Advanced Application of Polymer Nanocarriers in Delivery of Active Ingredients from Traditional Chinese Medicines
Abstract
1. Introduction
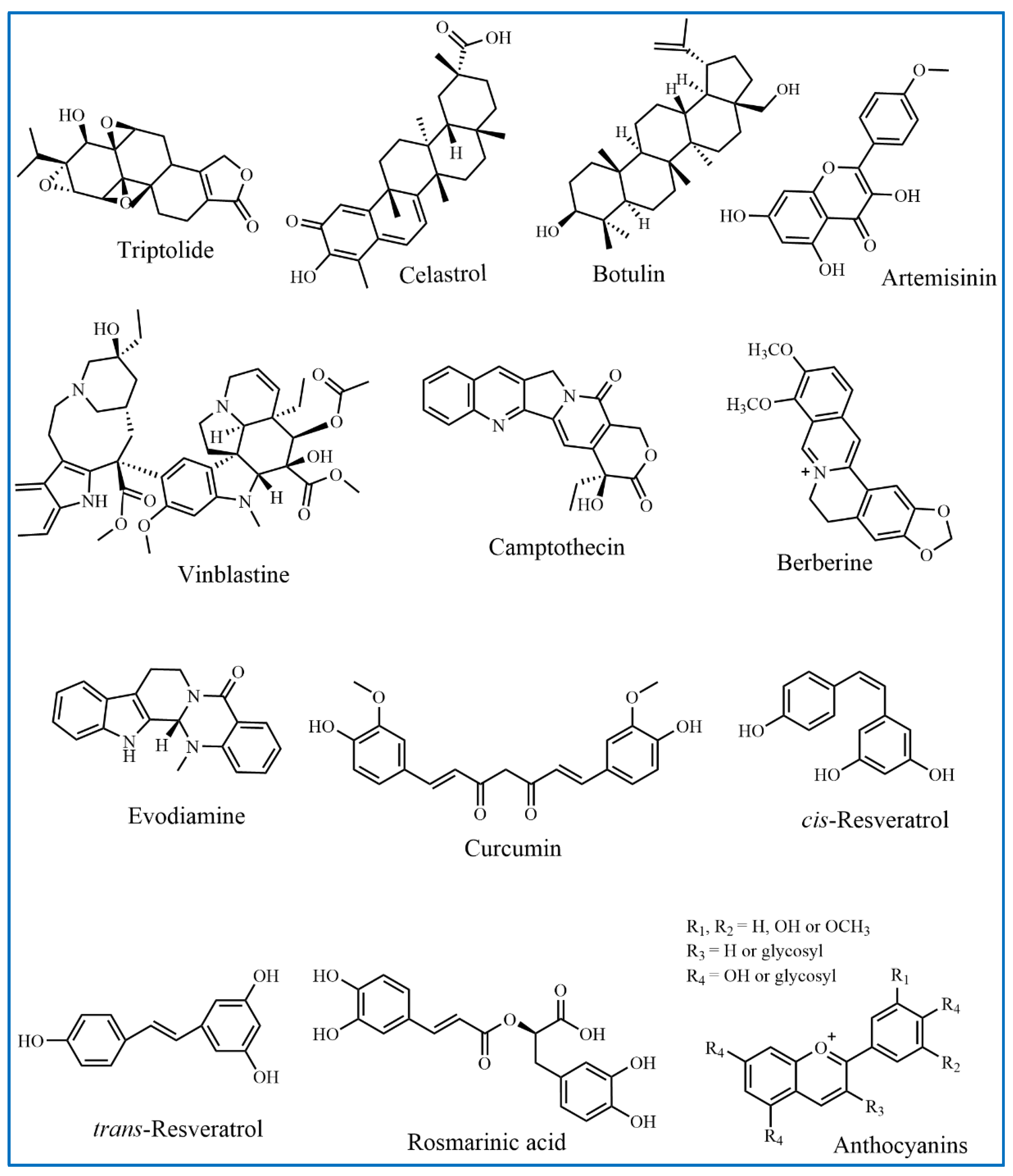
2. Active Ingredients from TCMs
2.1. Terpenoids
2.2. Flavonoids
2.3. Alkaloids
2.3.1. Vinblastine and Its Derivatives
2.3.2. Camptothecin and Its Derivatives
2.3.3. Berberine
2.3.4. Evodiamine
2.4. Polyphenols
2.4.1. Curcumin
2.4.2. Resveratrol
2.4.3. Rosmarinic Acid
2.4.4. Anthocyanins
3. Polymer Nanocarriers in Delivery of Active Ingredients from TCMs
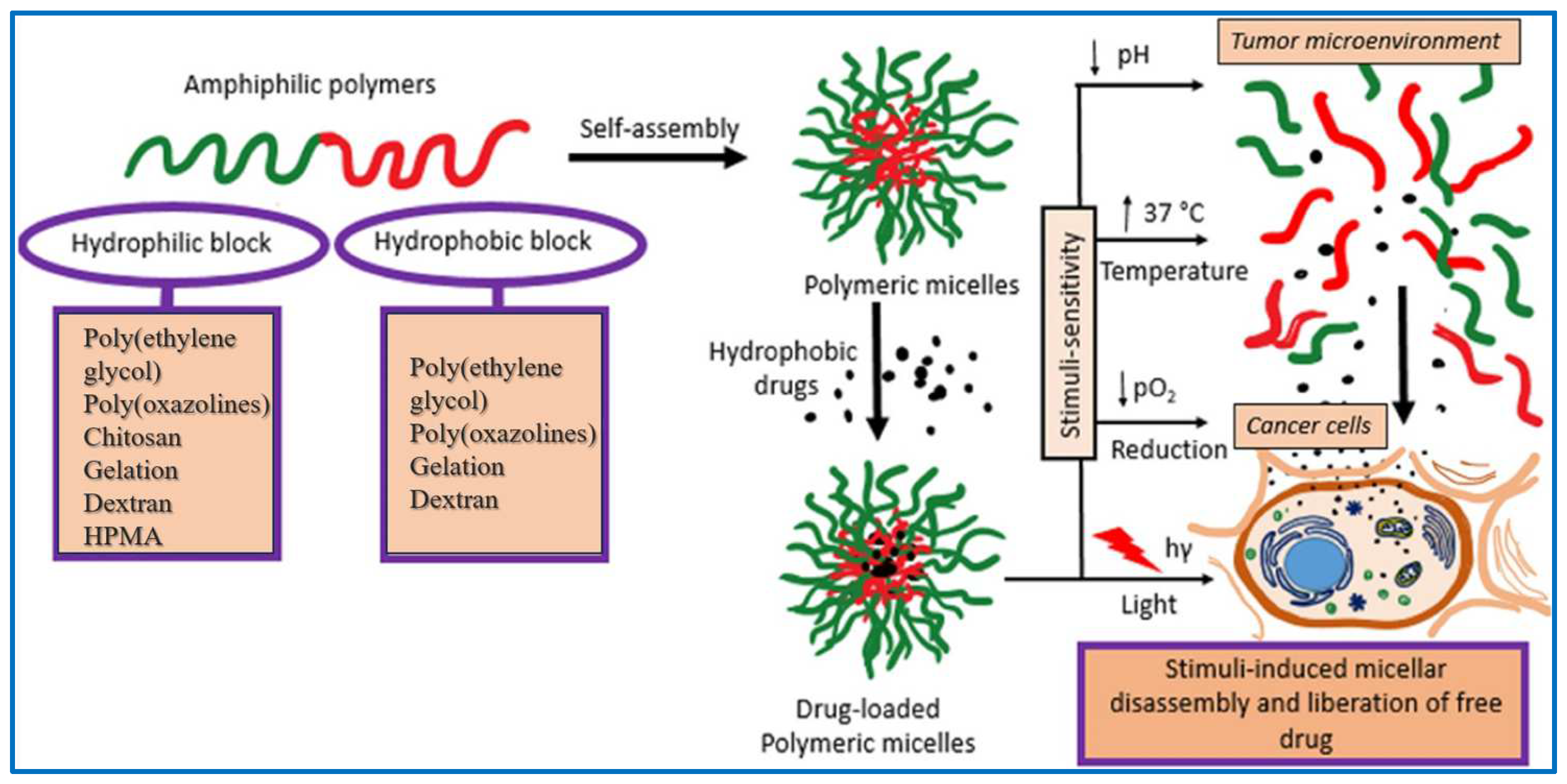
3.1. Polymer Micelles
3.2. Polymer Vesicles
3.3. Polymer Hydrogels
3.4. Polymer Drug Conjugates
4. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.C.; Zhong, X.K.; Zeng, Z.P.; Jiang, J.G.; Li, L.; Zhao, M.M.; Yang, X.Q.; Chen, J.; Zhang, B.S.; Zhao, Q.Z.; et al. Application of targeted drug delivery system in Chinese medicine. J. Control. Release 2009, 138, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Fan, Y.; Wu, Y.; Kebebe, D.; Zhang, B.; Lu, P.; Pi, J.; Liu, Z. Traditional Chinese medicine-combination therapies utilizing nanotechnology-based targeted delivery systems: A new strategy for antitumor treatment. Int. J. Nanomed. 2019, 14, 2029–2053. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Guo, D.; Fernandez-Varo, G.; Zhang, X.; Fu, S.; Ju, S.; Yang, H.; Liu, X.; Wang, Y.C.; Zeng, Y.; et al. The Integration of Nanomedicine with Traditional Chinese Medicine: Drug Delivery of Natural Products and Other Opportunities. Mol. Pharm. 2023, 20, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhou, H.; Wang, Y.F.; Sang, B.S.; Liu, L. Current Policies and Measures on the Development of Traditional Chinese Medicine in China. Pharmacol. Res. 2021, 163, 105187. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv. Colloid. Interface Sci. 2015, 221, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, B.; Fan, Y.; Wang, M.; Kebebe, D.; Li, J.; Liu, Z. Traditional Chinese medicine combined with hepatic targeted drug delivery systems: A new strategy for the treatment of liver diseases. Biomed. Pharmacother. 2019, 117, 109128. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Liu, L.; Bian, Y.; Azami, N.L.B.; Song, J.; Huang, Y.; Sun, M. Traditional Chinese medicine targets on hepatic stellate cells for the treatment of hepatic fibrosis: A mechanistic review. Portal. Hypertens. Cirrhosis 2023, 2, 16–31. [Google Scholar] [CrossRef]
