New Glycotoxin Inhibitor from Sesuvium sesuvioides Mitigates Symptoms of Insulin Resistance and Diabetes by Suppressing AGE-RAGE Axis in Skeletal Muscle
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of Compounds from S. sesuvioides
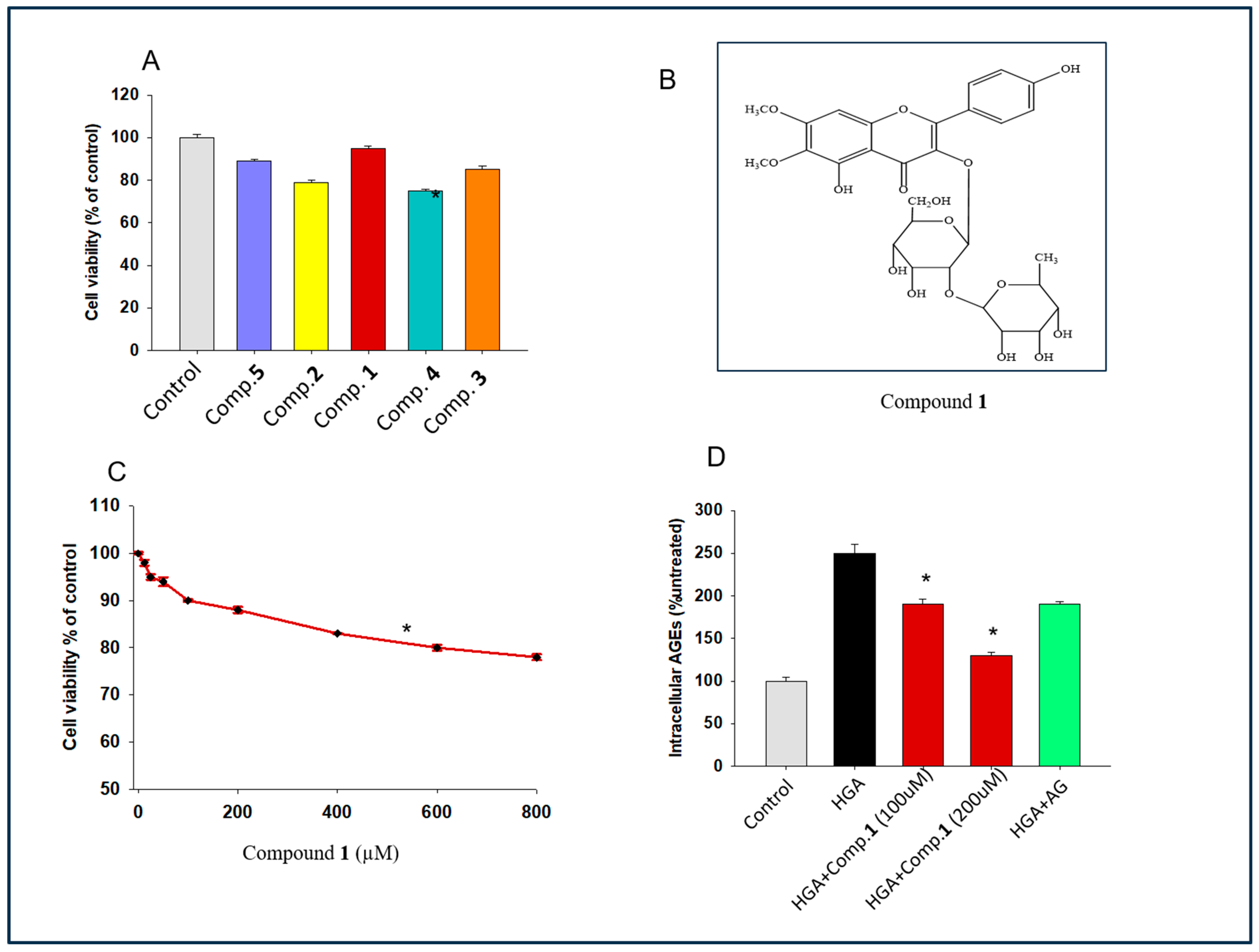
2.2. Exploring the Effect of Isolated Flavonoid Glycoside on Cell Proliferation
2.3. Determination of the Effect of Compound 1 on Intracellular Glycotoxin Formation
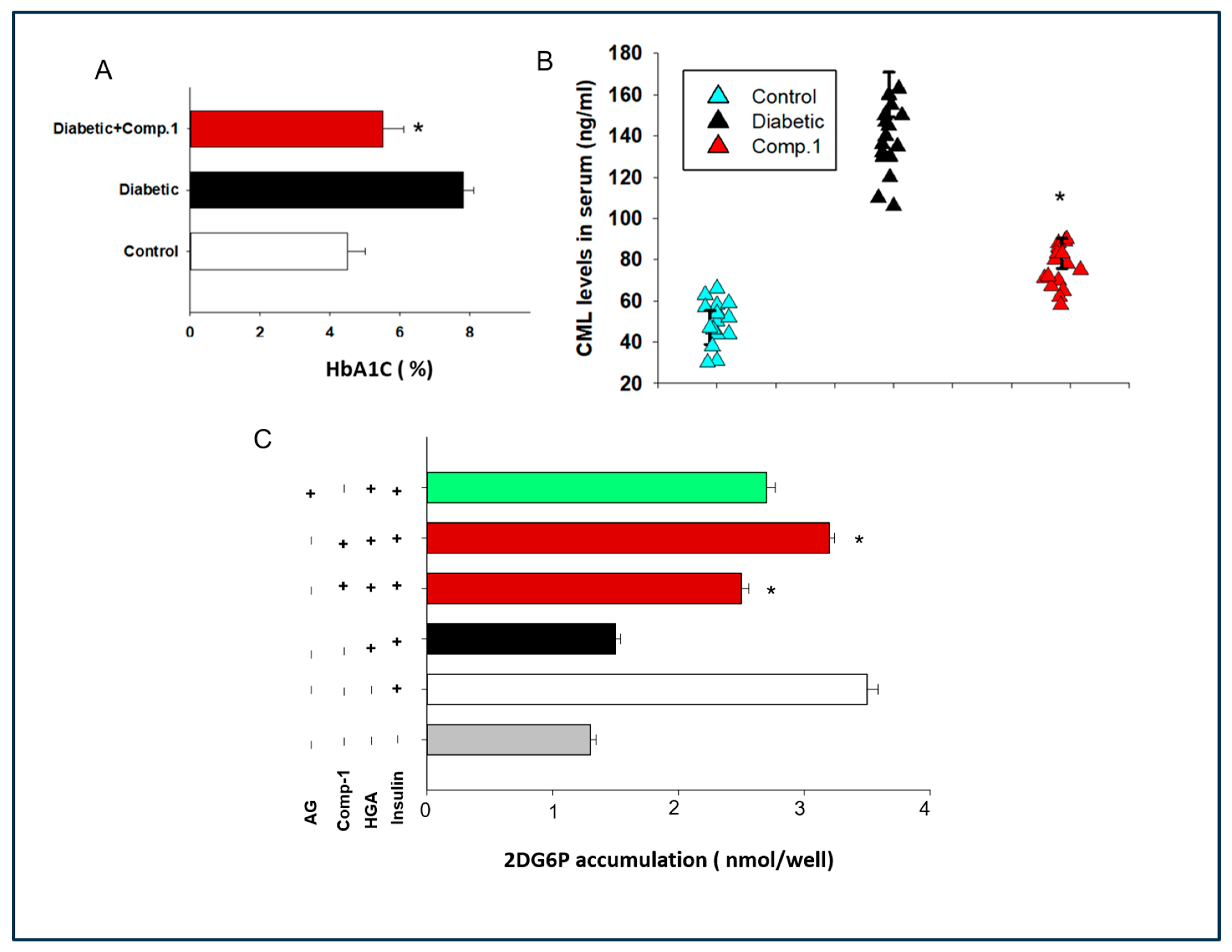
2.4. Determination of the Role of 1 against Serum AGEs Levels and HBA1c Levels in Mice
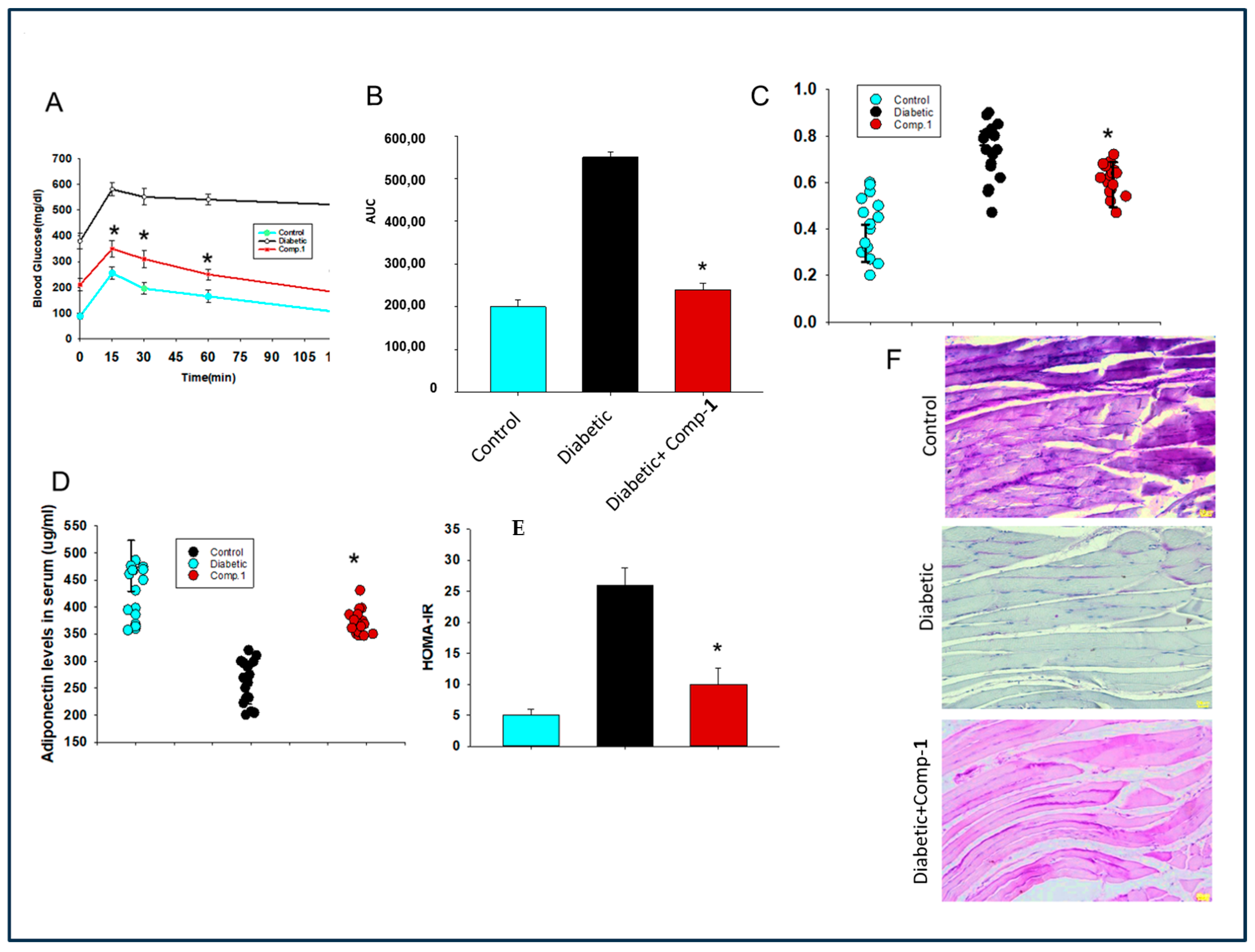
2.5. Assessment of Effect of Anti-AGE Compound on Glucose Tolerance and Insulin Secretion in a Mouse Model of Diabetes
2.6. Evaluation of Effect of 1 on Insulin Resistance and Hypo-Adiptonectinemia
2.7. Assessment of the Effect of the Anti-AGE Compound on Glycogen Synthesis in Muscle Tissue of Diabetic Mice
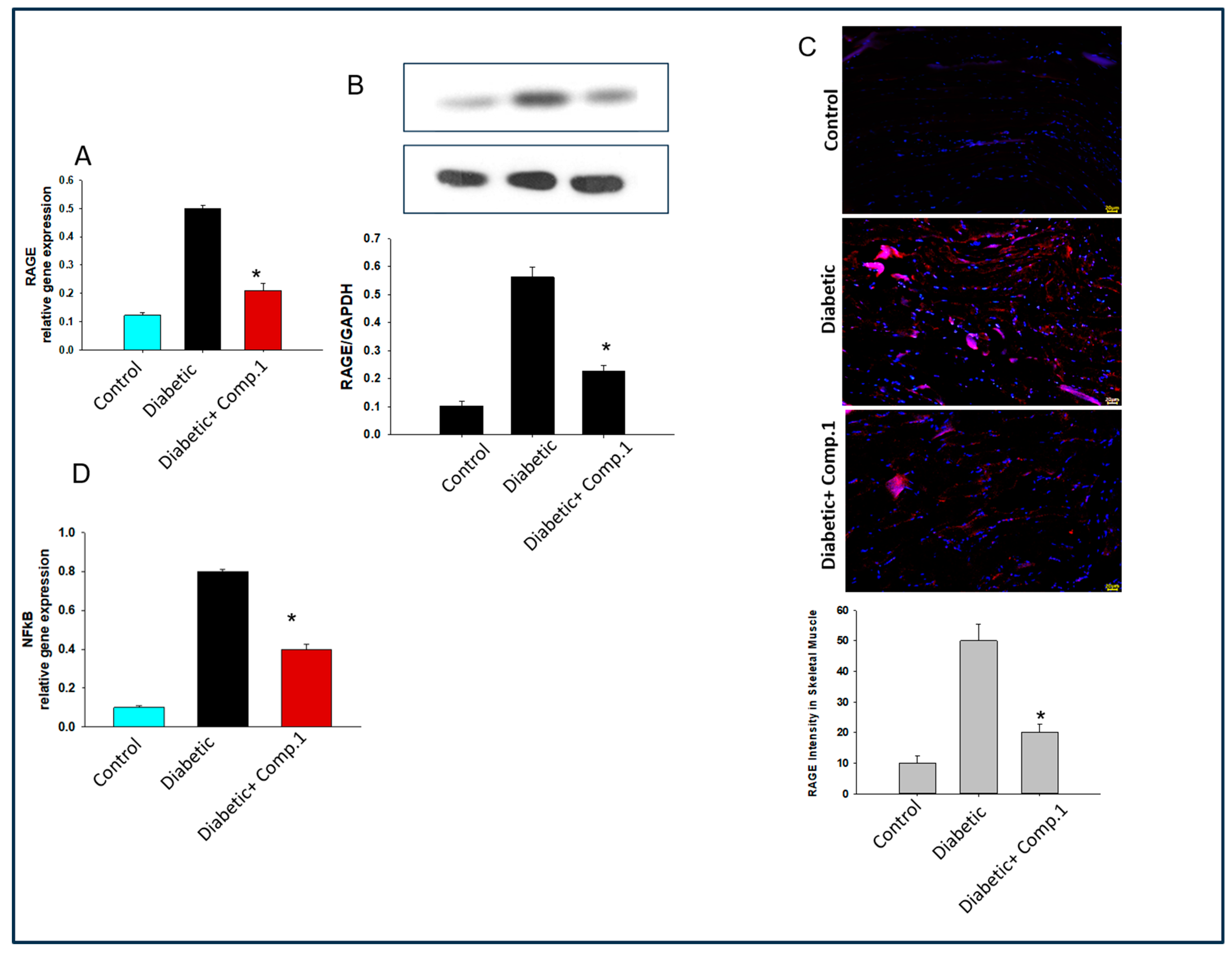
2.8. Deciphering the Molecular Mechanism behind the Role of 1 Mediated Improvement in Insulin Resistance
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Extraction and Isolation of Compounds from S. sesuvioides
4.4. Cell Culture
4.4.1. Cell Viability Assay
4.4.2. Anti-AGEs Assay
4.5. In Vivo Study
4.5.1. Sample Collection
4.5.2. Immunological Assays
4.5.3. Glucose Uptake Assay
4.5.4. Periodic Acid–Schiff (PAS) Staining for Glycogen Synthesis
4.5.5. Gene Expression by Real-Time PCR
4.5.6. Western Blot
4.5.7. Immunohistochemistry
4.6. Measurement of Parameters of Oxidative Stress
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; Scientific Committee: Brussels, Belgium, 2021. [Google Scholar]
