β-Galactosidase- and Photo-Activatable Fluorescent Probes for Protein Labeling and Super-Resolution STED Microscopy in Living Cells
Abstract
1. Introduction
2. Results and Discussion
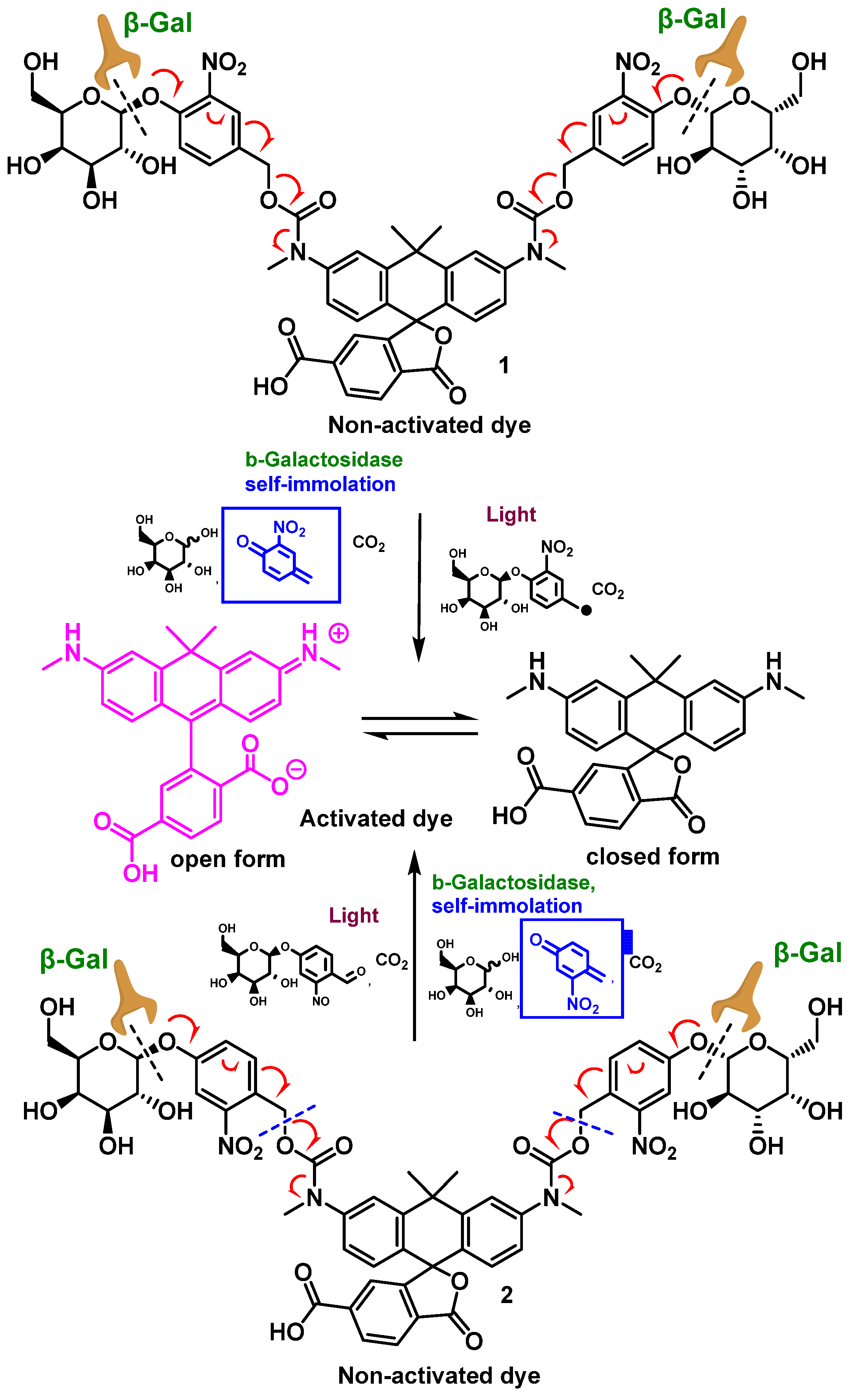
2.1. Design and Synthesis
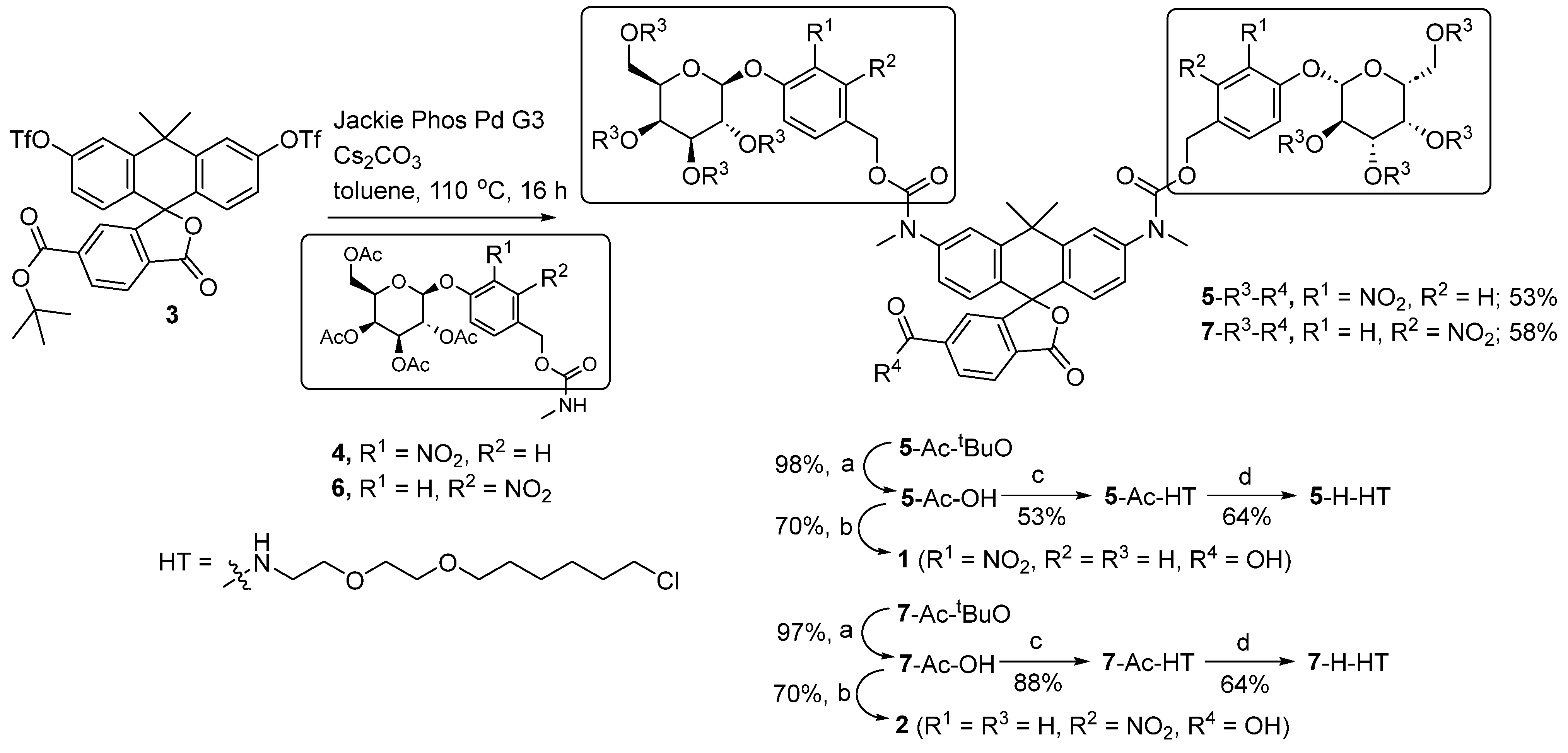
2.2. Synthesis of Probes 1 and 2
2.3. Activation and Optical Characterization
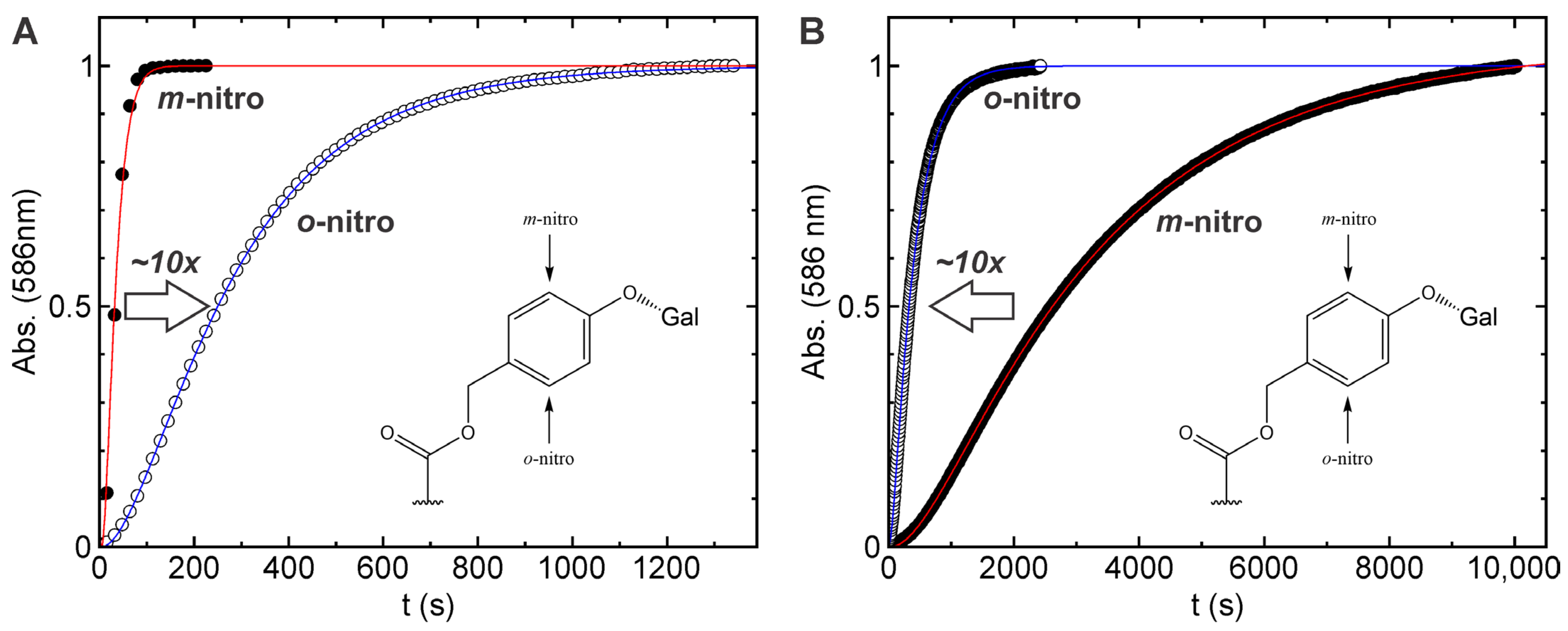
2.4. Comparison of Enzymatic activation and Photoactivation of Probes 1 and 2
2.5. Labeling and Bio-Imaging
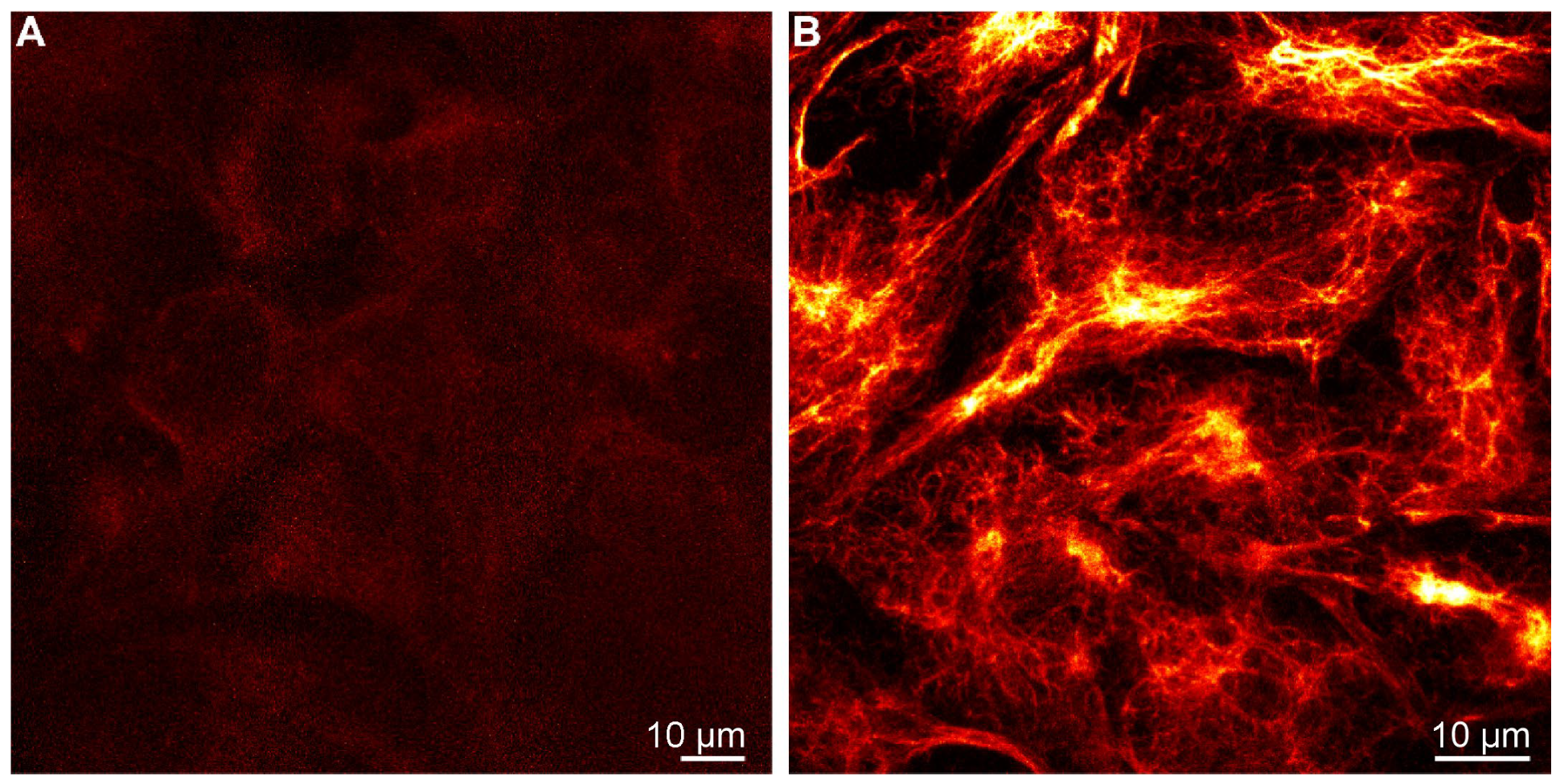
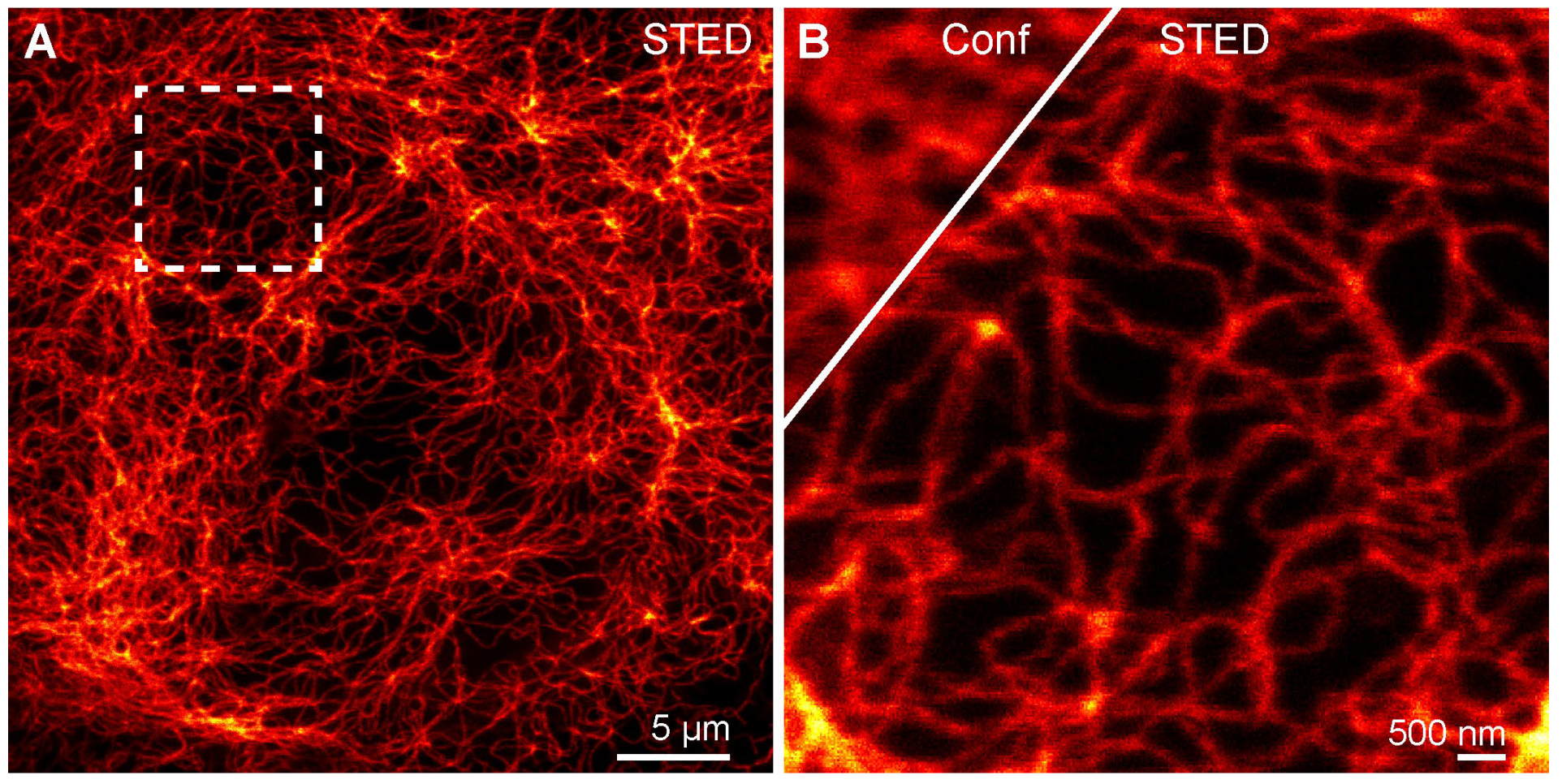
Confocal and STED Imaging with Probe 5-H-HT
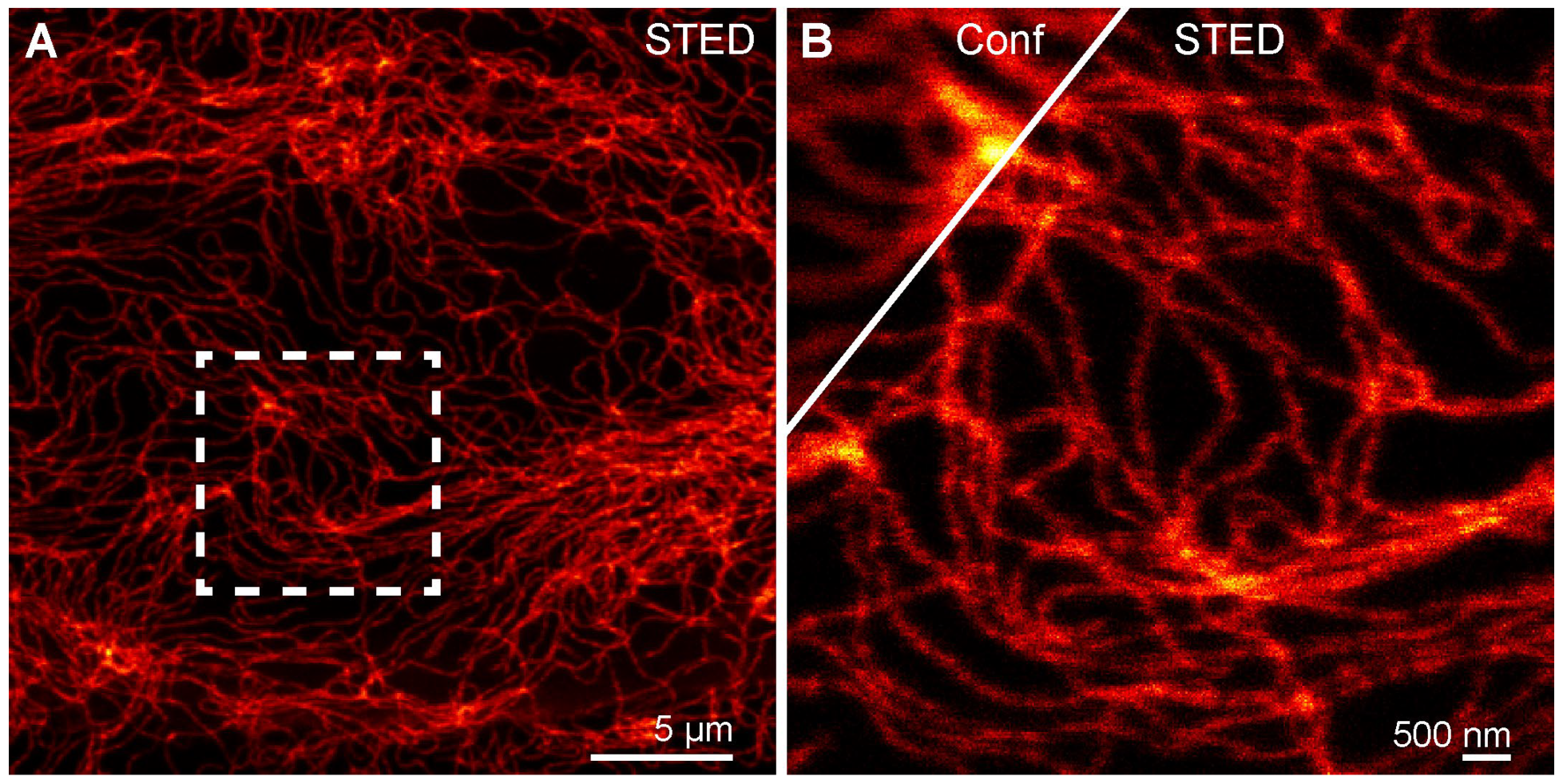
2.6. Confocal and STED Super-Resolution Imaging with Probe 7-H-HT
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Synthetic Procedures
4.3. Probe 1
4.3.1. Compound 5-Ac-tBuO
4.3.2. Compound 5-Ac-OH
4.4. Compound 1
4.5. Enzymatic Activation and Optical Characterization
4.6. Photoactivation and Optical Characterization
4.7. Labeling and Bio-Imaging
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Kim, D.; Yao, Q.; Ge, H.; Chung, J.; Fan, J.; Wang, J.; Peng, X.; Yoon, J. Activity-Based NIR Enzyme Fluorescent Probes for the Diagnosis of Tumors and Image-Guided Surgery. Angew. Chem. Int. Ed. 2021, 60, 17268–17289. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, R.; Kwon, N.; Ma, H.; Yoon, J. Activatable fluorescent probes for in situ imaging of enzymes. Chem. Soc. Rev. 2022, 51, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Li, H.; Choi, J.; Boo, J.; Jo, H.; Hyun, J.Y.; Shin, I. Glycosidase-targeting small molecules for biological and therapeutic applications. Chem. Soc. Rev. 2023, 52, 7036–7070. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, D.; Sakabe, M.; Kamiya, M.; Yamamoto, K.; Hiratake, J.; Ogawa, M.; Kosaka, N.; Choyke, P.L.; Nagano, T.; Kobayashi, H.; et al. Sensitive b-galactosidase-targeting fluorescence probe for visualizing small peritoneal metastatic tumours in vivo. Nat. Commun. 2015, 6, 6463. [Google Scholar]
- Yao, Y.; Zhang, Y.; Yan, C.; Zhu, W.-H.; Guo, Z. Enzyme-activatable fluorescent probes for β-galactosidase: From design to biological applications. Chem. Sci. 2021, 12, 9885–9894. [Google Scholar] [CrossRef]
- Kim, W.Y.; Won, M.; Salimi, A.; Sharma, A.; Lim, J.H.; Kwon, S.-H.; Jeon, J.-Y.; Lee, J.Y.; Kim, J.S. Monoamine oxidase-A targeting probe for prostate cancer imaging and inhibition of metastasis. Chem. Commun. 2019, 55, 13267–13270. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Yeo, D.C.; Wiraja, C.; Zhang, J.; Ning, X.; Xu, C.; Pu, K. Near-Infrared Fluorescent Molecular Probe for Sensitive Imaging of Keloid. Angew. Chem. Int. Ed. 2018, 57, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, J.; Lyu, Y.; Huang, J.; Pu, K. Near-Infrared Fluorescent Macromolecular Reporters for Real-Time Imaging and Urinalysis of Cancer Immunotherapy. J. Am. Chem. Soc. 2020, 142, 7075–7082. [Google Scholar] [CrossRef] [PubMed]