- Xu, W.; Xing, F.J.; Dong, K.; You, C.; Yan, Y.; Zhang, L.; Zhao, G.; Chen, Y.; Wang, K. Application of traditional Chinese medicine preparation in targeting drug delivery system. Drug. Deliv. 2015, 22, 258–265. [Google Scholar] [CrossRef]
- Rao, S.; Lin, Y.; Lin, R.; Liu, J.; Wang, H.; Hu, W.; Chen, B.; Chen, T. Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J. Nanobiotechnolo. 2022, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Wang, Y.; Han, X.; Zhang, Q.; Xie, H.; Chen, J.; Ji, D.; Mao, C.; Lu, T. A Modern Technology Applied in Traditional Chinese Medicine: Progress and Future of the Nanotechnology in TCM. Dose Response 2019, 17, 155932. [Google Scholar] [CrossRef]
- Qiao, L.; Han, M.; Gao, S.; Shao, X.; Wang, X.; Sun, L.; Fu, X.; Wei, Q. Research progress on nanotechnology for delivery of active ingredients from traditional Chinese medicines. J. Mater. Chem. B 2020, 8, 6333–6351. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yao, J.; Zhu, X.; Qi, Y. Nanomedicines: Redefining traditional medicine. Biomed. Pharmacother. 2021, 134, 111103. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Zhao, H.C.; Huang, J.Y.; Wang, H.L.; Li, J.S.; Lu, Y.; Di, L.Q. Challenges and strategies in progress of drug delivery system for traditional Chinese medicine Salviae Miltiorrhizae Radix et Rhizoma (Danshen). Chin. Herb. Med. 2021, 13, 78–89. [Google Scholar] [CrossRef]
- Wei, D.; Yang, H.; Zhang, Y.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Nano-traditional Chinese medicine: A promising strategy and its recent advances. J. Mater. Chem. B 2022, 10, 2973–2994. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Z.; Li, J.; Liu, S. Research progress on nanotechnology of traditional Chinese medicine to enhance the therapeutic effect of osteoarthritis. Drug Deliv. Transl. Res. 2024, 14, 1517–1534. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Wang, J.-F.; Wang, M.-X.; Peng, J.; Kong, X.-D.; Tian, J. Nano-based drug delivery systems for active ingredients from traditional Chinese medicine: Harnessing the power of nanotechnology. Front. Pharmacol. 2024, 15, 1405252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Chen, J.; Gao, T.; Zhang, Y.; Yu, N. Traditional Chinese medicine polysaccharide in nano-drug delivery systems: Current progress and future perspectives. Biomed. Pharmacother. 