Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines
Abstract
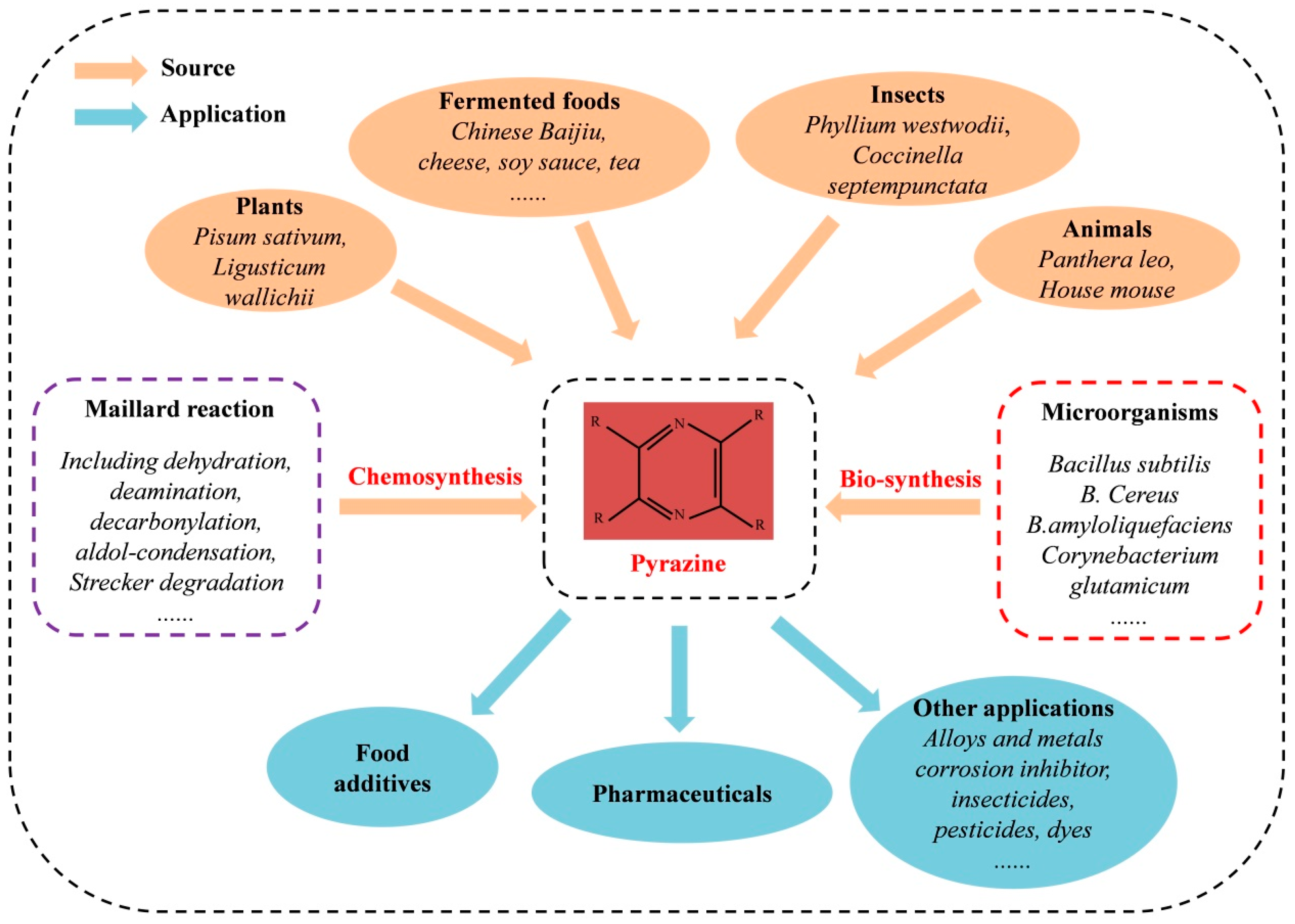
1. Introduction
2. Naturally Occurring Pyrazines and Their Pharmacological Applications
2.1. Tetramethylpyrazine
The Potential Medical Effects of TTMP
2.2. Dimethylpyrazine
The Potential Medical Effects of 2,5-DMP
2.3. Trimethylpyrazine
The Potential Functions of TMP
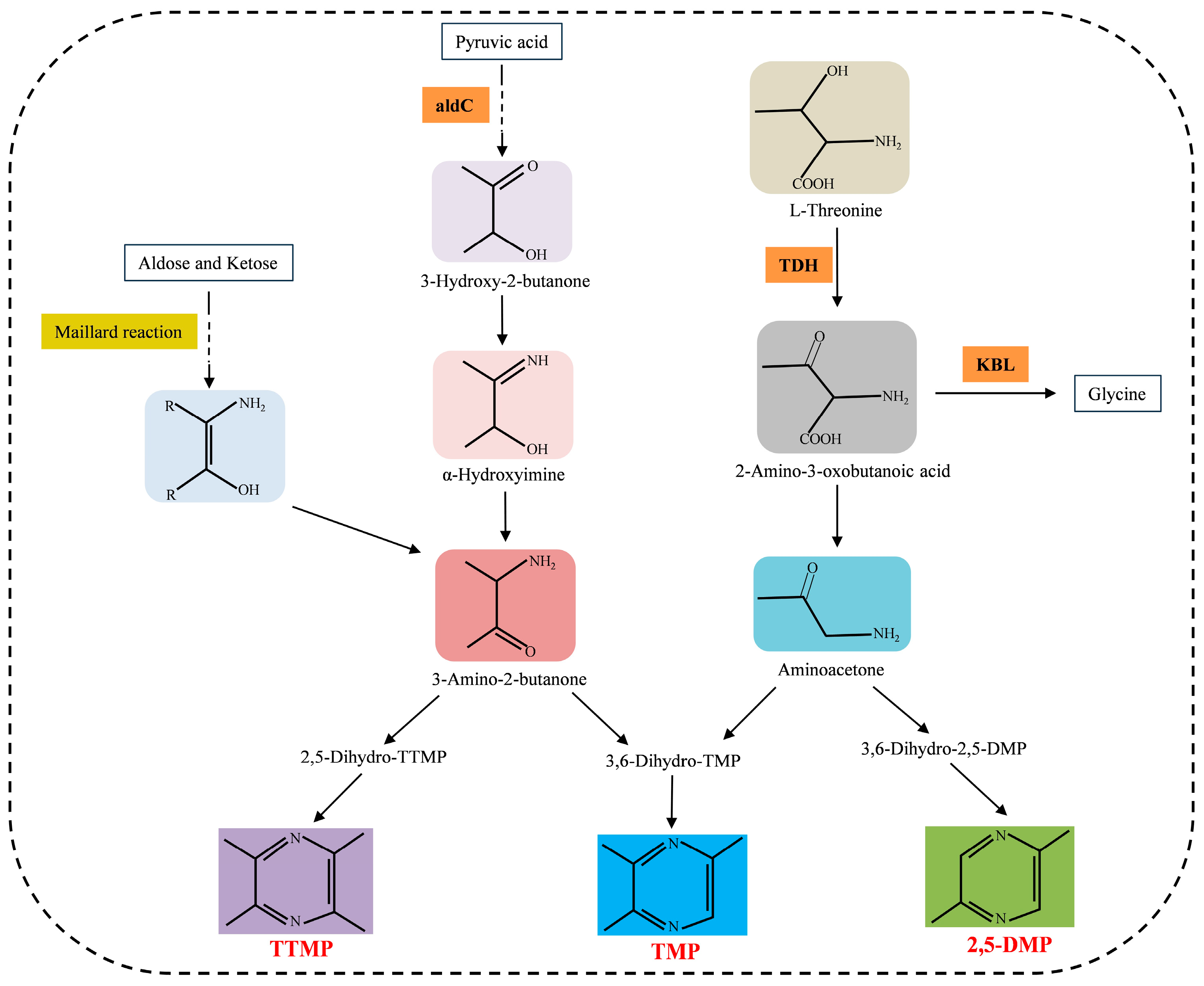
3. Synthesis Pathways of Naturally Occurring Pyrazines
3.1. The Synthesis of TTMP
3.2. The Synthesis of 2,5-DMP
3.3. The Synthesis of TMP
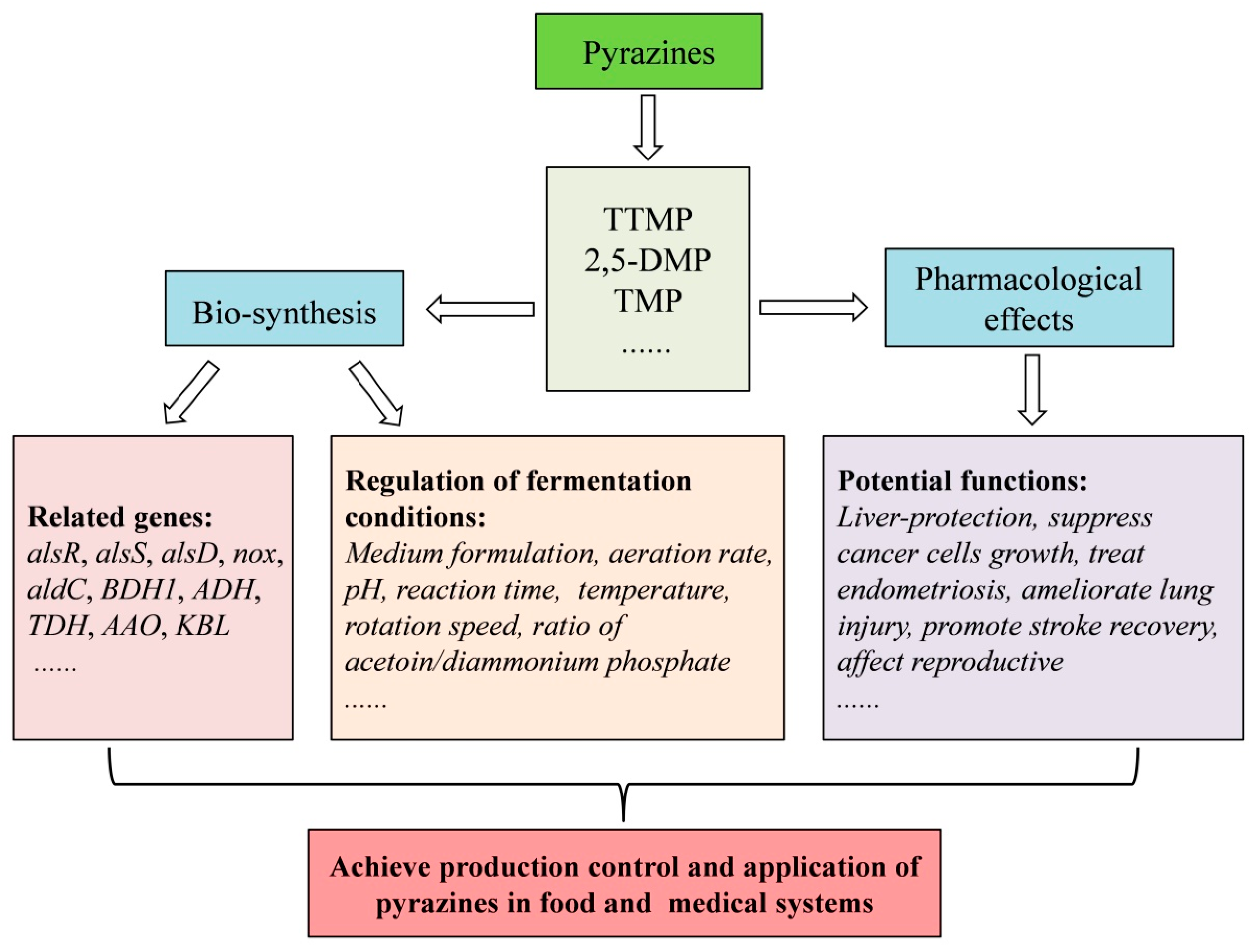
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wolfe, B.E.; Dutton, R.J. Fermented foods as experimentally tractable microbial ecosystems. Cell 2015, 161, 49–55. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef]
- Fayek, N.M.; Xiao, J.; Farag, M.A. A multifunctional study of naturally occurring pyrazines in biological systems; formation mechanisms, metabolism, food applications and functional properties. Crit. Rev. Food Sci. 2021, 63, 5322–5338. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X.; Yu, H. Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef]
- Ma, Y.J.; Wu, J.H.; Li, X.; Xu, X.B.; Wang, Z.Y.; Wu, C.; Du, M.; Song, L. Effect of alkyl distribution in pyrazine on pyrazine flavor release in bovine serum albumin solution. RSC Adv. 2019, 9, 36951–36959. [Google Scholar] [CrossRef]
- Amrani-Hemaimi, M.; Cerny, C.; Fay, L.B. Mechanisms of formation of alkylpyrazines in the Maillard reaction. J. Agric. Food Chem. 1995, 43, 2818–2822. [Google Scholar] [CrossRef]
- Besson, I.; Creuly, C.; Gros, J.B.; Larroche, C. Pyrazine production by Bacillus subtilis in solid-state fermentation on soybeans. Appl. Microbiol. Biotechnol. 1997, 47, 489–495. [Google Scholar] [CrossRef]
- Larroche, C.; Besson, I.; Gros, J.B. High pyrazine production by Bacillus subtilis in solid substrate fermentation on ground soybeans. Process Biochem. 1999, 34, 667–674. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, H.; Xiao, J. Pyrazines in Food. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 1–25. ISBN 978-981-13-1745-3. [Google Scholar]
- Scalone, G.L.L.; Ioannidis, A.G.; Lamichhane, P.; Devlieghere, F.; De Kimpe, N.; Cadwallader, K.; De Meulenaer, B. Impact of whey protein hydrolysates on the formation of 2,5-dimethylpyrazine in baked food products. Food Res. Int. 2020, 132, 109089. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Hashem, C.; Vranková, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and industrial application of these valuable flavor and fragrance compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Shu, C.K. Pyrazine formation from serine and threonine. J. Agric. Food Chem. 1999, 47, 4332–4335. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Pielech-Przybylska, K. Pyrazines biosynthesis by Bacillus strains isolated from natto fermented soybean. Biomolecules 2021, 11, 1736. [Google Scholar] [CrossRef]
- Dickschat, J.S.; Wickel, S.; Bolten, C.J.; Nawrath, T.; Schulz, S.; Wittmann, C. Pyrazine biosynthesis in Corynebacterium glutamicum. Eur. J. Org. Chem. 2010, 2010, 2687–2695. [Google Scholar] [CrossRef]
- Silva-Junior, E.A.; Ruzzini, A.C.; Paludo, C.R.; Nascimento, F.S.; Currie, C.R.; Clardy, J.; Pupo, M.T. Pyrazines from bacteria and ants: Convergent chemistry within an ecological niche. Sci. Rep. 2018, 8, 2595. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, S.; He, Y.; Nie, Y.; Xu, Y. Quantitation of pyrazines in Baijiu and during production process by a rapid and sensitive direct injection UPLC-MS/MS approach. LWT 2020, 128, 109371. [Google Scholar] [CrossRef]
