Abstract
The objective of this study was to analyze the chemical composition and evaluate the biological capabilities of the essential oils (EOs) extracted from leaves and stems of wild Aeschynomene indica L. plants by the hydrodistillation method. By using GC-FID/MS, fifty-six and fifty-five compounds, representing 95.1 and 97.6% of the essential oils in the leaves and stems, respectively, were characterized. The predominant constituents of A. indica EOs were (E)-caryophyllene, linalool, viridiflorol, phytol, hexadecanoic acid, trans-verbenol, and α-guaiene. The antibacterial and synergistic activities of the EOs were assessed by microdilution and checkerboard assays. The results revealed a potent inhibition and bactericidal activity against Staphylococcus aureus and Bacillus subtilis with MICs of 0.312–0.625 mg/mL. When combined with traditional antibiotics, the essential oils of A. indica possessed excellent synergistic effects against all tested bacteria. Additionally, the EOs of A. indica leaves showed higher antioxidant activity (IC50 = 0.11 ± 0.01 µg/mL) compared to the stem oil (IC50 = 0.19 ± 0.01 µg/mL) using the ABTS radical scavenging assay. The in vitro cytotoxicity of EOs against human cancer cell lines HepG2, MCF-7, A-549, and HCT-116 was examined, and MTT assays showed that the EOs possessed a significant cytotoxic potential against MCF-7 breast cancer cells, with IC50 values of 10.04 ± 1.82 and 15.89 ± 1.66 μg/mL, and a moderate cytotoxic activity against other tested cells. In conclusion, the A. indica EOs could be considered a potential source of pharmacologically active compounds.
1. Introduction
Plants are susceptible to invasion by numerous infections that may affect different parts of the plant. In order to restrict pathogen attacks, plants produce a variety of secondary metabolites to inhibit the growth of pathogens, such as terpenoids, phenolics, and nitrogen compounds [1]. Essential oils are a complex mixture of several lipophilic and highly volatile constituents that are produced by specific secretory tissues of the plant’s flowers, seeds, leaves, wood, and bark as secondary metabolites. These constituents exhibit an extensive range of pharmacological properties, such as analgesic, antioxidant, antimicrobial, anti-inflammatory, anticancer, anti-virus, neuroprotective, and anti-diabetic effects [2,3]. Since ancient times, medicinal and aromatic plants have been utilized for their wide variety of physiologically active secondary metabolites [4]. The growing interest in pharmacologically potent plant-based natural compounds as alternative therapeutics for treating infections has heightened the attention of scientists worldwide [4]. Essential oils could be interesting candidates for alternative antibiotics since studies have shown that specific essential oils and their isolated components exhibit antibacterial effects against a wide spectrum of pathogens [5]. Furthermore, the utilization of essential oils in combination therapy with traditional antibacterial drugs has demonstrated efficacy in reducing the emergence of resistant strains [6]. When these interactions lead to synergistic effects, they can also be used to enhance the efficiency of therapy. Another advantage of such combinations is the utilization of lower doses, resulting in a decrease in side effects and treatment expenses [7]. Therefore, essential oils are being increasingly investigated as a substitute for reducing the side effects of traditional treatments.
The genus Aeschynomene belongs to the Leguminosae family and comprises about 250 species. Plants belonging to the genus Aeschynomene have been found to possess hepatoprotective [8], antioxidant [9], antimicrobial [10], anti-inflammatory [11], and anthelmintic properties [12]. In preliminary phytochemical studies of different Aeschynomene species, the presence of flavonoid glycosides, pterocarpans, saponins, and chalcones was identified [13,14]. In previous studies, the compounds from Aeschynomene fascicularis were examined and demonstrated selective cytotoxic and antiproliferative activities [13]. An extract from the root bark of A. fascicularis was found to have a pronounced cytotoxic effect on KB and Hela cells [15]. Moreover, in another research, the extracts of A. fascicularis also showed effective cytotoxic activity against DU-145, KB, and Hep-2 cell lines and antiproliferative activity against the KB cell line [16].
Aeschynomene indica L., an annual herb of the genus Aeschynomene, is native to tropical Asia, North Korea, Africa, Oceania, Japan, and China [17,18]. The aerial parts of Aeschynomene indica L. have been utilized in Chinese folk medicine to cure a variety of conditions, including urticaria, furuncle, nyctalopia, hepatitis, enteritis, and dysentery [18,19]. Previous research demonstrated that the leaves of A. indica and its active ingredient efficiently enhanced wound healing and reduced scar formation. This effectiveness may be attributed to their ability to accelerate the transition of macrophage phenotypes and suppress the expression of TGF-β1 and α-SMA [19]. Additionally, preliminary phytochemical screening has identified flavonoid glycosides, phenols, and anthocyanidins in A. indica [18].
To our knowledge, no research has been conducted on the chemical composition and biological effects of the essential oils extracted from different parts of Aeschynomene indica L. This study is the first one to report the chemical composition and antibacterial, antioxidant, and cytotoxic activities of the essential oils obtained from leaves (LEOs) and stems (SEOs) of A. indica.
