Evaluation of the Impact of an Enzymatic Preparation Catalyzing the Decomposition of Raffinose from Poor-Quality Beets during the White Sugar Production Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Model Test with Enzyme Preparation
2.2. Technological Tests
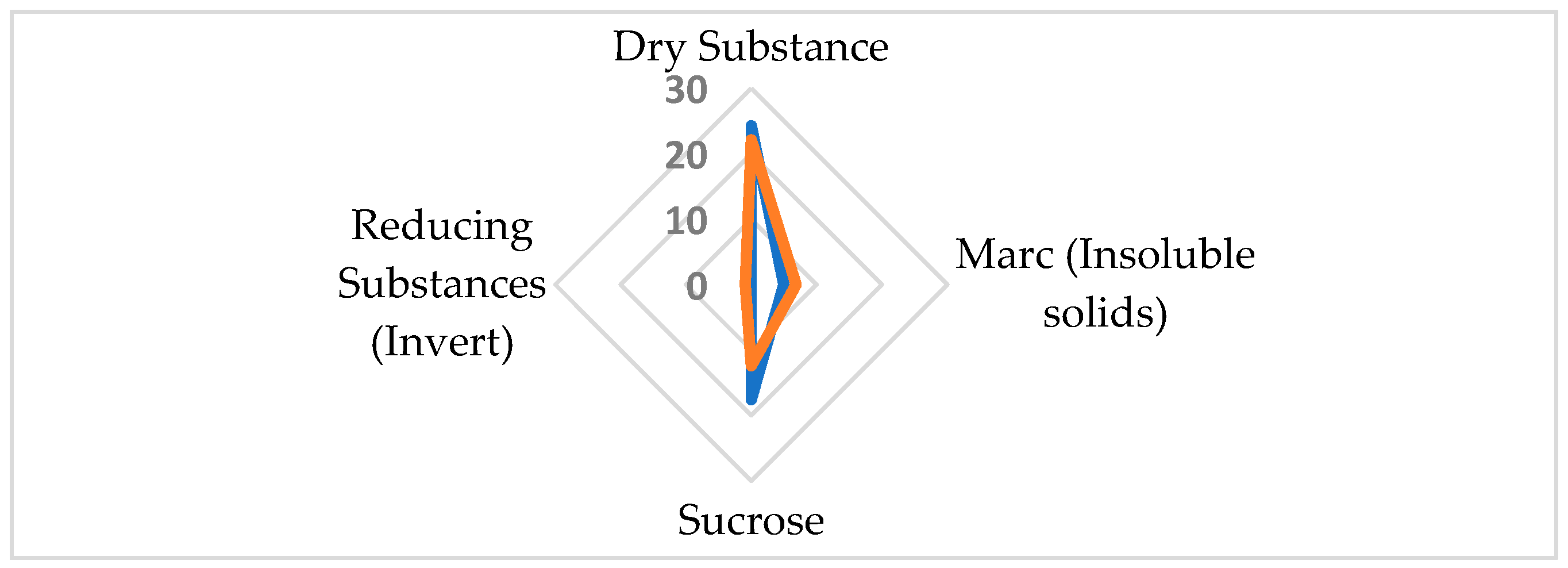
2.2.1. Chemical Quality of Sugar Beet
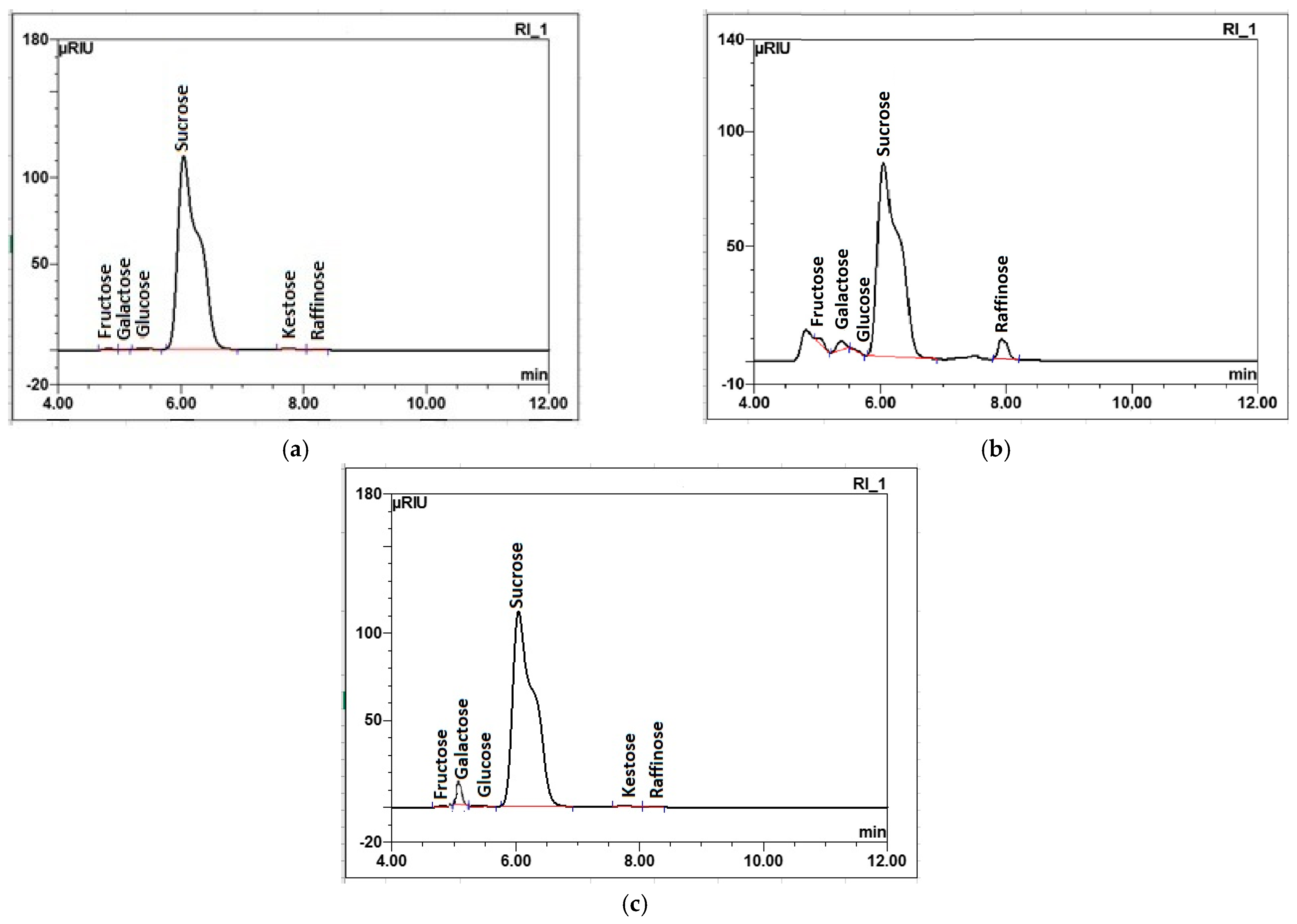
2.2.2. Chemical Quality of Raw Juices
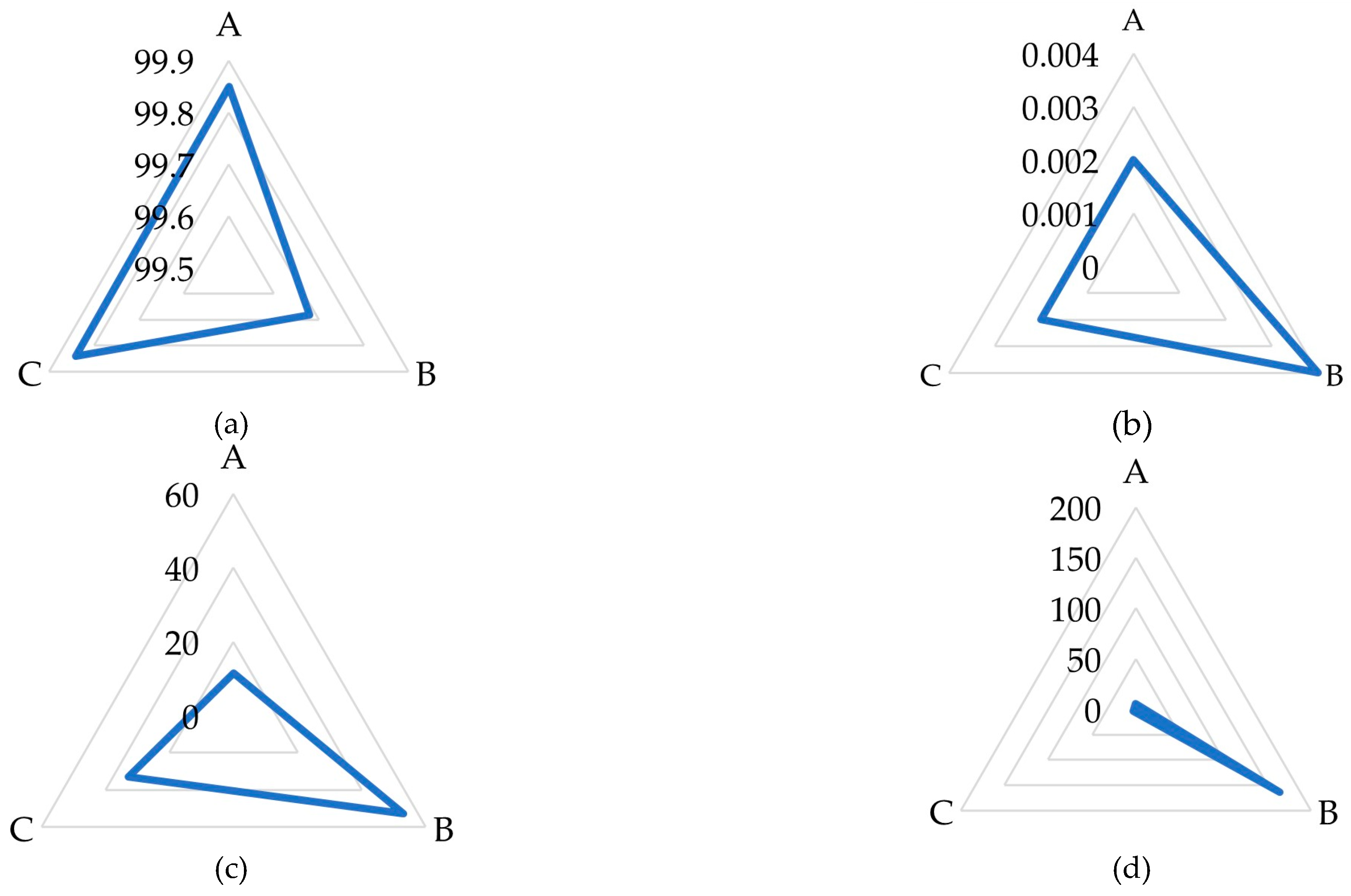
2.2.3. Chemical Quality of White Sugar
2.3. Confirmation of Experimental Data
2.4. Effectiveness of the Use of Enzyme Preparation in the Industrial Scale Application
- ○
- Average sugar production: 7716 tons/day × 14.3% = 1103.38 tons/day.
- ○
- Sugar uplift from raffinose hydrolysis: 1.68% × 0.68 = 1.14%.
- ○
- Daily sugar gain from hydrolysis, including “draft ratio”: 1103.38 tons/day × 1.1 (draft ratio) × 1.14% = 13.84 tons/day.
- ○
- Total sugar gain during enzymatic application period: 13.84 tons/day × 40 days = 553.60 tons.
- ○
- Value of additional sugar produced: 553.6 tons × 775 EUR/ton = 429,040 EUR.
- ○
- Cost of the enzyme preparation (according to data received from the producer):
- ▪
- 1.5 kg × 24 h × 40 days = 1440 kg.
- ▪
- 1440 kg × 81 EUR = 116,640 EUR.
- ○
- Net profit: 429,040 − 116,640 = 312,400 EUR.
