Synthesis of Pd-Doped SnO2 and Flower-like Hierarchical Structures for Efficient and Rapid Detection of Ethanolamine
Abstract
1. Introduction
2. Results and Discussion
3. Gas-Sensing Performance
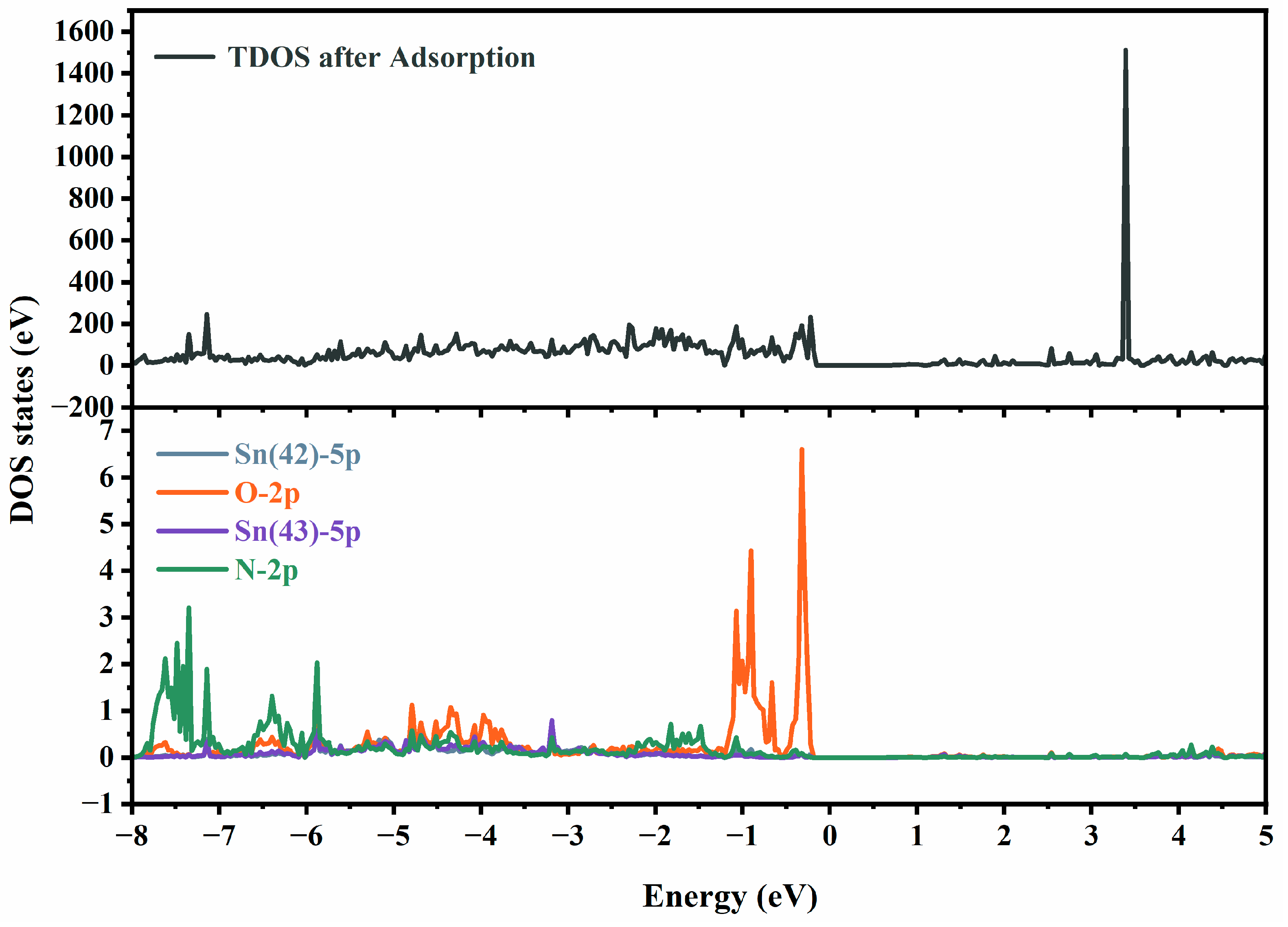
4. Gas-Sensing Mechanism
5. Experimental Section
5.1. Materials
5.2. Preparation of the Pd-SnO2 Flower-like Hierarchical Structure
5.3. Sensor Fabrication and Gas-Sensing Properties Test
5.4. Characterization
5.5. DFT Calculation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zheng, M.; Yang, M.; Zhang, X.; Cheng, X.; Zhou, X.; Gao, S.; Xu, Y.; Huo, L. Three-in-one Ni doped porous SnO2 nanorods sensor: Controllable oxygen vacancies content, surface site activation and low power consumption for highly selective NO2 monitoring. Sens. Actuators B Chem. 2023, 382, 133550. [Google Scholar] [CrossRef]
- Yu, D.J.; Oum, W.; Mirzaei, A.; Shin, K.Y.; Kim, E.B.; Kim, H.M.; Kim, S.S.; Kim, H.W. Enhancement of xylene gas sensing by using Au core structures in regard to Au@SnO2 core-shell nanocomposites. Sens. Actuators B Chem. 2023, 392, 134018. [Google Scholar] [CrossRef]
- Cao, P.F.; Ma, S.Y.; Xu, X.L.; Yang, T.T.; Han, T.; Xu, X.H.; Wen, D.P.; Wang, L.; Alhadi, A. Synthesis of popcorn-shaped yeast@WO3 microspheres assisted by yeast cells and their potential application as an ethanolamine gas sensor. Sens. Actuators A Phys. 2021, 318, 112516. [Google Scholar] [CrossRef]
- Chao, J.; Yu, H.; Zhang, K.; Zhou, Y.; Meng, D.; Sun, Y. Integration of ZnO and Au/ZnO Nanostructures into Gas Sensor Devices for Sensitive Ethanolamine Detection. ACS Appl. Nano Mater. 2023, 6, 5994–6001. [Google Scholar] [CrossRef]
- Bi, W.; Xiao, W.; Liu, S. Synthesis of Pt-doped SnO2 flower-like hierarchical structure and its gas sensing properties to isopropanol. J. Mater. Sci. 2021, 56, 6095–6109. [Google Scholar] [CrossRef]
- Si, R.; Li, Y.; Tian, J.; Tan, C.; Chen, S.; Lei, M.; Guo, X.; Zhang, S. The stability of SnO2 and In2O3 gas sensors to water under temperature modulation mode. Sens. Actuators B Chem. 2023, 393, 134222. [Google Scholar] [CrossRef]
- He, K.; Jin, Z.; Chu, X.; Bi, W.; Wang, W.; Wang, C.; Liu, S. Fast response–recovery time toward acetone by a sensor prepared with Pd doped WO3 nanosheets. RSC Adv. 2019, 9, 28439–28450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Bi, W.; Jin, Z.; Liu, S. Synthesis of hierarchical shell-core SnO2 microspheres and their gas sensing properties. Chin. Chem. Lett. 2020, 31, 2083–2086. [Google Scholar] [CrossRef]