2. Results
2.1. Chemical Composition
The leaves and stems of A. indica were broken into fragments and hydrodistilled to give leaf essential oils (LEOs) and stem oils (SEOs) in yields of 0.12% and 0.07% from air-dried parts of the leaves and stems of A. indica, respectively. Table 1 displays the discovered volatiles in the LEOs and SEOs of A. indica, together with the percentages of their contents, their calculated retention indices, and their retention indices from the literature. The qualitative analysis of EOs by GC-FID/MS revealed fifty-six and fifty-five compounds, representing 95.1% and 97.6% of the total essential oils in leaf and stem samples, respectively.

Table 1.
Chemical composition of LEOs and SEOs of A. indica.
Oxygenated monoterpenes (21.0% and 32.9%), sesquiterpene hydrocarbons (34.4% and 20.4%), and oxygenated sesquiterpenes (16.2% and 18.6%) were dominant in the leaf and stem essential oils, respectively. It is widely known that the promising potential of EOs is due to the presence of valuable active ingredients. (E)-caryophyllene was the most abundant compound among all constituents, by 17.3% and 10.8% in LEOs and SEOs, respectively. The main constituents of the LEOs were (E)-caryophyllene (17.3%), viridiflorol (8.1%), phytol (5.2%), trans-verbenol (4.9%), α-guaiene (4.7%), linalool (4.1%), n-octadecanol (3.5%), and pentadecanal (3.4%). In the stem essential oils, the main identified constituents were linalool (11.8%), (E)-caryophyllene (10.8%), viridiflorol (9.5%), phytol (7.0%), hexadecanoic acid (6.3%), (E)-β-ionone (3.3%), α-terpineol (3.1%), and α-guaiene (3.1%).
2.2. Antibacterial Activity
Two Gram-positive and two Gram-negative bacteria were tested for the antibacterial properties of LEOs and SEOs extracted from A. indica as well as the standard antibiotic chloramphenicol. The recorded minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) are presented in Table 2. For most of the tested bacterial strains, the bactericidal activities of the LEOs and SEOs showed no noticeable differences in MIC and MBC values, which ranged from 0.312 mg/mL to 2.500 mg/mL. Meanwhile, the test results showed that the A. indica EOs possessed moderate antibacterial activity against Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis, with MICs and MBCs ranging from 0.312 to 0.625 mg/mL, while the EOs showed weak antibacterial activity against Gram-negative bacteria tested (MICs = 1.250 mg/mL and MBCs = 2.500 mg/mL). Both EOs exhibited weaker antibacterial activities compared to the positive control chloramphenicol.

Table 2.
Antibacterial activities of LEOs and SEOs of A. indica.
2.3. Association Test
The synergistic effect between the A. indica EOs and conventional antibiotic agents (streptomycin and chloramphenicol) was investigated by testing the MIC values individually and in combination using a checkerboard assay [20]. The values of the fractional inhibitor concentration index (FICI) that were determined based on the results of the checkerboard assays are summarized in Table 3 and Table 4, respectively. The synergistic effect of both EOs with antibiotics resulted in a total synergistic effect against all tested bacterial strains (FICI ≤ 0.50), and the reduction of antibiotics MICs ranged from 4- to 16-fold when combined with A. indica EOs. Moreover, the best synergistic effect was recorded in the combination of LEOs and streptomycin against Staphylococcus aureus and Pseudomonas aeruginosa, with FICI values of 0.10 and 0.13, respectively, and the MIC values of streptomycin were reduced 16-fold.

Table 3.
Effect of the combination of EOs with chloramphenicol.

Table 4.
Effect of the combination of EOs with streptomycin.
2.4. Antioxidant Activity
Antioxidation is a complicated process that typically occurs in multiple mechanisms, and its assessment is commonly conducted through several test methods [21]. The ferric reducing power assay, ABTS cation, and DPPH radical scavenging assay were used to assess the antioxidant properties of the essential oils derived from A. indica. Trolox and butylated hydroxytoluene (BHT) served as positive controls. In the radical scavenging assay, leaf essential oils were more active, with IC50 values of 1.35 ± 0.06 and 0.11 ± 0.01 mg/mL, followed by stem essential oils, with IC50 values of 1.66 ± 0.05 and 0.19 ± 0.01 mg/mL (Table 5). Trolox equivalents (TEs) in the FRAP system were shown by the leaf essential oils of 103.78 ± 10.23 μmol Trolox × g−1, followed by stem essential oils of 57.75 ± 7.63 μmol Trolox × g−1. Thus, leaf essential oils demonstrated greater potential for antioxidants than plant stem essential oils. A. indica essential oils demonstrated considerably lower radical scavenging power and reducing ability compared to the positive control. Neither of the two oils was as effective as the reference antioxidants BHT and Trolox. These findings suggested that A. indica essential oils displayed moderate antioxidant effects.

Table 5.
Results of the antioxidant activity (DPPH, ABTS, and FRAP) of the EOs from different parts of A. indica.