2024, 173, 116330. [Google Scholar] [CrossRef]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, H.Y.; Shi, J.G.; Zhi, Y.W.; Yuan, T.T.; Cong, C.; Chun, Q.Z.; Xian, J.F. Self-assembled nanodrug delivery systems for anti-cancer drugs from traditional Chinese medicine. Biomater. Sci. 2024, 12, 1662–1692. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Li, C.; Zhang, K.; Hou, Z.; Sun, S.; Yang, L. Recent advances in drug delivery systems based on natural and synthetic polymes for treating obesity. Int. J. Biol. Macromol. 2024, 260, 129311. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.; Garbeva, P.; Vader, L.; van Wezel, G.P.; Dickschat, J.S.; Ulanova, D. Biosynthesis, evolution and ecology of microbial terpenoids. Nat. Prod. Rep. 2022, 39, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X2090355. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Shambilova, G.K.; Bukanova, A.S.; Kalauova, A.S.; Kalimanova, D.Z.; Abilkhairov, A.I.; Makarov, I.S.; Vinogradov, M.I.; Makarov, G.I.; Yakimov, S.A.; Koksharov, A.V.; et al. An Experimental Study on the Solubility of Betulin in the Complex Solvent Ethanol-DMSO. Processes 2024, 12, 1179. [Google Scholar] [CrossRef]
- Makarov, I.; Vinogradov, M.; Gromovykh, T.; Lutsenko, S.; Feldman, N.; Shambilova, G.; Sadykova, V. Antifungal Composite Fibers Based on Cellulose and Betulin. Fib. Polym. 2018, 6, 23. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [PubMed]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 1200. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of In Vitro and In Vivo Antioxidant Activities of Six Flavonoids with Similar Structures. Antioxidants 2020, 9, 5560. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V. Special Issue “Flavonoids and Their Disease Prevention and Treatment Potential”: Recent Advances and Future Perspectives. Molecules 2020, 25, 3460. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hansen, L.G.; Gudich, O.; Viehrig, K.; Lassen, L.M.M.; Schrubbers, L.; Adhikari, K.B.; Rubaszka, P.; Carrasquer-Alvarez, E.; Chen, L.; et al. A microbial supply chain for production of the anti-cancer drug vinblastine. Nature 2022, 609, 341–347. [Google Scholar] [CrossRef]
- Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T. Stereocontrolled Total Synthesis of (+)-Vinblastine. J. Am. Chem. Soc. 2002, 124, 2137–2139. [Google Scholar] [CrossRef]
