Ethyl Acetate Fraction from Eleutherococcus divaricatus Root Extract as a Promising Source of Compounds with Anti-Hyaluronidase, Anti-Tyrosinase, and Antioxidant Activity but Not Anti-Melanoma Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Panel
2.2. Anti-Enzymatic Panel
2.2.1. Inhibition of Bovine Hyaluronidase (bHYAL) and Fungal Tyrosinase (mTYR) by Crude Extracts and Fractions
2.2.2. Inhibition of Human Hyaluronidase (hHYAL) and Human Tyrosinase (hTYR) in Blood Samples from Children Diagnosed with Acute Lymphoblastic Leukemia by Ethyl Acetate Fraction
2.3. Antioxidant Panel
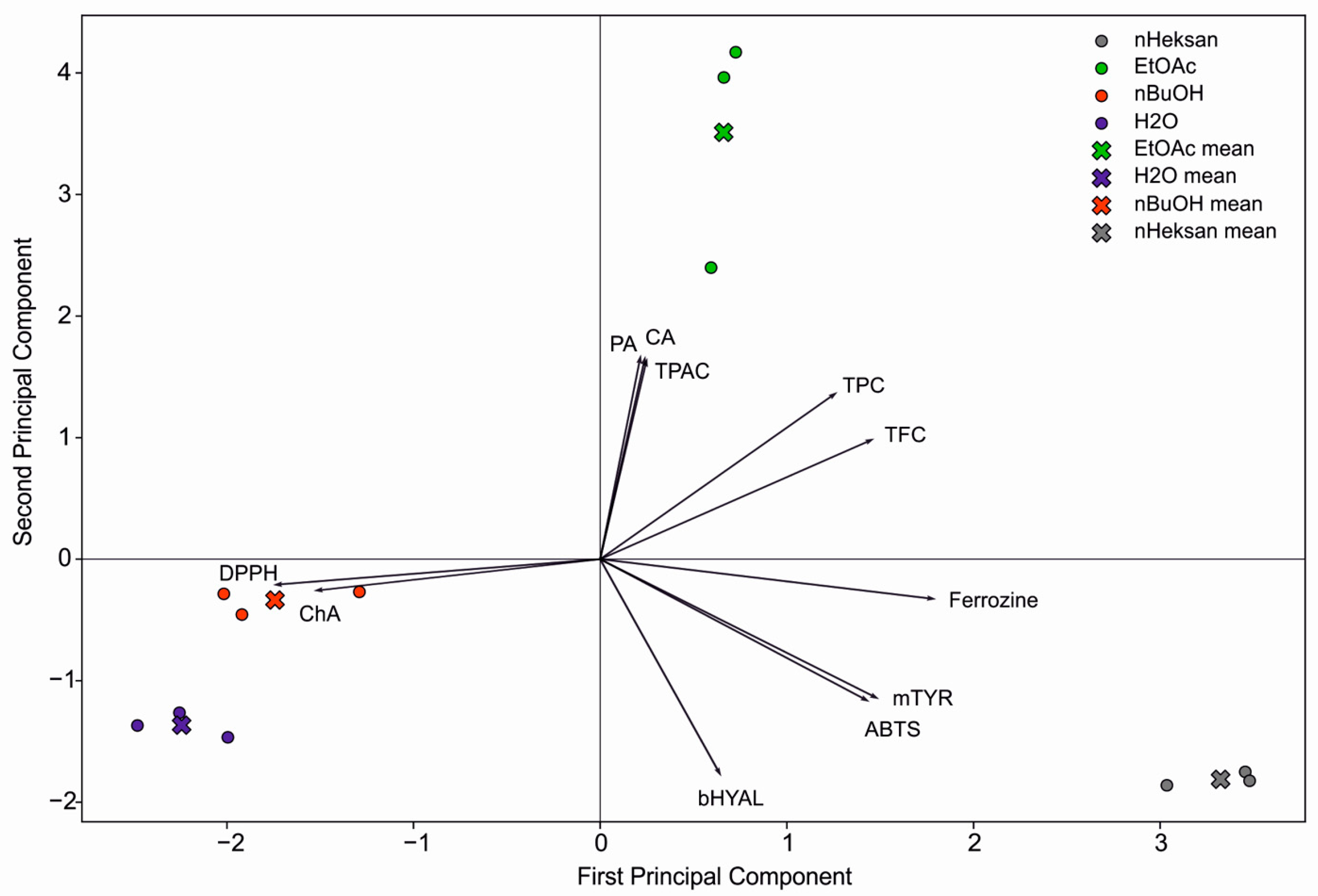
2.4. Principal Component Analysis (PCA)
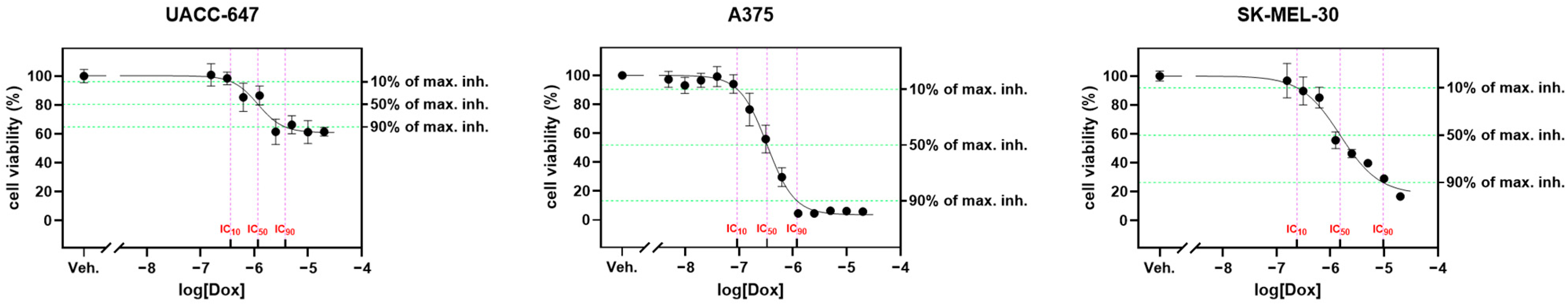
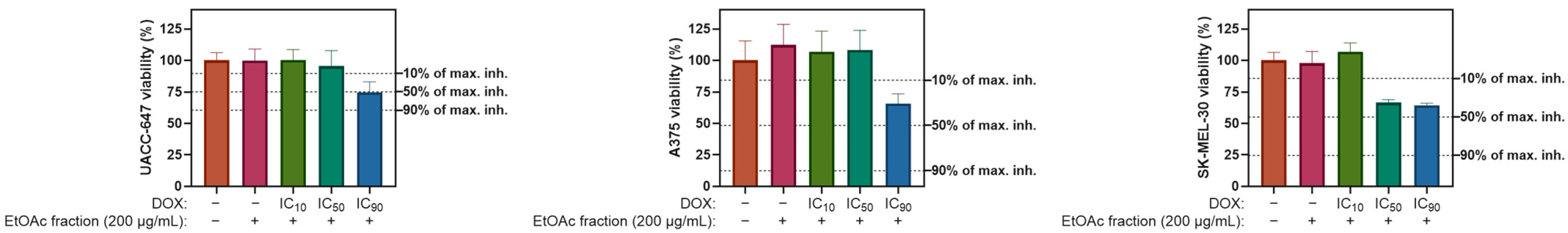
2.5. Cytotoxicity Panel
Ethyl Acetate Fraction of E. divaricatus Does Not Affect the Viability of Normal and Cancerous Skin Cells
3. Materials and Methods
3.1. Chemicals and Reagents
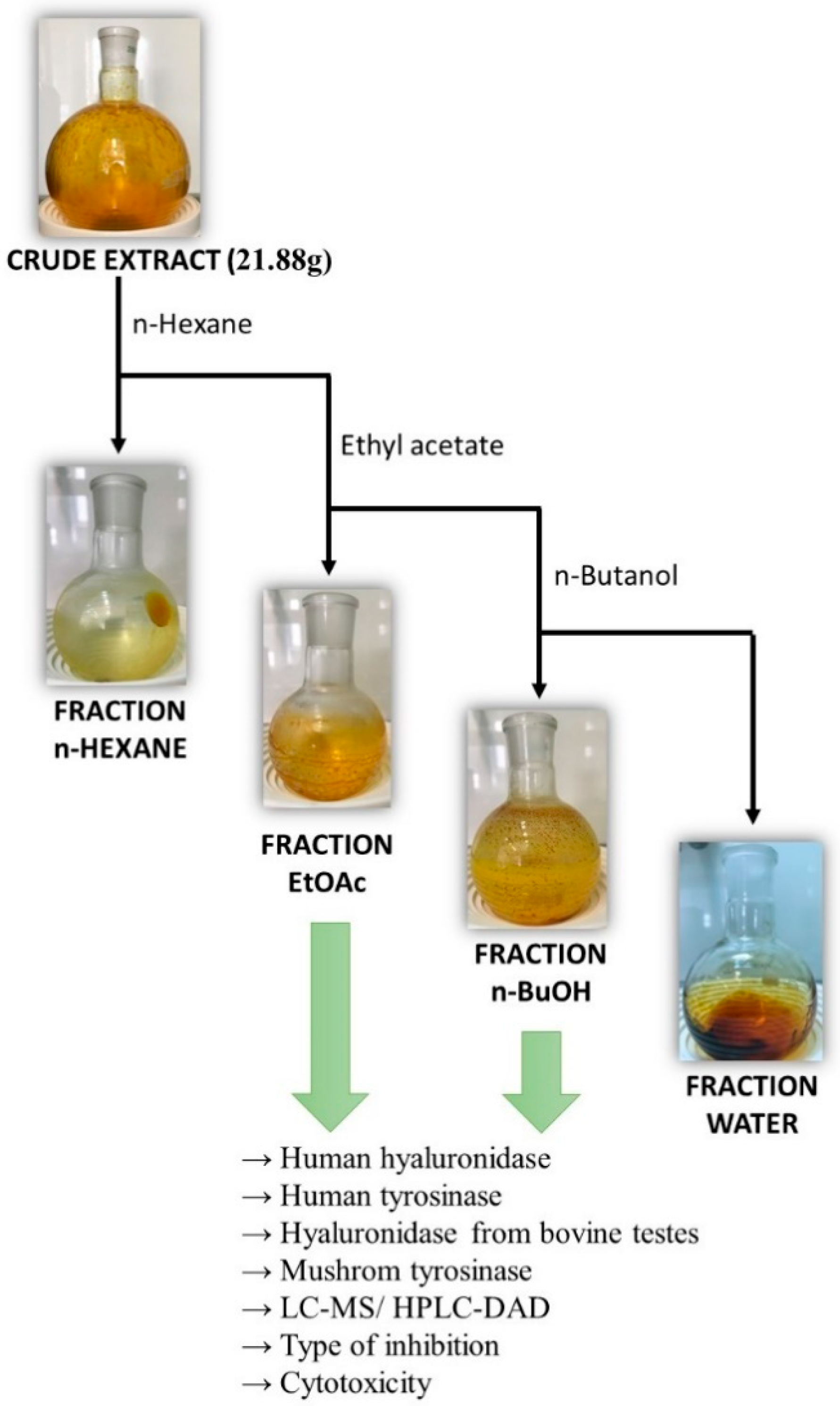
3.2. Extraction and Plant Material
Liquid–Liquid Extraction of Polyphenols
3.3. Phytochemical Panel
3.3.1. Chemical Composition
Determination of Total Phenolic Content (TPC)
Determination of Total Flavonoid Content (TFC)
Determination of Total Phenolic Acid Content (TPAC)
3.3.2. Chromatographic Analysis
3.4. Enzymatic Panel
3.4.1. Bovine Hyaluronidase Inhibition Assay
3.4.2. Human Serum Hyaluronidase from Children Diagnosed with Acute Lymphoblastic Leukemia
Blood Samples
Level of Human Serum Hyaluronidase
Human Serum Hyaluronidase Inhibition by the Ethyl Acetate Fraction
3.4.3. Tyrosinase Inhibition Assay
3.5. Antioxidant Panel
3.5.1. ABTS Free Radical Scavenging Activity
3.5.2. DPPH Free Radical Scavenging Activity
3.5.3. Iron (II) Ion Chelation Assay
3.6. Cytotoxicity Panel
Cell Culture and Cytotoxicity Assessment
3.7. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry 2007, 46, 6911–6920. [Google Scholar] [CrossRef] [PubMed]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2020, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and function of human tyrosinase and tyrosinase-related proteins. Chem.—Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- M Casanola-Martin, G.; Le-Thi-Thu, H.; Marrero-Ponce, Y.; A Castillo-Garit, J.; Torrens, F.; Rescigno, A.; Abad, C.; Tareq Hassan Khan, M. Tyrosinase enzyme: 1. An overview on a pharmacological target. Curr. Top. Med. Chem. 2014, 14, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Roulier, B.; Pérès, B.; Haudecoeur, R. Advances in the design of genuine human tyrosinase inhibitors for targeting melanogenesis and related pigmentations. J. Med. Chem. 2020, 63, 13428–13443. [Google Scholar] [CrossRef] [PubMed]
- Gębalski, J.; Graczyk, F.; and Załuski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure–activity relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Kumar Singla, R.; Mitsugu Ishiki, H.; Mendonca, J.B.; Sobral da Silva, M.; Barbosa Filho, M.; Tullius Scotti, M. Recent advancement in natural hyaluronidase inhibitors. Curr. Top. Med. Chem. 2016, 16, 2525–2531. [Google Scholar] [CrossRef]
- Frost, G.I. Recombinant human hyaluronidase (rHuPH20): An enabling platform for subcutaneous drug and fluid administration. Expert. Opin. Drug Deliv. 2007, 4, 427–440. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Mio, K.; Stern, R. Inhibitors of the hyaluronidases. Matrix Biol. 2002, 21, 31–37. [Google Scholar] [CrossRef]
- Khan, N.; Niazi, Z.R.; Akhtar, A.; Khan, M.M.; Khan, S.; Baloch, N.; Khan, S. Hyaluronidases: A therapeutic enzyme. Protein Pept. Lett. 2018, 25, 663–676. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, G.; Zeng, Q.H.; Li, Y.; Liu, H.; Wang, J.J.; Zhao, Y. A systematic review of synthetic tyrosinase inhibitors and their structure-activity relationship. Crit. Rev. Food Sci. Nutr. 2022, 62, 4053–4094. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, W.; Nazir, Y.; Fercher, C.; Blaskovich, M.A.; Cooper, M.A.; Ziora, Z.M. Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem. 2020, 187, 111892. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis. Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.I.; Hebert, J.H.; Reddy, P. The effects of Panax ginseng on quality of life. J. Clin. Pharm. Ther. 2003, 28, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. FCT 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Cho, I.H. Effects of Panax ginseng in neurodegenerative diseases. JGR 2012, 36, 342. [Google Scholar] [CrossRef] [PubMed]
