Agrocybe cylindracea Dietary Fiber Modification: Sodium Hydroxide Treatment Outperforms High-Temperature, Cellulase, and Lactobacillus Fermentation
Abstract
1. Introduction
2. Results and Discussion
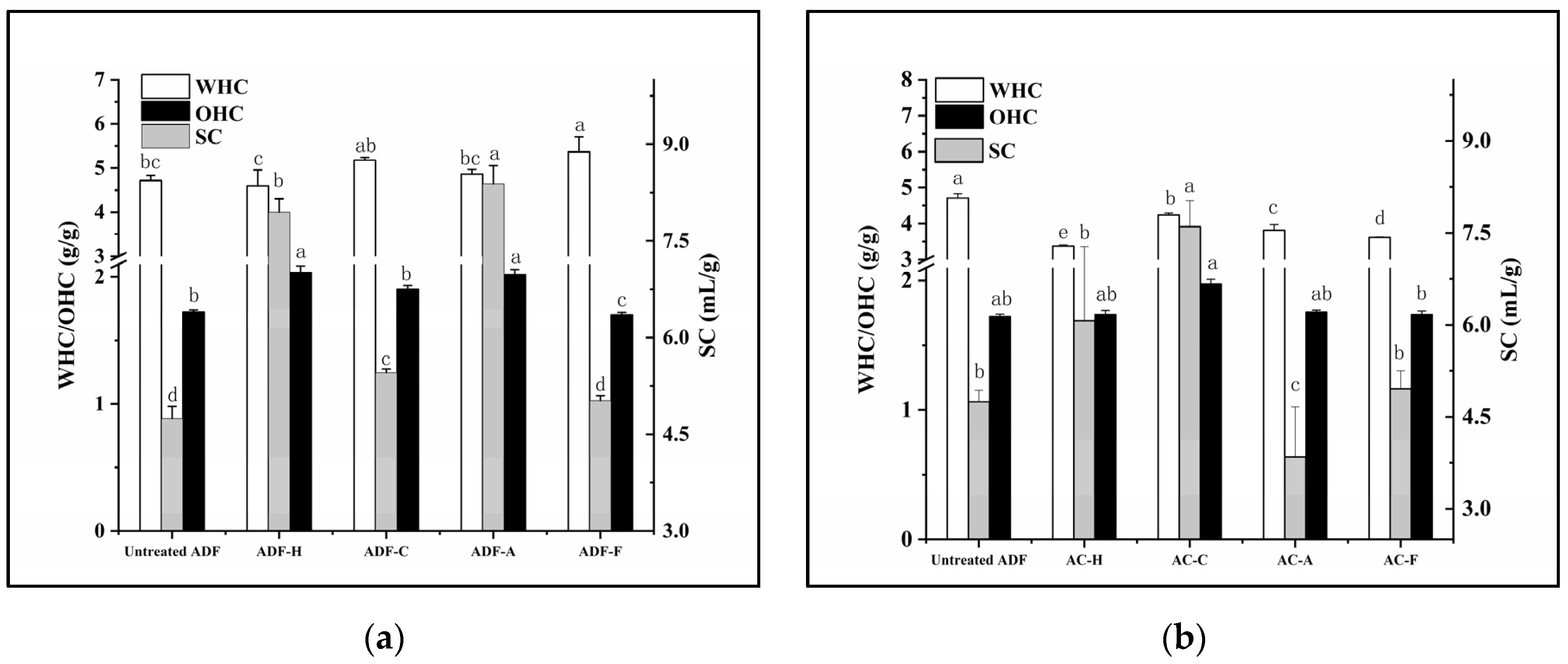
2.1. Physicochemical Properties
2.2. Adsorption Capacity
2.3. Structural Properties
2.3.1. Monosaccharide Composition
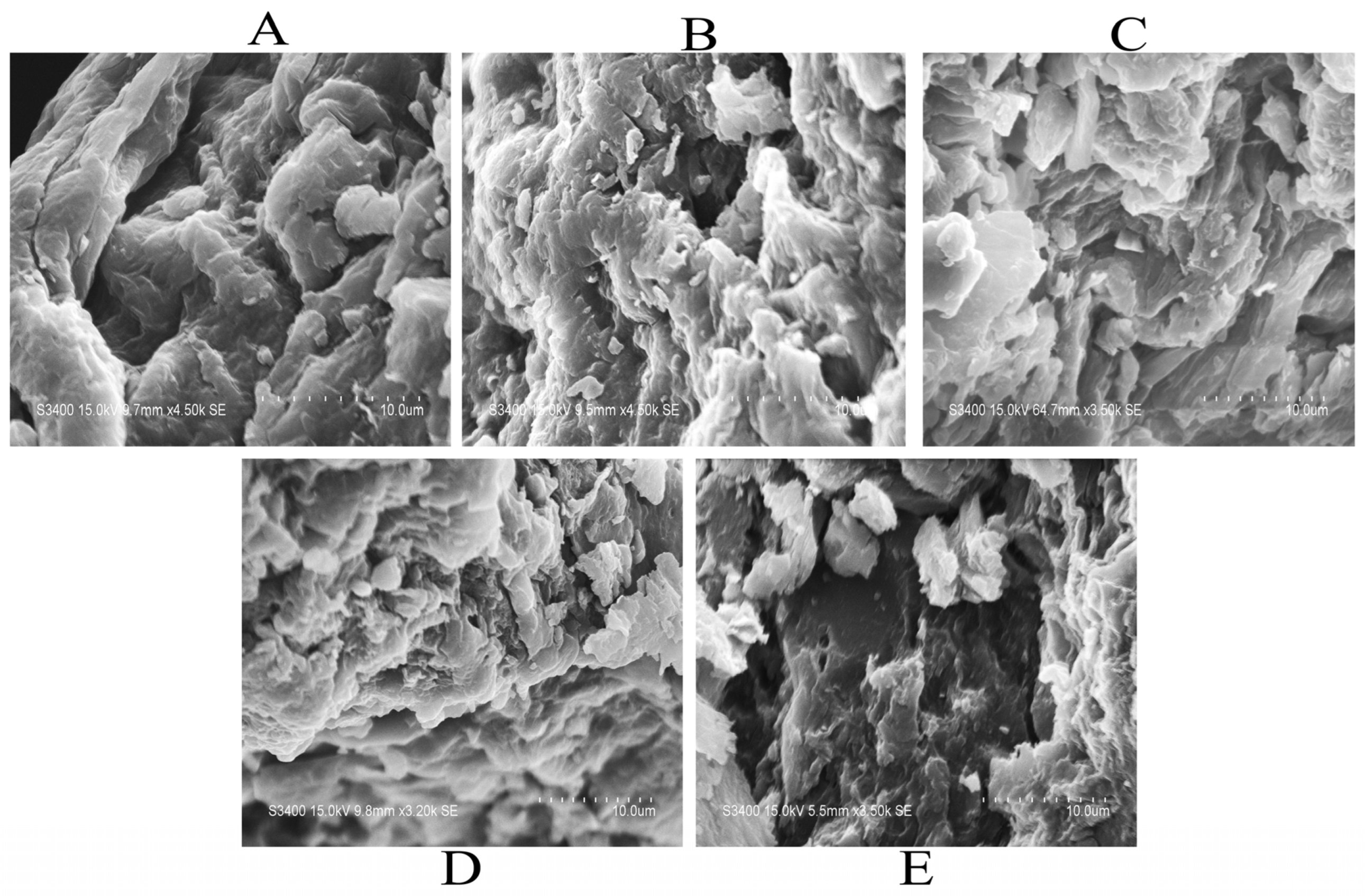
2.3.2. Microstructure
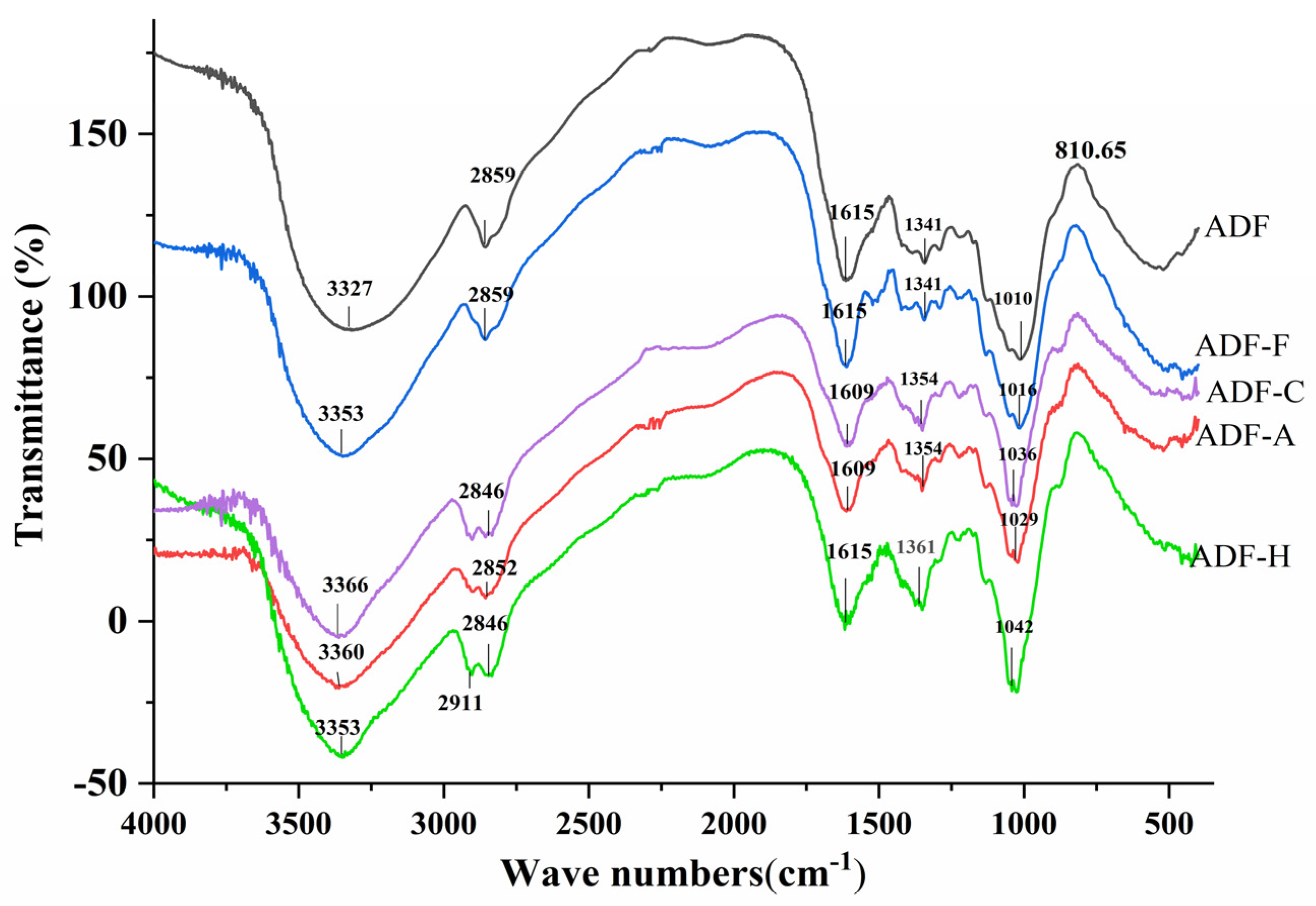
2.3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Functional Characteristics
2.4.1. Antioxidant Activity In Vitro
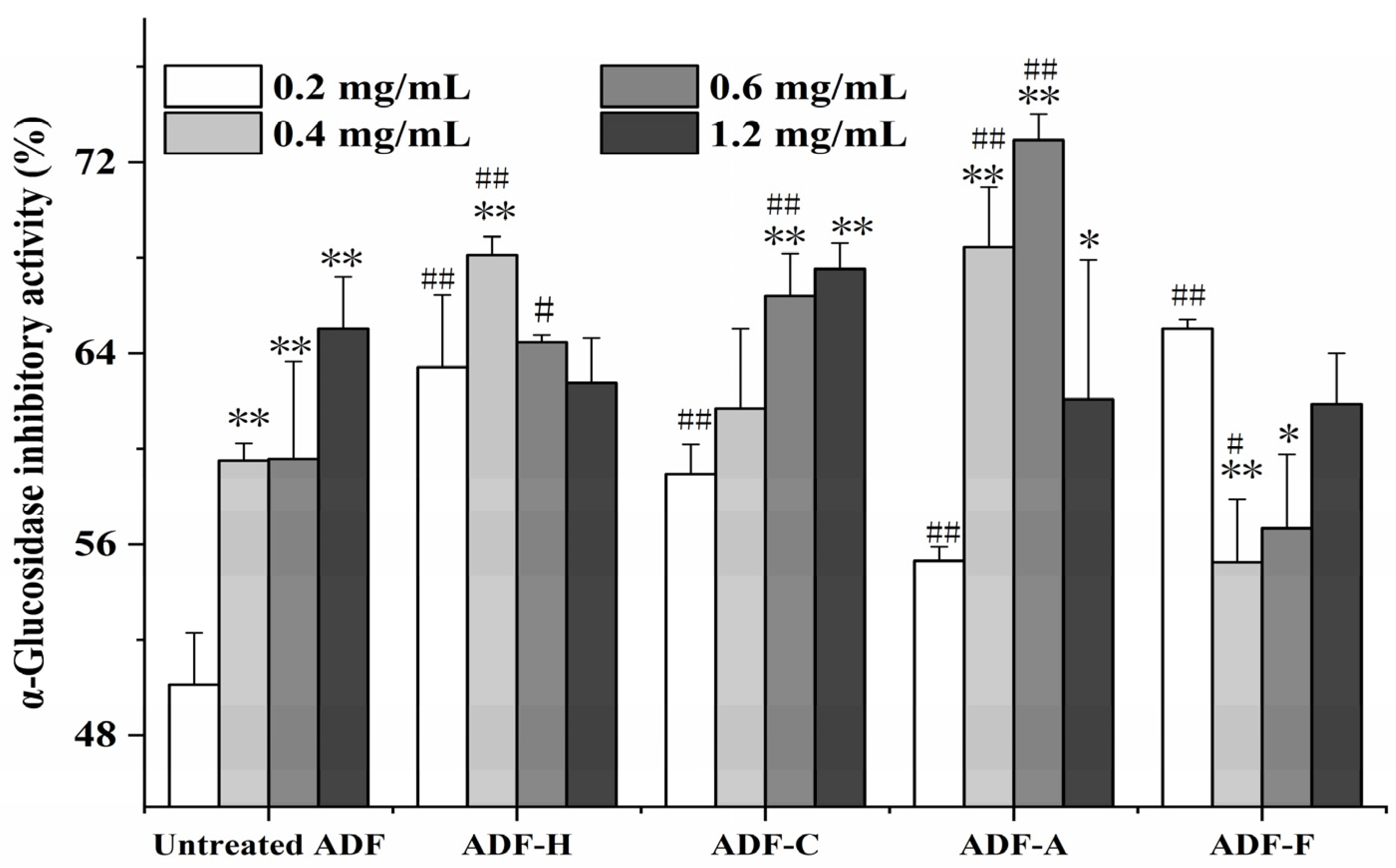
2.4.2. α-Glucosidase Inhibitory Activity
3. Materials and Methods
3.1. Materials
3.2. ADF Extraction
3.3. ADF Modification
3.3.1. Cellulase Treatment
3.3.2. High-Temperature Treatment
3.3.3. Sodium Hydroxide Hydrolysis
3.3.4. Fermentation Treatment
3.4. AC Powder Modification
3.5. Physicochemical Properties
3.5.1. The Water-Holding Capacity and Oil-Holding Capacity of ADF
3.5.2. DF Swelling Capacity
3.5.3. Adsorption Capacity
3.6. Structural Properties
3.6.1. Monosaccharide Composition
3.6.2. Scanning Electron Microscopy (SEM)
3.6.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.7. Functional Characteristics
3.7.1. Antioxidant Activity In Vitro
3.7.2. α-Glucosidase Inhibitory Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, H.; Sultan, Z.; Yousuf, O.; Malik, M.; Younis, K. A review of the health benefits, functional properties, and ultrasound-assisted dietary fiber extraction. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100356. [Google Scholar] [CrossRef]
- Howlett, J.F.; Betteridge, V.A.; Champ, M.; Craig, S.A.; Meheust, A.; Jones, J.M. The definition of dietary fiber—Discussions at the Ninth Vahouny Fiber Symposium: Building scientific agreement. Food Nutr. Res. 2010, 54, 5750. [Google Scholar] [CrossRef] [PubMed]
