Rational Fabrication of Nickel Vanadium Sulfide Encapsulated on Graphene as an Advanced Electrode for High-Performance Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
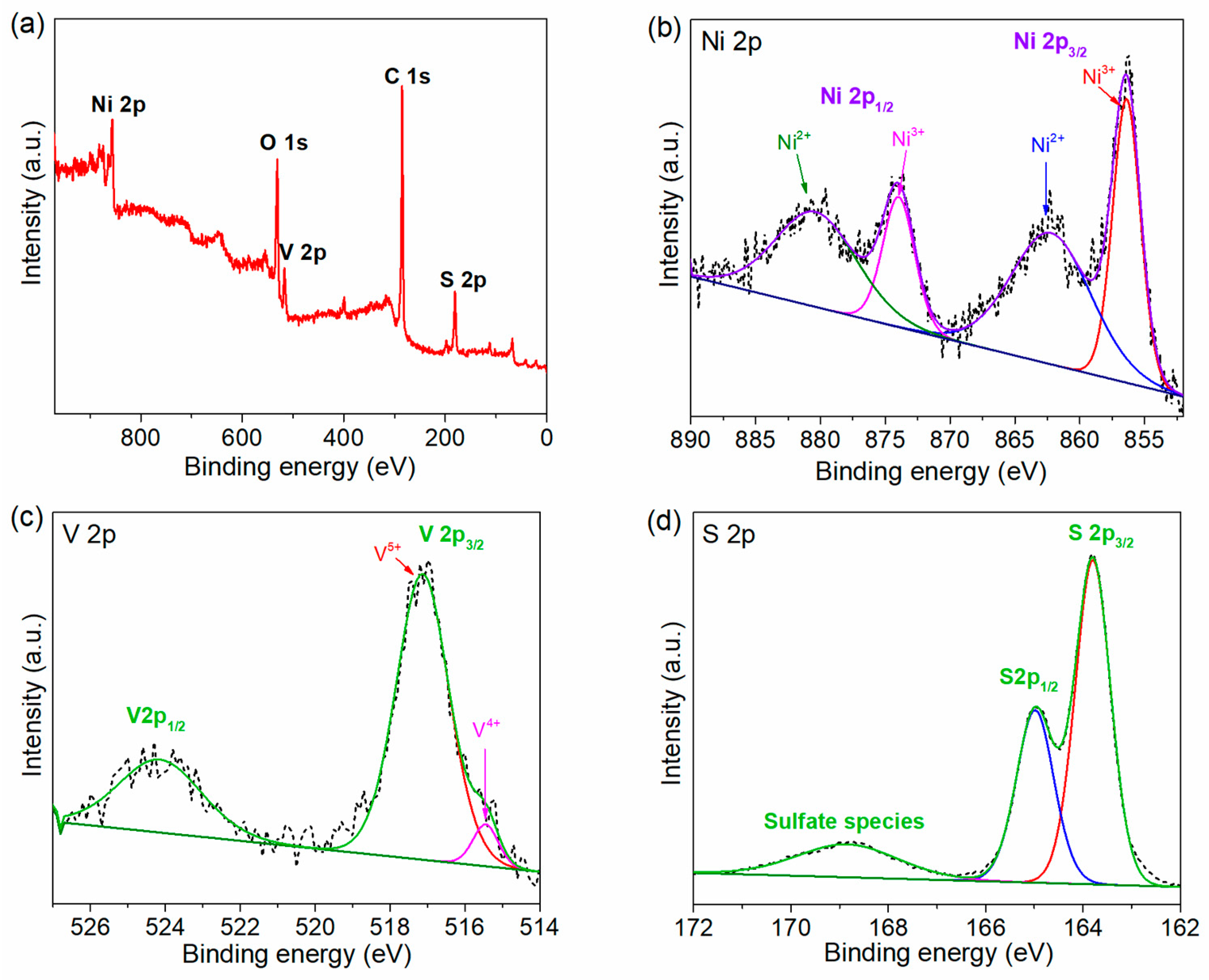
2.1. Morphology and Structural Analysis
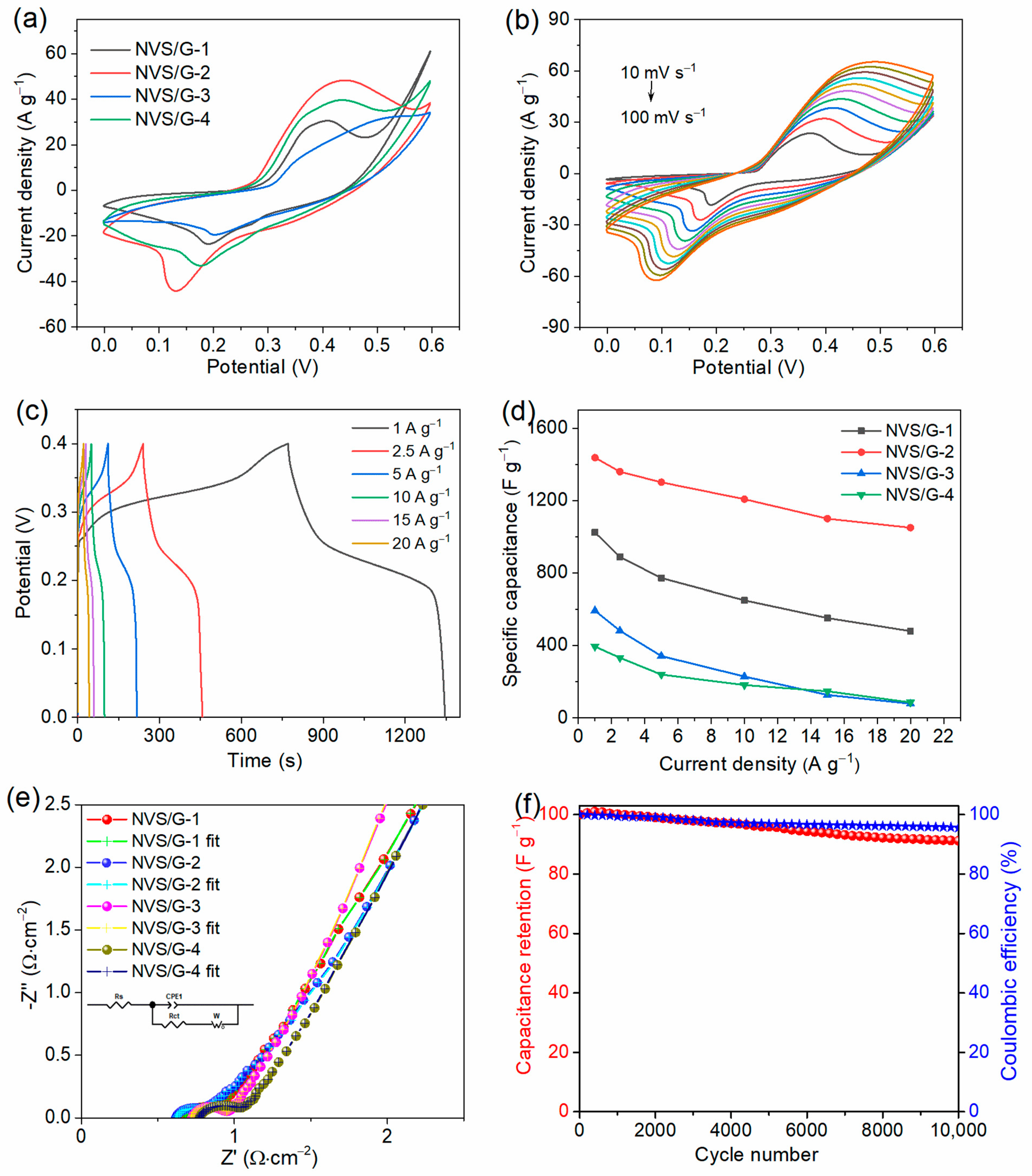
2.2. Electrochemical Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of the NVS/G Composite
3.3. Characterization of Materials
3.4. Electrochemical Analysis
3.5. Fabrication of the Asymmetric Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youssry, S.M.; Elkodous, M.A.; Kumar, R.; Kawamura, G.; Tan, W.K.; Matsuda, A. Thermal-assisted synthesis of reduced graphene oxide-embedded Ni nanoparticles as high-performance electrode material for supercapacitor. Electrochim. Acta 2023, 463, 142814. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Ma, Y.; Li, G.; Yuan, X.; Huang, Y.; Liu, G.; Guo, M.; Zheng, W. M2+-doped nickel selenide nanoflowers (M=Co, Cu, and Zn) for supercapacitors. ACS Appl. Nano Mater. 2024, 7, 8880–8889. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Ansari, S.A.; Kumar, P. Nanostructured titanium nitride and its composites as high-performance supercapacitor electrode material. Nanomaterials 2023, 13, 105. [Google Scholar] [CrossRef]
- Hao, Z.; Mahfooz, A.; Wu, Y.; Zeng, Y.; Gandi, A.N.; Zheng, J.; Zhu, W.; Wang, Z.; Liang, H. Synergy of VN and Fe2O3 Enables high performance anodes for asymmetricsupercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 18819–18827. [Google Scholar]
- Miah, M.; Hota, P.; Mondal, T.K.; Chen, R.; Saha, S.K. Mixed metal sulfides (FeNiS2) nanosheets decorated reduced graphene oxide for efficient electrode materials for supercapacitors. J. Alloys Compd. 2023, 933, 167648. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Abbasi, U.; Alzaid, M. Cobalt manganese phosphate and sulfate electrode materials for potential applications of battery-supercapacitor hybrid devices. J. Energy Storage 2022, 50, 104632. [Google Scholar] [CrossRef]
- Buldu-Akturk, M.; Toufani, M.; Tufanic, A.; Erdem, E. ZnO and reduced graphene oxide electrodes for all-in-one supercapacitor devices. Nanoscale 2022, 14, 3269. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, Z.; Cui, Z.; Hua, Y.; Wang, C.; Zhao, X.; Liu, X. Hierarchical copper cobalt sulfide nanobelt arrays for high performance asymmetric supercapacitors. Inorg. Chem. Front. 2021, 8, 3025–3036. [Google Scholar] [CrossRef]
- Zhou, A.; Chi, R.; Liu, R.; Shi, Y.; Zhang, Z.; Che, H.; Wang, G.; Mu, J.; Wang, Y.; Zhang, Z. Se-doped nickel-cobalt sulfide nanotube arrays with 3D networks for high-performance hybrid supercapacitor. Cream. Int. 2022, 48, 30536–30545. [Google Scholar] [CrossRef]