- Mochida, A.; Ogata, F.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Activatable fluorescent probes in fluorescence-guided surgery: Practical considerations. Bioorg. Med. Chem. 2018, 26, 925–930. [Google Scholar] [CrossRef]
- Chen, C.; Tian, R.; Zeng, Y.; Chu, C.; Liu, G. Activatable Fluorescence Probes for “Turn-On” and Ratiometric Biosensing and Bioimaging: From NIR-I to NIR-II. Bioconjugate Chem. 2020, 31, 276–292. [Google Scholar] [CrossRef]
- Halabi, E.A.; Thiel, Z.; Trapp, N.; Pinotsi, D.; Rivera-Fuentes, P. A Photoactivatable Probe for Super-Resolution Imaging of Enzymatic Activity in Live Cells. J. Am. Chem. Soc. 2017, 139, 13200–13207. [Google Scholar] [CrossRef]
- Li, M.; Yanga, M.; Zhu, W.-H. Advances in fluorescent sensors for β-galactosidase. Mater. Chem. Front. 2021, 5, 763–774. [Google Scholar] [CrossRef]
- Li, X.; Pan, Y.; Chen, H.; Duan, Y.; Zhou, S.; Wu, W.; Wang, S.; Liu, B. Specific Near-Infrared Probe for Ultrafast Imaging of Lysosomal β-Galactosidase in Ovarian Cancer Cells. Anal. Chem. 2020, 92, 5772–5779. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Sosinska, P.; Mikuła-Pietrasik, J.; Ryzek, M.; Naumowicz, E.; Ksiazek, K. Specificity of cytochemical and fluorescence methods of senescence associated β-galactosidase detection. Biogerontology 2014, 15, 407–413. [Google Scholar] [CrossRef]