- Masuo, S.; Tsuda, Y.; Namai, T.; Minakawa, H.; Shigemoto, R.; Takaya, N. Enzymatic cascade in Pseudomonas that produces pyrazine from α-amino acids. ChemBioChem 2020, 21, 353–359. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, S.; Nie, Y.; Xu, Y. Quantitative analysis of pyrazines and their perceptual interactions in soy sauce aroma type Baijiu. Foods 2021, 10, 441. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Shu, L.; He, Y. Study on metabolites of Bacillus producing soy sauce-like aroma in Jiang-flavor Chinese spirits. Food Sci. Nutr. 2020, 8, 97–103. [Google Scholar] [CrossRef]
- Dossey, A.T.; Gottardo, M.; Whitaker, J.M.; Roush, W.R.; Edison, A.S. Alkyldimethylpyrazines in the defensive spray of Phyllium westwoodii: A first for order Phasmatodea. J. Chem. Ecol. 2009, 35, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Rigling, M.; Kanter, J.P.; Zhang, Y. Application of a direct immersion—Stir bar sorptive extraction (DI-SBSE) combined GC–MS method for fingerprinting alkylpyrazines in tea and tea-like infusions. Eur. Food Res. Technol. 2022, 248, 1179–1189. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A. Pyrazines as potential corrosion inhibitors for industrial metals and alloys: A review. J. Bio- Tribo-Corros. 2018, 4, 18. [Google Scholar] [CrossRef]
- Cherniienko, A.; Pawełczyk, A.; Zaprutko, L. Antimicrobial and odour qualities of alkylpyrazines occurring in chocolate and cocoa products. Appl. Sci. 2022, 12, 11361. [Google Scholar] [CrossRef]
- Zou, J.; Gao, P.; Hao, X.; Xu, H.; Zhan, P.; Liu, X. Recent progress in the structural modification and pharmacological activities of ligustrazine derivatives. Eur. J. Med. Chem. 2018, 147, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.I.; Pickard, S.; Stadlmair, L.F.; Glaß-Theis, A.; Buckel, L.; Bakuradze, T.; Eisenbrand, G.; Richling, E. Alkylpyrazines from coffee are extensively metabolized to pyrazine carboxylic acids in the human body. Mol. Nutr. Food Res. 2019, 63, 1801341. [Google Scholar] [CrossRef] [PubMed]
- Miniyar, P.; Murumkar, P.; Patil, P.; Barmade, M.; Bothara, K. Unequivocal role of pyrazine ring in medicinally important compounds: A review. Mini-Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Hou, X.; Lyu, X.; Xi, L.; Zhao, J.Y. Accelerated green process of tetramethylpyrazine production from glucose and diammonium phosphate. Biotechnol. Biofuels 2014, 7, 106. [Google Scholar] [CrossRef]
- Rizzi, G.P. The biogenesis of food-related pyrazines. Food Rev. Int. 1988, 4, 375–400. [Google Scholar] [CrossRef]
- Zhang, W.; Si, G.; Li, J.; Ye, M.; Feng, S.; Mei, J.; Yang, Q.; Wang, J.; Zhou, P. Tetramethylpyrazine in Chinese sesame flavour liquor and changes during the production process. J. Inst. Brew. 2019, 125, 155–161. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, H.; Du, M.; Song, L.; Xu, X. Dispersive liquid–liquid microextraction for rapid and inexpensive determination of tetramethylpyrazine in vinegar. Food Chem. 2019, 286, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, C.; Li, X.; Sun, B.; Eldin, A.A.; Jia, Y. A combinational optimization method for efficient synthesis of tetramethylpyrazine by the recombinant Escherichia coli. Biochem. Eng. J. 2018, 129, 33–43. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Ma, Z.; Li, J.; Chen, X.; Diao, M.; Xie, N. A green route for high-yield production of tetramethylpyrazine from non-food raw materials. Front. Bioeng. Biotechnol. 2021, 9, 792023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, W.; Yang, K.; Duan, X.; Li, M.; Chen, X.; Zhang, J.; Kuang, M.; Liu, S.; Wu, X.; et al. Tetramethylpyrazine: A promising drug for the treatment of pulmonary hypertension. Br. J. Pharmacol. 2020, 177, 2743–2764. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, J.; Huang, X.; Zhang, Z.; Liu, J.; Zou, L.; Yang, X. Tetramethylpyrazine improves cognitive impairment and modifies the hippocampal proteome in two mouse models of Alzheimer’s disease. Front. Cell Dev. Biol. 2021, 9, 632843. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Acosta, M.F.; Gomez, A.I.; Grijalva, C.; Tang, H.; Yuan, J.X.J.; Mansour, H.M. Design and comprehensive characterization of tetramethylpyrazine (TMP) for targeted lung delivery as inhalation aerosols in pulmonary hypertension (PH): In vitro human lung cell culture and in vivo efficacy. Antioxidants 2021, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Qiu, J.; Zhang, Y.; Yang, Q.; Yin, X.; Li, J.; Yang, G. Tetramethylpyrazine alleviates behavioral and psychological symptoms of dementia through facilitating hippocampal synaptic plasticity in rats with chronic cerebral hypoperfusion. Front. Neurosci. 2021, 15, 646537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Si, G.; Rao, Z.; Zong, S.; Li, J.; Zhang, X.; Gao, C.; Ping, Z.; Ye, M. Hepatoprotective ability of tetramethylpyrazine produced by Bacillus amyloliquefaciens. Syst. Microbiol. Biomanuf. 2021, 1, 223–233. [Google Scholar] [CrossRef]
- Huang, S.; Xiao, F.; Guo, S.W.; Zhang, T. Tetramethylpyrazine retards the progression and fibrogenesis of endometriosis. Reprod. Sci. 2022, 29, 1170–1187. [Google Scholar] [CrossRef]
- Zou, L.; Liu, X.; Li, J.; Li, W.; Zhang, L.; Li, J.; Zhang, J. Tetramethylpyrazine enhances the antitumor effect of paclitaxel by inhibiting angiogenesis and inducing apoptosis. Front. Pharmacol. 2019, 10, 445053. [Google Scholar] [CrossRef]
- Zou, L.; Liu, X.; Li, J.; Li, W.; Zhang, L.; Fu, C.; Zhang, J.; Gu, Z. Redox-sensitive carrier-free nanoparticles self-assembled by disulfide-linked paclitaxel-tetramethylpyrazine conjugate for combination cancer chemotherapy. Theranostics 2021, 11, 4171. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, J.; Wan, Y.; Du, X.; Bai, X.; Li, C.; Lin, Y.; Liu, Z.; Zhou, M.; Zhong, Z. TAT-modified tetramethylpyrazine-loaded nanoparticles for targeted treatment of spinal cord injury. J. Control. Release 2021, 335, 103–116. [Google Scholar] [CrossRef]
- Lin, J.; Hao, C.; Gong, Y.; Zhang, Y.; Li, Y.; Feng, Z.; Xu, X.; Huang, H.; Liao, W. Effect of tetramethylpyrazine on neuroplasticity after transient focal cerebral ischemia reperfusion in rats. Evid.-Based Complement. Altern. 2021, 2021, 1587241. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.F.; Li, M.C.; Lin, Z.Y.; Li, M.Z.; Lu, Y.; Zhuang, Y.M.; Lei, J.F.; Wang, L.; Zhao, H. Tetramethylpyrazine promotes stroke recovery by inducing the restoration of neurovascular unit and transformation of A1/A2 reactive astrocytes. Front. Cell. Neurosci. 2023, 17, 1125412. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Tao, W.; Ding, D.; Zhang, X.; Zhao, S.; Zhang, Y.; Liu, X.; Gao, K.; Liu, S.; Li, L.; et al. Tetramethylpyrazine ameliorates acute lung injury by regulating the Rac1/LIMK1 signaling pathway. Front. Pharmacol. 2023, 13, 1005014. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, S.; Dai, W.; Pang, L.; Xie, Y.; Ren, T.; Zhang, X.; Bi, S.; Zheng, Y.; Wang, J.; et al. Tetramethylpyrazine: A review of its antitumor potential and mechanisms. Front. Pharmacol. 2021, 12, 764331. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, S.; Zheng, C.; Wang, F.; Luo, Y.; Wu, L.; Cao, J.; Guo, B.; Yu, P.; Zhang, G.; et al. Tetramethylpyrazine nitrone improves motor dysfunction and pathological manifestations by activating the PGC-1α/Nrf2/HO-1 pathway in ALS mice. Neuropharmacology 2021, 182, 108380. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Tian, J.H.; Li, H.Y.; Wan, X.; Zou, Y. Synthesis and evaluation of novel nitric oxide-donating ligustrazine derivatives as potent antiplatelet aggregation agents. Molecules 2023, 28, 3355. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Xu, D.Q.; Yue, S.J.; Chen, Y.Y.; Tang, Y.P. Neuroprotection by tetramethylpyrazine and its synthesized analogues for central nervous system diseases: A review. Mol. Biol. Rep. 2024, 51, 159. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume I, ISBN 9780429150838. [Google Scholar]
- Cerny, C.; Grosch, W. Quantification of character-impact odour compounds of roasted beef. Z. Lebensm.-Unters.-Forsch. 1993, 196, 417–422. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Zhang, Y. Characterization of pyrazines in some Chinese liquors and their approximate concentrations. J. Agric. Food Chem. 2007, 55, 9956–9962. [Google Scholar] [CrossRef]