- Tian, Z.; Chen, S.; Shi, Y.; Wang, P.; Wu, Y.; Li, G. Dietary Advanced Glycation End Products (dAGEs): An Insight between Modern Diet and Health. Food Chem. 2023, 415, 135735. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.J.M.; Hanssen, N.M.J.; van Greevenbroek, M.M.; Van der Kallen, C.J.; Feskens, E.J.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. Dietary Intake of Advanced Glycation Endproducts Is Associated with Higher Levels of Advanced Glycation Endproducts in Plasma and Urine: The CODAM Study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Fu, Z.; Liu, D. Development of a nongenetic mouse model of type 2 diabetes. Exp. Diabetes Res. 2011, 2011, 416254. [Google Scholar] [CrossRef] [PubMed]
- Pepe, D.; Elliott, C.G.; Forbes, T.L.; Hamilton, D.W. Detection of Galectin-3 and Localization of Advanced Glycation End Products (AGE) in Human Chronic Skin Wounds. Histol. Histopathol. 2014, 29, 251–258. [Google Scholar] [PubMed]
- Beeri, M.S.; Uribarri, J.; Cai, W.; Buchman, A.S.; Haroutunian, V. Human Brain and Serum Advanced Glycation End Products Are Highly Correlated: Preliminary Results of Their Role in Alzheimer Disease and Type 2 Diabetes. Endocr. Pract. 2020, 26, 576–577. [Google Scholar] [CrossRef]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, Cross-Linking, and Advanced Glycation End Products in Aging Human Skeletal Muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Blood and Tissue Advanced Glycation End Products as Determinants of Cardiometabolic Disorders Focusing on Human Studies. Nutrients 2023, 15, 2002. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Yamada, K.; Hamaguchi, K.; Nishimura, M.; Hatakeyama, E.; Tsuchida, H.; Sakamoto, K.; Kashiwabara, H.; Yokoyama, T.; Ikeda, K.; et al. Immunohistochemical Study of Human Advanced Glycation End-Products (AGE) and Growth Factors in Cardiac Tissues of Patients on Maintenance Dialysis and with Kidney Transplantation. Clin. Nephrol. 