- Ktenioudaki, A.; Gallagher, E. Recent advances in the development of high-fibre baked products. Trends Food Sci. Technol. 2012, 28, 4–14. [Google Scholar] [CrossRef]
- Wong, K.-H.; Lai, C.K.M.; Cheung, P.C.K. Immunomodulatory activities of mushroom sclerotial polysaccharides. Food Hydrocoll. 2011, 25, 150–158. [Google Scholar] [CrossRef]
- Bai, Q.; Xu, J.; Zhu, W.Y.; Huang, C.; Ni, X.M.; Zhao, H.; Feng, X.Q.; Li, L.; Du, S.S.; Fan, R.; et al. Effects of consumption of a low glycaemic index formula on glycaemic control in patients with type 2 diabetes managed by medical nutrition therapy. Food Sci. Technol. 2021, 41, 768–774. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.C.K. Mini-review on edible mushrooms as source of dietary fiber: Preparation and health benefits. Food Sci. Hum. Wellness 2013, 2, 162–166. [Google Scholar] [CrossRef]
- Wang, C.; Lin, M.; Li, Y.; Guo, Z. Improvement of soluble dietary fiber quality in Tremella fuciformis stem by steam explosion technology: An evaluation of structure and function. Food Chem. 2024, 437, 137867. [Google Scholar] [CrossRef]
- Dang, T.T.; Vasanthan, T. Modification of rice bran dietary fiber concentrates using enzyme and extrusion cooking. Food Hydrocoll. 2019, 89, 773–782. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Li, C.; Chen, J. Physicochemical, functional, and antioxidant properties of dietary fiber from Rosa roxburghii Tratt fruit modified by physical, chemical, and biological enzyme treatments. J. Food Process. Preserv. 2020, 44, e14858. [Google Scholar] [CrossRef]
- Si, J.; Yang, C.; Chen, Y.; Xie, J.; Tian, S.; Cheng, Y.; Hu, X.; Yu, Q. Structural properties and adsorption capacities of Mesona chinensis Benth residues dietary fiber prepared by cellulase treatment assisted by Aspergillus niger or Trichoderma reesei. Food Chem. 2023, 407, 135149. [Google Scholar] [CrossRef]