3. Materials and Methods
3.1. Sugar Beets, Raw Juices, and White Sugar
3.2. Chemicals and Reagents
3.3. Enzyme
3.4. Laboratory Model Test on Raw Sugar Beet Juice
3.5. Experiment Design in a Sugar Factory
3.6. Chemical Analysis of Sugar Beet Roots
3.7. Determination of Free Carbohydrates in Raw Sugar Beet Juice
3.8. Chemical Analysis of Sugar
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, L.G.; Klotz, K.L. Postharvest Storage Losses Associated with Aphanomyces Root Rot in Sugarbeet. J. Sugarbeet Res. 2006, 43, 113–128. [Google Scholar] [CrossRef]
- Campbell, L.G.; Klotz, K.L.; Smith, L.J. Postharvest Storage Losses Associated with Rhizomania in Sugar Beet. Plant. Dis. 2008, 92, 575–580. [Google Scholar] [CrossRef][Green Version]
- Al-Abdallah, A.; Othman, M.; Al-Jbawi, E.M. The Deterioration in Yield and Quality Traits of Post Harvested Sugar Beet (Beta Vulgaris, L.) Grown in Summer Time. J. Al-furat Univ. Sci. Res. Stud. 2010, 5, 211–223. [Google Scholar]
- Kenter, C.; Hoffmann, C. Qualitätsveränderungen Bei Der Lagerung Frostgeschädigter Zuckerrüben in Abhängigkeit von Temperatur Und Sorte. Zuckerindustrie 2006, 131, 85–91. [Google Scholar]
- Hoffmann, C.; Schnepel, K. Susceptibility to Root Tip Breakage Increases Storage Losses of Sugar Beet Genotypes. Sugar Ind. 2016, 141, 625–632. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Leijdekkers, M.; Ekelöf, J.; Vancutsem, F. Patterns for Improved Storability of Sugar Beet–Importance of Marc Content and Damage Susceptibility of Varieties in Different Environments. Eur. J. Agron. 2018, 101, 30–37. [Google Scholar] [CrossRef]
- Draycott, A.P. Sugar Beet. Wiley: Hoboken, NJ, USA, 2006; ISBN 9781405119115. [Google Scholar]
- Asadi, M. Beet-Sugar Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 9780471763475. [Google Scholar]
- Gruska, R.M.; Baryga, A.; Kunicka-Styczyńska, A.; Brzeziński, S.; Rosicka-Kaczmarek, J.; Miśkiewicz, K.; Sumińska, T. Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides. Molecules 2022, 27, 5125. [Google Scholar] [CrossRef] [PubMed]
- Gippert, A.-L.; Madritsch, S.; Woryna, P.; Otte, S.; Mayrhofer, M.; Eigner, H.; Garibay-Hernández, A.; D’Auria, J.C.; Molin, E.M.; Mock, H.-P. Unraveling Metabolic Patterns and Molecular Mechanisms Underlying Storability in Sugar Beet. BMC Plant Biol. 2022, 22, 430. [Google Scholar] [CrossRef]
- Keller, I.; Müdsam, C.; Rodrigues, C.M.; Kischka, D.; Zierer, W.; Sonnewald, U.; Harms, K.; Czarnecki, O.; Fiedler-Wiechers, K.; Koch, W.; et al. Cold-Triggered Induction of ROS- and Raffinose Metabolism in Freezing-Sensitive Taproot Tissue of Sugar Beet. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Han, Q.; Qi, J.; Hao, G.; Zhang, C.; Wang, C.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. ZmDREB1A Regulates RAFFINOSE SYNTHASE Controlling Raffinose Accumulation and Plant Chilling Stress Tolerance in Maize. Plant Cell Physiol. 2020, 61, 331–341. [Google Scholar] [CrossRef]
- Haagenson, D.M.; Klotz, K.L.; Campbell, L. Impact of Storage Temperature, Storage Duration, and Harvest Date on Sugarbeet Raffinose Metabolism. Postharvest Biol. Technol. 2008, 49, 221–228. [Google Scholar] [CrossRef]
- Coca, M.; Garcia, M.; Gonzalez, G.; Pena, M.; Garcia, J.A. Study of Coloured Components Formed in Sugar Beet Processing. Food Chem. 2004, 86, 421–433. [Google Scholar] [CrossRef]
- Naqvi, A.Z. Kabir-ud-Din Clouding Phenomenon in Amphiphilic Systems: A Review of Five Decades. Colloids Surfaces B Biointerfaces 2018, 165, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, G.; Mei, Y.; Li, R.; Zhang, H.; Liu, Y. Present Status on Removal of Raffinose Family Oligosaccharides–a Review. Czech, J. Food Sci. 2019, 37, 141–154. [Google Scholar] [CrossRef]
- Hassan, I.; Mostafa, S. Influence of Sugar Beet Nitrogen Content on Quality and Efficiency of Sugar Extraction. J. Food Dairy Sci. 2018, 9, 111–116. [Google Scholar] [CrossRef]
- Kulneva, N.G.; Surin, P.Y.; Fedoruk, V.A.; Matvienko, N.A. Substantiation of a Method for Producing Sugar during Deep Processing of Beet Molasses. Proc. Vor. State Univ. Eng. Technol. 2022, 84, 58–65. [Google Scholar] [CrossRef]
- Wieczorek, C.; Sionek, B.; Przybylski, W.; Lahuta, L.B. The Influence of Culinary Processing of Legume Seeds on the Content of Soluble Carbohydrates. Probl. Notebooks Adv. Agric. Sci. 2016, 584, 139–150. [Google Scholar]
- Berlowska, J.; Cieciura-Wloch, W.; Kalinowska, H.; Kregiel, D.; Borowski, S.; Pawlikowska, E.; Binczarski, M.; Witonska, I. Enzymatic Conversion of Sugar Beet Pulp: A Comparison of Simultaneous Saccharification and Fermentation and Separate Hydrolysis and Fermentation for Lactic Acid Production. Food Technol. Biotechnol. 2018, 56, 188–196. [Google Scholar] [CrossRef]
- Kolakowski, E. Application of Enzymatic Hydrolysis and Resinthesis for Protein Modyfication and Obtaining of Bioactive Peptides. Adv. Agric. Sci. 2006, 53, 33–78. [Google Scholar]
- Schaffer, A.; Zhifang, G. Plant-Derived Alkaline Alpha-Galactosidase. US Patent US20060084163A1, 20 April 2006. Available online: https://Patents.Google.Com/Patent/US20060084163A1/En (accessed on 23 July 2024).