- Smith, A.L.; Barringer, S.A. Color and volatile analysis of peanuts roasted using oven and microwave technologies. J. Food Sci. 2014, 79, C1895–C1906. [Google Scholar] [CrossRef]
- Van Durme, J.; Ingels, I.; De Winne, A. Inline roasting hyphenated with gas chromatography-mass spectrometry as an innovative approach for assessment of cocoa fermentation quality and aroma formation potential. Food Chem. 2016, 205, 66–72. [Google Scholar] [CrossRef]
- Guo, X.; Song, C.; Ho, C.T.; Wan, X. Contribution of L-theanine to the formation of 2,5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018, 263, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Q.; Wang, X.C.; Tao, N.P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Tian, H.; Xu, T.; Dou, Y.; Li, F.; Yu, H.; Ma, X. Optimization and characterization of shrimp flavor nanocapsules containing 2,5-dimethylpyrazine using an inclusion approach. J. Food Process. Preserv. 2017, 41, e13015. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Nsor-Atindana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Optimization of key aroma compounds for dog food attractant. Anim. Feed Sci. Technol. 2017, 225, 173–181. [Google Scholar] [CrossRef]
- Yang, C.; You, J.; Hu, M.; Yi, G.; Zhang, R.; Xu, M.; Shao, M.; Yang, T.; Zhang, X.; Rao, Z. Redistribution of intracellular metabolic flow in E. coli improves carbon atom economy for high-yield 2,5-dimethylpyrazine production. J. Agric. Food Chem. 2021, 69, 2512–2521. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, H.; Ohta, A. Effects of 2,5-dimethylpyrazine on reproductive and accessory reproductive organs in female rats. Res. Commun. Chem. Pathol. Pharmacol. 1992, 75, 99–107. [Google Scholar]
- Yamada, K.; Shimizu, A.; Ohta, A. Effects of dimethylpyrazine isomers on reproductive and accessory reproductive organs in male rats. Biol. Pharm. Bull. 1993, 16, 203–206. [Google Scholar] [CrossRef][Green Version]
- Yamada, K.; Shimizu, A.; Komatsu, H.; Sakata, R.; Ohta, A. Effects of 2,5-dimethylpyrazine on plasma testosterone and polyamines-and acid phosphatase-levels in the rat prostate. Biol. Pharm. Bull. 1994, 17, 730–731. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamada, K.; Watanabe, Y.; Aoyagi, Y.; Ohta, A. Effect of alkylpyrazine derivatives on the duration of pentobarbital- induced sleep, picrotoxicin-induced convulsion and g-aminobutyric acid (GABA) levels in the mouse brain. Biol. Pharm. Bull 2001, 24, 1068–1071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamada, K.; Sano, M.; Fujihara, H.; Ohta, A. Effect of 2,5-dimethylpyrazine on uterine contraction in late stage of pregnant female rats. Biol. Pharm. Bull. 2003, 26, 1614–1617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daev, E.V.; Dukelskaya, A.V. The female pheromone 2,5-dimethylpyrazine induces sperm-head abnormalities in male CBA mice. Russ. J. Genet. 2003, 39, 811–815. [Google Scholar] [CrossRef]
- Kagami, K.; Onda, K.; Hirano, T.; Oka, K. Comparison of the lipid-lowering effects of nicotinic acid and 2,5-dimethylpyrazine in rats. J. Food Lipids 2008, 15, 444–452. [Google Scholar] [CrossRef]
- Kagami, K.; Onda, K.; Oka, K.; Hirano, T. Suppression of blood lipid concentrations by volatile Maillard reaction products. Nutrition 2008, 24, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Apfelbach, R.; Soini, H.A.; Vasilieva, N.Y.; Novotny, M.V. Behavioral responses of predator-naïve dwarf hamsters (Phodopus campbelli) to odor cues of the European ferret fed with different prey species. Physiol. Behav. 2015, 146, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Miao, Z.; Novotny, M.V. Role of the adrenal gland and adrenal-mediated chemosignals in suppression of estrus in the house mouse: The lee-boot effect revisited. Biol. Reprod. 1998, 59, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Soso, S.B.; Koziel, J.A. Characterizing the scent and chemical composition of Panthera leo marking fluid using solid-phase microextraction and multidimensional gas chromatography–mass spectrometry-olfactometry. Sci. Rep. 2017, 7, 5137. [Google Scholar] [CrossRef]