- Tan, Y.; Li, S.; Li, C.; Liu, S. Glucose adsorption and alpha-amylase activity inhibition mechanism of insoluble dietary fiber: Comparison of structural and microrheological properties of three different modified coconut residue fibers. Food Chem. 2023, 418, 135970. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Ge, Y.; Liu, D.; Zhao, S.; Wei, M.; Jiliu, J.; Hu, X.; Quan, Z.; Wu, Y.; Su, Y.; et al. Effects of High-Temperature, High-Pressure, and Ultrasonic Treatment on the Physicochemical Properties and Structure of Soluble Dietary Fibers of Millet Bran. Front. Nutr. 2021, 8, 820715. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y. Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Liu, X.; Gong, Z.; Cui, W.; Wang, Y.; Wang, W. Extraction, modification, and property characterization of dietary fiber from Agrocybe cylindracea. Food Sci. Nutr. 2020, 8, 6131–6143. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Z.; Wang, W.; Mu, J.; Liu, Y.; Wang, J.; Stipkovits, L.; Hui, X.; Wu, G.; Sun, J. The effects of enzymatic modification on the functional ingredient—Dietary fiber extracted from potato residue. LWT 2022, 153, 112511. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef] [PubMed]
- Carlo, M.D.; Sacchetti, G.; Mattia, C.D.; Compagnone, D.; Mastrocola, D.; Liberatore, L.; Cichelli, A. Contribution of the Phenolic Fraction to the Antioxidant Activity and Oxidative Stability of Olive Oil. J. Agric. Food Chem. 2004, 52, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Q.; Wang, L.; Zha, S.; Zhang, L.; Zhao, B. Physicochemical and functional properties of dietary fiber from maca (Lepidium meyenii Walp.) liquor residue. Carbohydr. Polym. 2015, 132, 509–512. [Google Scholar] [CrossRef]
- Valencia-Espinosa, I.; Welti-Chanes, J.; Garcia-Amezquita, L.E.; Tejada-Ortigoza, V. Green Extraction and Modification of Dietary Fiber from Traditional and Novel Sources. In Sustainable Food Science: A Comprehensive Approach; Elsevier: Amsterdam, The Netherlands, 2023; pp. 254–270. [Google Scholar]