2.5. Cytotoxic Activity
Four human cancer cell lines (HepG2, MCF-7, A-549, and HCT-116) and a human normal hepatocyte (LO2) cell line were subjected to an MTT cell viability experiment in order to assess the cytotoxic effects of extracted LEOs and SEOs. Doxorubicin was tested as a reference. The results obtained with various doses of A. indica EOs extracted from two different organs after 24 h, 48 h, and 72 h of exposure are presented in Figure 1, and the IC50 values are presented in Table 6. The histograms reported in Figure 1 showed that essential oils reduce cell viability in a dose- and time-dependent manner. The results indicate that the essential oils from the leaves and stems of A. indica have promising cytotoxic capabilities against MCF-7 cells, with IC50 values of 10.04 ± 1.82 and 15.89 ± 1.66 μg/mL after 72 h of treatment, respectively. Interestingly, the leaf EOs exhibited more cytotoxicity against all the cell lines tested compared to the stem EOs. However, both LEOs and SEOs exhibited weaker growth inhibitory activity against cell lines compared to doxorubicin. The selectivity indices (SIs) were determined by taking the ratio of the IC50 values (48 h) of LO2, representing non-cancerous cells, to the IC50 values (48 h) of the cancer cells. The selectivity index of EOs was calculated in the range of 0.95 to 3.38 for LEOs and 0.80 to 1.72 for SEOs. The LEOs presented the best selectivity index (3.38) against the MCF-7 cell lines.
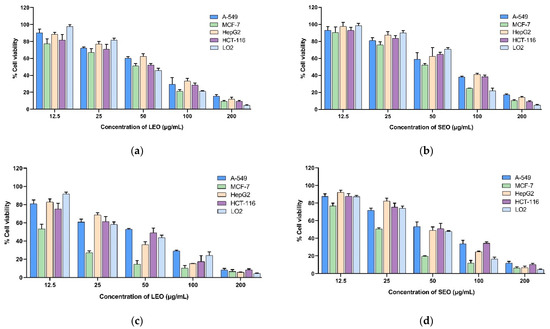
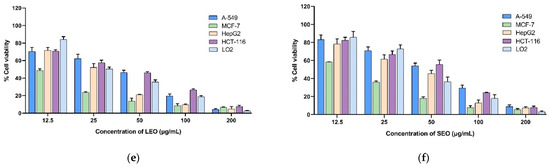
Figure 1.
(a) Cytotoxic activity of LEOs for 24 h; (b) cytotoxic activity of SEOs for 24 h; (c) cytotoxic activity of LEOs for 48 h; (d) cytotoxic activity of SEOs for 48 h; (e) cytotoxic activity of LEOs for 72 h; and (f) cytotoxic activity of SEOs for 72 h. Doxorubicin was used as a positive control. IC50: concentration reducing cell growth by 50%.

Table 6.
Cytotoxicity (IC50, μg/mL) of the LEOs and SEOs of A. indica.
3. Discussion
Differences in the constituents of the leaf and stem EOs derived from A. indica have been observed. The predominant compounds in the LEOs and SEOs were linalool (4.1% and 11.8%), (E)-caryophyllene (17.3% and 10.8%), α-guaiene (4.7% and 3.1%), viridiflorol (8.1% and 9.5%), etc. trans-Verbenol was detected in LEOs at a concentration of 4.9%; however, it was not detected in SEOs. In SEOs, hexadecanoic acid accounted for 6.3% of the total, whereas in LEOs, it was less than 1%. The essential oil constituents of other legume plants have been reported to be rich in similar compounds, such as (E)-caryophyllene [22,23,24,25,26], phytol [22,26], (E)-nerolidol [27], pentadecanal [27], and δ-cadinene [24]. However, cis-verbenol and trans-verbenol have not been reported.
Recent research demonstrated that the major compounds observed in A. indica EOs possess potent bioactive capabilities. For example, (E)-caryophyllene, which was found in both SEOs and LEOs, is well-known for its antibacterial [28,29], antifungal [29], anti-inflammatory [30], and anticancer activities [29,31]. Furthermore, viridiflorol demonstrated strong anticancer activity against the Daoy cells and MCF-7 [32] and is widely utilized as an antioxidant, anti-tuberculosis, and anti-inflammatory agent [33]. Linalool has also been found to exhibit anti-inflammatory [34,35], antimicrobial [36,37,38,39], anticancer [40], and neuroprotective activities [41].