- Janamatti, A.T.; Kumar, A.; Kaur, C.; Gogoi, R.; Varghese, E.; Kumar, S. Fumigation by bacterial volatile 2,5-dimethylpyrazine enhances anthracnose resistance and shelf life of mango. Eur. J. Plant Pathol. 2022, 164, 209–227. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Tong, J.; Xu, Y. An alkylpyrazine synthesis mechanism involving L-threonine-3-dehydrogenase describes the production of 2,5-dimethylpyrazine and 2,3,5-trimethylpyrazine by Bacillus subtilis. Appl. Environ. Microbiol. 2019, 85, e01807–e01819. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, B.; Wang, L.Z.; Zhang, G.J.; Li, X.; Guo, G.; Chen, X.; Lu, P.; Zhang, K. Progress in synthesis of 2,3,5-trimethylpyrazine. Shangdong Chem. Ind. 2014, 43, 32–34. [Google Scholar]
- Worben, H.J.; Timmer, R.; Ter Heide, R.; De Valois, P.J. Nitrogen compounds in rum and whiskey. J. Food Sci. 1971, 36, 464–465. [Google Scholar] [CrossRef]
- Perkins, M.V.; Fletcher, M.T.; Kitching, W.; Drew, R.A.; Moore, C.J. Chemical studies of rectal gland secretions of some species of Bactrocera dorsalis complex of fruit flies (diptera: Tephritidae). J. Chem. Ecol. 1990, 16, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Poramarcom, R.; Boake, C.R.B. Behavioural influences on male mating success in the Oriental fruit fly, Dacus dorsalis Hendel. Anim. Behav. 1991, 42, 453–460. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Yan, C.M.; Chen, C.; Zhao, X.Q.; Li, T.; Sun, B.W. Three new cocrystals derived from liquid pyrazine spices: X-ray structures and hirshfeld surface analyses. Res. Chem. Intermed. 2019, 45, 5745–5760. [Google Scholar] [CrossRef]
- Birk, F.; Brescia, F.F.; Fraatz, M.A.; Pelzer, R.; Zorn, H. Aroma active alkylated pyrazines are produced by Basfia succiniciproducens as by-products of succinic acid production. Flavour Frag. J. 2021, 36, 605–612. [Google Scholar] [CrossRef]
- Cao, Y.L. The Study of 2,5-Dimethylpyrazine Biosynthetic Pathway by Bacillus subtilis and Construction of a High-Yield Strain; Jiangnan University: Wuxi, China, 2019. [Google Scholar]
- Liu, X.; Yang, W.; Gu, H.; Bughio, A.A.; Liu, J. Optimization of fermentation conditions for 2,3,5-trimethylpyrazine produced by Bacillus amyloliquefaciens from Daqu. Fermentation 2024, 10, 112. [Google Scholar] [CrossRef]
- Jia, R.B.; Guo, W.L.; Zhou, W.B.; Jiang, Y.J.; Zhu, F.F.; Chen, J.H.; Li, Y.; Liu, B.; Chen, S.J.; Chen, J.C.; et al. Screening and identification of Monacus strain with high TMP production and statistical optimization of its culture medium composition and liquid state fermentation conditions using response surface methodology (RSM). Biotechnol. Biotechnol. Equ. 2017, 31, 828–838. [Google Scholar]
- Meng, W.; Wang, R.; Xiao, D. Metabolic engineering of Bacillus subtilis to enhance the production of tetramethylpyrazine. Biotechnol. Lett. 2015, 37, 2475–2480. [Google Scholar] [CrossRef]
- Zhu, B.F.; Xu, Y. A feeding strategy for tetramethylpyrazine production by Bacillus subtilis based on the stimulating effect of ammonium phosphate. Bioproc. Biosyst. Eng. 2010, 33, 953–959. [Google Scholar] [CrossRef]
- Meng, W.; Ding, F.; Wang, R.M.; Wang, T.F. Enhanced production of tetramethylpyrazine in Bacillus licheniformis BL1 through aldC over-expression and acetaldehyde supplementation. Sci. Rep. 2020, 10, 3544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Si, G.; Rao, Z.; Li, J.; Zhang, X.; Mei, J.; Wang, J.; Ye, M.; Zhou, P. High yield of tetramethylpyrazine in functional Fuqu using Bacillus amyloliquefaciens. Food Biosci. 2019, 31, 100435. [Google Scholar] [CrossRef]