- Frodin, D.G. Araliaceae. In A Revised Handbook to the Flora of Ceylon; Routledge: London, UK, 2017; Volume 10, pp. 1–20. [Google Scholar]
- Liu, L.; Xu, F.R.; Wang, Y.Z. Traditional uses, chemical diversity and biological activities of Panax L. (Araliaceae): A review. J. Ethnopharmacol. 2020, 263, 112792. [Google Scholar] [CrossRef] [PubMed]
- Oh, O.J.; Chang, S.Y.; Kim, T.H.; Yang, K.S.; Yook, C.S.; Park, S.Y.; Nohara, T. Constituents of Acanthopanax divaricatus var. albeofructus. Nat. Med. 2000, 54, 29–32. [Google Scholar]
- Lee, J.H.; Sun, Y.N.; Kim, Y.H.; Lee, S.K.; Kim, H.P. Inhibition of lung inflammation by acanthopanax divaricatus var. albeofructus and its constituents. Biomol. Ther. 2016, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Yook, C.S.; Kim, H.C.; Ko, S.K. Measurement of characteristic phytochemical levels in different Acan-thopanax Species by HPLC. Yakhak Hoeji. 2017, 61, 90–95. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, M.H.; Pae, S.B.; Oh, K.W.; Jung, C.S.; Baek, I.Y.; Lee, S. Analysis of yield of eleutherosides B and E in Acanthopanax divaricatus and A. koreanum Grown with varying cultivation methods. Sci. World J. 2014, 2014, 515291. [Google Scholar]
- Huang, Y.H.; Li, J.T.; Zan, K.; Wang, J.; Fu, Q. The traditional uses, secondary metabolites, and pharmacology of Eleutherococcus species. Phytochem. Rev. 2022, 21, 1081–1184. [Google Scholar] [CrossRef]
- Załuski, D.; Mendyk, E.; Smolarz, H.D. Identification of MMP-1 and MMP-9 inhibitors from the roots of Eleutherococcus divaricatus, and the PAMPA test. Nat. Prod. Res. 2016, 30, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Załuski, D.; Smolarz, H.D.; and Gawlik-Dziki, U. Bioactive compounds and antioxidative, antileukemic and anti-MMPs activity of Eleutherococcus species cultivated in Poland. Nat. Prod. Commun. 2012, 7, 1934578X1200701118. [Google Scholar]
- Adamczyk, K.; Olech, M.; Abramek, J.; Pietrzak, W.; Kuźniewski, R.; Bogucka-Kocka, A.; Ptaszyńska, A.A.; Rapacka-Gackowska, A.; Skalski, T.; Strzemski, M.; et al. Eleutherococcus species cultivated in Europe: A new source of compounds with antiacetylcholinesterase, antihyaluronidase, anti-DPPH, and cytotoxic activities. Oxidative Med. Cell. Longev. 2019, 2019, 8673521. [Google Scholar] [CrossRef]