- Gu, K.; Qiu, W.; Guo, Z.; Yan, C.; Zhu, S.; Yao, D.; Shi, P.; Tian, H.; Zhu, W.-H. An enzyme-activatable probe liberating AIEgens: On-site sensing and long-term tracking of β-galactosidase in ovarian cancer cells. Chem. Sci. 2019, 10, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, M.; Asanuma, D.; Kamiya, M.; Iwatate, R.J.; Hanaoka, K.; Terai, T.; Nagano, T.; Urano, Y. Rational Design of Highly Sensitive Fluorescence Probes for Protease and Glycosidase Based on Precisely Controlled Spirocyclization. J. Am. Chem. Soc. 2013, 135, 409–414. [Google Scholar] [CrossRef]
- Wang, W.; Vellaisamy, K.; Li, G.; Wu, C.; Ko, C.-N.; Leung, C.-H.; Ma, D.-L. Development of a Long-Lived Luminescence Probe for Visualizing β-Galactosidase in Ovarian Carcinoma Cells. Anal. Chem. 2017, 89, 11679–11684. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Han, M.S.; Tung, C.-H. A fluorogenic probe for β-galactosidase activity imaging in living cells. Mol. BioSyst. 2013, 9, 3001–3008. [Google Scholar] [CrossRef]
- Doura, T.; Kamiya, M.; Obata, F.; Yamaguchi, Y.; Hiyama, T.Y.; Matsuda, T.; Fukamizu, A.; Noda, M.; Miura, M.; Urano, Y. Detection of LacZ-Positive Cells in Living Tissue with Single-Cell Resolution. Angew. Chem. Int. Ed. 2016, 55, 9620–9624. [Google Scholar] [CrossRef] [PubMed]
- Cecioni, S.; Ashmus, R.A.; Gilormini, P.