- Shaheen, N.; Aadil, M.; Zulfiqar, S.; Sabeeh, H.; Agboola, P.O.; Warsi, M.F.; Aboud MF, A.; Shakir, I. Fabrication of different conductive matrix supported binary metal oxides for supercapacitors applications. Ceram. Int. 2021, 47, 5273–5285. [Google Scholar] [CrossRef]
- Chen, X.; Sun, M.; Jaber, F.; Nezhad, E.Z.; Hui, K.S.; Li, Z.; Bae, S.; Ding, M. A flexible wearable self-supporting hybrid supercapacitor device based on hierarchical nickel cobalt sulfide@C electrode. Sci. Rep. 2023, 13, 15555. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, X.; Lu, S.; Han, E.; Chen, G.; Zhang, Z.; Zhang, H.; He, Y. Hydrothermal synthesis of vanadium doped nickel sulfide nanoflower for high-performance supercapacitor. J. Alloys Compd. 2022, 928, 167189. [Google Scholar] [CrossRef]
- Dai, J.; Soram, B.S.; Kim, N.H.; Lee, J.H. Achieving the high-energy-density asymmetric supercapacitor by hierarchical molybdenum-vanadium-sulphide@covalt sulphide core–shell nanoporous hybrid as cathode and iron oxide@carbon-nanofbers/nitrogen-doped reduced graphene oxide aerogel as anode. J. Energy Storage 2023, 69, 107939. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Miao, J.Y.; Yan, X.H.; Zhu, Y.H.; Li, Y.L.; Zhang, W.J.; Zhu, W.; Pan, J.M.; Javed, M.S.; Hussain, S. Vanadium disulfide nanosheets loaded on carbon cloth as electrode for flexible quasi-solid-state asymmetric supercapacitors: Energy storage mechanism and electrochemical performance. J. Mater. Chem. C 2022, 10, 640. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Liu, W.; Wang, S.; Wu, S.; Fang, S.; Cheng, F. Operando constructing vanadium tetrasulfide based heterostructures enabled by extrinsic adsorbed oxygen for enhanced zinc ion storage. J. Mater. Chem. A 2021, 9, 11433. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Cao, Y.; Zhou, W.; Chai, H. The ultralong cycle life of solid flexible asymmetric supercapacitors based on nickel vanadium sulfide nanospheres. CrystEngComm 2020, 22, 5226–5236. [Google Scholar] [CrossRef]
- Krishnan, S.; Gupta, A.K.; Singh, M.K.; Guha, N.; Rai, D.K. Nitrogen-rich Cu-MOF decorated on reduced graphene oxide nanosheets for hybrid supercapacitor applications with enhanced cycling stability. Chem. Eng. J. 2022, 435, 135042. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, H.; Cui, X. Design and fabrication of nickel vanadium sulfide directly grown on the basis of NiV-LDH nanosheets for high-performance supercapacitors. J. Energy Storage 2023, 63, 107117. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, Y.; Wan, L.; Du, C.; Zhang, Y.; Chen, J.; Xie, M. Structurally-stable Mg-Co-Ni LDH grown on reduced graphene by ball-milling and ion-exchange for highly-stable asymmetric supercapacitor. J. Colloid. Interf. Sci. 2023, 649, 519–527. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Pham, H.D.; Nanjundan, A.K.; Fernando, J.F.S.; Jayaramulu, K.; Golberg, D.; Han, Y.K.; Dubal, D.P. True meaning of pseudocapacitors and their performance metrics: Asymmetric versus hybrid supercapacitors. Small 2020, 16, 2002806. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Yao, F.; Yue, H. Controllable synthesis of vanadium-doped nickel-chalcogenide/graphene cathodes and MnV2O6·2H2O/graphene anode for high-energy asymmetric supercapacitors. J. Energy Storage 2023, 61, 106717. [Google Scholar] [CrossRef]
- Ochai-Ejeh, F.O.; Bello, A.; Dangbegnon, J.; Khaleed, A.A.; Madito, M.J.; Manyala, F.B.N. High electrochemical performance of hierarchical porous activated carbon derived from lightweight cork (Quercus suber). J. Mater. Sci. 2017, 52, 10600–10613. [Google Scholar] [CrossRef]
- Balu, R.; Dakshanamoorthy, A. One pot preparation of tin sulfide decorated graphene nanocomposite for high performance supercapacitor applications. Inorg. Chem. Commun. 2022, 136, 109148. [Google Scholar] [CrossRef]
- Kumar, S.; Riyajuddin, S.; Afshan, M.; Aziz, S.T.; Maruyama, T.; Ghosh, K. In-situ growth of urchin manganese sulfide anchored three-dimensional graphene (γ-MnS@3DG) on carbon cloth as a flexible asymmetric supercapacitor. J. Phys. Chem. Lett. 2021, 12, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Mao, J.; Niu, H.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. NiS2/MoS2 mixed phases with abundant active edge sites induced by sulfidation and graphene introduction towards high-rate supercapacitors. Chem. Eng. J. 2021, 406, 126713. [Google Scholar] [CrossRef]
- Manikandan, R.; Raj, C.J.; Yu, K.H.; Kim, B.C. Self-coupled nickel sulfide @ nickel vanadium sulfide nanostructure as a novel high capacity electrode material for supercapattery. Appl. Surf. Sci. 2019, 497, 143778. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, Y.; Li, Y. Nitrogen-doped nickel sulfide composite array electrode as an efficient electrocatalyst for hydrogen evolution reaction. J. Electron. Mater. 2021, 50, 5081–5089. [Google Scholar] [CrossRef]
- Singh, S.J.; Lim, Y.Y.; Hmar, J.J.L.; Chinnamuthu, P. Temperature dependency on Ce-doped CuO nanoparticles: A comparative study via XRD line broadening analysis. Appl. Phys. A 2022, 128, 188. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, M.; Zhao, J.; Shao, J.J.; Ding, Z. Iron-doped nickel sulfide nanospheres anchored on reduced graphene oxide for high performance supercapacitors. Mater. Chem. Front. 2024, 8, 1816. [Google Scholar] [CrossRef]
