Comparison of Cricket Protein Powder and Whey Protein Digestibility
Abstract
1. Introduction
2. Results
2.1. Nutritional Value
2.2. Protein Digestibility
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Nutrient Analysis
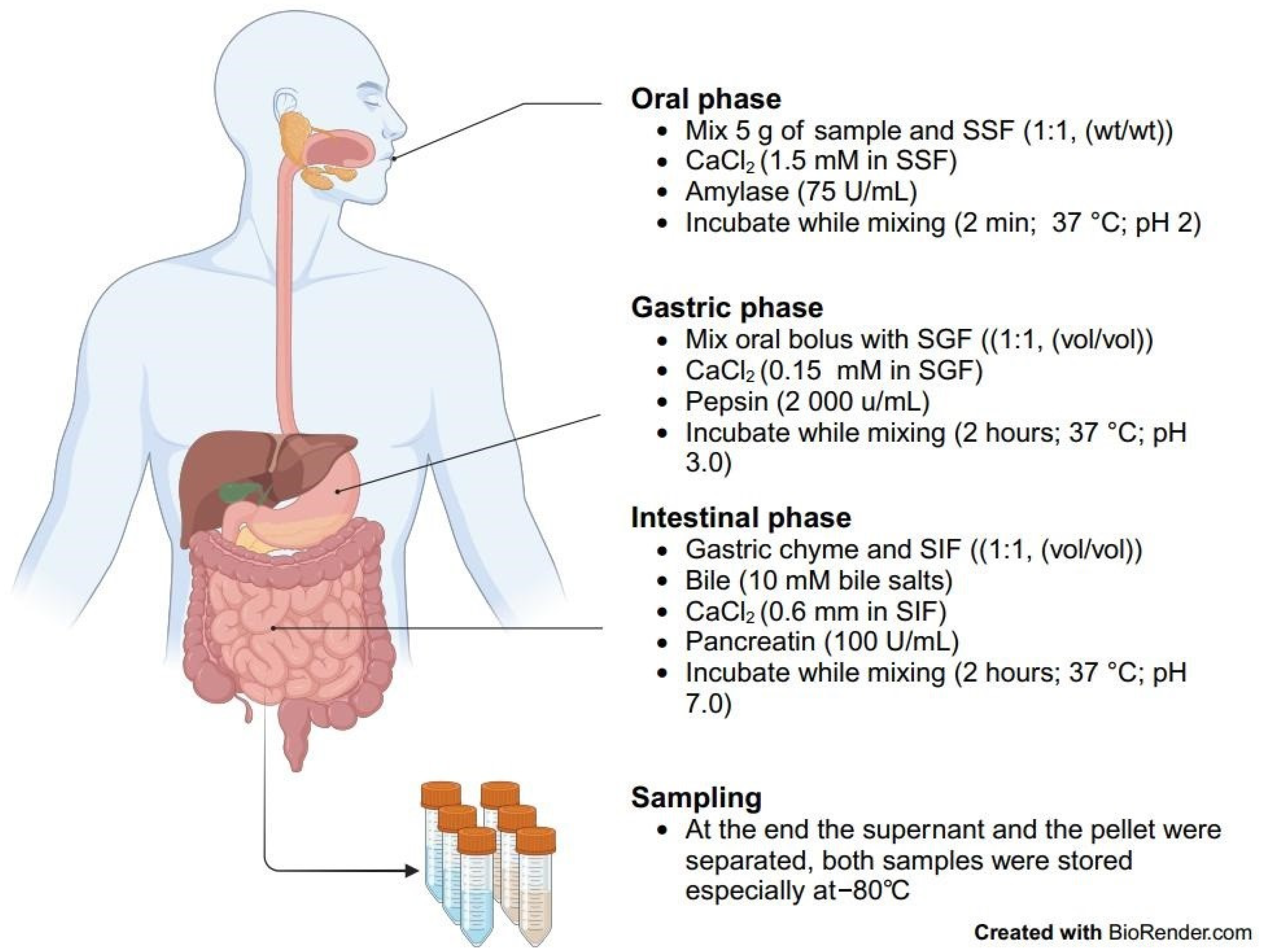
4.3. Static In Vitro Digestion Model
4.4. Determination of Protein Digestibility
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A Potential Source of Protein and Other Nutrients for Feed and Food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Ariëns, R.M.C.; Bastiaan-Net, S.; van den Berg-Somhorst, D.B.P.M.; El Bachrioui, K.; Boudewijn, A.; van den Dool, R.T.M.; de Jong, G.A.H.; Wichers, H.J.; Mes, J.J. Comparing nutritional and digestibility aspects of sustainable proteins using the INFOGEST digestion protocol. J. Funct. Foods 2021, 87, 104748. [Google Scholar] [CrossRef]
- Hermans, W.J.H.; Senden, J.M.; Churchward-Venne, T.A.; Paulussen, K.J.M.; Fuchs, C.J.; Smeets, J.S.J.; van Loon, J.J.A.; Verdijk, L.B.; van Loon, L.J.C. Insects are a viable protein source for human consumption: From insect protein digestion to postprandial muscle protein synthesis in vivo in humans: A double-blind randomized trial. Am. J. Clin. Nutr. 2021, 114, 934–944. [Google Scholar] [CrossRef]
- Oonincx, D.G.; van Itterbeeck, J.; Heetkamp, M.J.; van den Brand, H.; van Loon, J.J.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed]
- FAO. Expert Consultation Meeting: Assessing the Potential of Insects as Food and Feed in Assuring Food Security; FAO: Rome, Italy, 2012. [Google Scholar]
- Raheem, D.; Raposo, A.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Carrascosa, C. Entomophagy: Nutritional, ecological, safety and legislation aspects. Food Res. Int. 2019, 126, 108672. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Manditsera, F.A.; Luning, P.A.; Fogliano, V.; Lakemond, C.M.M. Effect of domestic cooking methods on protein digestibility and mineral bioaccessibility of wild harvested adult edible insects. Food Res. Int. 2019, 121, 404–411. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.; Barroso, F.G.; Fabrikov, D.; Sánchez-Muros, M.J. In Vitro Crude Protein Digestibility of Insects: A Review. Insects 2022, 13, 682. [Google Scholar] [CrossRef]
- Kröger, T.; Dupont, J.; Büsing, L.; Fiebelkorn, F. Acceptance of Insect-Based Food Products in Western Societies: A Systematic Review. Front. Nutr. 2021, 8, 759885. [Google Scholar] [CrossRef]