Bacteria are responsible for a number of detrimental effects on human health, the deterioration of food products, and a multitude of other issues. With the emergence of drug-resistant bacteria, there is a necessity to explore alternative sources of defense against pathogenic bacteria. EOs and their components play a pivotal role in inhibiting the growth of microorganisms [42]. It is well established that essential oils possess antibacterial activities, particularly against Gram-positive bacteria [43,44], such as Staphylococcus aureus. This bacterium is a significant pathogen responsible for various human illnesses, ranging from mild infections of skin and soft tissue to severe tissue and sepsis [45,46]. The LEOs and SEOs of A. indica showed inhibitory effects against both Gram-positive and -negative bacterial strains that were examined in this study. Compared to the stem essential oils, the LEOs showed higher activity against S. aureus and P. aeruginosa, with MICs of 0.312 and 1.250 mg/mL for LEOs and 0.625 and 2.500 mg/mL for SEOs, respectively. In addition, the essential oils exhibited similar antibacterial activities against B. subtilis and E. coli, with the same MIC values of 0.312 and 1.250 mg/mL, respectively. The variations revealed in the compositions of the two essential oils may explain their varying levels of biological activity in the current investigation [47]. The presence of bioactive compounds, including (E)-caryophyllene, viridiflorol, and linalool, was responsible for this activity; these compounds have been reported to possess antibacterial potential [29,48,49,50,51,52]. In contrast to tested Gram-positive bacterial strains, the oils exhibited lower efficacy against Gram-negative bacteria. The discrepancies in antibacterial efficacy may be attributed to the net repulsion of the two outer complex membranes’ structure in the Gram-negative bacterial cell wall, which is absent in Gram-positive bacteria [53]. Essential oils exert biological activities through a variety of mechanisms due to their diversity in chemical composition [54]. The antibacterial action of essential oils is multifaceted and consists of a combination of disrupting cell membranes, inducing oxidative stress, damaging genetic material, and inhibiting enzyme activity [54]. Among these, the most significant mechanism of action is the disruption of bacterial cell membranes. Essential oils’ lipophilic character makes them easily penetrable through bacterial cell membranes, which breaks membrane integrity and increases permeability, causing disruption to many cellular activities, including energy production (membrane-coupled), membrane transport, and other metabolic regulating functions [55].
Recently, the combination of conventional antibiotics with medicinal natural products has become a popular strategy to reverse the antibacterial activity of failed antibiotics in treating infections that are resistant to these drugs via drug–drug interactions involving the action of multiple antibacterial mechanisms. These strategies may prevent the emergence of novel resistance mechanisms in bacteria, minimize the use of antibiotics while maintaining current antibiotic classes for therapeutic benefits, and mitigate adverse effects [56]. The current investigation demonstrated that the combination of A. indica EOs and antibiotics possessed a synergistic interaction in all examined bacteria, as indicated by an FIC index value of less than 0.5. Moreover, the concentration of essential oils and antibiotics in the combined test was much lower than that of the single chemical concentration. The maximum synergistic effect was observed for S. aureus and P. aeruginosa, with a 16-fold gain compared to streptomycin’s MIC. It is noteworthy that chloramphenicol or streptomycin, in combination with EOs extracted from different parts of A. indica, showed a strong synergistic effect against both Gram-positive and Gram-negative bacteria tested. This observation suggests that A. indica essential oils may serve as promising effective natural adjuvant agents in conjunction with traditional antibiotics, with the aim of reducing the risk of developing antibiotic-resistant microorganisms.
The in vitro antioxidant activities of A. indica LEOs and SEOs were evaluated in three different models, including DPPH, ABTS, and FRAP. Overall, in the three tests, both LEOs and SEOs exhibited antiradical capacities against different oxidants and displayed moderate ferric reducing abilities. The differences noticed in the activities of the leaves and stem EOs towards the two distinct radicals (DPPH• and ABTS•+) can be assigned to several factors, such as the complications, polarity, and selectivity of isomers of the radicals [57]. Scavenging of ABTS•+ by the EOs from different parts of A. indica was higher than that of DPPH• radicals. This may be because ABTS•+ cation radicals are more sensitive to high-molecular-weight phenolics and more reactive than DPPH• radicals, wherein the reaction of ABTS•+ radicals with antioxidants is completed within 1 min [58]. Furthermore, the solvation ability of the oils towards the radical’s medium may vary, and these factors have been found to impact the effectiveness of volatile components in suppressing various types of radicals [57]. The high content of phytol in the essential oils in the present study is noteworthy and likely contributed to the improved antioxidant properties of these EOs. Phytol, a diterpenoid alcohol, has demonstrated strong in vitro antioxidant activity, effectively eliminating hydroxyl radicals and preventing the development of thiobarbituric acid reactive substances [59].
The utilization of medicinal plant chemicals for the development and treatment of a multitude of diseases, especially cancer, has been researched for decades [60]. In this study, we examined the in vitro cytotoxic effects of EOs and determined the IC50 values on A-549, MCF-7, HepG2, and HCT-116 cells. We present evidence demonstrating the cytotoxic effects of both LEOs and SEOs in tested cell lines of diverse origins. The data indicated that the MCF-7 cell line exhibited a more favorable response to A. indica EOs, demonstrating enhanced cytotoxicity. When MCF-7 cells were exposed to A. indica essential oils, a notable potential for cytotoxic effects was identified, which indicates that A. indica essential oils could be a promising therapy option for patients with breast cancer. Early research indicates that (E)-caryophyllene exhibits significant cytotoxic activity against various cancer cells, including A-549 (lung carcinoma), HeLa (cervical carcinoma), and HT-29 (colon adenocarcinoma) cells [29,31]. Additionally, it has been shown to potentiate the effects of conventional chemotherapy agents such as doxorubicin and paclitaxel, indicating its potential as an adjunct in chemotherapeutic regimens [61]. Furthermore, studies have demonstrated the effectiveness of viridiflorol in inducing apoptosis in different cell lines, including A549, Daoy, and MCF-7 [32].