- Hu, J.; Wang, T.; Wang, Y.; Huang, D.; He, G.; Han, Y.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Enhanced formaldehyde detection based on Ni doping of SnO2 nanoparticles by one-step synthesis. Sens. Actuators B Chem. 2018, 263, 120–128. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Fang, R.; Li, H.; He, W.; Du, C. UV-assisted room temperature NO2 sensor using monolayer graphene decorated with SnO2 nanoparticles. Ceram. Int. 2020, 46, 2255–2260. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, S.; Zhou, T.; Tu, J.; Zhang, T. Facile preparation of hierarchical structure based on p-type Co3O4 as toluene detecting sensor. Appl. Surf. Sci. 2020, 503, 144167. [Google Scholar] [CrossRef]
- Li, Y.; Luo, N.; Sun, G.; Zhang, B.; Jin, H.; Lin, L.; Bala, H.; Cao, J.; Zhang, Z.; Wang, Y. Synthesis of porous nanosheets-assembled ZnO/ZnCo2O4 hierarchical structure for TEA detection. Sens. Actuators B Chem. 2019, 287, 199–208. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, S.; Tie, Y.; Zhang, Q.; Wang, W.; Pei, S.; Xu, X. Highly sensitive formaldehyde gas sensors based on Y-doped SnO2 hierarchical flower-shaped nanostructures. J. Alloy. Compd. 2019, 792, 938–944. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Wang, W.; Xue, Y.; Sun, Y.; Li, P.; Lian, K.; Zhang, W.; Chen, L.; Shi, J.; et al. Synthesis and gas sensing properties of NiO/SnO2 hierarchical structures toward ppb-level acetone detection. Mater. Res. Bull. 2018, 102, 294–303. [Google Scholar] [CrossRef]
- Chu, L.; Zhang, J.; Wu, Z.; Wang, C.; Sun, Y.; Dong, S.; Sun, J. Solar-driven photocatalytic removal of organic pollutants over direct Z-scheme coral-branch shape Bi2O3/SnO2 composites. Mater. Charact. 2020, 159, 110036. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhao, L.; Liu, F.; Sun, X.; Hu, X.; Lu, G. Preparation of Ce-doped SnO2 cuboids with enhanced 2-butanone sensing performance. Sens. Actuators B Chem. 2021, 341, 130039. [Google Scholar] [CrossRef]
- Shang, W.; Wang, D.; Zhang, B.; Jiang, C.; Qu, F.; Yang, M. Aliovalent Fe(iii)-doped NiO microspheres for enhanced butanol gas sensing properties. Dalton Trans. 2018, 47, 15181–15188. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Li, S.; Zhao, F.; Dong, T.; He, P.; Yu, L.; Miao, J.; Fan, X. Cu-doped flower-like SnO2 architecture toward promoting SO2 detection: Fast equilibrium and low trace monitoring. Sens. Actuators B Chem. 2023, 390, 133953. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Wang, C.; Ma, J.; Xu, L.; Lv, C.; Sun, Y. Investigation of doping effects of different noble metals for ethanol gas sensors based on mesoporous In2O3. Nanotechnology 2021, 32, 305503. [Google Scholar] [CrossRef]