- Graczyk, F.; Gębalski, J.; Makuch-Kocka, A.; Gawenda-Kempczyńska, D.; Ptaszyńska, A.A.; Grzyb, S.; Bogucka-Kocka, A.; Załuski, D. Phenolic Profile, Antioxidant, Anti-Enzymatic and Cytotoxic Activity of the Fruits and Roots of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Molecules 2022, 27, 5579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Liu, X.Q.; Kim, Y.H.; Whang, W.K. Chemical constituents and their acetyl cholinesterase inhibitory and antioxidant activities from leaves of Acanthopanax henryi: Potential complementary source against Alzheimer’s disease. Arch. Pharmacal Res. 2014, 37, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Li, W.; Yan, X.T.; Yang, S.Y.; Kim, Y.H. Chemical constituents from the stems of Acanthopanax divaricatus var. albeofructus. Biochem. Syst. Ecol. 2014, 100, 164–168. [Google Scholar] [CrossRef]
- Gębalski, J.; Małkowska, M.; Gawenda-Kempczyńska, D.; Słomka, A.; Strzemski, M.; Styczyński, J.; Załuski, D. Eleutherococcus divaricatus Fruits Decrease Hyaluronidase Activity in Blood Serum and Protect from Antioxidative Damages in In Vitro Model. Int. J. Mol. Sci. 2024, 25, 2033. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Katagiri, T.; Osaka, M.; Yamauchi, S.; Yoshimura, K.; Kawada, M.; Fujii, Y.; Suzuki, Y.; Sasaki, K. Hyaluronidase and degranulation inhibitors from the edible roots of Oenanthe javanica including seric acids F and G that were obtained by heating. Biosci. Biotechnol. Biochem. 2021, 85, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, A.; Kusano, G.; Warashina, T.; Miyase, T. Phenolic constituents of the aerial parts of Cimicifuga simplex and Cimicifuga japonica. J. Nat. Prod. 2010, 73, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, F.; Gębalski, J.; Piskorska, E.; Małkowska, M.; Słomka, A.; Gawenda-Kempczyńska, D.; Kondrzycka-Dąda, A.; Olszewska-Słonina, D.; Styczyński, J.; Taglialatela-Scafati, O.; et al. The Eleutherococcus senticosus fruits’ intractum affects changes in the transepithelial electric potential in the distal section of the rabbit’s large intestine and inhibits hyaluronidase. J. Ethnopharmacol. 2024, 117847. [Google Scholar]