- Guiné, R.P.F. Textural Properties of Bakery Products: A Review of Instrumental and Sensory Evaluation Studies. Appl. Sci. 2022, 12, 8628. [Google Scholar] [CrossRef]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in grain, flour, amino acid composition, protein profiling, and proportion of total flour proteins of different wheat cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Amoah, I.; Cobbinah, J.C.; Yeboah, J.A.; Essiam, F.A.; Lim, J.J.; Tandoh, M.A.; Rush, E. Edible Insect Powder for Enrichment of Bakery Products—A Review of Nutritional, Physical Characteristics and Acceptability of Bakery Products to Consumers. Future Foods 2023, 8, 100251. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From Farm to Fork: Crickets as Alternative Source of Protein, Minerals, and Vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Romero, G.; Chisaguano-Tonato, A.M.; Herrera-Fontana, M.E.; Chimbo-Gándara, L.F.; Sharifi-Rad, M.; Giampieri, F.; Battino, M.; Vernaza, M.G.; Álvarez-Suárez, J.M. House cricket (Acheta domesticus): A review based on its nutritional composition, quality, and potential uses in the food industry. Trends Food Sci. Technol. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Ardoin, R.; Marx, B.D.; Boeneke, C.; Prinyawiwatkul, W. Effects of cricket powder on selected physical properties and US consumer perceptions of whole-wheat snack crackers. Int. J. Food Sci. Technol. 2021, 56, 4070–4080. [Google Scholar] [CrossRef]
- Sah, B.N.P.; McAinch, A.J.; Vasiljevic, T. Modulation of bovine whey protein digestion in gastrointestinal tract: A comprehensive review. Int. Dairy J. 2016, 62, 10–18. [Google Scholar] [CrossRef]
- Boirie, Y. Chapter 16—Whey Protein and Muscle Protection. In Nutrition and Skeletal Muscle; Walrand, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 271–281. [Google Scholar]
- Ververis, E.; Boué, G.; Poulsen, M.; Pires, S.M.; Niforou, A.; Thomsen, S.T.; Tesson, V.; Federighi, M.; Naska, A. A systematic review of the nutrient composition, microbiological and toxicological profile of Acheta domesticus (house cricket). J. Food Compos. Anal. 2022, 114, 104859. [Google Scholar] [CrossRef]