The additive or synergetic effects of the bioactive compounds identified in the EOs may be responsible for the greater biological activity of the LEOs in comparison to the SEOs. The biological activities of the LEOs may have been enhanced by other terpenoid constituents, even in small amounts, for example, by β-pinene, limonene, and β-elemene, which have previously been reported to possess remarkable bioactivities [62,63,64,65,66,67,68].
4. Materials and Methods
4.1. Plant Material
A. indica leaves and stems were obtained from Quzhou, located in Zhejiang Province, China, in September 2022. Dr. Hong Zhao, Shandong University, China, verified the classification of the plant and placed a voucher specimen (No. 022021) in the herbarium of the Department of Biological Sciences at Shandong University in Weihai, China.
4.2. Essential Oil Extraction
The plant materials (1 kg of leaves or stems of A. indica) were hydrodistilled for three hours using a Clevenger-type device to derive EOs and then kept at 4 °C for analysis. The experiments were conducted three times.
4.3. Identification of EO Components
GC and GC/MS were used to analyze the composition of EOs. Agilent Technologies 7890A (St. Clara, CA, USA) gas chromatography equipped with FID and a non-polar HP-5MS column, using helium as the carrier at a flow rate of 1.3 mL/min, was used to perform the GC analysis. The GC column oven was set from 60 °C (held for 1 min) to 250 °C (held for 14 min), with an increase of 8 °C/min. The injector temperature was 260 °C. The EOs were diluted with hexane to 1% (v/v) and injected into the GC system. Mass spectra were recorded at 70 eV. The mass scanning range was from 50 to 550 m/z.
The identification of LEO and SEO components was conducted by comparing their mass spectra to those in the NIST and WILEY libraries and by comparing their retention indices (retention indices relative to C10–C30 n-alkanes) to the standard RI provided in the literature [69,70,71].
4.4. Antibacterial Activity Assays
The method of microdilution of the broth was employed to assess the antibacterial potential of EOs [72]. The strains examined were P. aeruginosa, E. coli, B. subtilis, and S. aureus. EOs were diluted in 96-well microplates using a two-fold serial dilution method, with 100 μL added to each well. Subsequently, the dilutions were combined with a bacterial solution consisting of 100 μL with a concentration of 106 CFU/mL. The positive control utilized was chloramphenicol. After a 24-h incubation at 37 °C, the growth of the bacteria was evaluated using TTC as a growth indicator. The MIC of the sample was the lowest concentration with no bacterial growth. The MBC was identified by inoculating 100 μL of subculture from the well, with no visible growth of bacteria occurring after incubation at 37 °C for 24 h.
4.5. Synergistic Antibacterial Activity of EOs
The synergistic effects of the combination of chloramphenicol or streptomycin with EOs were assessed using the micro broth checkboard method [73]. Sample concentrations varied from 4 to 1/32 times the MIC, depending on previously determined MIC values. In summary, 50 μL of LEOs or SEOs was introduced at two-fold dilution concentrations into different microwells of the 96-well plates. These microwells already contained 50 µL of various antibiotic concentrations and 100 μL of bacterial suspension (106 CFU/mL). After incubation at 37 °C for 24 h, the MIC of EO or antibiotics tested alone and in combination were determined. The FICI value was calculated as a predictor of synergy by using the following formula:
Synergy is considered to exist if FICI ≤ 0.5 [74].
4.6. Antioxidant Activity Evaluation
4.6.1. DPPH Radical Scavenging
The DPPH test was conducted by the 96-well plate method, as described in reference [75]. Briefly, 150 μL of a 0.05 mg/mL DPPH solution was added to each well of a plate. Additionally, 50 μL of the sample at varying concentrations were added to the wells, excluding the blank test wells. The control test well received an addition of 200 µL of methanol. The solutions were shaken for 1 min and subsequently placed in darkness for 6 h. The absorbance was quantified at 517 nm. The standard reference compounds utilized were BHT and Trolox. Radical inhibition (I%) was calculated as follows:
I (%) = [1 − (Asample − Ablank)/Ablank)] × 100
A represents absorbance.
4.6.2. ABTS Radical Scavenging
The ABTS test was performed according to a previously published procedure with minor adjustments [75]. Briefly, 7 mM ABTS was mixed with 2.45 mM potassium persulfate to give an ABTS•+ solution, which was left in the darkness for 16 h. The ABTS•+ solution was diluted in PBS to an absorbance of about 0.700 (±0.02) at a wavelength of 734 nm. The EOs were mixed with methanol to obtain the desired concentrations. Afterward, 50µL of the sample solution was combined with 150 µL of the ABTS•+ solution in a 96-well plate. The mixture was placed in a lightless environment for 30 min, after which the absorbance was quantified at 734 nm. The ABTS•+ scavenging effect was determined using the same formula that was used for DPPH.
4.6.3. Ferric Reducing Power
The ferric reducing power was assayed using a previous method with minor modifications [75]. The FRAP reagent included 300 mM acetate buffer (pH 3.6), 10 mM TPTZ solution in 40 mM HCl, and FeCl3·6H2O (20 mM) in a proportion of 10:1:1. Afterwards, 20 μL serial dilutions of EOs and 180 μL of the FRAP reagent were combined in a 96-well plate in darkness and then incubated at 37 °C for 30 min. The absorbance was measured at 593 nm. The activity was quantified as the Trolox-equivalent antioxidant capacity.