- Yu, C.Y.; Kim, S.H.; Lim, J.D.; Kim, M.J.; Chung, I.M. Intraspecific relationship analysis by DNA markers and in vitro cytotoxic and antioxidant activity in Eleutherococcus senticosus. Toxicol. Vitr. 2003, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Horng, C.T.; Liu, I.M.; Kuo, D.H.; Tsai, Y.W.; Shieh, P. Comparison of xanthine oxidase-inhibiting and free radical-scavenging activities between plant adaptogens of Eleutherococcus senticosus and Rhodiola rosea. Drug Dev. Res. 2010, 71, 249–252. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Z.; Ren, J.; Liu, X.; Xu, Z.; Whang, W.; Liang, Z.; Mans, D.; Zhang, X. Identification of antioxidant ingredients by GC-MS from the essential oil of Purple Eleutherococcus simonii leaves. Food Sci. Technol. 2021, 42, e76821. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Jiang, M. Chemical Constituents of Eleutherococcus sessiliflorus (Rupr. and Maxim.). Nat. Prod. Commun. 2020, 15, 1934578X20905760. [Google Scholar] [CrossRef]
- Wang, H.C.; Tseng, Y.H.; Wu, H.R.; Chu, F.H.; Kuo, Y.H.; Wang, S.Y. Anti-proliferation effect on human breast cancer cells via inhibition of pRb phosphorylation by taiwanin E isolated from Eleutherococcus trifoliatus. Nat. Prod. Commun. 2014, 9, 1934578X1400900921. [Google Scholar] [CrossRef]
- Han, D.; Liu, Y.; Li, X.M.; Wang, S.Y.; Sun, Y.; Algradi, A.M.; Zou, H.-D.; Pan, J.; Guan, W.; Kuang, H.-X.; et al. Elesesterpenes A–K: Lupane-type Triterpenoids from the Leaves of Eleutherococcus sessiliflorus. Front. Chem. 2022, 9, 813764. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhu, M.Z.; Wu, W.; Jiao, L.L.; Yang, P.F.; Guo, M.Q. Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [PubMed]
- Polish Pharmacopoeia VI; Polish Pharmaceutical Society: Warszawa, The Netherlands, 2002; p. 150.
- Di Ferrante, N. Turbidimetric measurement of acid mucopoly-saccharides and hyaluronidase activity. J. Biol. Chem. 1956, 220, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Dudek-Makuch, M.; Chanaj-Kaczmarek, J.; Czepulis, N.; Korybalska, K.; Rutkowski, R.; Łuczak, J.; Grabowska, K.; Bylka, W.; Witowski, J. Anti-inflammatory Activity and Phytochemical Profile of Galinsoga Parviflora Cav. Molecules 2018, 23, 2133. [Google Scholar] [CrossRef] [PubMed]