- van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species—Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein quality and physicochemical properties of commercial cricket and mealworm powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.C.; Monteiro, M.L.G.; da Costa-Lima, B.R.C.; Alvares, T.S.; Conte-Junior, C.A. In vitro digestibility of commercial whey protein supplements. LWT-Food Sci. Technol. 2015, 61, 7–11. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Chen, Q.; Hu, A.; Li, T.; Guo, F.; Wang, Q. Preparation of Whole-Cut Plant-Based Pork Meat and Its Quality Evaluation with Animal Meat. Gels 2023, 9, 461. [Google Scholar] [CrossRef]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Ndiritu, A.; Kinyuru, J.; Kenji, G.; Njihia Gichuhi, P. Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Charact. 2017, 11, 2013–2021. [Google Scholar] [CrossRef]
- Poelaert, C.; Beckers, Y.; Despret, X.; Portetelle, D.; Francis, F.; Bindelle, J. In vitro evaluation of fermentation characteristics of two types of insects as potential novel protein feeds for pigs. J. Anim. Sci. 2016, 94, 198–201. [Google Scholar] [CrossRef]
- Marono, S.; Piccolo, G.; Loponte, R.; Meo, C.; Attia, Y.; Nizza, A.; Bovera, F. In Vitro Crude Protein Digestibility of Tenebrio molitor and Hermetia illucens Insect Meals and its Correlation with Chemical Composition Traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Huecker, M.; Sarav, M.; Pearlman, M.; Laster, J. Protein Supplementation in Sport: Source, Timing, and Intended Benefits. Curr. Nutr. Rep. 2019, 8, 382–396. [Google Scholar] [CrossRef]
- ISO 5983-1:2005; Animal Feeding Stuffs–Determination of Nitrogen Content and Calculation of Crude Protein Content–Part 1: Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 13903:2005; Animal Feeding Stuffs—Determination of Amino Acids Content. International Organization for Standardization: Geneva, Switzerland, 2005.
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
| Component (g/100 g DM) | Cricket Protein Powder | Whey Protein |
|---|---|---|
| Crude protein (TN × 6.25) | 72.41 ± 0.46 b | 82.89 ± 0.49 a |
| Total fat | 18.35 ± 0.53 b | 4.80 ± 0.23 a |
| Aspartic acid | 5.25 ± 0.11 b | 7.72 ± 0.11 a |
| Threonine | 2.58 ± 0.12 b | 5.21 ± 0.16 a |
| Serine | 2.85 ± 0.11 b | 5.04 ± 0.11 a |
| Glutamic acid | 5.60 ± 0.15 b | 12.59 ± 0.12 a |
| Proline | 3.81 ± 0.12 b | 4.49 ± 0.14 a |
| Glycine | 3.23 ± 0.13 a | 2.09 ± 0.12 b |
| Alanine | 6.27 ± 0.17 a | 3.98 ± 0.17 b |
| Valine | 4.03 ± 0.15 b | 4.49 ± 0.15 a |
| Isoleucine | 2.93 ± 0.11 b | 4.75 ± 0.12 a |
| Leucine | 5.14 ± 0.14 b | 7.72 ± 0.10 a |
| Tyrosine | 4.09 ± 0.13 a | 2.77 ± 0.12 b |