-A.; Zhu, S.; Chen, X.; Shan, X.; Gros, C.; Deen, M.C.; Wang, Y.; Britton, R.; et al. Quantifying lysosomal glycosidase activity within cells using bis-acetal substrates. Nat. Chem. Biol. 2022, 18, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Yim, J.J.; Bogyo, M.A. A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Butkevich, A.N.; Mitronova, G.Y.; Sidenstein, S.C.; Klocke, J.L.; Kamin, D.; Meineke, D.N.H.; D’Este, E.; Kraemer, P.-T.; Danzl, J.G.; Belov, V.N.; et al. Fluorescent Rhodamines and Fluorogenic Carbopyronines for Super-Resolution STED Microscopy in Living Cells. Angew. Chem. Int. Ed. 2016, 55, 3290–3294. [Google Scholar] [CrossRef] [PubMed]
- Aktalay, A.; Khan, T.A.; Bossi, M.L.; Belov, V.N.; Hell, S.W. Photoactivatable Carbo- and Silicon-Rhodamines and Their Application in MINFLUX Nanoscopy. Angew. Chem. Int. Ed. 2023, 62, e202302781. [Google Scholar] [CrossRef] [PubMed]
- Carl, P.L.; Chakravarty, P.K.; Katzenellenbogen, J.A. A novel connector linkage applicable in prodrug design. J. Med. Chem. 1981, 24, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; Klein, T.; Kopek, B.G.; Shtengel, G.; Hess, H.F.; Sauer, M.; Lavis, L.D. Synthesis of a Far-Red Photoactivatable Silicon-Containing Rhodamine for Super-Resolution Microscopy. Angew. Chem. Int. Ed. 2016, 55, 1723–1727. [Google Scholar] [CrossRef]
- Butkevich, A.N.; Weber, M.; Delgado, A.R.C.; Ostersehlt, L.M.; D’Este, E.; Hell, S.W. Photoactivatable Fluorescent Dyes with Hydrophilic Caging Groups and Their Use in Multicolor Nanoscopy. J. Am. Chem. Soc. 2021, 143, 18388–18393. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Zhang, Y.; Gao, G.; Liu, J.; Zhang, X.; Wang, M.; Hou, S. Ratiometric fluorescent probes with a self-immolative spacer for real-time detection of β-galactosidase and imaging in living cells. Anal. Chim. Acta 2018, 1033, 193–198. [Google Scholar] [CrossRef]
- Gaplovsky, M.; Il’ichev, Y.V.; Kamdzhilov, Y.; Kombarova, S.V.; Mac, M.; Schwörer, M.A.; Wirz, J. Photochemical reaction mechanisms of 2-nitrobenzyl compounds: 2-Nitrobenzyl alcohols form 2-nitroso hydrates by dual proton transfer. Photochem. Photobiol. Sci. 2005, 4, 33–42. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kumar, R.; Sharma, A.; Yoon, B.; Kim, H.M.; Lee, H.; Hong, K.S.; Kim, J.S. In vivo imaging of b-galactosidase stimulated activity in hepatocellular carcinoma using ligand-targeted fluorescent probe. Biomaterials 2017, 122, 83–90. [Google Scholar] [CrossRef]
- Hicks, J.D.; Hyde, A.M.; Martínez-Cuezva, A.; Buchwald, S.L. Pd-Catalyzed N-Arylation of Secondary Acyclic Amides: Catalyst Development, Scope, and Computational Study. J. Am. Chem. Soc. 2009, 131, 16720–16734. [Google Scholar] [CrossRef]
- Los, G.V.; Encell, L.P.; McDougall, M.G.; Hartzell, D.D.; Karassina, N.; Zimprich, C.; Wood, M.G.; Learish, R.; Ohana, R.F.; Urh, M.; et al. HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis. ACS Chem. Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef]
- Hauke, S.; von Appen, A.; Quidwai, T.; Ries, J.; Wombacher, R. Specific protein labeling with caged fluorophores for dual-color imaging and super-resolution microscopy in living cells. Chem. Sci. 2017, 8, 559–566. [Google Scholar] [CrossRef]