1998, 49, 273–280. [Google Scholar] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of Disease: Advanced Glycation End-Products and Their Receptor in Inflammation and Diabetes Complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285–293. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef]
- Al-Saoudi, E.; Christensen, M.M.B.; Nawroth, P.; Fleming, T.; Hommel, E.E.; Jørgensen, M.E.; Fleischer, J.; Hansen, C.S. Advanced Glycation End-Products Are Associated with Diabetic Neuropathy in Young Adults with Type 1 Diabetes. Front. Endocrinol. 2022, 13, 891442. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Yabuuchi, J.; Ueda, S.; Yamagishi, S.-i.; Nohara, N.; Nagasawa, H.; Wakabayashi, K.; Matsui, T.; Yuichiro, H.; Kadoguchi, T.; Otsuka, T.; et al. Association of Advanced Glycation End Products with Sarcopenia and Frailty in Chronic Kidney Disease. Sci. Rep. 2020, 10, 17647. [Google Scholar] [CrossRef]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral Advanced Glycation Endproducts (AGEs) Promote Insulin Resistance and Diabetes by Depleting the Antioxidant Defenses AGE Receptor-1 and Sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Yap, F.Y.T.; Tong, D.C.K.; Andrikopoulos, S.; Gasser, A.; Thallas-Bonke, V.; Webster, D.E.; Miyazaki, J.-I.; Kay, T.W.; Slattery, R.M.; et al. Advanced Glycation End Products Are Direct Modulators of Beta-Cell Function. Diabetes 2011, 60, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ma, Y.; Wang, X.; Zhang, Y.; Zhu, L.; Shi, S.; Pan, S.; Liu, Z. Advanced Glycation End Products Induce Skeletal Muscle Atrophy and Insulin Resistance via Activating ROS-Mediated ER Stress PERK/FOXO1 Signaling. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E279–E287. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Ramdas, M.; Goodman, S.; Pyzik, R.; Chen, X.; Zhu, L.; Striker, G.E.; Vlassara, H. Restriction of Advanced Glycation End Products Improves Insulin Resistance in Human Type 2 Diabetes: Potential Role of AGER1 and SIRT1. Diabetes Care 2011, 34, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Sajid-Ur-Rehman, M.; Ishtiaq, S.; Khan, M.S.; Jabeen, Q.; Youssef, F.S.; Ahmed, S.A.; Elhady, S.S.; Ashour, M.L. Characterization of Sesuvium sesuvioides Using High-Performance Liquid Chromatography and Validation of Its In Vivo Anti-Hyperthyroidism Effect via Suppressing Oxidative Stress and Inflammatory Markers. ACS Omega 2023, 8, 45896–45905. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Munawar, M.; Iqbal, S.; Sadaf, Z. Ethno-Medicinal Uses of Wild Herbs and Shrubs of Tehsil Yazman, Punjab, Pakistan. Pak. J. Agric. Sci. 2019, 56, 735–741. [Google Scholar]
- Qasim, M.; Gulzar, S.; Khan, M.A. Halophytes as Medicinal Plants. In Proceedings of the NAM Meeting, Denizli, Turkey, 27–29 June 2011; pp. 330–343. [Google Scholar]
- Qureshi, R.; Bhatti, G.R. Ethnobotany of Plants Used by the Thari People of Nara Desert, Pakistan. Fitoterapia 2008, 79, 468–473. [Google Scholar] [CrossRef]
- Ahmad, S.; Wariss, H.M.; Alam, K.; Anjum, S.; Mukhtar, M. Ethnobotanical Studies of Plant Resources of Cholistan Desert, Pakistan. Int. J. Sci. Res. 2014, 3, 1782–1788. [Google Scholar]
- Sajid-Ur-Rehman, M.; Ishtiaq, S.; Khan, M.A.; Alshamrani, M.; Younus, M.; Shaheen, G.; Abdullah, M.; Sarwar, G.; Khan, M.S.; Javed, F. Phytochemical Profiling, In Vitro and In Vivo Anti-Inflammatory, Analgesic and Antipyretic Potential of Sesuvium sesuvioides (Fenzl) Verdc. (Aizoaceae). Inflammopharmacology 2021, 29, 789–800. [Google Scholar] [CrossRef]