4.7. Cytotoxic Activity Evaluation
The cytotoxic effects of EOs on four different types of cancer cells (MCF-7, A-549, HCT-116, and HepG2), as well as non-cancer LO2 cells, were evaluated by an MTT assay, as previously reported [76]. The tested cell lines were obtained from the Shanghai Institute for Biological Sciences (SIBS, Shanghai, China). The cells were cultured in an RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/mL penicillin, and 100 μnits/mL streptomycin at 37 °C with a 5% CO2. A total of 5 × 104 cells were distributed into each well of a 96-well plate, along with 100 μL of an RPMI medium and 10% FBS. Following incubation for 24 h, the EOs were diluted in a two-fold serial dilution to a concentration of 6.25 to 400.00 μg/mL and then added to each well. After 48 h, 20 μL of MTT solution (5 mg/mL) was added to each well. The plates were subsequently incubated for an additional 4 h. The formazan blue produced in the cell was dissolved in 150 μL of DMSO solution. The absorbance was quantified at 570 nm.
4.8. Statistical Analysis
All the experiments were carried out in triplicate, and the data were presented as the mean ± SD. Statistical analysis of the data was carried out using the SPSS software package (IBM SPSS Statistics, v. 29). One-way ANOVA was used to test the statistical significance of the data. Differences were considered statistically significant at a significance level of p ≤ 0.05.
5. Conclusions
This study evaluated the composition and antioxidant, antibacterial, synergistic antibacterial, and cytotoxicity effects of EOs. The primary constituents of EOs included (E)-caryophyllene, linalool, viridiflorol, phytol, hexadecanoic acid, trans-verbenol, and α-guaiene, among others. By utilizing three distinct methodologies to assess antioxidant activity, the essential oils demonstrate a promising capacity to eliminate ABTS radicals. In addition, the essential oils of A. indica showed stronger inhibitory effects against S. aureus compared to other types of microorganisms. Furthermore, a synergistic impact was detected when essential oils were combined with antibiotics, resulting in the effective inhibition of all tested bacterial strains. MTT assays demonstrated the cytotoxic effects of both LEOs and SEOs in tested cell lines of diverse origins, especially MCF-7 cells. These findings indicate that these oils possess the capacity to serve as a natural antibacterial agent, offering prospective applications in the food, pharmaceutical, and cosmetics sectors.
Author Contributions
Conceptualization: P.L.; Data curation: L.F., F.X., S.Q., C.S. and P.L.; Formal analysis: L.F.; Funding acquisition: P.L.; Investigation: L.F., F.X., S.Q., C.S. and P.L.; Methodology: L.F. and P.L.; Project administration: P.L.; Resources: L.F. and P.L.; Software: L.F., F.X. and S.Q.; Supervision: P.L.; Validation: L.F., F.X., S.Q., C.S. and P.L.; Visualization: L.F.; Writing—original draft, L.F. and P.L.; and Writing—review and editing: L.F. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M. Plant secondary metabolites: The weapons for biotic stress management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Shah, M.; Bibi, S.; Kamal, Z.; Al-Sabahi, J.N.; Alam, T.; Ullah, O.; Murad, W.; Rehman, N.U.; Al-Harrasi, A. Bridging the chemical profile and biomedical effects of Scutellaria edelbergii essential oils. Antioxidants 2022, 11, 1723. [Google Scholar] [CrossRef]
- Goodier, M.C.; Zhang, A.J.; Goldfarb, N.I.; Warshaw, E.M.; Nikle, A.B.; Hylwa, S.A. Use of essential oils: A general population survey. Contact Dermat. 2019, 80, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent advances in the application of antibacterial complexes using essential oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Visan, D.C.; Oprea, E.; Radulescu, V.; Voiculescu, I.; Biris, I.A.; Cotar, A.I.; Saviuc, C.; Chifiriuc, M.C.; Marinas, I.C. Original contributions to the chemical composition, microbicidal, virulence-arresting and antibiotic-enhancing activity of essential oils from four coniferous species. Pharmaceuticals 2021, 14, 1159. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, P.; Pandae, V. Hepatoprotective activity of Aeschynomene aspera. Linn. Pharmacol. 2011, 3, 297–304. [Google Scholar]
- Bharathi, M.P.; Rao, D.M. Evaluation of in vitro antioxidant activity of Aeschynomene indica. J. Pharm. Res. 2015, 9, 21–26. [Google Scholar]
- Achika, J.; Ndukwe, G.; Ayo, R. Isolation, characterization and antimicrobial activity of 3β, 22E-stigmasta-5, 22-dien-3-ol from the aerial part of Aeschynomene uniflora E. Mey. Br. J. Pharm. Res. 2016, 11, 1–8. [Google Scholar] [CrossRef]
- Ignoato, M.C.; Fabrão, R.M.; Schuquel, I.T.A.; Botelho, M.F.P.; Santin, S.M.d.O.; Arruda, L.L.M.d.; Bersani-Amado, C.A.; Souza, M.C.d. Phytochemical study and evaluation of anti-inflammatory activity of Aeschynomene fluminensis vell. (Fabaceae). Quim. Nova 2012, 35, 2241–2244. [Google Scholar] [CrossRef]