- Gębalski, J.; Małkowska, M.; Graczyk, F.; Słomka, A.; Piskorska, E.; Gawenda-Kempczyńska, D.; Kondrzycka-Dąda, A.; Bogucka-Kocka, A.; Strzemski, M.; Sowa, I.; et al. Phenolic Compounds and Antioxidant and Anti-Enzymatic Activities of Selected Adaptogenic Plants from South America, Asia, and Africa. Molecules 2023, 28, 6004. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Żmudzki, P.; Podolak, I. Anti-melanoma potential of two benzoquinone homologues embelin and rapanone-a comparative in vitro study. Toxicol. Vitr. 2020, 65, 104826. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, Z.; Qie, X.; Chen, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Interaction of soy protein isolate hydrolysates with cyanidin-3-O-glucoside and its effect on the in vitro antioxidant capacity of the complexes under neutral condition. Molecules 2021, 26, 1721. [Google Scholar] [CrossRef]
- Naseer, S.; Iqbal, J.; Naseer, A.; Kanwal, S.; Hussain, I.; Tan, Y.; Aguilar-Marcelino, L.; Cossio-Bayugar, R.; Zajac, Z.; Bin Jardan, Y.A. Deciphering chemical profiling, pharmacological responses and potential bioactive constituents of Saussurea lappa Decne. Extracts through in vitro approaches. Saudi J. Biol. Sci. 2022, 29, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
| Fraction | TPC [mgGAE/g] | TFC [mgQE/g] | TPAC [mgCAE/g] | Mass of Fraction [g] |
|---|---|---|---|---|
| n-Hexane | 68.16 ± 1.32 ab | 21.80 ± 1.53 ab | 1.03 ± 0.18 ab | 2.02 |
| Ethyl acetate (EtOAc) | 110.89 ± 6.32 b | 27.95 ± 4.11 b | 2.81 ± 0.48 b | 1.02 |
| n-Butanol (n-BuOH) | 22.03 ± 0.77 ab | 0.65 ± 0.77 a | 1.74 ± 0.24 ab | 5.11 |
| Water | 4.02 ± 2.88 a | 3.36 ± 2.17 ab | 0.55 ± 0.033 a | 9.26 |
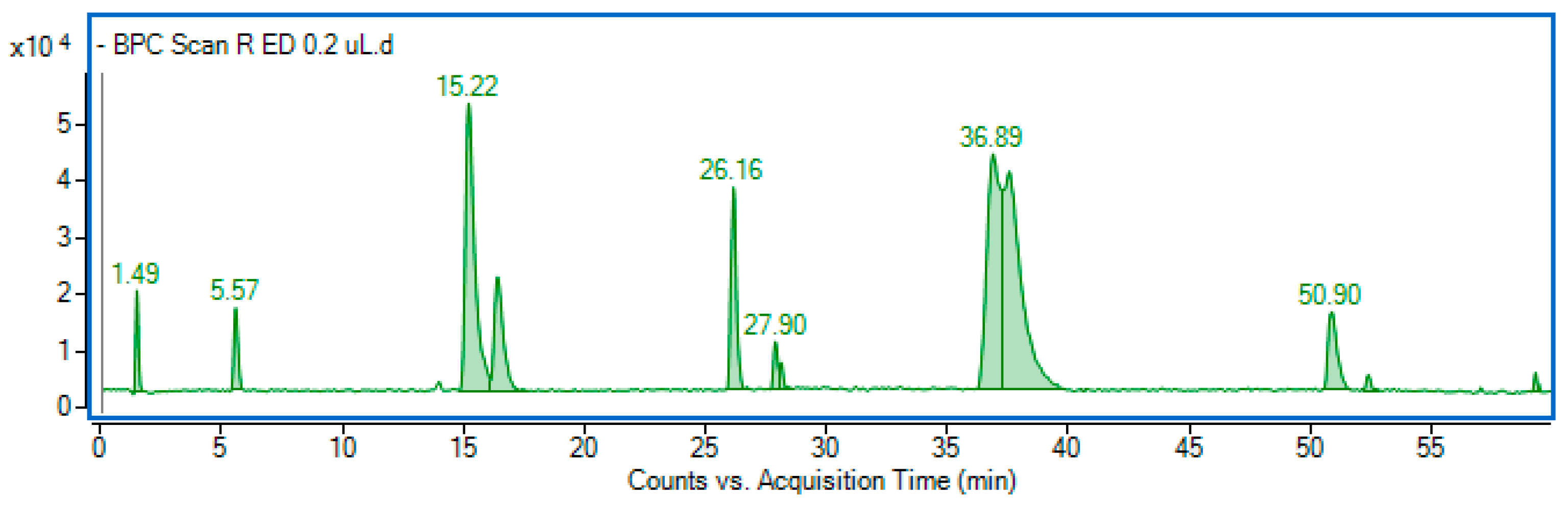
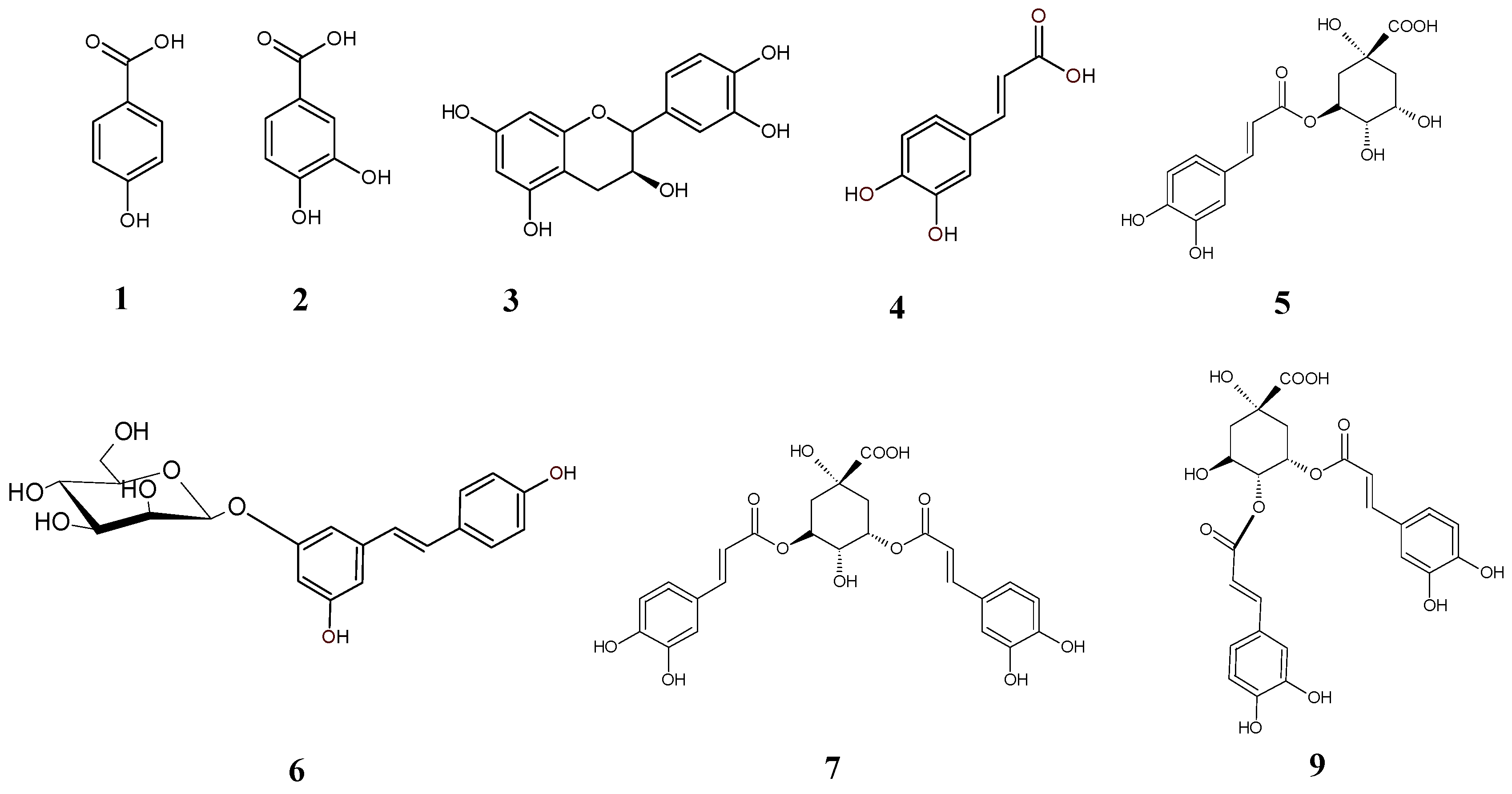
| No | Rt (min) | Observed Ion Mass [M − H]−/(Fragments) | Δ ppm | Formula | Identified |
|---|---|---|---|---|---|
| 1 | 5.57 | 153.01973 | 2.58 | C7H6O4 | Protocatechuic acid * |
| 2 | 8.73 | 137.02481 | 2.84 | C7H6O3 | Hydroxybenzoic acid |
| 3 | 14.00 | 289.07203 | 0.92 | C15H14O6 | Catechin * |
| 4 | 15.20 | 179.03505 (135, 191) | 0.38 | C9H8O4 | Caffeic acid * |
| 5 | 16.42 | 353.08835 (191, 179) | 1.54 | C16H18O9 | Chlorogenic acid * |