- Zong, H.; Zhang, A.; Dong, J.; He, J.; Fu, H.; Guo, H.; Liu, F.; Xu, J.; Liu, J. Flexible asymmetric supercapacitor based on open-hollow nickel-MOFs/reduced graphene oxide aerogel electrodes. Chem. Eng. J. 2023, 475, 146088. [Google Scholar] [CrossRef]
- Gan, Z.; Ren, X.; Sun, Y.; Yan, Y.; Cao, B.; Shen, W.; Yu, H.; Li, Z.; Fu, Y. Highly porous nanocomposites of Mn doped cobalt-based hydroxide/sulfide as high-performance electrode materials for hybrid supercapacitors. J. Energy Storage 2023, 69, 107934. [Google Scholar] [CrossRef]
- Guo, M.; Balamurugan, J.; Li, X.; Kim, N.H.; Lee, J.H. Hierarchical 3D cobalt-doped Fe3O4 nanospheres@NG hybrid as an advanced anode material for high-performance asymmetric supercapacitors. Small 2017, 13, 1701275. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Park, S.; Kim, B.J.; Heo, S.B.; Yu, J.H.; Shin, J.S.; Hong, J.-A.; Kim, B.-S.; Kim, Y.D.; Park, Y.; et al. Dual-functional quantum-dots light emitting diodes based on solution processable vanadium oxide hole injection layer. Sci. Rep. 2021, 11, 1700. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Liu, X.; Pan, H.; Wu, K.; Xue, Y.; Li, B.; Cao, Y. Facile fabrication of iron cobalt sulfide nanoparticles within N-doped graphene for high-performance supercapacitors. ACS Appl. Nano Mater. 2022, 5, 16553–16563. [Google Scholar] [CrossRef]
- Guo, M.; Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Facile fabrication of Co2CuS4 nanoparticle anchored N-doped graphene for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 17560. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, X.; Tian, B.; Guo, P.; Liang, J.; Wu, W. Hollow heterogeneous CuSe@MnSe for high-performance printed flexible supercapacitor. Chem. Eng. J. 2023, 471, 144590. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Yang, Z.; Zhang, X.; Zhang, Q. One-pot hydrothermal synthesis of nitrogen and phosphorus Co-doped graphene decorated with flower-like molybdenum sulfide for enhanced supercapacitor performance. Electrochim. Acta 2020, 331, 135265. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Bose, A.C. Recent advances and future perspectives of VS4 and its nanostructure composites for supercapacitor applications: A review. Energy Fuels 2023, 37, 10799–10826. [Google Scholar]
- Ng, S.; Ghosh, K.; Vyskocil, J.; Pumera, M. Two-dimensional vanadium sulfide flexible graphite/polymer films for near-infrared photoelectrocatalysis and electrochemical energy storage. Chem. Eng. J. 2022, 435, 135131. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Liu, Q.; Liu, L.; Zha, R.; Zhang, Y.; Li, Q.; Fang, L. An integrated cathode engineered by hierarchical honeycomb-like copper-molybdenum sulfide nanosheets for hybrid supercapacitor with improved energy storage capability. ChemElectroChem 2022, 9, e202200612. [Google Scholar]