- Alekhya, C.; Yasodamma, N.; Chaithra, D.; Job Roger Binny, A. Anthelmintic activity of Aeschynomene aspera and Aeschynomene indica. Int. J. Pharm. Pharm. Sci. 2013, 5, 386–388. [Google Scholar]
- Caamal-Fuentes, E.; Moo-Puc, R.; Torres-Tapia, L.W.; Peraza-Sanchez, S.R. Pterocarpans from the root bark of Aeschynomene fascicularis. Nat. Prod. Commun. 2013, 8, 1421–1422. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.M.; Salama, M.M.; Mohammed, F.F.; Tohamy, A.F.; El Deeb, K.S. Metabolic profile and hepatoprotective effect of Aeschynomene elaphroxylon (Guill. & Perr.). PLoS ONE 2019, 14, e0210576. [Google Scholar]
- Caamal-Fuentes, E.; Torres-Tapia, L.W.; Simá-Polanco, P.; Peraza-Sánchez, S.R.; Moo-Puc, R. Screening of plants used in Mayan traditional medicine to treat cancer-like symptoms. J. Ethnopharmacol. 2011, 135, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Caamal-Fuentes, E.E.; Peraza-Sánchez, S.R.; Torres-Tapia, L.W.; Moo-Puc, R.E. Isolation and identification of cytotoxic compounds from Aeschynomene fascicularis, a Mayan medicinal plant. Molecules 2015, 20, 13563–13574. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1995; Volume 41, p. 351. [Google Scholar]
- Aruna, C.; Chaithra, D.; Alekhya, C.; Yasodamma, N. Pharmacognostic studies of Aeschynomene indica L. Int. J. Pharm. Pharm. Sci. 2012, 4, 76–77. [Google Scholar]
- Lei, Z.Y.; Chen, J.J.; Cao, Z.J.; Ao, M.Z.; Yu, L.J. Efficacy of Aeschynomene indica L. leaves for wound healing and isolation of active constituent. J. Ethnopharmacol. 2019, 228, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Najda, A.; Michalak, I. Investigation of chemical constituents and antioxidant activity of biologically active plant-derived natural products. Molecules 2023, 28, 5572. [Google Scholar] [CrossRef]
- Noudogbessi, J.; Sessou, P.; Wotto, V.; Figueredo, G.; Chalard, P.; Chalchat, J.; Dansou, K.; Sohounhloué, D. Chemical compositions and preventive activity of essential oils extracted from the leaves of two varieties of Tephrosia (Leguminosae-papilionoideae) collected in Benin on Callosobruchus maculatus (Fabricius). Asian J. Res. Chem. 2012, 5, 1431–1436. [Google Scholar]
- Zoghbi, M.d.G.B.; Andrade, E.H.; Martins-da-Silva, R.C.; Trigo, J.R. Chemical variation in the volatiles of Copaifera reticulata Ducke (Leguminosae) growing wild in the states of Pará and Amapá, Brazil. J. Essent. Oil Res. 2009, 21, 501–503. [Google Scholar] [CrossRef]
- Zoghbi, M.d.G.B.; Martins-da-Silva, R.C.; Trigo, J.R. Volatiles of oleoresins of Copaifera paupera (Herzog) Dwyer C. piresii Dwyer and C. pubiflora Benth. (Leguminosae). J. Essent. Oil Res. 2009, 21, 403–404. [Google Scholar] [CrossRef]
- Gurgel, E.S.C.; de Oliveira, M.S.; Souza, M.C.; da Silva, S.G.; de Mendonca, M.S.; da Silva Souza Filho, A.P. Chemical compositions and herbicidal (phytotoxic) activity of essential oils of three Copaifera species (Leguminosae-Caesalpinoideae) from Amazon-Brazil. Ind. Crops. Prod. 2019, 142, 111850. [Google Scholar] [CrossRef]
- de Almeida, M.C.; Souza, L.G.; Ferreira, D.A.; Monte, F.J.; Braz-Filho, R.; de Lemos, T.L. Chemical composition of the essential oil and fixed oil Bauhinia pentandra (Bong.) D. Dietr. Pharmacogn. Mag. 2015, 11, S362–S364. [Google Scholar]
- Al-Qudah, M.A. Chemical composition of essential oil from Jordanian Lupinus varius L. Arab. J. Chem. 2013, 6, 225–227. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topcu, G.; Eri, C.; Sönmez, U.; Kartal, M.; Kurucu, S.; Bozok-Johansson, C. Terpenoids from Salvia sclarea. Phytochemistry 1994, 36, 971–974. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- de Morais Oliveira-Tintino, C.D.; Pessoa, R.T.; Fernandes, M.N.M.; Alcântara, I.S.; da Silva, B.A.F.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; da Silva, M.d.S.; Tintino, S.R.; Rodrigues, F.F.G. Anti-inflammatory and anti-edematogenic action of the Croton campestris A. St.-Hil (Euphorbiaceae) essential oil and the compound β-caryophyllene in in vivo models. Phytomedicine 2018, 41, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Akiel, M.A.; Alshehri, O.Y.; Aljihani, S.A.; Almuaysib, A.; Bader, A.; Al-Asmari, A.I.; Alamri, H.S.; Alrfaei, B.M.; Halwani, M.A. Viridiflorol induces anti-neoplastic effects on breast, lung, and brain cancer cells through apoptosis. Saudi J. Biol. Sci. 2022, 29, 816–821. [Google Scholar] [CrossRef]
- Trevizan, L.N.F.; do Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; do Carmo Vieira, M.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar] [PubMed]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochem. Res. 2015, 40, 1520–1525. [Google Scholar] [CrossRef]