- Wang, C.; Lin, M.; Li, Y.; Zhuang, W.; Guo, Z. Effect of steam explosion modified soluble dietary fiber from Tremella fuciformis stem on the quality and digestibility of biscuits. Int. J. Biol. Macromol. 2024, 265, 130905. [Google Scholar] [CrossRef]
- Ahmed, F.; Sairam, S.; Urooj, A. In vitro hypoglycemic effects of selected dietary fiber sources. J. Food Sci. Technol. 2010, 48, 285–289. [Google Scholar] [CrossRef]
- Ma, M.-m.; Mu, T.-H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Liu, X.; Suo, K.; Wang, P.; Li, X.; Hao, L.; Zhu, J.; Yi, J.; Kang, Q.; Huang, J.; Lu, J. Modification of wheat bran insoluble and soluble dietary fibers with snail enzyme. Food Sci. Hum. Wellness 2021, 10, 356–361. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, W.; Cao, J.; He, K.; Liu, Y.; Bai, X. Microstructure characteristics of tea seed dietary fibre and its effect on cholesterol, glucose and nitrite ion adsorption capacities in vitro: A comparison study among different modifications. Int. J. Food Sci. Technol. 2019, 55, 1781–1791. [Google Scholar] [CrossRef]
- Lin, D.; Long, X.; Huang, Y.; Yang, Y.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Effects of microbial fermentation and microwave treatment on the composition, structural characteristics, and functional properties of modified okara dietary fiber. LWT 2020, 123, 109059. [Google Scholar] [CrossRef]
- Sang, J.; Li, L.; Wen, J.; Liu, H.; Wu, J.; Yu, Y.; Xua, Y.; Gu, Q.; Fu, M.; Lin, X. Chemical composition, structural and functional properties of insoluble dietary fiber obtained from the Shatian pomelo peel sponge layer using different modification methods. LWT 2022, 165, 113737. [Google Scholar] [CrossRef]