- Huang, J.; Li, Z.; Liaw, B.Y.; Zhang, J. Graphical analysis of electrochemical impedance spectroscopy data in Bode and Nyquist representations. J. Power Sources 2016, 309, 82–98. [Google Scholar] [CrossRef]
- Ahmad, R.; Iqbal, N.; Noor, T.; Ali, G.; Ali, M.; Shahzad, N.; Raza, M.A. Zeolitic imidazolate frameworks derived Co-Zn-nanoporous carbon-sulfide material for supercapacitors. Electrochim. Acta 2022, 404, 139739. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, M.; Wang, A.; Liu, H.; Wang, Z.; Huang, C.; Yue, S.; Xie, J.; Hu, Z. Bimetallic organic framework in situ fabrication nanoflower-like cobalt nickel sulfide and ultrathin layered double hydroxide arrays for high-efficient asymmetric hybrid supercapacitor. J. Alloys Compd. 2023, 938, 168565. [Google Scholar] [CrossRef]
- Hassan, H.U.; Iqbal, M.W.; Zfzal, A.M.; Abbas, T.; Zaka, A.; Yasmeen, A.; Noor, N.A.; Aftab, S.; Ullah, H. Highly stable binary composite of nickel silver sulfide (NiAg2S) synthesized using the hydrothermal approach for high-performance supercapattery applications. Int. J. Energy Res. 2022, 46, 11346–11358. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Vallem, S.; Nallapureddy, R.R.; Adem, S.; Joo, S.W. Self-assembled hierarchical silkworm-type bimetallic sulfde (NiMo3S4) Nanostructures developed on SgC3N4 sheets: Promising electrode material for supercapacitors. ACS Appl. Energy Mater. 2023, 6, 812–821. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Y.; Li, C.; Chen, W.; Zhu, W.; Wang, L. Staggered nickel–vanadium layered double hydroxide nanosheets on reduced graphene oxide via in-situ growth for enhanced supercapacitor performance. J. Alloys Compd. 2023, 935, 168048. [Google Scholar] [CrossRef]
- Ndiaye, B.M.; Diop, M.; Gning, I.; Ngom, B.D.; Chaker, M. Synthesis of Ni-VO2@C/SO42− composite using cashew leaves for supercapacitor application. J. Energy Storage 2024, 81, 110307. [Google Scholar] [CrossRef]
- Tu, Q.; Zhang, Q.; Sun, X.; Wang, J.; Lin, B.; Chen, L.; Liu, J.; Deng, Z. Construction of three-dimensional nickel-vanadium hydrotalcite with ball-flower architecture for screen-printed asymmetric supercapacitor. Appl. Surf. Sci. 2023, 615, 156347. [Google Scholar] [CrossRef]