- Sajid-Ur-Rehman, M.; Ishtiaq, S.; Aati, H.Y.; Sherif, A.E.; Khan, M.A.; Hussain, M.; Khan, M.S.; Ahmed, M.; Naseem, M.J.; Khan, K.-u.-R. Antiarthritic Potential of the Butanol Fraction of Sesuvium sesuvioides: An In Vitro, In Vivo, and In Silico Evaluation. Front. Pharmacol. 2023, 14, 1136459. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef]
- Bonina, F.; Puglia, C.; Ventura, D.; Aquino, R.P.; Tortora, S.; Sacchi, A.; Saija, A.; Tomanio, A.; Pellegrino, M.L.; Capraraiis, P. In Vitro Antioxidant and In Vivo Photoprotective Effects of a Lyophilized Extract of Capparis spinosa L. Buds. J. Cosmet. Sci. 2002, 53, 321–335. [Google Scholar] [PubMed]
- Yamamoto, N.; Sato, T.; Kawasaki, K.; Murosaki, S.; Yamamoto, Y. A Nonradioisotope, Enzymatic Assay for 2-Deoxyglucose Uptake in L6 Skeletal Muscle Cells Cultured in a 96-Well Microplate. Anal. Biochem. 2006, 351, 139–145. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Afridi, S.K.; Aftab, M.F.; Murtaza, M.; Ghaffar, S.; Karim, A.; Mughal, U.R.; Khan, K.M.; Waraich, R.S. A New Glycotoxins Inhibitor Attenuates Insulin Resistance in Liver and Fat Cells. Biochem. Biophys. Res. Commun. 2016, 476, 188–195. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in Inflammatory Diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Soni, P. RAGE Inhibitors in Neurodegenerative Diseases. Biomedicines 2023, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Bertics, P.J. Human Eosinophils Express RAGE, Produce RAGE Ligands, Exhibit PKC-Delta Phosphorylation and Enhanced Viability in Response to the RAGE Ligand, S100B. Int. Immunol. 