- Gigant, B.; Wang, C.; Ravelli, R.B.G.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Colby, D.A.; Seto, S.; Va, P.; Tam, A.; Kakei, H.; Rayl, T.J.; Hwang, I.; Boger, D.L. Total Synthesis of Vinblastine, Vincristine, Related Natural Products, and Key Structural Analogues. J. Am. Chem. Soc. 2009, 131, 4904–4916. [Google Scholar] [CrossRef]
- Keglevich, P.; Hazai, L.; Kalaus, G.; Szantay, C. Modifications on the basic skeletons of vinblastine and vincristine. Molecules 2012, 17, 5893–5914. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, L.; Ma, X.; Wang, J.; Yang, J.; Zhou, X.; Yang, Y.; Liu, H. RIP1/RIP3/MLKL-mediated necroptosis contributes to vinblastine-induced myocardial damage. Mol. Cell. Biochem. 2021, 476, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Hamada, S.; Sakai, T.; Koike, H.; Yoshida, M.; Nishida, Y. Efficacy of low-dose chemotherapy with methotrexate and vinblastine for patients with extra-abdominal desmoid-type fibromatosis: A systematic review. Jpn. J. Clin. Oncol. 2019, 50, 419–424. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Phytochemicals Derived from Catharanthus roseus and Their Health Benefits. Technologies 2020, 8, 80. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C. Camptothecin and Taxol: Discovery to Clinic—Thirteenth Bruce F. Cain Memorial Award Lecture1. Cancer Res. 1995, 55, 753–760. [Google Scholar] [PubMed]
- Rivory, L.P.; Robert, J. Molecular, cellular, and clinical aspects of the pharmacology of 20(S)camptothecin and its derivatives. Pharmacol. Ther. 1995, 68, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Baiju, S.; Afzal, A.; Shahin Thayyil, M.; Al-Otaibi, J.S.; Kashif Ali, S. Computational Studies on Anticancerous Camptothecin and it’s derivative Camp-10 by Density Functional Theory. Res. Chem. 2023, 5, 100837. [Google Scholar] [CrossRef]
- Lorence, A.; Nessler, C.L. Camptothecin, over four decades of surprising findings. Phytochemistry 2004, 65, 2735–2749. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, M.K.; Alshehri, M.M.; Alshehri, A.M.; Alshlali, O.M.; Mahzari, A.M.; Almalki, H.H.; Kulaybi, O.Y.; Alghazwni, M.K.; Kamal, M.; Imran, M. Camptothecin loaded nano-delivery systems in the cancer therapeutic domains: A critical examination of the literature. J. Drug Deliv. Sci. Technol. 