- Yu, G.Y.; Bei, J.; Zhao, J.; Li, Q.H.; Cheng, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhao, T.; Zhang, Y.; Tan, Y.; Han, X.; Tang, Y.; Chen, G. Isolated compounds from Dracaena angustifolia Roxb and acarbose synergistically/additively inhibit α-glucosidase and α-amylase: An in vitro study. BMC Complement. Med. Ther. 2022, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Ma, Q.; Chen, Y.; Lu, Y.; Wang, Y.; Jia, Y.; Zhang, M.; Chen, H. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020, 129, 108870. [Google Scholar] [CrossRef]
- Yang, X.; Dai, J.; Zhong, Y.; Wei, X.; Wu, M.; Zhang, Y.; Huang, A.; Wang, L.; Huang, Y.; Zhang, C.; et al. Characterization of insoluble dietary fiber from three food sources and their potential hypoglycemic and hypolipidemic effects. Food Funct. 2021, 12, 6576–6587. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Shkrob, I.; Dembitsky, V.M. Lipids and fatty acids of wild edible mushrooms of the genus Boletus. J. Food Lipids 2008, 15, 370–383. [Google Scholar] [CrossRef]
- Wei, Z.-J.; Thakur, K.; Zhang, J.-G.; Ren, Y.-F.; Wang, H.; Zhu, D.-Y.; Ma, Y.-L.; Wang, C.-H. Physicochemical and Functional Properties of Dietary Fiber from Bamboo Shoots (Phyllostachys praecox). Emir. J. Food Agric. 2017, 29, 509–517. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Fu, X.; Huang, Q.; Chen, Q. Physicochemical, functional and biological properties of soluble dietary fibers obtained from Rosa roxburghii Tratt pomace using different extraction methods. Process Biochem. 2023, 128, 40–48. [Google Scholar] [CrossRef]
- Mohan, S.; Pinto, B.M. Zwitterionic glycosidase inhibitors: Salacinol and related analogues. Carbohydr. Res. 2007, 342, 1551–1580. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.-Y.; Bai, Y.-Y.; Tang, J.; Chen, W. Antioxidation and α-glucosidase inhibitory activities of barley polysaccharides modified with sulfation. LWT—Food Sci. Technol. 2015, 64, 104–111. [Google Scholar] [CrossRef]
