances in Olive Leaves Using UPLC-MS
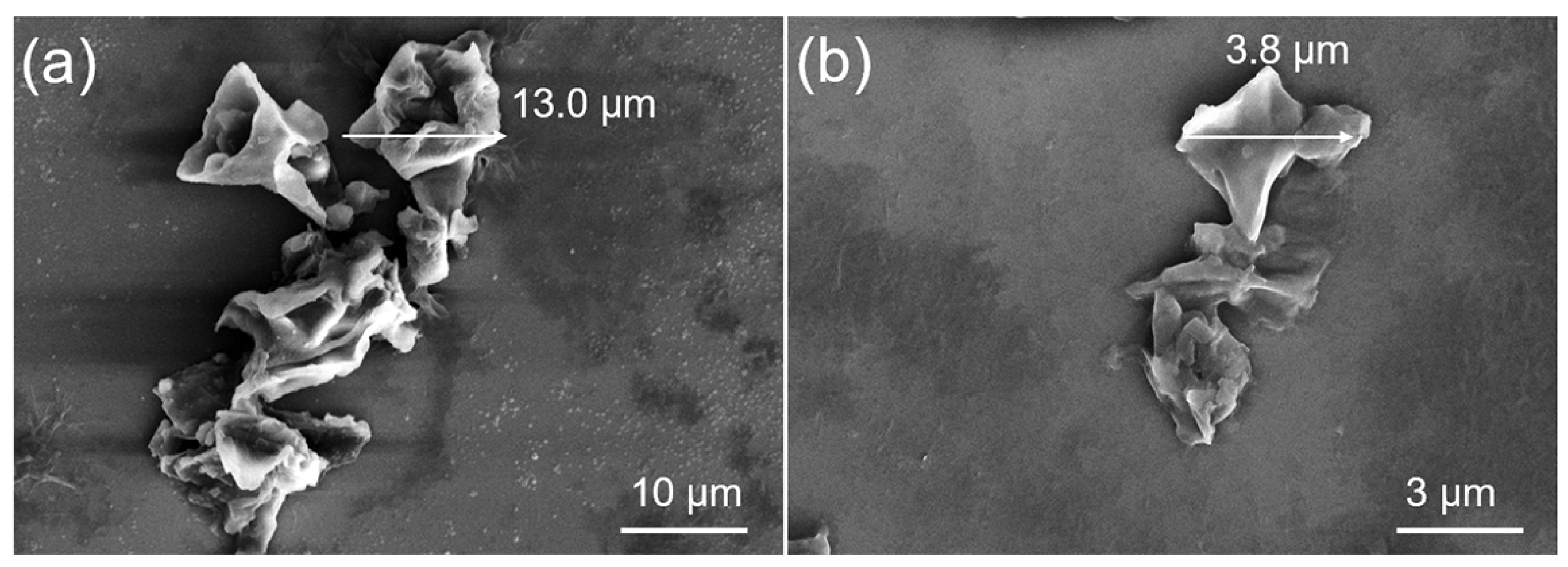
3.6. Morphology Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Taamalli, A.; Maria Gomez-Caravaca, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; Zhang, Y.L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, B.; Ballini, R.; Ciceri, D.; Allegrini, P.; Palmieri, A. A Practical and Efficient Conversion of Luteolin into Luteoloside. Synthesis 2021, 53, 4075–4078. [Google Scholar]
- Laguerre, M.; Giraldo, L.J.L.; Piombo, G.; Figueroa-Espinoza, M.C.; Pina, M.; Benaissa, M.; Combe, A.; Castera, A.R.; Lecomte, J.; Villeneuve, P. Characterization of Olive-Leaf Phenolics by ESI-MS and Evaluation of their Antioxidant Capacities by the CAT Assay. J. Am. Oil Chem. Soc. 2009, 86, 1215–1225. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Wani, I.A.; Ahmad, M. Olive oil and its principal bioactive compound: Hydroxytyrosol—A review of the recent literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Stupans, I.; Murray, M.; Kirlich, A.; Tuck, K.L.; Hayball, P.J. Inactivation of cytochrome P450 by the food-derived complex phenol oleuropein. Food Chem. Toxicol. 2001, 39, 1119–1124. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, R.; Giorno, L.; Piacentini, E.; Mazzuca, S.; Drioli, E. Kinetic study of a biocatalytic membrane reactor containing immobilized β-glucosidase for the hydrolysis of oleuropein. J. Membr. Sci. 2009, 339, 215–223. [Google Scholar] [CrossRef]
- Hannachi, H.; Elfalleh, W. Enrichment of Olive Oil with Polyphenols from Oleaster Leaves Using Central Composite Design for the Experimental Measurements. Anal. Lett. 2020, 54, 590–607. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; De Montijo-Prieto, S.; Jiménez-Valera, M.; Carrasco-Pancorbo, A.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Comparative Extraction of Phenolic Compounds from Olive Leaves Using a Sonotrode and an Ultrasonic Bath and the Evaluation of Both Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Jafari, S.M.; Aalami, M.; Khomeiri, M. Microwave-assisted extraction of phenolic compounds from olive leaves; a comparison with maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Montenegro, Z.J.S.; Lvarez-Rivera, G.; Mendiola, J.A.; Ibáez, E.; Cifuentes, A. Extraction and Mass Spectrometric Characterization of Terpenes Recovered from Olive Leaves Using a New Adsorbent-Assisted Supercritical CO2 Process. Foods 2021, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Domínguez-Rodríguez, G.; Castro-Puyana, M.; Marina, M.L. Chapter 6: Polyphenols analysis and related challenges. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Amsterdam, The Netherlands, 2018; pp. 177–232. [Google Scholar]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Poklar Ulrih, N. Enhanced yield of oleuropein from olive leaves using ultrasound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Lucas, A.D.; Ossa, E.M.D.L.; Rincón, J.; Blanco, M.A.; Gracia, I. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Supercrit. Fluids 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Park, J.Y.; Gu, Y.M.; Chun, J.; Sang, B.I.; Lee, J.H. Pilot-scale continuous biogenic silica extraction from rice husk by one-pot alkali hydrothermal treatment and ball milling. Chem. Biol. Technol. Agric 2023, 10, 102. [Google Scholar] [CrossRef]
- Chen, X.; Guo, M.; Sang, Y.; Sun, J. Effect of ball-milling treatment on the structure, physicochemical properties and allergenicity of proteins from oyster (Crassostrea gigas). Lebensm. Wiss. Technol. 2022, 166, 113803. [Google Scholar] [CrossRef]
- Julakanti, S.; Charles, A.P.R.; Zhao, J.; Bullock, F.; Syed, R.; Myles, Y.; Wu, Y. Hempseed protein (Cannabis sativa L.): Influence of extraction pH and ball milling on physicochemical and functional properties. Food Hydrocoll. 2023, 143, 108835. [Google Scholar] [CrossRef]
- Ronca, C.L.; Marques, S.S.; Ritieni, A.; Giménez-Martínez, R.; Barreiros, L.; Segundo, M.A. Olive Oil Waste as a Source of Functional Food Ingredients: Assessing Polyphenolic Content and Antioxidant Activity in Olive Leaves. Foods 2024, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Carrara, M.; Kelly, M.T.; Griffin, L.; Margout-Jantac, D. Development and cross-validation of simple HPLC-fluorescence and UPLC-MS-UV methods for rapid determination of oleuropein in olive leaves. Phytochem. Anal. 2023, 35, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Ahin, S.; Lbay, Z.; Krbalar, S. Study on Optimum Extraction Conditions for Olive Leaf Extracts Rich in Polyphenol and Flavonoid. Sep. Sci. Technol. 2015, 5, 1181–1189. [Google Scholar]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, L.; Gao, S.; Zhu, C.; Yan, Y.; Liu, X.; Li, L.; Chen, H.Y. A Value-Added Utilization Method of Sugar Production By-Products from Rice Straw: Extraction of Lignin and Evaluation of Its Antioxidant Activity. Processes 2022, 10, 1210. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, T.; Peng, F.; Xu, F.; Sun, R. Separation and Structural Characterization of Lignin from Hybrid Poplar Based on Complete Dissolution in DMSO/LiCl. Sep. Sci. Technol. 