2011, 23, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Bouzakri, K.; Karlsson, H.K.R.; Vestergaard, H.; Madsbad, S.; Christiansen, E.; Zierath, J.R. IRS-1 Serine Phosphorylation and Insulin Resistance in Skeletal Muscle from Pancreas Transplant Recipients. Diabetes 2006, 55, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Bross, P. A Cellular Viability Assay to Monitor Drug Toxicity. Methods Mol. Biol. 2010, 648, 303–311. [Google Scholar] [PubMed]
- Sultan, K.R.; Henkel, B.; Terlou, M.; Haagsman, H.P. Quantification of Hormone-Induced Atrophy of Large Myotubes from C2C12 and L6 Cells: Atrophy-Inducible and Atrophy-Resistant C2C12 Myotubes. Am. J. Physiol. Cell Physiol. 2006, 290, C650–C659. [Google Scholar] [CrossRef] [PubMed]
- Weigert, C.; Hennige, A.M.; Brischmann, T.; Beck, A.; Moeschel, K.; Schauble, M.; Brodbeck, K.; Haring, H.U.; Schleicher, E.D.; Lehmann, R. The phosphorylation of Ser318 of insulin receptor substrate 1 is not per se inhibitory in skeletal muscle cells but is necessary to trigger the attenuation of the insulin-stimulated signal. J. Biol. Chem. 2005, 280, 37393–37399. [Google Scholar] [CrossRef]
- Waraich, R.S.; Zaidi, N.; Moeschel, K.; Beck, A.; Weigert, C.; Voelter, W.; Kalbacher, H.; Lehmann, R. Development and Precise Characterization of Phospho-Site-Specific Antibody of Ser357 of IRS-1: Elimination of Cross Reactivity with Adjacent Ser358. Biochem. Biophys. Res. Commun. 2008, 376, 26–31. [Google Scholar] [CrossRef]


| Position | δH (mult., J in Hz) | δc |
|---|---|---|
| 2 | - | 160.4 |
| 3 | - | 133.1 |
| 4 | - | 178.0 |
| 5 | - | 152.0 |
| 6 | - | 132.0 |
| 7 | - | 159.0 |
| 8 | 6.85 (s) | 91.7 |
| 9 | - | 152.1 |
| 10 | - | 105.7 |
| 1′ | - | 121.2 |
| 2′ & 6′ | 8.10 (d, 8.5) | 131.3 |
| 3′ & 5′ | 6.89 (d, 8.5) | 115.5 |
| 4′ | - | 156.9 |
| 1′ | 5.70 (d, 7.6) | 98.6 |
| 2′ | 3.45 (dd, 8.0, 7.8) | 78.0 |