- Li, D.; Huang, W.; Wang, C.; Qiu, S. The complete genome sequence of the thermophilic bacterium Laceyella sacchari FBKL4.010 reveals the basis for tetramethylpyrazine biosynthesis in Moutai-flavor Daqu. MicrobiologyOpen 2019, 8, e922. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.Y.; Wei, Y.N.; Lin, L.C.; Chen, S.J.; Feng, P.P.; Xiao, D.G.; Lin, X.; Zhang, C.Y. Increasing yield of 2,3,5,6-tetramethylpyrazine in Baijiu through Saccharomyces cerevisiae metabolic engineering. Front. Microbiol. 2020, 11, 596306. [Google Scholar] [CrossRef]
- Eng, T.; Sasaki, Y.; Herbert, R.A.; Lau, A.; Trinh, J.; Chen, Y.; Mirsiaghi, M.; Petzold, C.J.; Mukhopadhyay, A. Production of tetra-methylpyrazine using engineered Corynebacterium glutamicum. Metab. Eng. Commun. 2020, 10, e00115. [Google Scholar] [CrossRef]
- Fujita, K.I.; Wada, T.; Shiraishi, T. Reversible interconversion between 2,5-dimethylpyrazine and 2,5-dimethylpiperazine by iridium-catalyzed hydrogenation/dehydrogenation for efficient hydrogen storage. Angew. Chem. Int. Edit. 2017, 56, 10886–10889. [Google Scholar] [CrossRef]
- Xu, J.; Yu, H.; Chen, X.; Liu, L.; Zhang, W. Accelerated green process of 2,5-dimethylpyrazine production from glucose by genetically modified Escherichia coli. ACS Synth. Biol. 2020, 9, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, J.; Liu, L.; Zhang, W. Biosynthesis of 2,5-dimethylpyrazine from L-threonine by whole-cell biocatalyst of recombinant Escherichia coli. Chin. J. Biotechnol. 2021, 37, 228–241. [Google Scholar]
- Liu, X.X.; Xi, X.Q.; Shen, T.S.; Shi, H.L.; Zhang, Y.J.; Kan, Y.C.; Yao, L.G.; Tang, C.D. Enhancing biosynthesis efficiency of 2,5-dimethylpyrazine by overexpressing L-threonine dehydrogenase and NADH oxidase in Escherichia coli. Mol. Catal. 2023, 550, 113550. [Google Scholar] [CrossRef]
- Liu, X.X.; Wang, Y.; Zhang, J.H.; Lu, Y.F.; Dong, Z.X.; Yue, C.; Huang, X.Q.; Zhang, S.P.; Li, D.D.; Yao, L.G.; et al. Engineering Escherichia coli for high-yielding 2,5-Dimethylpyrazine synthesis from L-Threonine by reconstructing metabolic pathways and enhancing cofactors regeneration. Biotechnol. Biofuels Bioprod. 2024, 17, 44. [Google Scholar] [CrossRef]
- Zeng, M.; Wu, H.; Han, Z.; Du, Z.; Yu, X.; Luo, W. Metabolic engineering of Escherichia coli for production of 2,5-Dimethylpyrazine. J. Agric. Food Chem. 2024, 72, 4267–4276. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Hao, Z.; Payne, R.; Ho, C.T. Effects of water content on volatile generation and peptide degradation in the Maillard reaction of glycine, diglycine, and triglycine. J. Agric. Food Chem. 2005, 53, 6443–6447. [Google Scholar] [CrossRef] [PubMed]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Formation of pyrazines in Maillard model systems of lysine-containing dipeptides. J. Agric. Food Chem. 2010, 58, 2470–2478. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Impact of the N-terminal amino acid on the formation of pyrazines from peptides in Maillard model systems. J. Agric. Food Chem. 2012, 60, 4697–4708. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of pyrazines in Maillard model systems: Effects of structures of lysine-containing dipeptides/tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Ma, Y.; Du, M.; Wu, C.; Xu, X. Mechanism of carbon skeleton formation of 2,3,5-trimethylpyrazine via a conversion reaction between methylglyoxal and glyoxal. J. Agric. Food Chem. 2023, 71, 5337–5344. [Google Scholar] [CrossRef]
- Yin, X.; Wei, Y.; Li, T.; Zhang, J.; Zou, L.; Cui, Q.; Lu, C.; Ning, J. Heterocyclic compounds formation in large-leaf yellow tea induced by the Maillard reaction at different roasting temperatures. LWT 2023, 182, 114856. [Google Scholar] [CrossRef]
- Ming, H.; Guo, Z.; Zhou, J.; Chen, M.; Xu, D.; Yao, X. Optimization of flavor components fermentation conditions of Bacillus licheniformis from Daqu by central composite design. Sci. Technol. Food Ind. 2015, 36, 182–186. [Google Scholar]