2010, 45, 2497–2506. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the Aqueous Extraction of Phenolic Compounds from Olive Leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Ilbay, Z.; Sahin, S.; Bueyuekkabasakal, K. A novel approach for olive leaf extraction through ultrasound technology: Response surface methodology versus artificial neural networks. Korean J. Chem. Eng. 2014, 31, 1661–1667. [Google Scholar] [CrossRef]
- Niu, H.; Li, Y.; Lei, Y.; Zhang, L.; Peng, J.; Guo, S. Microwave Drying of Anthracite: A Parameter Optimized by Response Surface Methodology. Arab. J. Sci. Eng. 2012, 37, 65–73. [Google Scholar] [CrossRef]



| Independent Variable | Experimental Response | |||||
|---|---|---|---|---|---|---|
| Runs | Temperature (A) (°C) | Solvent-to-Solid Ratio (B) (mL/g) | Milling Speed (C) (rpm) | Recovery of Oleuropein (%) | Recovery of Luteoloside (%) | Recovery of Hydroxytyrosol (%) |
| 1 | 40 | 20 | 350 | 58.4 ± 0.2 | 54.2 ± 0.5 | 39.1 ± 0.4 |
| 2 | 40 | 40 | 350 | 61.9 ± 0.1 | 65.5 ± 0.2 | 35.7 ± 0.2 |
| 3 | 40 | 30 | 250 | 55.5 ± 0.6 | 58.2 ± 0.1 | 39.2 ± 0.7 |
| 4 | 40 | 30 | 450 | 65.7 ± 0.3 | 51.1 ± 0.3 | 33.7 ± 0.5 |
| 5 | 50 | 20 | 250 | 61.7 ± 0.3 | 64.9 ± 0.6 | 42.2 ± 0.1 |
| 6 | 50 | 40 | 250 | 68.1 ± 0.4 | 68.2 ± 0.5 | 42.6 ± 0.4 |
| 7 | 50 | 20 | 450 | 71.2 ± 0.1 | 54.7 ± 0.1 | 40.2 ± 0.7 |
| 8 | 50 | 40 | 450 | 76.9 ± 0.3 | 59.7 ± 0.3 | 41.3 ± 0.3 |
| 9 | 50 | 30 | 350 | 73.7 ± 0.2 | 73.2 ± 0.2 | 43.5 ± 0.2 |
| 10 | 50 | 30 | 350 | 74.2 ± 0.3 | 72.1 ± 0.7 | 43.4 ± 0.6 |
| 11 | 50 | 30 | 350 | 74.3 ± 0.3 | 71.5 ± 0.2 | 42.9 ± 0.1 |
| 12 | 50 | 30 | 350 | 72.9 ± 0.1 | 71.2 ± 0.6 | 43.4 ± 0.2 |
| 13 | 50 | 30 | 350 | 73.6 ± 0.7 | 70.8 ± 0.4 | 43.1 ± 0.2 |
| 14 | 60 | 20 | 350 | 71.4 ± 0.2 | 71.1 ± 0.1 | 38.4 ± 0.5 |
| 15 | 60 | 40 | 350 | 77.0 ± 0.4 | 73.0 ± 0.2 | 41.1 ± 0.2 |
| 16 | 60 | 30 | 250 | 67.1 ± 0.2 | 69.5 ± 0.8 | 39.1 ± 0.7 |
| 17 | 60 | 30 | 450 | 75.9 ± 0.1 | 64.7 ± 0.5 | 39.4 ± 0.1 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Inference |
|---|---|---|---|---|---|---|
| Model | 0.0702 | 9 | 0.0078 | 73.78 | <0.0001 | significant |
| A—Temperature | 0.0311 | 1 | 0.0311 | 294.45 | <0.0001 | significant |
| B—Solvent-to-solid ratio | 0.0056 | 1 | 0.0056 | 53.15 | 0.0002 | significant |
| C—Milling speed | 0.0174 | 1 | 0.0174 | 164.52 | <0.0001 | significant |
| AB | 0.0001 | 1 | 0.0001 | 1.04 | 0.3411 | not significant |
| AC | 0 | 1 | 0 | 0.4635 | 0.5179 | not significant |
| BC | 0 | 1 | 0 | 0.1159 | 0.7435 | not significant |