| 3′ | 3.42 (t, 7.8) | 77.6 |
| 4′ | 3.76, overlapped | 71.0 |
| 5′ | 3.10, overlapped | 77.8 |
| 6′a | 3.56, m | 61.1 |
| 6′b | 3.28, (d, 11.4) | |
| 1′ | 5.10 (s) | 101.1 |
| 2′ | 3.09 (dd, 8.0, 7.8) | 70.6 |
| 3′ | 3.47 (t, 9.0) | 70.9 |
| 4′ | 3.15, overlapped | 72.2 |
| 5′ | 3.75, overlapped | 68.8 |
| 6′ | 0.79 (s) | 17.7 |
| 6-OCH3 | 3.71 (s) | 60.5 |
| 7-OCH3 | 3.89 (s) | 56.9 |
| 5-OH | 12.60 (s) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffar, S.; Waraich, R.S.; Orfali, R.; Al-Taweel, A.; Aati, H.Y.; Kamran, S.; Perveen, S. New Glycotoxin Inhibitor from Sesuvium sesuvioides Mitigates Symptoms of Insulin Resistance and Diabetes by Suppressing AGE-RAGE Axis in Skeletal Muscle. Molecules 2024, 29, 3649. https://doi.org/10.3390/molecules29153649
Ghaffar S, Waraich RS, Orfali R, Al-Taweel A, Aati HY, Kamran S, Perveen S. New Glycotoxin Inhibitor from Sesuvium sesuvioides Mitigates Symptoms of Insulin Resistance and Diabetes by Suppressing AGE-RAGE Axis in Skeletal Muscle. Molecules. 2024; 29(15):3649. https://doi.org/10.3390/molecules29153649
Chicago/Turabian StyleGhaffar, Safina, Rizwana Sanaullah Waraich, Raha Orfali, Areej Al-Taweel, Hanan Y. Aati, Sonia Kamran, and Shagufta Perveen. 2024. "New Glycotoxin Inhibitor from Sesuvium sesuvioides Mitigates Symptoms of Insulin Resistance and Diabetes by Suppressing AGE-RAGE Axis in Skeletal Muscle" Molecules 29, no. 15: 3649. https://doi.org/10.3390/molecules29153649
APA StyleGhaffar, S., Waraich, R. S., Orfali, R., Al-Taweel, A., Aati, H. Y., Kamran, S., & Perveen, S. (2024). New Glycotoxin Inhibitor from Sesuvium sesuvioides Mitigates Symptoms of Insulin Resistance and Diabetes by Suppressing AGE-RAGE Axis in Skeletal Muscle. Molecules, 29(15), 3649. https://doi.org/10.3390/molecules29153649