- Meng, L.; Li, Y.; Yang, M.; Chuai, X.; Zhou, Z.; Hu, C.; Sun, P.; Liu, F.; Yan, X.; Lu, G. Temperature-controlled resistive sensing of gaseous H2S or NO2 by using flower-like palladium-doped SnO2 nanomaterials. Mikrochim Acta 2020, 187, 297. [Google Scholar] [CrossRef]
- RG, A.; Ahirwar, S.; Singh, K.; Andhiwal, A.; Basu, P.K. Design, Fabrication, and Packaging of an Optothermally Activated Nanocrystalline Pd–ZnO-Based Selective CO Sensor on a Screen-Printed In-Plane Heater. ACS Appl. Electron. Mater. 2022, 4, 1651–1668. [Google Scholar] [CrossRef]
- Bi, W.; Wang, W.; Liu, S. Synthesis of Rh–SnO2 nanosheets and ultra-high triethylamine sensing performance. J. Alloy. Compd. 2020, 817, 152730. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Z.; Chen, C.; Wen, T.; Zhao, X.; Xie, L. Highly sensitive H2S gas sensors based on Pd-doped CuO nanoflowers with low operating temperature. Sens. Actuators B Chem. 2017, 253, 809–817. [Google Scholar] [CrossRef]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: A combined experimental and first-principle study. Sens. Actuators B Chem. 2019, 296, 126710. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B Chem. 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Khan, R.; Zulfiqar; Zaman, Y. Effect of annealing on structural, dielectric, transport and magnetic properties of (Zn, Co) co-doped SnO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2015, 27, 4003–4010. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Han, B.; Xiao, X.; Chen, G.; Djerdj, I.; Wang, Y. Nanoparticle cluster gas sensor: Pt activated SnO2 nanoparticles for NH3 detection with ultrahigh sensitivity. Nanoscale 2015, 7, 14872–14880. [Google Scholar] [CrossRef]
- Bharathi, P.; Krishna Mohan, M.; Shalini, V.; Harish, S.; Navaneethan, M.; Archana, J.; Ganesh Kumar, M.; Dhivya, P.; Ponnusamy, S.; Shimomura, M.; et al. Growth and influence of Gd doping on ZnO nanostructures for enhanced optical, structural properties and gas sensing applications. Appl. Surf. Sci. 2020, 499, 143857. [Google Scholar] [CrossRef]
- He, P.; Fu, H.; Gong, Y.; Chen, J.; Yang, X.; Han, D.; Xiong, S.; Li, S.; An, X. SnO2@ZnS core-shell hollow spheres with enhanced room-temperature gas-sensing performance. Sens. Actuators B Chem. 2023, 388, 133809. [Google Scholar] [CrossRef]
- Lu, W.; Ding, D.; Xue, Q.; Du, Y.; Xiong, Y.; Zhang, J.; Pan, X.; Xing, W. Great enhancement of CH4 sensitivity of SnO2 based nanofibers by heterogeneous sensitization and catalytic effect. Sens. Actuators B Chem. 2018, 254, 393–401. [Google Scholar] [CrossRef]