- Xie, C.; Guo, H.; Wu, Z.; Guo, Y.; Gao, Y.; Yuan, J.; Gu, Z. Optimization of high-quality dietary fiber production in submerged fermentation by Agrocybe chaxingu. Ann. Microbiol. 2013, 63, 1169–1175. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Zhao, X.; Xu, C.; Zhou, Y.; Peng, J. Soluble Fiber with High Water-Binding Capacity, Swelling Capacity, and Fermentability Reduces Food Intake by Promoting Satiety Rather Than Satiation in Rats. Nutrients 2016, 8, 615. [Google Scholar] [CrossRef]
- Wutsqa, Y.U.; Suratman, S.; Sari, S.L.A. Detection of terpenoids and steroids in Lindsaea obtusa with thin layer chromatography. Asian J. Nat. Prod. Biochem. 2021, 19, 66–69. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, Y.; Leng, F.; Wang, S.; Wang, F.; Zhuang, Y.; Liu, X.; Wang, X.; Ma, X. Establishment and Application of a Method for Rapid Determination of Total Sugar Content Based on Colorimetric Microplate. Sugar Tech 2016, 19, 424–431. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Huang, K.; Liu, R.; Zhang, Y.; Guan, X. Characteristics of two cedarwood essential oil emulsions and their antioxidant and antibacterial activities. Food Chem. 2021, 346, 128970. [Google Scholar] [CrossRef] [PubMed]
- Zouirech, O.; Alyousef, A.A.; Barnossi, A.E.; Moussaoui, A.E.; Bourhia, M.; Salamatullah, A.M.; Ouahmane, L.; Giesy, J.P.; Aboul-soud, M.A.M.; Lyoussi, B.; et al. Phytochemical Analysis and Antioxidant, Antibacterial, and Antifungal Effects of Essential Oil of Black Caraway (Nigella sativa L.) Seeds against Drug-Resistant Clinically Pathogenic Microorganisms. BioMed Res. Int. 2022, 2022, 5218950. [Google Scholar] [CrossRef] [PubMed]



| Name | ADF-C | ADF-H | ADF-A | ADF-F | The Untreated ADF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molar Ratio (%) | µg/mg | Molar Ratio (%) | µg/mg | Molar Ratio (%) | µg/mg | Molar Ratio (%) | µg/mg | Molar Ratio (%) | µg/mg | |