| A2 | 0.0105 | 1 | 0.0105 | 99.38 | <0.0001 | significant |
| B2 | 0.001 | 1 | 0.001 | 9.82 | 0.0165 | significant |
| C2 | 0.0031 | 1 | 0.0031 | 28.93 | 0.001 | significant |
| Residual | 0.0007 | 7 | 0.0001 | - | - | - |
| Lack of Fit | 0.0006 | 3 | 0.0002 | 6.55 | 0.0505 | not significant |
| Pure Error | 0.0001 | 4 | 0 | - | - | - |
| Cor Total | 0.0709 | 16 | - | - | - | - |
| Std. Dev. | 0.0103 | - | - | - | - | - |
| Mean | 0.6938 | - | - | - | - | - |
| C.V. % | 1.48 | - | - | - | - | - |
| R2 | 0.9896 | - | - | - | - | - |
| R2Adj | 0.9762 | - | - | - | - | - |
| R2Pred | 0.8586 | - | - | - | - | - |
| Adeq. Precision | 27.6616 | - | - | - | - | - |
| PRESS | 0.01 | - | - | - | - | - |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Inference |
|---|---|---|---|---|---|---|
| Model | 0.0838 | 9 | 0.0093 | 52.95 | <0.0001 | significant |
| A—Temperature | 0.0304 | 1 | 0.0304 | 172.84 | <0.0001 | significant |
| B—Solvent-to-solid ratio | 0.0058 | 1 | 0.0058 | 32.87 | 0.0007 | significant |
| C—Milling speed | 0.0117 | 1 | 0.0117 | 66.59 | <0.0001 | significant |
| AB | 0.0022 | 1 | 0.0022 | 12.57 | 0.0094 | significant |
| AC | 0.0001 | 1 | 0.0001 | 0.7524 | 0.4145 | not significant |
| BC | 0.0001 | 1 | 0.0001 | 0.411 | 0.5419 | not significant |
| A2 | 0.0049 | 1 | 0.0049 | 27.77 | 0.0012 | significant |
| B2 | 0.0024 | 1 | 0.0024 | 13.85 | 0.0074 | significant |
| C2 | 0.0236 | 1 | 0.0236 | 134.02 | <0.0001 | significant |
| Residual | 0.0012 | 7 | 0.0002 | - | - | - |
| Lack of Fit | 0.0009 | 3 | 0.0003 | 3.36 | 0.1359 | not significant |
| Pure Error | 0.0003 | 4 | 0.0001 | - | - | - |
| Cor Total | 0.085 | 16 | - | - | - | - |
| Std. Dev. | 0.0133 | - | - | - | - | - |
| Mean | 0.6551 | - | - | - | - | - |
| C.V. % | 2.02 | - | - | - | - | - |
| R2 | 0.9855 | - | - | - | - | - |
| R2Adj | 0.9669 | - | - | - | - | - |
| R2Pred | 0.8277 | - | - | - | - | - |
| Adeq. Precision | 21.7706 | - | - | - | - | - |
| PRESS | 0.0146 | - | - | - | - | - |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Inference |
|---|---|---|---|---|---|---|
| Model | 0.0126 | 9 | 0.0014 | 69.43 | <0.0001 | significant |
| A—Temperature | 0.0013 | 1 | 0.0013 | 65.86 | <0.0001 | significant |
| B—Solvent-to-solid ratio | 8 × 10−6 | 1 | 8 × 10−6 | 0.3973 | 0.5485 | not significant |
| C—Milling speed | 0.0009 | 1 | 0.0009 | 44.85 | 0.0003 | significant |
| AB | 0.0009 | 1 | 0.0009 | 46.2 | 0.0003 | significant |
| AC | 0.0008 | 1 | 0.0008 | 41.77 | 0.0003 | significant |
| BC | 0 | 1 | 0 | 0.6084 | 0.461 | not significant |