- Meguro, S.; Imafuk, S.; Kawamura, K.; Hashimoto, S.; Narita, S. Process for Beet Sugar Production. US Patent US4036694A, 19 July 1977. Available online: https://Patents.Google.Com/Patent/US4036694A/En (accessed on 23 July 2024).
- Naghipouzade Mahani, M.; Aghkhani, M.H.; Behzad, K.; Rohani, A. Study of Effect of Blade Edge on Sucrose Extraction of Sugar Beet and Quality of Raw Juice. Iran. Food Sci. Technol. Res. J. 2017, 13, 191–201. [Google Scholar]
- Borji, A.; Borji, F.-E.; Jourani, A. Sugar Industry: Effect of Dextran Concentrations on the Sucrose Crystallization in Aqueous Solutions. J. Eng. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Eggleston, G.; Monge, A. Optimization of Sugarcane Factory Application of Commercial Dextranases. Process Biochem. 2005, 40, 1881–1894. [Google Scholar] [CrossRef]
- Jiménez, E.R. Dextranase in Sugar Industry: A Review. Sugar Tech 2009, 11, 124–134. [Google Scholar] [CrossRef]
- Korneeva, O.S.; Yakovleva, S.F.; Matvienko, N.A.; Frolova, L.N.; Motina, E.A.; Shuvaeva, G.P. Promising Methods for Intensifying the Process of Sucrose Extraction from Beet Chips. Proc. Vor. State Univ. Eng. Technol. 2023, 85, 149–155. [Google Scholar] [CrossRef]
- Ademakinwa, A.N.; Agboola, F.K. Biochemical Characterization and Kinetic Studies on a Purified Yellow Laccase from Newly Isolated Aureobasidium Pullulans NAC8 Obtained from Soil Containing Decayed Plant Matter. J. Genet. Eng. Biotechnol. 2016, 14, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Lahuta, L.B.; Goszczyńska, J. Inhibition of Raffinose Family Oligosaccharides and Galactosyl Pinitols Breakdown Delays Germination of Winter Vetch (Vicia Villosa Roth.) Seeds. Acta Soc. Bot. Pol. 2011, 78, 203–208. [Google Scholar] [CrossRef]
- Divakar, S.; Anila, H. Enzymatic Treatment (α Galactosidase) to Reduce Antinutrient Content in Raw Jackfruit Bulb Flour and Seed Flour. Int. J. Pure Appl. Biosci. 2019, 7, 98–104. [Google Scholar] [CrossRef]
- Mabinya, L.V.; Brand, J.M.; Muyima, N.Y.O.; Pironcheva, G.L. Kinetic Properties of α-Galactosidase and the Localization of Total Proteins in Erwinia Chrysanthemi. Food Technol. Biotechnol. 2004, 42, 23–26. [Google Scholar]
- Pui, L.P.; Saleena, L.A.K. Enzyme-Aided Treatment of Fruit Juice: A Review. Food Process. Tech. Technol. 2023, 53, 38–48. [Google Scholar] [CrossRef]
- Yeo, S.; Liong, M. Effect of Prebiotics on Viability and Growth Characteristics of Probiotics in Soymilk. J. Sci. Food Agric. 2010, 90, 267–275. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Root Quality of Sugarbeet. Sugar Tech. 2010, 12, 276–287. [Google Scholar] [CrossRef]
- Baryga, A. Studies on the Technological Value of Sugar Beet and the Quality of Sugar in the Context of Using Post-Fermentation Digestate from Biogas Plants in Cultivation. Habilitation Thesis, UMW Olsztyn, Olsztyn, Poland, 2019. [Google Scholar]
- Baryga, A.; Poleć, B. Effect of Sugar Beet Roots Harvesting Date and Storage Method on Processing Value of the Raw Material. Adv. Sci. Technol. Agri-Food Ind. 2020, 75, 34–55. [Google Scholar]
- Baryga, A.; Połeć, B. Studies on Technological Quality of Sugar Beets and Soil Parameters in Relation to Method of Soil Fertilization. Int. J. Environ. Agric. Res. 2016, 2, 42–53. [Google Scholar]
- Hoffmann, C.M.; Koch, H.-J.; Märländer, B. Sugar Beet. In Crop Physiology Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 634–672. ISBN 978-0-12-819194-1. [Google Scholar]
- Keller, I.; Müdsam, C.; Rodrigues, C.M.; Kischka, D.; Zierer, W.; Sonnewald, U.; Harms, K.; Czarnecki, O.; Fiedler-Wiechers, K.; Koch, W.; et al. Cold-Triggered Induction of ROS- and Raffinose-Related Metabolism in Freezing-Sensitive Taproot Tissue of Sugar Beet. bioRxiv 2021. [Google Scholar] [CrossRef]
- Fugate, K.K.; Eide, J.D.; Lafta, A.M.; Tehseen, M.M.; Chu, C.; Khan, M.F.R.; Finger, F.L. Transcriptomic and Metabolomic Changes in Postharvest Sugarbeet Roots Reveal Widespread Metabolic Changes in Storage and Identify Genes Potentially Responsible for Respiratory Sucrose Loss. Front. Plant Sci. 2024, 15, 1320705. [Google Scholar] [CrossRef] [PubMed]
- Iztayev, A.; Kulazhanov, T.K.; Yakiyayeva, M.A.; Zhakatayeva, A.N.; Baibatyrov, T.A. Method for the Safe Storage of Sugar Beets Using an Ion-Ozone Mixture. Acta Sci. Pol. Technol. Aliment. 2021, 20, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, P.; Stropek, Z.; Gołacki, K. Mechanical Properties of Sugar Beet Roots under Impact Loading Conditions. Materials 2023, 16, 1281. [Google Scholar] [CrossRef]
- Loginova, K.; Loginov, M.; Vorobiev, E.; Lebovka, N.I. Better Lime Purification of Sugar Beet Juice Obtained by Low Temperature Aqueous Extraction Assisted by Pulsed Electric Field. LWT–Food Sci. Technol. 2012, 46, 371–374. [Google Scholar] [CrossRef]
- Bahrami, M.E.; Honarvar, M.; Ansari, K.; Jamshidi, B. Measurement of Quality Parameters of Sugar Beet Juices Using Near-Infrared Spectroscopy and Chemometrics. J. Food Eng. 2020, 271, 109775. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Chen, W.; Xu, J.; Wu, B. Identification and Characterization of a Thermostable GH36 α-Galactosidase from Anoxybacillus Vitaminiphilus WMF1 and Its Application in Synthesizing Isofloridoside by Reverse Hydrolysis. Int. J. Mol. Sci. 2021, 22, 10778. [Google Scholar] [CrossRef]
- Álvarez-Cao, M.-E.; Cerdán, M.-E.; González-Siso, M.-I.; Becerra, M. Bioconversion of Beet Molasses to Alpha-Galactosidase and Ethanol. Front. Microbiol. 2019, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, B.A.; EL-Taib, R.A.G. Quality of Some Sugar Beet Varieties as Affected by Toshka Region Conditions, Aswan (Egypt). Suez Canal Univ. J. Food Sci. 2019, 6, 13–18. [Google Scholar] [CrossRef]
- Mesele, T.L. Review on Physico-Chemical Properties of Honey in Eastern Africa. J. Apic. Res. 2021, 60, 33–45. [Google Scholar] [CrossRef]
- Peterbauer, T.; Richter, A. Biochemistry and Physiology of Raffinose Family Oligosaccharides and Galactosyl Cyclitols in Seeds. Seed Sci. Res. 2001, 11, 185–197. [Google Scholar]
- Fabris, E.; Bulfoni, M.; Nencioni, A.; Nencioni, E. Intra-Laboratory Validation of Alpha-Galactosidase Activity Measurement in Dietary Supplements. Molecules 2021, 26, 1566. [Google Scholar] [CrossRef]
- Wojtczak, M.; The Course of the 2022/23 Sugar Campaign in Poland. Presentation at the 35th Post-Campaign Technical and Raw Materials Conference. Available online: https://stc.pl/konferencja2023.php (accessed on 29 February 2024).