| Glucosamine hydrochloride | 1.74 ± 0.00 a | 6.21 ± 0.01 b | 1.74 ± 0.01 a | 7.36 ± 0.03 a | 1.52 ± 0.00 b | 5.92 ± 0.03 c | 1.73 ± 0.00 a | 6.26 ± 0.01 b | 1.43 ± 0.00 c | 5.50 ± 0.05 d |
| Galactose | 2.99 ± 0.00 c | 8.92 ± 0.01 c | 2.09 ± 0.05 d | 7.36 ± 0.14 d | 2.11 ± 0.00 d | 6.87 ± 0.04 e | 3.44 ± 0.04 a | 10.39 ± 0.10 a | 3.10 ± 0.02 b | 9.98 ± 0.03 b |
| Glucose | 81.83 ± 0.91 c | 244.04 ± 0.07 d | 82.59 ± 0.01 b | 291.36 ± 0.83 a | 84.45 ± 0.02 a | 275.16 ± 2.05 b | 81.79 ± 0.05 c | 247.01 ± 0.20 d | 84.31 ± 0.15 a | 271.16 ± 2.64 c |
| Xylose | 5.00 ± 0.01 c | 12.55 ± 0.00 c | 5.59 ± 0.07 a | 16.45 ± 0.25 a | 4.50 ± 0.02 c | 12.23 ± 0.15 d | 5.62 ± 0.01 a | 14.14 ± 0.02 b | 4.23 ± 0.04 d | 11.34 ± 0.00 e |
| Mannose | 8.40 ± 0.02 a | 25.02 ± 0.01 b | 7.98 ± 0.00 b | 28.15 ± 0.09 a | 7.42 ± 0.01 c | 24.16 ± 0.22 c | 7.41 ± 0.01 c | 22.39 ± 0.01 d | 6.93 ± 0.1 d | 22.27 ± 0.13 d |
| Total | 296.74 ± 0.05 d | 350.68 ± 0.99 a | 324.34 ± 2.49 b | 300.19 ± 0.05 d | 320.25 ± 2.58 c | |||||
| Name | Glucosamine Hydrochloride | Galactose | Glucose | Xylose | Mannose | DPPH | ABTS | FRAP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucosamine hydrochloride | Pearson correlation | 1 | 0.059 | −0.923 * | 0.920 * | 0.786 | 0.422 | −0.606 | 0.864 | |
| Sig. (two-tailed) | 0.925 | 0.025 | 0.027 | 0.115 | 0.479 | 0.279 | 0.059 | |||
| Galactose | Pearson correlation | 0.059 | 1 | −0.421 | 0.029 | −0.216 | 0.491 | 0.034 | −0.053 | |
| Sig. (two-tailed) | 0.925 | 0.48 | 0.963 | 0.728 | 0.401 | 0.956 | 0.933 | |||
| Glucose | Pearson correlation | −0.923 * | −0.421 | 1 | −0.821 | −0.676 | −0.635 | 0.61 | −0.711 | |
| Sig. (two-tailed) | 0.025 | 0.48 | 0.089 | 0.21 | 0.25 | 0.275 | 0.178 | |||
| Xylose | Pearson correlation | 0.920 * | 0.029 | −0.821 | 1 | 0.527 | 0.118 | −0.299 | 0.953 * | |
| Sig. (two-tailed) | 0.027 | 0.963 | 0.089 | 0.361 | 0.851 | 0.625 | 0.012 | |||