- Bu, W.; Zhang, Y.; Qin, Q.; Li, Y.; Chuai, X.; Zhou, Z.; Hu, C.; Wang, T.; Sun, P.; Liu, F.; et al. Improved ppb-level NO2 conductometric sensor induced by trace Au on SnO2 nanosheet. Sens. Actuators B Chem. 2023, 379, 133237. [Google Scholar] [CrossRef]
- Xu, S.; Wang, M.; Chen, C.-P.; Feng, S. Sea urchin-like SnO2/α-Fe2O3 heterostructural microspheres for enhanced acetone gas sensing: Materials preparation, performance evaluation, and mechanism investigation. Sens. Actuators B Chem. 2023, 379, 133288. [Google Scholar] [CrossRef]
- Qiao, L.; Bing, Y.; Wang, Y.; Yu, S.; Liang, Z.; Zeng, Y. Enhanced toluene sensing performances of Pd-loaded SnO2 cubic nanocages with porous nanoparticle-assembled shells. Sens. Actuators B Chem. 2017, 241, 1121–1129. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Xie, N.; Chen, F.; Wang, T.; Guo, L.; Wang, C.; Wang, Q.; Ma, J.; Sun, Y.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B Chem. 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Kou, H.; Shao, T.; Dong, J.; Cheng, Y.; Zhang, F.; Guo, J.; Liu, X.; Wang, X. Ethanol sensor built on a SnO2/In2O3 composite generated from MOF. Sens. Actuators B Chem. 2023, 396, 134628. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Liu, Y.; Enyashin, A.N.; Yang, X.; Zhou, J.; Jin, W.; Chen, W. Metal cations doped vanadium oxide nanotubes: Synthesis, electronic structure, and gas sensing properties. Sens. Actuators B Chem. 2018, 256, 1021–1029. [Google Scholar] [CrossRef]
- Ahmed, A.; Siddique, M.N.; Ali, T.; Tripathi, P. Defect assisted improved room temperature ferromagnetism in Ce doped SnO2 nanoparticles. Appl. Surf. Sci. 2019, 483, 463–471. [Google Scholar] [CrossRef]
- Dai, H.; Ding, J.; Chen, H.; Fu, H. Improvement of ethanolamine sensing performance based on Au-modified ZnO rod-like nanoflowers. Mater. Lett. 2023, 340, 134183. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, W.; Gao, N.; Zhao, R.; Ahmed, M.M.; Zhang, T.; Li, J.; Du, J.; Asefa, T. Highly sensitive and selective gas-phase ethanolamine sensor by doping sulfur into nanostructured ZnO. Sens. Actuators B Chem. 2019, 296, 126633. [Google Scholar] [CrossRef]
- Tomer, V.K.; Devi, S.; Malik, R.; Nehra, S.P.; Duhan, S. Highly sensitive and selective volatile organic amine (VOA) sensors using mesoporous WO3–SnO2 nanohybrids. Sens. Actuators B Chem. 2016, 229, 321–330. [Google Scholar] [CrossRef]
- Kulandaisamy, A.J.; Elavalagan, V.; Shankar, P.; Mani, G.K.; Babu, K.J.; Rayappan, J.B.B. Nanostructured Cerium-doped ZnO thin film—A breath sensor. Ceram. Int. 2016, 42, 18289–18295. [Google Scholar] [CrossRef]