- Pathak, M.; Mane, P.; Chakraborty, B.; Cho, J.S.; Jeong, S.M.; Rout, C.S. Construction of nickel molybdenum sulfide/black phosphorous 3D hierarchical structure toward high performance supercapacitor electrodes. Small 2024, 20, 2310120. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, A.; Fu, H.; Zong, H.; Jin, F.; Zhao, K.; Liu, J. In Situ Generation of CeCoSx bimetallic sulfide derived from “Egg-Box” sea-weed biomass on S/N Co-doped graphene aerogels for flexible all solid-state supercapacitors. Chem. Eng. J. 2023, 453, 139633. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Vikraman, D.; Santhoshkumar, P.; Bathula, C.; Palem, R.R.; Kathalingam, A.; Kim, H.-S.; Kim, J.-H.; Kim, H.S. Sheet-like morphology CuCo2O4 bimetallic nanoparticles adorned on graphene oxide composites for symmetrical energy storage applications. J. Alloys Compd. 2021, 892, 162182. [Google Scholar] [CrossRef]
- Bokhari, S.W.; Wei, S.; Gao, W. Synthesis of bimetallic MoS2/VS2 nano-urchins-reduced graphene oxide hybrid nanocomposite for high performance supercapacitor application. Electrochim. Acta 2021, 398, 139300. [Google Scholar] [CrossRef]
- Basavaraju, S.K.; Chavati, G.B.; Yanjerappa, A.N.; Venkatesh, K.; Venkateswarlu, M.; Muralidhara, H.B. Synthesis of cauliflower-like reduced graphene oxide nickel sulfide nanocomposites for the vanadium redox battery and supercapacitor application. ACS Appl. Electron. Mater. 2023, 5, 3698–3707. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Hao, S.; Wu, H. Bimetal metal–organic framework-derived Ni–Mn@carbon/reduced graphene oxide as a cathode for an asymmetric supercapacitor with high energy density. Langmuir 2023, 39, 12510–12519. [Google Scholar] [CrossRef]
- Guo, M.; Balamurugan, J.; Kim, N.H.; Lee, J.H. High-energy solid-state asymmetric supercapacitor based on nickel vanadium oxide/NG and iron vanadium oxide/NG electrodes. Appl. Catal. B—Environ. 2018, 239, 290–299. [Google Scholar] [CrossRef]
- Devi, R.K.; Ganesan, M.; Chen, T.-W.; Chen, S.-M.; Akilarasan, M.; Rwei, S.-P.; Yu, J.; Elayappan, T.; Shaju, A. A facile strategy for the synthesis of manganese-doped nickel sulfide nanosheets and oxygen, nitrogen-enriched 3D-graphene-like porous carbon for hybrid supercapacitor. J. Alloys Compd. 2023, 944, 169261. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Vikraman, D.; Hussain, S.; Thirumalraj, B.; Santhoshkumar, P.; Parangusan, H.; Park, H.-C.; Jung, J.; Kim, H.-S. Unveiling the redox electrochemistry of 1D, urchin-like vanadium sulfide electrodes for high-performance hybrid supercapacitors. J. Energy Chem. 2023, 79, 569–580. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Wei, Z.; Zhang, X.; Wang, R. Ultrafast microwave synthesis of nickel-cobalt sulfide/graphene hybrid electrodes for high-performance asymmetrical supercapacitors. ACS Appl. Energy Mater. 2021, 4, 8262–8274. [Google Scholar] [CrossRef]