- Muir, B.M. Sugar Beet Processing to Sugars. In Sugar Beet Cultivation, Management and Processing; Misra, V., Srivastava, S., Mall, A.K., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 837–862. ISBN 978-981-19-2729-4. [Google Scholar]
- Average Sugar Prices in the European Union and in Poland. Available online: https://kzpbc.com.pl/srednie-ceny-cukru-w-unii-europejskiej-i-w-polsce,32,pl.html (accessed on 9 March 2024).
- PN-EN 12880:2004; Characterization of Sludges–Determination of Dry Residue and Water Content. Polish Committee for Standardization: Warsaw, Poland, 2004.
- ICUMSA Method GS6-3; Polarimetric Sucrose Content of Sugar Beet by the Macerator or Cold Aqueous Digestion Method Using Aluminium Sulphate as Clarifying Agent–Official. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 1994.
- PN-EN 13342:2002; Characterisation of Sewage Sludge–Determination of Kjeldahl Nitrogen. Polish Committee for Standardization: Warsaw, Poland, 2002.
- ICUMSA Method GS6-5; α-Amino Nitrogen in Sugar Beet by the Copper Method (‘Blue Number’) after Defecation with Aluminium Sulphate–Official. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2007.
- Ravn, H.C.; Bandsholm Sørensen, O.; Meyer, A.S. Time of Harvest Affects the Yield of Soluble Polysaccharides Extracted Enzymatically from Potato Pulp. Food Bioprod. Process. 2015, 93, 77–83. [Google Scholar] [CrossRef]
- Shodex NH2P-50 Series Columns. In Analysis of Saccharides in Food Industry; Technical Notebook No. 2; Showa Denko Europe GmbH: Wiesbaden, Germany, 2015.
- ICUMSA Method GS2/3-1; Polarization of White Sugar by Polarimetry (Braunschweig Method). International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2011.
- ICUMSA Method GS2/1/3/9-15; Sugar Moisture by Loss on Drying. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2007.
- ICUMSA Method GS2/3/9-5; Reducing Sugars in Purified Sugar by the Knight and Allen EDTA Method. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2011.
- ICUMSA Method GS2/3-10; White Sugar Solution Colour. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2011.
- ICUMSA Method GS2-13; Reflectance of White Sugar. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2011.
- ICUMSA Method GS2/3/9-17; Conductivity Ash in Refined Sugar Products and in Plantation White Sugar. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2011.
- ICUMSA Method GS2/1/7/9-33; Sulphite in White Sugar, Cane Sugar Juices and Syrups, VVHP Raw Sugar and in Plantation White Sugar by the Rosaniline Colorimetric Method. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2011.
- ICUMSA Method GS2/3/9-19; Insoluble Matter in White Sugar. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2007.
- ICUMSA Method GS2/3-18; Turbidity of White Sugar Solutions. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2013.
- ICUMSA Method GS1-23; Determination of PH. International Commission for Uniform Methods of Sugar Analysis (ICUMSA): Brussels, Belgium, 2009.
- PN-EN PN-A-74855-10:1987; Sugar–Methods of Test–Determination of Ferromagnetic Impurities Content. Polish Committee for Standardization: Brussels, Belgium, 1987.