- Bi, W.; Chen, H.; Yang, S.; Wang, X.; Liu, A.; Ma, X.; Xie, H.; Liu, S. The Three-Dimensional Hierarchical Structure of Bix-Sn1–xO2 Used for Ultrasensitive Detection of Butanone under UV Irradiation. ACS Omega 2024, 9, 27932–27944. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, S.; Chen, Y.; Liu, W.; Wang, X.; Jiang, H.; Ma, S.; Yun, P. CsPbBr3-Based Nanostructures for Room-Temperature Sensing of Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2022, 14, 39524–39534. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, H.; Liu, W.; Wang, S.; Wang, X.; Wang, M.; Ma, W.; Sun, G.; Liu, J. Design of Ti3C2Tx/SnO2-Selective Ethanolamine Sensor. ACS Appl. Nano Mater. 2024, 7, 4324–4335. [Google Scholar] [CrossRef]
- Li, X.; Ge, W.; Wang, P.; Han, K.; Zhao, H.; Zhang, Q.; Diwu, H.; Liu, Z. Near-infrared enhanced SnO2/SnSe2 heterostructures for room-temperature NO2 detection: Experiments and DFT calculations. Sens. Actuators B Chem. 2023, 397, 134643. [Google Scholar] [CrossRef]
- Yang, W.; Fang, B.; Xiao, X.; Meng, H.; Liu, S. Hierarchical core-shell heterostructures of α-MoO3 nanorods@NiO nanosheets for significant detection of ethyl acetate vapor. Sens. Actuators B Chem. 2022, 358, 131457. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Zhang, B.; Xue, Z.; Mu, T. Substrate molecule adsorption energy: An activity descriptor for electrochemical oxidation of 5-Hydroxymethylfurfural (HMF). Chem. Eng. J. 2022, 433, 133842. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Liu, S.; Zhang, J.; Fu, X.Z.; Luo, J.L. Folic Acid Self-Assembly Enabling Manganese Single-Atom Electrocatalyst for Selective Nitrogen Reduction to Ammonia. Nanomicro Lett. 2021, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Lu, J.; Yang, B.; San, X.; Wu, Z.-S. 2D intrinsically defective RuO2/Graphene heterostructures as All-pH efficient oxygen evolving electrocatalysts with unprecedented activity. Nano Energy 2020, 78, 105185. [Google Scholar] [CrossRef]
- Yan, F.; Shen, G.; Yang, X.; Qi, T.; Sun, J.; Li, X.; Zhang, M. Low operating temperature and highly selective NH3 chemiresistive gas sensors based on Ag3PO4 semiconductor. Appl. Surf. Sci. 2019, 479, 1141–1147. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]







| Space Group | P42/mnm | |
|---|---|---|
| Lattice parameters | a (Å) | 4.7427 |
| b (Å) | 4.7427 | |
| c (Å) | 3.1907 | |
| Unit cell volume (Å3) | 71.7674 | |
| Element coordinates Sn | x | 0 |
| y | 0 | |
| z | 0 | |
| O | x | 0.3024 |
| y | 0.3024 | |
| z | 0 | |
| Average grain size (nm) | 9.3810 | |
| Average maximum microstrain | 0.0022 | |