| Phenylalanine | 2.93 ± 0.09 a | 2.43 ± 0.12 b |
| Methionine | 2.83 ± 0.13 b | 2.84 ± 0.13 a |
| Histidine | 2.73 ± 0.10 b | 6.18 ± 0.09 a |
| Lysine | 4.07 ± 0.12 b | 7.61 ± 0.12 a |
| Arginine | 3.93 ± 0.12 a | 2.30 ± 0.13 b |
| Sum of AA | 62.29 ± 1.62 b | 82.21 ± 1.64 a |
| Sample | Cricket Protein Powder | Whey Protein | ||
|---|---|---|---|---|
| Crude Protein | Sum of AA | Crude Protein | Sum of AA | |
| Threonine | 69.99 ± 2.58 c | 81.36 ± 3.00 b | 123.30 ± 3.09 a | 124.32 ± 3.12 a |
| Valine | 76.25 ± 2.32 b | 88.63 ± 2.69 a | 74.16 ± 2.02 b | 74.77 ± 2.04 b |
| Isoleucine | 61.31 ± 1.82 c | 71.27 ± 2.12 b | 86.86 ± 1.79 a | 87.58 ± 1.81 a |
| Leucine | 80.62 ± 1.84 c | 93.72 ± 2.14 b | 105.85 ± 1.08 a | 106.72 ± 1.09 a |
| Phenylalanine | 69.77 ± 1.81 b | 81.10 ± 2.11 a | 50.50 ± 2.04 c | 50.92 ± 2.05 c |
| Methionine | 102.13 ± 4.58 b | 141.98 ± 5.33 a | 107.07 ± 4.00 b | 107.96 ± 4.03 b |
| Lysine | 87.82 ± 2.17 c | 102.09 ± 2.53 b | 143.37 ± 1.80 a | 144.55 ± 1.81 a |
| EAAI (%) | 79.31 ± 2.36 b | 92.19 ± 2.74 a | 94.06 ± 2.33 a | 94.84 ± 2.35 a |
| Amino Acid Digestibility (%) | Time of Intestinal Phase In Vitro Digestion | ||||||
|---|---|---|---|---|---|---|---|
| 0 min | 20 min | 40 min | 60 min | 80 min | 100 min | 120 min | |
| Aspartic acid | 52.03 ± 0.17 f | 65.48 ± 0.15 e | 73.00 ± 0.16 d | 73.02 ± 0.16 d | 73.61 ± 0.17 c | 75.17 ± 0.13 b | 80.40 ± 0.18 a |
| Threonine | 49.63 ± 0.15 f | 63.99 ± 0.18 e | 70.71 ± 0.17 d | 70.78 ± 0.15 d | 71.42 ± 0.17 c | 72.91 ± 0.19 b | 78.84 ± 0.17 a |
| Serine | 49.39 ± 0.20 e | 64.03 ± 0.19 d | 71.24 ± 0.20 c | 71.42 ± 0.18 c | 71.76 ± 0.17 c | 73.33 ± 0.20 b | 78.92 ± 0.20 a |
| Glutamic acid | 53.05 ± 0.20 e | 66.67 ± 0.19 d | 73.45 ± 0.20 c | 73.73 ± 0.18 c | 73.99 ± 0.20 c | 75.06 ± 0.21 b | 80.31 ± 0.22 a |
| Proline | 58.78 ± 0.18 e | 70.99 ± 0.16 d | 75.87 ± 0.17 c | 76.64 ± 0.18 b | 76.59 ± 0.19 b | 77.04 ± 0.18 b | 82.78 ± 0.17 a |
| Glycine | 51.92 ± 0.20 e | 65.97 ± 0.22 d | 70.59 ± 0.21 c | 70.63 ± 0.22 c | 71.28 ± 0.20 c | 72.88 ± 0.23 b | 78.32 ± 0.23 a |
| Alanine | 52.87 ± 0.21 f | 66.51 ± 0.23 e | 71.13 ± 0.22 d | 71.42 ± 0.21 cd | 72.02 ± 0.23 c | 73.90 ± 0.22 b | 78.67 ± 0.23 a |
| Valine | 40.05 ± 0.19 f | 68.14 ± 0.21 e | 71.65 ± 0.19 d | 72.64 ± 0.21 c | 72.89 ± 0.22 bc | 73.37 ± 0.21 b | 79.27 ± 0.20 a |
| Isoleucine | 50.61 ± 016 f | 65.54 ± 0.18 e | 71.04 ± 0.17 d | 71.70 ± 0.18 c | 72.78 ± 0.18 b | 72.84 ± 0.19 b | 78.91 ± 0.18 a |