- Lin, H.; Liu, Y.; He, Q.; Liu, P.; Che, Z.; Wang, X.; Huang, J. Characterization of odor components of Pixian Douban (broad bean paste) by aroma extract dilute analysis and odor activity values. Int. J. Food Prop. 2019, 22, 1223–1234. [Google Scholar] [CrossRef]
- Ren, L.; Ma, Y.; Xie, M.; Lu, Y.; Cheng, D. Rectal bacteria produce sex pheromones in the male oriental fruit fly. Curr. Biol. 2021, 31, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
| Roles of TTMP | Sources of TTMP | Mechanisms | Experimental Species | References |
|---|---|---|---|---|
| Alleviate the symptoms of Alzheimer’s disease | ND 1 | Reduce beta-amyloid levels and tau phosphorylation; change the expression of oxidative phosphorylation complex proteins in the hippocampus | Alzheimer’s disease transgenic mice | [36] |
| Attenuate pulmonary hypertension | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Antioxidant properties | Monocrotaline-induced pulmonary hypertension rats; pulmonary cells from humans | [37] |
| Alleviate behavioral and psychological symptoms of dementia | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Alleviate dendritic and spine deficits; up-regulate the expression of synapse-related proteins in the hippocampus; activate signaling pathways to promote synaptic remodeling | Male Sprague Dawley rats | [38] |
| Liver-protective activity | Produced by Bacillus amyloliquefaciens XJB-104 with distillers’ grains | Decrease serum levels of biochemical indicators of liver injury and suppress inflammatory cytokines; alleviating the decrease in SOD 2, CAT 3, GSH 4, and MDA 5 | Kunming male mice | [39] |
| Treat endometriosis | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Decrease lesional fibrosis and lesion weight; improve hyperalgesia | Virgin female Balb/C mice; endometriotic tissue from 5 premenopausal cycling women | [40] |
| Suppress ovarian tumor cells’ proliferation | Purchased from Energy Chemical Co., Ltd. (Shanghai, China) | Suppress angiogenesis; promote apoptosis of tumor cells; augment the antitumor effects of paclitaxel | Female BALB/c nude mice | [41] |
| Suppress cancer cells’ growth | Purchased from Saeng Chemical Co., Ltd. (Shanghai, China) | Higher cytotoxicity, apoptosis rate, and cell-cycle arrest (conjugated with paclitaxel); promote cellular uptake and intracellular drug release | Female nu/nu nude mice | [42] |
| Treat spinal cord injury | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Anti-inflammatory, antioxidant, and neuroprotective activity | Male specific pathogen-free Sprague Dawley rats | [43] |
| Promote functional outcome after ischemic stroke | Purchased from Aladdin Chemistry Co. (Shanghai, China) | Ameliorate synaptic interface and postsynaptic density; partially participate in regulation of neuroplasticity | Male Sprague Dawley rats | [44] |
| Promote stroke recovery | Purchased from Harbin Medisan Pharmaceutical Co., Ltd. (Lot No. 090923A, Harbin, Heilongjiang, China) | Induce the restoration of neurovascular unit and transformation of A1/A2 reactive astrocytes | Male Sprague Dawley rats | [45] |
| Ameliorate acute lung injury | Obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) | Regulate the Rac1/LIMK1 signaling pathway | C57/BL6 male mice | [46] |
| Effects of 2,5-DMP | Sources of 2,5-DMP | Mechanisms | Experimental Species | References |
|---|---|---|---|---|
| Affect reproductive and accessory reproductive organs | ND 1 | Reduce uterus weight | Female rats | [61] |
| Affect reproductive and accessory reproductive organs | ND | Decrease the weight of prostate, seminal vesicles, and testosterone levels in plasma | Male rats | [62] |
| Affect plasma testosterone and polyamines and acid phosphatase levels | ND | Decrease plasma testosterone and levels of polyamines and acid phosphatase in the prostate | Juvenile male rats | [63] |
| Affect uterine contraction | ND | Inhibit the late phase of pregnant uterine movements | Female rats | [65] |