| 6 | 26.16 | 389.12423 (227) | 0.10 | C20H22O8 | Piceid (Resveratrol der.) |
| 7 | 36.84 | 515.12021 (353) | 1.38 | C25H24O12 | 3,5-dicaffeoylquinic acid * |
| 8 | 37.63 | 515.12048 (353) | 1.90 | C25H24O12 | Dicaffeoylquinic acid |
| 9 | 50.90 | 515.12035 (353) | 1.65 | C25H24O12 | 4,5-dicaffeoylquinic acid * |
| 10 | 59.33 | 577.13521 (198, 385) | 0.10 | C30H26O12 | Diferulic acid derivative |
| PA | CA | ChA | 3,5-DCA | DCA | 4,5-DCA | |
|---|---|---|---|---|---|---|
| n-Hexane | 0.017 ± 0.001 | 0.077 ± 0.006 | 0.593 ± 0.020 | 0.283 ± 0.001 | 0.334 ± 0.001 | 0.150 ± 0.009 |
| Ethyl acetate | 9.293 ± 0.105 | 24.018 ± 0.045 | 16.653 ± 0.055 | 126.97 ± 3.08 | 150.63 ± 3.65 | 26.615 ± 0.253 |
| n-Butanol | 0.492 ± 0.001 | 0.595 ± 0.003 | 59.198 ± 0.153 | 24.87 ± 0.109 | 29.51 ± 0.129 | 6.383 ± 0.061 |
| Water | 0.169 ± 0.008 | 0.281 ± 0.004 | 44.360 ± 0.102 | 1.294 ± 0.011 | 1.540 ± 0.013 | 0.129 ± 0.009 |
| Type of Extract | bHYAL | mTYR |
|---|---|---|
| Chloroform (CHCl3) | 111.73 ± 0.75 a | 188.50 ± 1.83 ab |
| Ethyl acetate | 104.13 ± 2.51 a | 274.37 ± 3.69 b |
| 75% methanol | 100.80 ± 0.9 a | 103.60 ± 4.23 a |
| CHCl3:MeOH:H2O | 181.27 ± 0.92 a | 221.83 ± 2.21 ab |
| Type of Fraction | bHYAL | mTYR |
|---|---|---|
| n-Hexane | 94.44 ± 0.80 ab | 207.50 ± 3.63 b |
| Ethyl acetate | 27.50 ± 0.65 a | 65.50 ± 1.35 ab |
| n-Butanol | 56.10 ± 6.86 ab | 85.40 ± 2.51 ab |
| Water | 71.60 ± 3.87 ab | 81.10 ± 5.32 ab |
| Aescin | 388.8 ± 1.81 b | |
| Kojic acid | 4.44 ± 0.06 a |
| Standard | bHYAL | mTYR |
|---|---|---|
| Eleutheroside B | NA | NA |
| Eleutheroside E | NA | NA |
| Eleutheroside E1 | NA | NA |
| Caffeic acid | 111.34 ± 3.59 a | 56.22 ± 0.67 a |
| Chlorogenic acid | 519.14 ± 17.94 ab | 107.52 ± 3.46 ab |
| Protocatechuic acid | 920.20 ± 87.71 b | 134.57 ± 3.46 b |
| N° | Level of hHYAL [ng/mL] | hHYAL [%] | Level of hTYR [ng/mL] | hTYR [%] |
|---|---|---|---|---|
| EtOAc | EtOAc | |||
| Mean ± SD | Mean ± SD | |||
| 1 | 95.27 | 53.47 ± 12.37 ab | 4.68 | NA |
| 2 | 116.90 | 89.85 ± 7.73 b | 9.31 | NA |
| 3 | 162.15 | 66.67 ± 20.00 ab | 15.26 | NA |
| 4 | 81.86 | 38.71 ± 5.59 ab | 78.94 | NA |
| 5 | 25.20 | 30.43 ± 3.07 a | 52.17 | NA |
| Mean value | 96.27 | 55.82 | 32.07 | - |