- Wombacher, R.; Cornish, V.W. Chemical tags: Applications in live cell fluorescence imaging. J. Biophotonics 2011, 4, 391–402. [Google Scholar] [CrossRef]
- Thevathasan, J.V.; Kahnwald, M.; Cieslinski, K.; Hoess, P.; Peneti, S.K.; Reitberger, M.; Heid, D.; Kasuba, K.C.; Hoerner, S.J.; Li, Y.; et al. Nuclear pores as versatile reference standards for quantitative superresolution microscopy. Nat. Methods 2019, 16, 1045–1053. [Google Scholar] [CrossRef]
- Franke, W.W.; Schmid, E.; Osborn, M.; Weber, K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc. Nat. Acad. Sci. USA 1978, 75, 5034–5038. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Dechat, T.; Grin, B.; Helfand, B.; Mendez, M.; Pallari, H.M.; Goldman, R.D. Introducing intermediate filaments: From discovery to disease. J. Clin. Investig. 2009, 119, 1763–1771. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Araújo, H.C.; Lautenschläger, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Bossi, M.L.; Konen, T.; Belov, V.N.; Irie, M.; Hell, S.W. Asymmetric Diarylethenes with Oxidized 2-Alkylbenzothiophen-3-yl Units: Chemistry, Fluorescence, and Photoswitching. Adv. Optical Mater. 2019, 7, 1801746. [Google Scholar] [CrossRef]
- Ratz, M.; Testa, I.; Hell, S.W.; Jakobs, S. CRISPR/Cas9-mediated endogenous protein tagging for RESOLFT super-resolution microscopy of living human cells. Sci. Rep. 2015, 5, 9592. [Google Scholar] [CrossRef] [PubMed]