| Induce sperm-head abnormalities | Purchased from Sigma-Aldrich (St. Louis, MO, USA); released by female CBA mice | Induce genetic damage during meiotic divisions | Highly inbred male CBA mice | [66] |
| Affect the concentration of plasma non-esterified fatty acid | ND | Suppress the non-esterified fatty acid rebound induced by nicotinic acid | Male Wistar rats | [67] |
| Affect the concentration of blood lipids | ND | Decrease blood lipid concentrations | Male Wistar rats | [68] |
| Genes | Experimental Species | Measures | TTMP Yield | References |
|---|---|---|---|---|
| Transcriptional regulator (alsR), acetolactate synthase (alsS), acetolactate decarboxylase (alsD), NADH oxidase (nox) | Escherichia coli BL15 | Regulate medium formulation and aeration rate; adjust pH values and ammonium salt for fermentation | 16.10 g/L | [33] |
| Diacetyl reductase gene, fumarate reductase gene, lactate dehydrogenase gene, etc. | E. coli | Impair or block genes of by-products metabolic pathways; optimize molar ratio of acetoin/diammonium phosphate, reaction time, temperature and rotation speed of the microreactor | 56.72 g/L | [34] |
| α-acetolactate decarboxylase (aldC) | Bacillus licheniformis BL1 | Overexpress aldC gene; supply exogenous acetaldehyde in fermentation medium | 43.75 g/L | [85] |
| ND 1 | B. amyloliquefaciens XJB-104 | Adjust the amount of distillers’ grains during solid-state fermentation; adjust pH; improve Fuqu medium | 1.28 g/kg | [86] |
| Amino acid dehydrogenase (ADH), transaminase gene | Laceyella sacchari FBKL4.010 | Analyze TTMP metabolism related gene | ND | [87] |
| 2,3-butanediol dehydrogenase 1 (BDH1) and BDH2 | Saccharomyces cerevisiae | Disrupt BDH1 and overexpress BDH2 | 9.47 mg/L | [88] |
| mk and hmgR homologs | Corynebacterium glutamicum ATCC 13032 | Express mk homologs (from Staphylococcus aureus and Corynebacterium kroppenstedtii) and hmgR from S. aureus; Feed with ionic liquids | 5.00 g/L | [89] |
| Genes | Experimental Species | Measures | 2,5-DMP Yield | References |
|---|---|---|---|---|
| ND 1 | Aspergillus melleus | Add two whey protein hydrolysates | ND | [11] |
| ND | Oolong Tea (Camellia sinensis) | Add additional L-theanine | ND | [56] |
| L-threonine-3-dehydrogenase (TDH), aminoacetone oxidase (AAO), 2-Amino-3-ketobutyrate coenzyme A (CoA) ligase (KBL) | Escherichia coli | Overexpress TDH and AAO; knock out KBL gene | 1682.00 mg/L | [60] |
| TDH, KBL | Bacillus subtilis 168 | Heterologously express TDH; inactivate KBL; supply with L-threonine | 2.82 mM/L | [73] |
| TDH; AAO; NADH oxidase (Nox); L-threonine transporter protein (SstT) | E. coli | Rewrite the de novo 2,5-DMP biosynthesis pathway; supply with L-threonine; balance the intracellular NADH/NAD+ ratio; control the transmembrane transport of L-threonine | 1.43 g/L | [91] |
| TDH, Nox | E. coli BL21(DE3) | Overexpress TDH gene; optimize the expression mode of Nox; disrupt shunt metabolic pathway to reduce by-products | 1095.70 mg/L | [92] |
| TDH, Nox | E. coli BL21(DE3) | Overexpress EcTDH and EfNox; add additional L-threonine | 2009.31 mg/L | [93] |
| TDH, SstT, NoX, AAO | E. coli BL21(DE3) | Overexpress EcTDH, EcSstT, EhNox, and ScAAO; optimize reaction conditions | 2897.30 mg/L | [94] |
| TDH | E. coli | Enhance TDH gene expression; modify the L-threonine transport system | 3.1 g/L | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Quan, W. Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines. Molecules 2024, 29, 3597. https://doi.org/10.3390/molecules29153597
Liu X, Quan W. Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines. Molecules. 2024; 29(15):3597. https://doi.org/10.3390/molecules29153597
Chicago/Turabian StyleLiu, Xun, and Wenli Quan. 2024. "Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines" Molecules 29, no. 15: 3597. https://doi.org/10.3390/molecules29153597
APA StyleLiu, X., & Quan, W. (2024). Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines. Molecules, 29(15), 3597. https://doi.org/10.3390/molecules29153597