2023, 79, 104034. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Deng, Y.; Liu, M.; Liao, L.; Dai, X.; Guo, C.; Zhao, X.; He, L.; Peng, C.; Li, Y. The pharmacological activity of berberine, a review for liver protection. Eur. J. Pharmacol. 2021, 890, 173655. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Emran, T.B.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.M.; et al. Berberine as a Potential Anticancer Agent: A Comprehensive Review. Molecules 2021, 26, 12340. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.F.; Wang, B.X.; Li, T.J.; Tashiro, S.I.; Minami, M.; Xing, D.J.; Ikejima, T. Evodiamine, a constituent of Evodiae Fructus, induces anti-proliferating effects in tumor cells. Cancer Sci. 2003, 94, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Gavaraskar, K.; Dhulap, S.; Hirwani, R.R. Therapeutic and cosmetic applications of Evodiamine and its derivatives—A patent review. Fitoterapia 2015, 106, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, C. Evodiamine: A Novel Anti-Cancer Alkaloid from Evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Deng, J.; Zhang, H.; Liang, Z.; Lei, F.; Wang, Y.; Yang, X.; Wang, Z. Design, synthesis and bioactivity evaluation of novel N-phenyl-substituted evodiamine derivatives as potent anti-tumor agents. Bioorg. Med. Chem. 2021, 55, 116595. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, L.; Song, J.; Li, X. Evodiamine: A review of its pharmacology, toxicity, pharmacokinetics and preparation researches. J. Ethnopharmacol. 2020, 262, 113164. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, J.B.; Lee, P.; Kim, S.H. Evodiamine Inhibits Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation. Int. J. Mol. Sci. 2021, 22, 3385. [Google Scholar] [CrossRef]
- Gutierrez-Del-Rio, I.; Lopez-Ibanez, S.; Magadan-Corpas, P.; Fernandez-Calleja, L.; Perez-Valero, A.; Tunon-Granda, M.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 3540. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; Volume 6, pp. 3–44. [Google Scholar]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2016, 20, 1689–1699. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 6540. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2330. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Imran, M.; Moussa, A.Y.; Mostafa, N.M.; El-Shazly, M.; et al. Resveratrol’ biotechnological applications: Enlightening its antimicrobial and antioxidant properties. J. Herbal. Med. 2022, 32, 4560. [Google Scholar] [CrossRef]