- Butkevich, A.N.; Ta, H.; Ratz, M.; Stoldt, S.; Jakobs, S.; Belov, V.N.; Hell, S.W. Two-Color 810 nm STED Nanoscopy of Living Cells with Endogenous SNAP-Tagged Fusion Proteins. ACS Chem. Biol. 2018, 13, 475–480. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.A.; Stoldt, S.; Bossi, M.L.; Belov, V.N.; Hell, S.W. β-Galactosidase- and Photo-Activatable Fluorescent Probes for Protein Labeling and Super-Resolution STED Microscopy in Living Cells. Molecules 2024, 29, 3596. https://doi.org/10.3390/molecules29153596
Khan TA, Stoldt S, Bossi ML, Belov VN, Hell SW. β-Galactosidase- and Photo-Activatable Fluorescent Probes for Protein Labeling and Super-Resolution STED Microscopy in Living Cells. Molecules. 2024; 29(15):3596. https://doi.org/10.3390/molecules29153596
Chicago/Turabian StyleKhan, Taukeer A., Stefan Stoldt, Mariano L. Bossi, Vladimir N. Belov, and Stefan W. Hell. 2024. "β-Galactosidase- and Photo-Activatable Fluorescent Probes for Protein Labeling and Super-Resolution STED Microscopy in Living Cells" Molecules 29, no. 15: 3596. https://doi.org/10.3390/molecules29153596
APA StyleKhan, T. A., Stoldt, S., Bossi, M. L., Belov, V. N., & Hell, S. W. (2024). β-Galactosidase- and Photo-Activatable Fluorescent Probes for Protein Labeling and Super-Resolution STED Microscopy in Living Cells. Molecules, 29(15), 3596. https://doi.org/10.3390/molecules29153596