- Konwar, M.; Singh, R.S.K.; Borthakur, L.J. Zinc cobalt sulfide/reduced graphene oxide nanocomposite for high-performance asymmetric supercapacitor: A microwave-assisted synthesis. Energy Technol. 2024, 12, 2301524. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, X.; Ma, Y.; Zhao, Q.; Sui, Y.; Song, J.; Ma, W.; Zhang, P.; Qin, C. Synthesis of polypyrrole nanotubes@nickel-molybdenum sulfide core–shell composites for aqueous high-performance asymmetric supercapacitors. Electroanal. Chem. 2021, 897, 115588. [Google Scholar] [CrossRef]
- Guo, M.; Du, J.; Liu, X.; Cao, Y.; Li, X.; Wu, K.; Li, Z. Cobalt-doped vanadium sulfide nanorods anchored on graphene for high-performance supercapacitors. ACS Appl. Nano Mater. 2024, 7, 15469–15477. [Google Scholar] [CrossRef]





| Materials | Specific Capacitance | Electrolyte | Stability | Ref. |
|---|---|---|---|---|
| C-Zn/Co/S | 815 F g−1 at 2 mV s−1 | 2 M KOH | 83.9% (5000 cycles) | [42] |
| NiCo2S4@NC | 1021.6 F g−1 at 1 A g−1 | 6 M KOH | 92.7% (5000 cycles) | [43] |
| NiV-LDH | 465.33 F g−1 at 1 A g−1 | 3 M KOH | 90.7% (5000 cycles) | [18] |
| NiAg2S | 571.2 C/g at 1.4 A g−1 | 1 M KOH | 86% (1000 cycles) | [44] |
| NMS@S-gC | 934.2 F g−1 at 1 A g−1 | 2 M KOH | 89.5% (5000 cycles) | [45] |
| Ni4V1-LDH@rGO | 1511.1 F g−1 at 1 A g−1 | 2 M KOH | 85.3% (1000 cycles) | [46] |
| Ni-VO2@C/SO42− | 355 F g−1 at 1 A g−1 | _ | 88% (10,000 cycles) | [47] |
| 3D NiV-LDHs | 1069 F g−1 at 1 A g−1 | 6 M KOH | 68% (1500 cycles) | [48] |
| NiMo3S4/BP | 830 F·g−1 at 1 A·g−1 | 0.5 M K2SO4 | 86% (6000 cycles) | [49] |
| CeCoSx-SA/GF | 873.3 F·g−1 at 1 A·g−1 | 6 M KOH | 87.1% (5000 cycles) | [50] |
| NVS/G | 1437 F·g−1 at 1 A·g−1 | 3 M KOH | 91.2% (10,000 cycles) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Du, J.; Liu, X.; Liu, W.; Zhao, M.; Wang, J.; Li, X. Rational Fabrication of Nickel Vanadium Sulfide Encapsulated on Graphene as an Advanced Electrode for High-Performance Supercapacitors. Molecules 2024, 29, 3642. https://doi.org/10.3390/molecules29153642
Guo M, Du J, Liu X, Liu W, Zhao M, Wang J, Li X. Rational Fabrication of Nickel Vanadium Sulfide Encapsulated on Graphene as an Advanced Electrode for High-Performance Supercapacitors. Molecules. 2024; 29(15):3642. https://doi.org/10.3390/molecules29153642
Chicago/Turabian StyleGuo, Meng, Jia Du, Xueguo Liu, Wentao Liu, Mingjian Zhao, Jianqi Wang, and Xuyang Li. 2024. "Rational Fabrication of Nickel Vanadium Sulfide Encapsulated on Graphene as an Advanced Electrode for High-Performance Supercapacitors" Molecules 29, no. 15: 3642. https://doi.org/10.3390/molecules29153642
APA StyleGuo, M., Du, J., Liu, X., Liu, W., Zhao, M., Wang, J., & Li, X. (2024). Rational Fabrication of Nickel Vanadium Sulfide Encapsulated on Graphene as an Advanced Electrode for High-Performance Supercapacitors. Molecules, 29(15), 3642. https://doi.org/10.3390/molecules29153642