| Leucine | 50.68 ± 0.19 e | 66.49 ± 0.20 d | 70.89 ± 0.21 c | 71.40 ± 0.21 c | 71.44 ± 0.19 c | 73.22 ± 0.18 b | 78.26 ± 0.20 a |
| Tyrosine | 48.31 ± 0.18 f | 63.09 ± 0.19 e | 69.28 ± 0.21 d | 69.32 ± 0.20 d | 70.40 ± 0.18 c | 71.46 ± 0.18 b | 77.86 ± 0.19 a |
| Phenylalanine | 53.81 ± 0.17 f | 65.75 ± 0.17 e | 72.03 ± 0.14 d | 72.51 ± 0.16 d | 73.77 ± 0.15 c | 74.83 ± 0.15 b | 80.56 ± 0.13 a |
| Methionine | 52.10 ± 0.17 f | 66.53 ± 0.18 e | 72.1 ± 0.18 d | 72.36 ± 0.20 cd | 72.94 ± 0.19 c | 74.35 ± 0.21 b | 79.65 ± 0.17 a |
| Histidine | 57.16 ± 0.17 g | 66.34 ± 0.16 f | 69.66 ± 0.17 e | 71.31 ± 0.16 d | 71.88 ± 0.15 c | 74.87 ± 0.15 b | 79.35 ± 0.16 a |
| Lysine | 58.60 ± 0.18 f | 70.28 ± 0.18 e | 75.16 ± 0.18 d | 75.36 ± 0.17 cd | 75.90 ± 0.16 c | 78.13 ± 0.16 b | 81.97 ± 0.17 a |
| Arginine | 54.84 ± 0.18 f | 67.11 ± 0.19 e | 72.48 ± 0.17 d | 72.49 ± 0.17 d | 73.38 ± 0.18 c | 75.50 ± 0.17 b | 79.94 ± 0.19 a |
| Total digestibility (%) | 52.11 ± 2.14 c | 66.54 ± 2.18 b | 72.00 ± 2.18 ab | 72.38 ± 2.14 ab | 72.93 ± 2.17 ab | 74.37 ± 2.17 ab | 79.64 ± 2.16 a |
| Amino Acid Digestibility (%) | Time of Intestinal Phase In Vitro Digestion | ||||||
|---|---|---|---|---|---|---|---|
| 0 min | 20 min | 40 min | 60 min | 80 min | 100 min | 120 min | |
| Aspartic acid | 62.93 ± 0.19 f | 76.46 ± 0.18 e | 81.90 ± 0.17 d | 92.46 ± 0.19 c | 97.61 ± 0.19 b | 98.36 ± 0.17 a | 98.81 ± 0.18 a |
| Threonine | 63.07 ± 0.21 f | 76.50 ± 0.20 e | 81.92 ± 0.21 d | 92.38 ± 0.21 c | 97.43 ± 0.18 b | 97.92 ± 0.20 ab | 98.32 ± 0.19 a |
| Serine | 62.66 ± 0.19 f | 76.18 ± 0.21 e | 81.84 ± 0.19 d | 92.33 ± 0.20 c | 97.49 ± 0.19 b | 97.84 ± 0.18 ab | 98.28 ± 0.17 a |
| Glutamic acid | 62.43 ± 0.17 f | 75.98 ± 0.18 e | 81.50 ± 0.14 d | 92.17 ± 0.18 c | 97.28 ± 0.18 b | 97.67 ± 0.16 ab | 98.13 ± 0.17 a |
| Proline | 64.68 ± 0.22 f | 76.18 ± 0.23 e | 82.23 ± 0.21 d | 92.45 ± 0.24 c | 98.36 ± 0.24 b | 98.54 ± 0.21 ab | 99.26 ± 0.23 a |
| Glycine | 62.73 ± 0.19 f | 76.28 ± 0.18 e | 81.72 ± 0.17 d | 92.26 ± 0.18 c | 97.50 ± 0.16 b | 97.79 ± 0.17 ab | 98.19 ± 0.18 a |
| Alanine | 63.61 ± 0.21 f | 76.63 ± 0.20 e | 82.10 ± 0.21 d | 92.58 ± 0.20 c | 98.08 ± 0.22 b | 98.44 ± 0.23 ab | 98.81 ± 0.21 a |
| Valine | 63.71 ± 0.19 f | 76.78 ± 0.21 e | 82.66 ± 0.19 d | 92.64 ± 0.21 c | 97.74 ± 0.22 b | 98.13 ± 0.21 ab | 98.50 ± 0.20 a |