| Mannose | Pearson correlation | 0.786 | −0.216 | −0.676 | 0.527 | 1 | 0.638 | −0.930 * | 0.439 | |
| Sig. (two-tailed) | 0.115 | 0.728 | 0.21 | 0.361 | 0.246 | 0.022 | 0.46 | |||
| DPPH | Pearson correlation | 0.422 | 0.491 | −0.635 | 0.118 | 0.638 | 1 | −0.838 | −0.065 | |
| Sig. (two-tailed) | 0.479 | 0.401 | 0.25 | 0.851 | 0.246 | 0.076 | 0.917 | |||
| ABTS | Pearson correlation | −0.606 | 0.034 | 0.61 | −0.299 | −0.930 * | −0.838 | 1 | −0.141 | |
| Sig. (two-tailed) | 0.279 | 0.956 | 0.275 | 0.625 | 0.022 | 0.076 | 0.821 | |||
| FRAP | Pearson correlation | 0.864 | −0.053 | −0.711 | 0.953 * | 0.439 | −0.065 | −0.141 | 1 | |
| Sig. (two-tailed) | 0.059 | 0.933 | 0.178 | 0.012 | 0.46 | 0.917 | 0.821 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Wang, L.; Dong, L.; Yin, M.; Wei, S.; Luo, P. Agrocybe cylindracea Dietary Fiber Modification: Sodium Hydroxide Treatment Outperforms High-Temperature, Cellulase, and Lactobacillus Fermentation. Molecules 2024, 29, 3519. https://doi.org/10.3390/molecules29153519
Kang J, Wang L, Dong L, Yin M, Wei S, Luo P. Agrocybe cylindracea Dietary Fiber Modification: Sodium Hydroxide Treatment Outperforms High-Temperature, Cellulase, and Lactobacillus Fermentation. Molecules. 2024; 29(15):3519. https://doi.org/10.3390/molecules29153519
Chicago/Turabian StyleKang, Jingjing, Li Wang, Ling Dong, Mingyue Yin, Shaofeng Wei, and Peng Luo. 2024. "Agrocybe cylindracea Dietary Fiber Modification: Sodium Hydroxide Treatment Outperforms High-Temperature, Cellulase, and Lactobacillus Fermentation" Molecules 29, no. 15: 3519. https://doi.org/10.3390/molecules29153519
APA StyleKang, J., Wang, L., Dong, L., Yin, M., Wei, S., & Luo, P. (2024). Agrocybe cylindracea Dietary Fiber Modification: Sodium Hydroxide Treatment Outperforms High-Temperature, Cellulase, and Lactobacillus Fermentation. Molecules, 29(15), 3519. https://doi.org/10.3390/molecules29153519