- Park, S.-N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.-K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef]
- Carson, C.; Riley, T. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef]
- Pattnaik, S.; Subramanyam, V.R.; Bapaji, M.; Kole, C.R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 1997, 89, 39–46. [Google Scholar]
- Pitarokili, D.; Couladis, M.; Petsikos-Panayotarou, N.; Tzakou, O. Composition and antifungal activity on soil-borne pathogens of the essential oil of Salvia sclarea from Greece. J. Agric. Food Chem. 2002, 50, 6688–6691. [Google Scholar] [CrossRef]
- Iwasaki, K.; Zheng, Y.-W.; Murata, S.; Ito, H.; Nakayama, K.; Kurokawa, T.; Sano, N.; Nowatari, T.; Villareal, M.O.; Nagano, Y.N. Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 2016, 22, 9765–9774. [Google Scholar] [CrossRef]
- Park, H.; Seol, G.H.; Ryu, S.; Choi, I.-Y. Neuroprotective effects of (−)-linalool against oxygen-glucose deprivation-induced neuronal injury. Arch. Pharm. Res. 2016, 39, 555–564. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Deyno, S.; Mtewa, A.G.; Abebe, A.; Hymete, A.; Makonnen, E.; Bazira, J.; Alele, P.E. Essential oils as topical anti-infective agents: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 47, 102224. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Alternat. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.-P.; Molle, V. Staphylococcus aureus toxins: An update on their pathogenic properties and potential treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Swee-Hua-Erin, L.; Lai, K.-S. Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The biological activities of 20 nature identical essential oil constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Duman, A.D.; Telci, I.; Dayisoylu, K.S.; Digrak, M.; Demirtas, İ.; Alma, M.H. Evaluation of bioactivity of linalool-rich essential oils from Ocimum basilucum and Coriandrum sativum varieties. Nat. Prod. Commun. 2010, 5, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Ireland, B.; Hibbert, D.; Goldsack, R.; Doran, J.; Brophy, J. Chemical variation in the leaf essential oil of Melaleuca quinquenervia (Cav.) ST Blake. Biochem. Syst. Ecol. 2002, 30, 457–470. [Google Scholar] [CrossRef]
- Barbouchi, M.; Benzidia, B. Chemical variability in essential oils isolated from roots, stems, leaves and flowers of three Ruta species growing in Morocco. J. King Saud Univ. Sci. 2021, 33, 101634. [Google Scholar] [CrossRef]
- Probst, I.; Sforcin, J.; VLM, R.; Fernandes, A.; Fernandes Júnior, A. Antimicrobial activity of propolis and essential oils and synergism between these natural products. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 159–167. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Mohdaly, A.A.A.; Ramadan, M.F. Characteristics, composition and functional properties of seeds, seed cake and seed oil from different Brassica carinata genotypes. Food Bioscience 2022, 48, 100752. [Google Scholar]
- Santos, C.C.d.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.; Dirsch, V.; Supuran, C. International natural product Sciences taskforce. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Abu Zahra, H.; Ammar, R.B.; Mohamed, M.E.; Ibrahim, H.-I.M. Beta-caryophyllene enhances the anti-tumor activity of cisplatin in lung cancer cell lines through regulating cell cycle and apoptosis signaling molecules. Molecules 2022, 27, 8354. [Google Scholar] [CrossRef]
- da Silva Rivas, A.C.; Lopes, P.M.; de Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar]
- Cascaes, M.M.; Carneiro, O.d.S.; Nascimento, L.D.d.; de Moraes, Â.A.B.; de Oliveira, M.S.; Cruz, J.N.; Guilhon, G.M.S.P.; Andrade, E.H.d.A. Essential oils from Annonaceae species from Brazil: A systematic review of their phytochemistry, and biological activities. Int. J. Mol. Sci. 2021, 22, 12140. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- d’Alessio, P.A.; Ostan, R.; Bisson, J.-F.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Meeran, M.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic studies on cancer cell interaction and its chemosensitization effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Qu, J.; Xu, L.; Hou, K.; Zhang, J.; Qu, X.; Liu, Y. β-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer 2011, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov/chemistry (accessed on 18 March 2023).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; ISBN 978-1-68440-220-5. [Google Scholar]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Hogg, G.; Barr, J.; Webb, C. In-vitro activity of the combination of colistin and rifampicin against multidrug-resistant strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 1998, 41, 494–495. [Google Scholar] [CrossRef][Green Version]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).