| A2 | 0.0074 | 1 | 0.0074 | 369.74 | <0.0001 | significant |
| B2 | 0.0001 | 1 | 0.0001 | 4.82 | 0.0642 | not significant |
| C2 | 0.0006 | 1 | 0.0006 | 30.36 | 0.0009 | significant |
| Residual | 0.0001 | 7 | 0 | - | - | - |
| Lack of Fit | 0.0001 | 3 | 0 | 6.12 | 0.0562 | not significant |
| Pure Error | 0 | 4 | 6.3 × 10−6 | - | - | - |
| Cor Total | 0.0127 | 16 | - | - | - | - |
| Std. Dev. | 0.0045 | - | - | - | - | - |
| Mean | 0.4049 | - | - | - | - | - |
| C.V. % | 1.11 | - | - | - | - | - |
| R2 | 0.9889 | - | - | - | - | - |
| R2Adj | 0.9747 | - | - | - | - | - |
| R2Pred | 0.8514 | - | - | - | - | - |
| Adeq. Precision | 26.7609 | - | - | - | - | - |
| PRESS | 0.0019 | - | - | - | - | - |
| Temperature (A) (°C) | Solvent-to-Solid Ratio (B) (mL/g) | Milling Speed (C) (rpm) | Predicted Recovery | Experimental Recovery | |
|---|---|---|---|---|---|
| Oleuropein | 56.4 | 39.1 | 429 | 78.8% | 79.0% ± 0.9% |
| Luteoloside | 58.4 | 31.3 | 328 | 74.9% | 74.6% ± 1.2% |
| Hydroxytyrosol | 51.5 | 32.7 | 317 | 43.6% | 43.1% ± 1.3% |
| Code Level | ||||
|---|---|---|---|---|
| Low | Center | High | ||
| Independent variable | Code variable | −1 | 0 | +1 |
| Temperature (°C) | A | 40 | 50 | 60 |
| Solvent-to-solid ratio (mL/g) | B | 20 | 30 | 40 |
| Milling speed (rpm) | C | 250 | 350 | 450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Q.; Wang, J.; Tao, K.; Huang, H.; Zhao, Y.; Jia, J.; Tan, H.; Chang, H. Optimization of Phenolic-Enriched Extracts from Olive Leaves via Ball Milling-Assisted Extraction Using Response Surface Methodology. Molecules 2024, 29, 3658. https://doi.org/10.3390/molecules29153658
Xiang Q, Wang J, Tao K, Huang H, Zhao Y, Jia J, Tan H, Chang H. Optimization of Phenolic-Enriched Extracts from Olive Leaves via Ball Milling-Assisted Extraction Using Response Surface Methodology. Molecules. 2024; 29(15):3658. https://doi.org/10.3390/molecules29153658
Chicago/Turabian StyleXiang, Qixuan, Jingyi Wang, Kan Tao, Hu Huang, Yaping Zhao, Jinping Jia, Huijun Tan, and Huailong Chang. 2024. "Optimization of Phenolic-Enriched Extracts from Olive Leaves via Ball Milling-Assisted Extraction Using Response Surface Methodology" Molecules 29, no. 15: 3658. https://doi.org/10.3390/molecules29153658
APA StyleXiang, Q., Wang, J., Tao, K., Huang, H., Zhao, Y., Jia, J., Tan, H., & Chang, H. (2024). Optimization of Phenolic-Enriched Extracts from Olive Leaves via Ball Milling-Assisted Extraction Using Response Surface Methodology. Molecules, 29(15), 3658. https://doi.org/10.3390/molecules29153658