| Rwp (%) | 8.48% | |
| GOF-index | 1.37 |
| Materials | Con. (ppm) | T (°C) | Res. | Ref. |
|---|---|---|---|---|
| Popcorn-shaped yeast@WO3 | 100 | 180 | 6.80 | [3] |
| Au-ZnO hollow microsphere | 100 | 240 | 68.8 | [4] |
| Au/ZnO rod-like nanoflower | 100 | 210 | 138.3 | [39] |
| S-ZnO nanoflower | 100 | 240 | 84.0 | [40] |
| WO3-SnO2 nanocomposite | 50 | 220 | 13.0 | [41] |
| Ce-doped ZnO thin films | 100 | 25 | 65.0 | [42] |
| Pd-SnO2 nanoflower | 100 | 150 | 106 | This work |
| Adsorption Models | Adsorbents | EA(g) (eV) | Esurf (eV) | EA/surf (eV) | Eads (eV) |
|---|---|---|---|---|---|
| Optimized SnO2 (110) surface | ethanol | −46.940 | −580.882 | −629.756 | −1.934 |
| acetone | −56.041 | −580.882 | −638.256 | −1.333 | |
| formaldehyde | −22.163 | −580.882 | −604.159 | −1.114 | |
| ether | −79.841 | −580.882 | −661.198 | −0.475 | |
| ethylamine | −58.630 | −580.882 | −641.902 | −2.390 | |
| Pd atoms replacing Sn5c atoms | ethanol | −46.940 | −886.404 | −934.035 | −0.691 |
| acetone | −56.041 | −886.404 | −944.191 | −1.746 | |
| formaldehyde | −22.163 | −886.404 | −910.178 | −1.610 | |
| ether | −79.841 | −886.404 | −966.752 | −0.507 | |
| ethylamine | −58.630 | −886.404 | −948.017 | −2.983 | |
| Pd atoms replacing Sn6c atoms | ethanol | −46.940 | −886.714 | −935.727 | −2.073 |
| acetone | −56.041 | −886.714 | −944.239 | −1.484 | |
| formaldehyde | −22.163 | −886.714 | −910.938 | −2.061 | |
| ether | −79.841 | −886.714 | −967.050 | −0.495 | |
| ethylamine | −58.630 | −886.714 | −949.151 | −3.807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, W.; Zhu, J.; Zheng, B.; Liu, S.; Zhang, L. Synthesis of Pd-Doped SnO2 and Flower-like Hierarchical Structures for Efficient and Rapid Detection of Ethanolamine. Molecules 2024, 29, 3650. https://doi.org/10.3390/molecules29153650
Bi W, Zhu J, Zheng B, Liu S, Zhang L. Synthesis of Pd-Doped SnO2 and Flower-like Hierarchical Structures for Efficient and Rapid Detection of Ethanolamine. Molecules. 2024; 29(15):3650. https://doi.org/10.3390/molecules29153650
Chicago/Turabian StyleBi, Wenjie, Jinmiao Zhu, Bin Zheng, Shantang Liu, and Lilong Zhang. 2024. "Synthesis of Pd-Doped SnO2 and Flower-like Hierarchical Structures for Efficient and Rapid Detection of Ethanolamine" Molecules 29, no. 15: 3650. https://doi.org/10.3390/molecules29153650
APA StyleBi, W., Zhu, J., Zheng, B., Liu, S., & Zhang, L. (2024). Synthesis of Pd-Doped SnO2 and Flower-like Hierarchical Structures for Efficient and Rapid Detection of Ethanolamine. Molecules, 29(15), 3650. https://doi.org/10.3390/molecules29153650