| Fraction | IC50 ABTS | IC50 DPPH | Ferrozine * |
|---|---|---|---|
| n-Hexane | 80.20 ± 5.40 b | NA | 80.15 ± 0.73 |
| Ethyl acetate | 9.69 ± 0.035 ab | 36.83 ± 2.43 ab | 34.44 ± 3.99 |
| n-Butanol | 10.10 ± 0.21 ab | 61.49 ± 1.87 ab | 23.52 ± 3.24 |
| Water | 24.99 ± 0.095 ab | 106.10 ± 4.51 b | 4.09 ± 2.48 |
| BHA | 2.35 ± 0.11 a | 62.52 ± 4.13 ab | |
| AA | 2.27 ± 0.07 a | 24.93 ± 0.28 ab | |
| TROLOX | 2.85 ± 0.18 ab | 13.68 ± 3.53 a | |
| EDTA | 98.9 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gębalski, J.; Małkowska, M.; Wnorowska, S.; Gawenda-Kempczyńska, D.; Strzemski, M.; Wójciak, M.; Słomka, A.; Styczyński, J.; Załuski, D. Ethyl Acetate Fraction from Eleutherococcus divaricatus Root Extract as a Promising Source of Compounds with Anti-Hyaluronidase, Anti-Tyrosinase, and Antioxidant Activity but Not Anti-Melanoma Activity. Molecules 2024, 29, 3640. https://doi.org/10.3390/molecules29153640
Gębalski J, Małkowska M, Wnorowska S, Gawenda-Kempczyńska D, Strzemski M, Wójciak M, Słomka A, Styczyński J, Załuski D. Ethyl Acetate Fraction from Eleutherococcus divaricatus Root Extract as a Promising Source of Compounds with Anti-Hyaluronidase, Anti-Tyrosinase, and Antioxidant Activity but Not Anti-Melanoma Activity. Molecules. 2024; 29(15):3640. https://doi.org/10.3390/molecules29153640
Chicago/Turabian StyleGębalski, Jakub, Milena Małkowska, Sylwia Wnorowska, Dorota Gawenda-Kempczyńska, Maciej Strzemski, Magdalena Wójciak, Artur Słomka, Jan Styczyński, and Daniel Załuski. 2024. "Ethyl Acetate Fraction from Eleutherococcus divaricatus Root Extract as a Promising Source of Compounds with Anti-Hyaluronidase, Anti-Tyrosinase, and Antioxidant Activity but Not Anti-Melanoma Activity" Molecules 29, no. 15: 3640. https://doi.org/10.3390/molecules29153640
APA StyleGębalski, J., Małkowska, M., Wnorowska, S., Gawenda-Kempczyńska, D., Strzemski, M., Wójciak, M., Słomka, A., Styczyński, J., & Załuski, D. (2024). Ethyl Acetate Fraction from Eleutherococcus divaricatus Root Extract as a Promising Source of Compounds with Anti-Hyaluronidase, Anti-Tyrosinase, and Antioxidant Activity but Not Anti-Melanoma Activity. Molecules, 29(15), 3640. https://doi.org/10.3390/molecules29153640