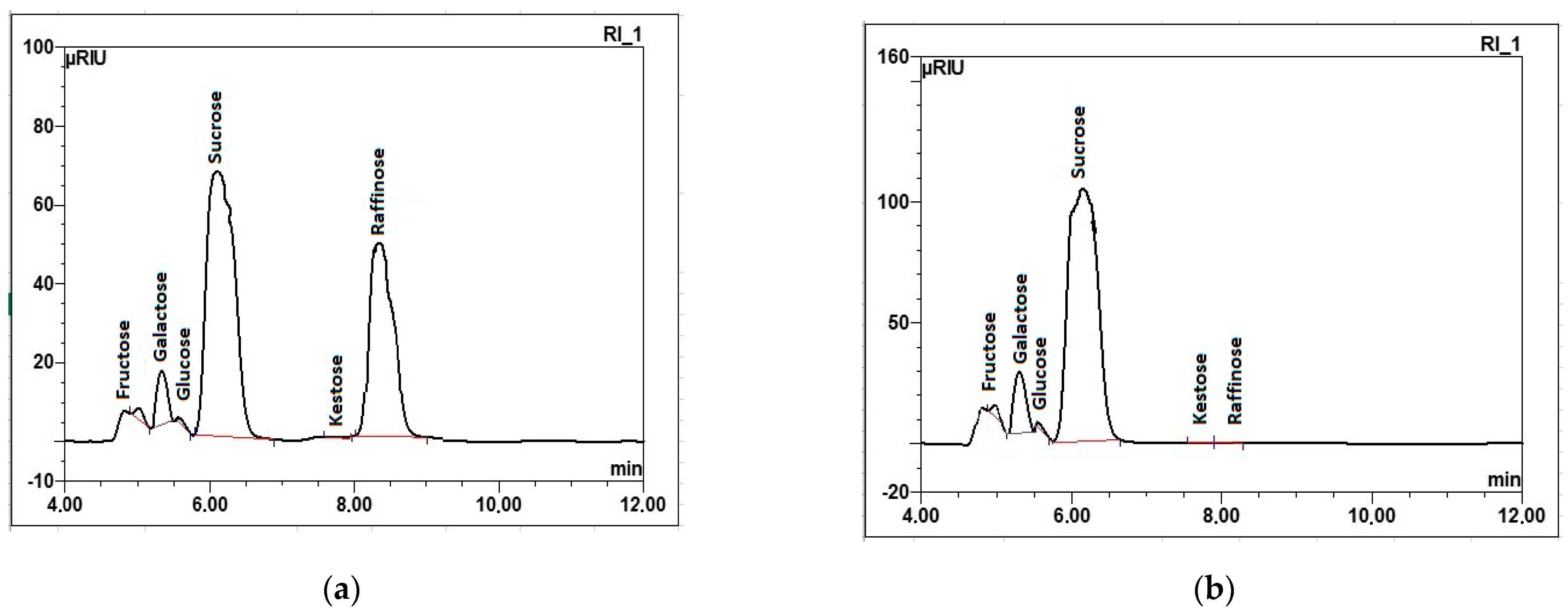
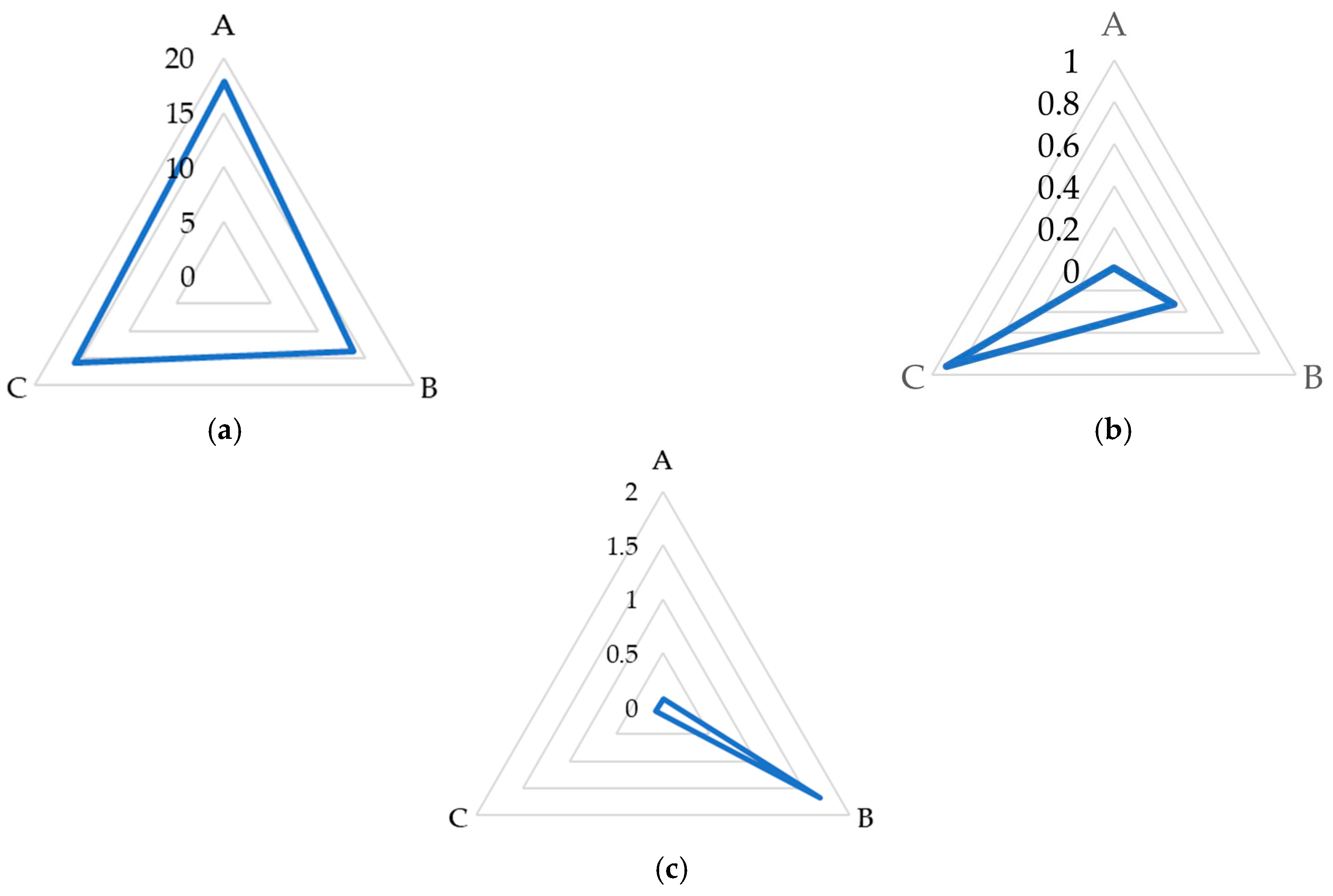

| Raw Sugar Beet Juice | ||
|---|---|---|
| Carbohydrates, % | With Raffinose 1 | With Raffinose after Enzymatic Preparation Treatment 2 |
| Fructose | 0.63 ± 0.01 a | 0.59 ± 0.03 a |
| Glucose | 0.17 ± 0.01 a | 0.15 ± 0.02 a |
| Galactose | 2.64 ± 0.02 b | 4.42 ± 0.05 a |
| Sucrose | 8.49 ± 0.02 b | 14.55 ± 0.03 a |
| Trehalose | ND | ND |
| Kestose | 0.02 ± 0.01 a | 0.02 ± 0.01 a |
| Raffinose | 8.69 ± 0.01 a | 0.04 ± 0.01 b |
| Sugar Beet Roots | Raw Sugar Beet Juice | White Sugar | |||||
| Parameter | % | Carbohydrates | % | Parameter | |||
| Good quality | Dry Substance | 24.31 ± 0.04 a | Fructose | 0.03 ± 0.02 b | Polarization (°Z) | 99.85 ± 0.00 a | |
| Marc (Insoluble solids) | 4.97 ± 0.12 b | Glucose | 0.06 ± 0.01 a | Moisture (%) | 0.007 ± 0.000 c | ||
| Sucrose | 17.56 ± 0.06 a | Galactose | 0.01 ± 0.01 c | Reducing Sugars (%) | 0.002 ± 0.000 b | ||
| Reducing Substances (Invert) | 0.08 ± 0.01 b | Sucrose | 17.81 ± 0.30 a | Ash (%) | 0.003 ± 0.000 c | ||
| Soluble Ash Content | 0.360 ± 0.003 b | Trehalose | ND | Color in comparison with dry standard (IU420) | 0.2 ± 0.0 c | ||
| Potassium | 0.167 ± 0.002 a | Kestose | 0.02 ± 0.01 b | Color of sugar solution (IU420) | 11.50 ± 0.14 c | ||
| Sodium | 0.0056 ± 0.0004 b | Raffinose | 0.08 ± 0.01 b | Sulfite (mg/kg) | 0.18 ± 0.00 c | ||
| Alpha-amino acid nitrogen | 0.010 ± 0.000 a | Insoluble Matter (mg/kg) | 5.7 ± 0.4 b | ||||
| Amide nitrogen | 0.013 ± 0.002 a | Turbidity (IU420) | 5.4 ± 0.1 c | ||||
| pH | 6.01 ± 0.02 c | ||||||
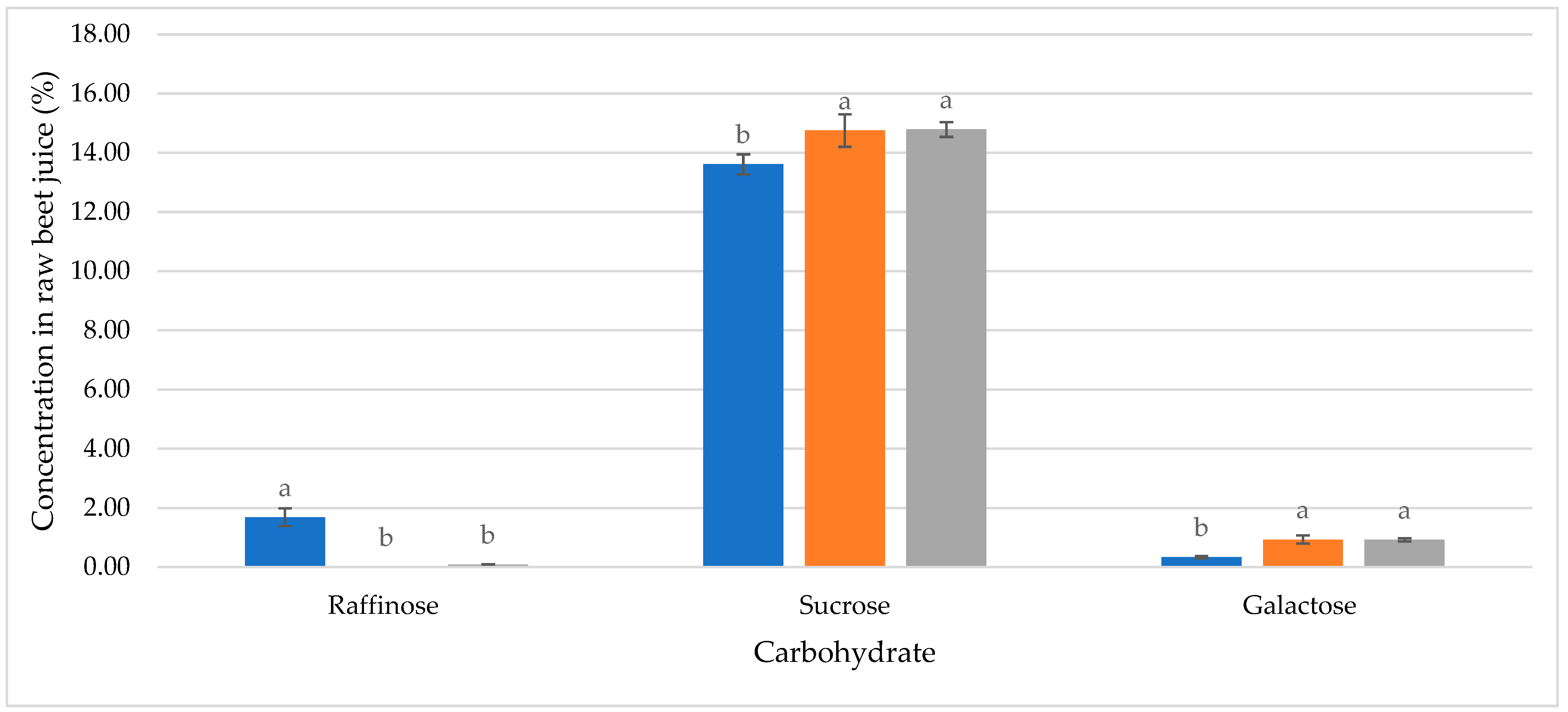
| Poor quality | Without enzyme preparation | Fructose | 0.10 ± 0.02 a | Polarization (°Z) | 99.68 ± 0.00 c | ||
| Glucose | 0.02 ± 0.01 b | Moisture (%) | 0.008 ± 0.000 b | ||||
| Galactose | 0.33 ± 0.04 b | Reducing Sugars (%) | 0.004 ± 0.000 a | ||||
| Sucrose | 13.61 ± 0.34 c | Ash (%) | 0.031 ± 0.001 a | ||||
| Dry Substance | 22.15 ± 0.03 b | Trehalose | ND | Color in comparison with dry standard (IU420) | 3.0 ± 0.1 a | ||
| Marc (Insoluble solids) | 6.82 ± 0.16 a | Kestose | ND | Color of sugar solution (IU420) | 53.00 ± 0.24 a | ||
| Sucrose | 12.35 ± 0.02 b | Raffinose | 1.68 ± 0.30 a | Sulfite (mg/kg) | 5.55 ± 0.09 a | ||