| Isoleucine | 63.02 ± 0.18 f | 76.08 ± 0.17 e | 81.78 ± 0.16 d | 92.35 ± 0.16 c | 97.55 ± 0.17 b | 97.86 ± 0.18 ab | 98.31 ± 0.18 a |
| Leucine | 61.38 ± 0.15 f | 75.20 ± 0.16 e | 81.02 ± 0.16 d | 91.64 ± 0.16 c | 96.93 ± 0.13 b | 97.32 ± 0.18 ab | 97.85 ± 0.17 a |
| Tyrosine | 63.33 ± 0.16 f | 76.78 ± 0.18 e | 81.80 ± 0.18 d | 92.57 ± 0.17 c | 97.84 ± 0.18 b | 97.84 ± 0.19 b | 98.71 ± 0.20 a |
| Phenylalanine | 64.42 ± 0.19 f | 77.20 ± 0.18 e | 82.73 ± 0.18 d | 93.16 ± 0.17 c | 98.20 ± 0.18 b | 98.55 ± 0.20 ab | 98.87 ± 0.18 a |
| Methionine | 60.89 ± 0.18 f | 73.61 ± 0.20 e | 79.01 ± 0.18 d | 89.16 ± 0.19 c | 94.16 ± 0.20 b | 94.53 ± 0.18 ab | 94.96 ± 0.21 a |
| Histidine | 64.83 ± 0.14 f | 77.04 ± 0.14 e | 82.16 ± 0.17 d | 92.60 ± 0.17 c | 97.60 ± 0.16 b | 97.93 ± 0.15 ab | 98.16 ± 0.16 a |
| Lysine | 62.53 ± 0.19 f | 75.57 ± 0.17 e | 81.75 ± 0.19 d | 92.33 ± 0.18 c | 97.33 ± 0.18 b | 97.74 ± 0.18 ab | 98.17 ± 0.20 a |
| Arginine | 63.77 ± 0.18 f | 76.75 ± 0.20 e | 82.41 ± 0.15 d | 92.73 ± 0.18 c | 97.81 ± 0.19 b | 98.14 ± 0.16 ab | 98.52 ± 0.18 a |
| Total digestibility (%) | 63.06 ± 2.18 c | 76.23 ± 2.17 b | 81.85 ± 2.13 b | 92.36 ± 2.18 a | 97.54 ± 2.18 a | 97.93 ± 2.21 a | 98.38 ± 2.19 a |
| Nutrition Information per 100 g | ||
|---|---|---|
| Cricket Protein Powder | Pure Whey Protein | |
| Energy value | 1939 kJ/463 kcal | 367 kJ/1554 kcal |
| Fat (g) | 20 | 5.0 |
| of which saturated fatty acids (g) | 5.2 | 0.3 |
| Carbohydrates (g) | 0.5 | 3.5 |
| of which sugars (g) | 0 | 3.5 |
| Fibre (g) | 9.5 | 0 |
| Protein (g) | 70 | 80 |
| Salt (g) | 0.8 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampová, B.; Doskočil, I.; Šmíd, P.; Kouřimská, L. Comparison of Cricket Protein Powder and Whey Protein Digestibility. Molecules 2024, 29, 3598. https://doi.org/10.3390/molecules29153598
Lampová B, Doskočil I, Šmíd P, Kouřimská L. Comparison of Cricket Protein Powder and Whey Protein Digestibility. Molecules. 2024; 29(15):3598. https://doi.org/10.3390/molecules29153598
Chicago/Turabian StyleLampová, Barbora, Ivo Doskočil, Petr Šmíd, and Lenka Kouřimská. 2024. "Comparison of Cricket Protein Powder and Whey Protein Digestibility" Molecules 29, no. 15: 3598. https://doi.org/10.3390/molecules29153598
APA StyleLampová, B., Doskočil, I., Šmíd, P., & Kouřimská, L. (2024). Comparison of Cricket Protein Powder and Whey Protein Digestibility. Molecules, 29(15), 3598. https://doi.org/10.3390/molecules29153598