| Reducing Substances (Invert) | 0.92 ± 0.02 a | Insoluble Matter (mg/kg) | 164.0 ± 4.1 a | ||||
| Soluble Ash Content | 0.450 ± 0.013 a | Turbidity (IU420) | 469.0 ± 0.9 a | ||||
| pH | 8.20 ± 0.08 a | ||||||
| Potassium | 0.161 ± 0.009 b | With enzyme preparation | Fructose | 0.11 ± 0.01 a | Polarization (°Z) | 99.84 ± 0.00 b | |
| Sodium | 0.0068 ± 0.0003 a | Glucose | 0.01 ± 0.01 b | Moisture (%) | 0.019 ± 0.002 a | ||
| Alpha-amino acid nitrogen | 0.009 ± 0.000 b | Galactose | 0.92 ± 0.05 a | Reducing Sugars (%) | 0.002 ± 0.000 b | ||
| Amide nitrogen | 0.006 ± 0.000 b | Sucrose | 14.79 ± 0.25 b | Ash (%) | 0.011 ± 0.000 b | ||
| Trehalose | ND | Color in comparison with dry standard (IU420) | 1.8 ± 0.1 b | ||||
| Kestose | 0.06 ± 0.02 a | Color of sugar solution (IU420) | 32.90 ± 0.21 b | ||||
| Raffinose | 0.08 ± 0.02 b | Sulfite (mg/kg) | 0.31 ± 0.01 b | ||||
| Insoluble Matter (mg/kg) | 3.1 ± 0.2 c | ||||||
| Turbidity (IU420) | 6.0 ± 0.2 b | ||||||
| pH | 6.69 ± 0.04 b | ||||||
| Parameter | Description | Methodology | Typical Range/Value, (%) [37] |
|---|---|---|---|
| Dry substance content | The amount of dry mass in sugar beet roots | ICUMSA GS2/3-1 (2011) [37,55] | 24–25 |
| Marc content | The amount of insoluble plant components remaining after juice extraction | Standard Method [37] | 4.0–6.0 |
| Sucrose content | The amount of sucrose | ICUMSA GS6-3 (1994) [56] | 14.–19 |
| Reducing sugars content (invert) | The level of reducing substances such as glucose and fructose, which are formed by the hydrolysis of sucrose | Berlin Institute Method [37] | 0.02–0.1 |
| Soluble ash content | The mineral content in beet juice | Conductometrically [37] | 0.5–0.6 |
| Amide nitrogen | Amide nitrogen content | National standard PN-EN 13342:2002 [57] | ≤0.015 |
| α-Amino acid nitrogen | The nitrogen content from amino acids | ICUMSA GS6-5 (2007) [58] | ≤0.03 |
| Metal concentration: sodium, potassium | Sodium and potassium content | ASA spectrophotometry, National Standard (FAAS) [38] | Na: 0.1–0.3, K: 0.01–0.10 |
| Parameter | Description | Methodology | Typical Range/Value [8,35] |
|---|---|---|---|
| Polarization | Optical rotation of sucrose | ICUMSA GS2/3-1 (2011) [61] | ≥99.7 °Z |
| Moisture | Water content | ICUMSA GS2/1/3/9-15 (2007) [62] | ≤0.06% |
| Reducing substance | Glucose and fructose levels | ICUMSA GS2/3/9-5 (2011) [63] | ≤0.04% |
| Color | White sugar solution color | ICUMSA GS2/3-10 (2011) [64] | ≤45 IU420 |
| Reflectance | Whiteness and purity of sugar crystals | ICUMSA GS2-13 (2011) [65] | ≥99.0% |
| Conductometric ash | Determines mineral content | ICUMSA GS2/3/9-17 (2011) [66] | ≤0.027% |
| Sulfite content | Sulfite levels | ICUMSA GS2/1/7/9-33 (2011) [67] | ≤10 mg/kg |
| Insoluble matter in water | Insoluble impurities | ICUMSA GS2/3/9-19 (2007) [68] | ≤20 mg/kg |
| Turbidity | Cloudiness of white sugar solution | ICUMSA GS2/3-18 (2013) [69] | ≤30 IU |
| pH | Acidity or alkalinity sugar water solution | ICUMSA GS1-23 (2009) [70] | 6.5–8.0 |
| Ferromagnetic contaminants | Ferromagnetic materials | PN-A-74855-10:1987 [71] | ≤0.5 mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśkiewicz, A.; Kunicka-Styczyńska, A.; Baryga, A.; Gruska, R.M.; Brzeziński, S.; Świącik, B. Evaluation of the Impact of an Enzymatic Preparation Catalyzing the Decomposition of Raffinose from Poor-Quality Beets during the White Sugar Production Process. Molecules 2024, 29, 3526. https://doi.org/10.3390/molecules29153526
Jaśkiewicz A, Kunicka-Styczyńska A, Baryga A, Gruska RM, Brzeziński S, Świącik B. Evaluation of the Impact of an Enzymatic Preparation Catalyzing the Decomposition of Raffinose from Poor-Quality Beets during the White Sugar Production Process. Molecules. 2024; 29(15):3526. https://doi.org/10.3390/molecules29153526
Chicago/Turabian StyleJaśkiewicz, Andrzej, Alina Kunicka-Styczyńska, Andrzej Baryga, Radosław Michał Gruska, Stanisław Brzeziński, and Beata Świącik. 2024. "Evaluation of the Impact of an Enzymatic Preparation Catalyzing the Decomposition of Raffinose from Poor-Quality Beets during the White Sugar Production Process" Molecules 29, no. 15: 3526. https://doi.org/10.3390/molecules29153526
APA StyleJaśkiewicz, A., Kunicka-Styczyńska, A., Baryga, A., Gruska, R. M., Brzeziński, S., & Świącik, B. (2024). Evaluation of the Impact of an Enzymatic Preparation Catalyzing the Decomposition of Raffinose from Poor-Quality Beets during the White Sugar Production Process. Molecules, 29(15), 3526. https://doi.org/10.3390/molecules29153526