- Kung, H.C.; Lin, K.J.; Kung, C.T.; Lin, T.K. Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease. Biomedicines 2021, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochem 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hucherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochem 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3427. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 2344. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules 2021, 26, 4352. [Google Scholar] [CrossRef]
- Tena, N.; Martin, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 3490. [Google Scholar] [CrossRef] [PubMed]
- Mthimkhulu, N.; Mosiane, K.S.; Nweke, E.E.; Balogun, M.; Fru, P.N. Prospects of Delivering Natural Compounds by Polymer-Drug Conjugates in Cancer Therapeutics. Anti-Cancer Agent. Med. Chem. 2022, 22, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Pei, Y.; Wang, Z.; Wang, C.; Hua, D. Recent advances on macromolecular medicinal materials for radioprotection. J. Drug Deliv. Sci. Technol. 2023, 81, 104224. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Dh, A.; Jdr, A.; Avka, B. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval—ScienceDirect. Adv. Drug Del. Rev. 2020, 156, 80–118. [Google Scholar]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, L.; Maryam Hosseinikhah, S.; Vahdat-Lasemi, F.; Sukhorukov, V.N.; Kesharwani, P.; Sahebkar, A. Polymeric micelles paving the Way: Recent breakthroughs in camptothecin delivery for enhanced chemotherapy. Int. J. Pharm. 2024, 659, 124292. [Google Scholar] [CrossRef]
- Cao, Z.; Zuo, X.; Liu, X.; Xu, G.; Yong, K.-T. Recent progress in stimuli-responsive polymeric micelles for targeted delivery of functional nanoparticles. Adv. Colloid. Interface Sci. 2024, 330, 103206. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
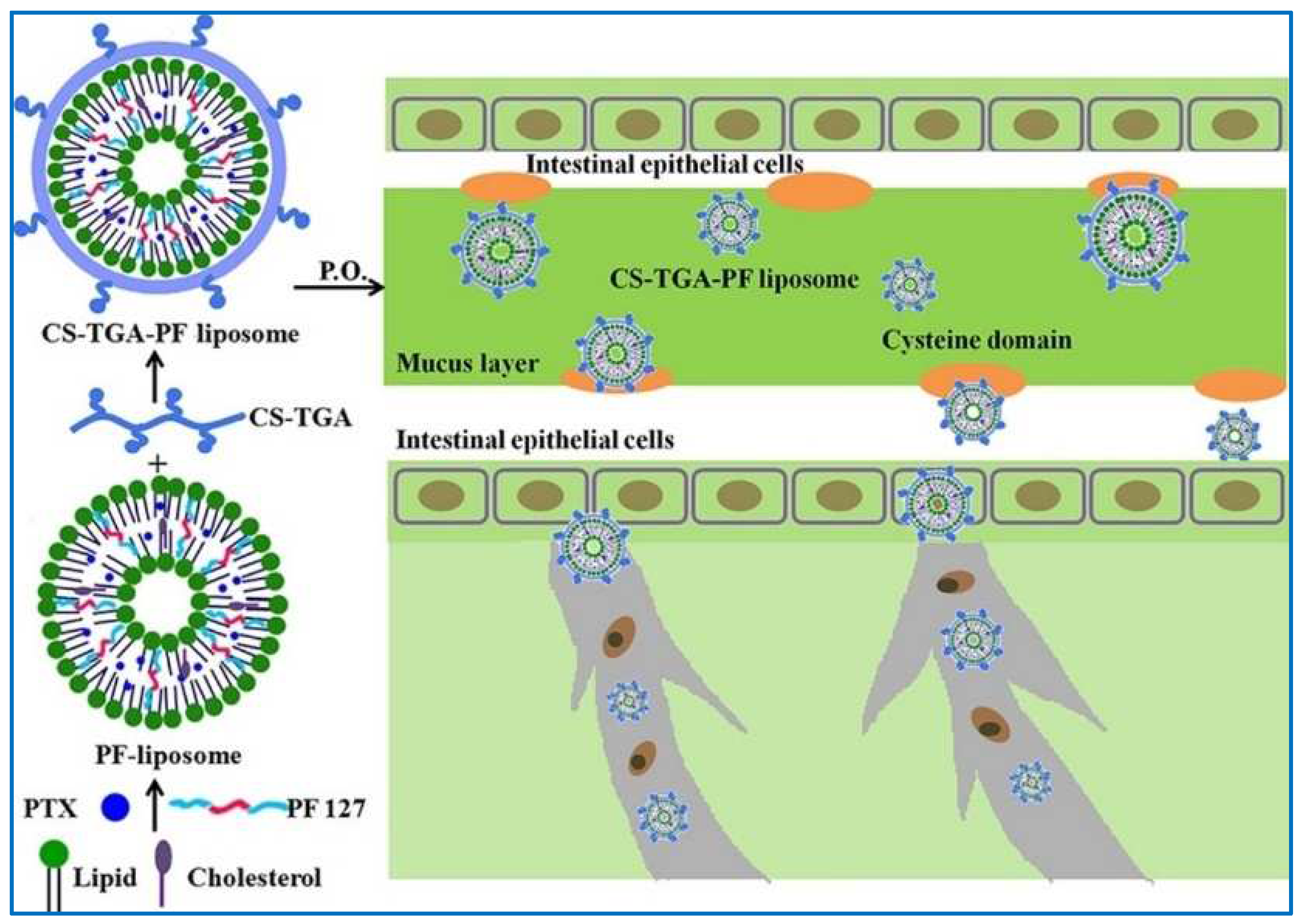
- Wang, X.; Qiu, L.; Wang, X.; Ouyang, H.; Li, T.; Han, L.; Zhang, X.; Xu, W.; Chu, K. Evaluation of intestinal permeation enhancement with carboxymethyl chitosan-rhein polymeric micelles for oral delivery of paclitaxel. Int. J. Pharm. 2020, 573, 118840. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Tanaka, K.; Ouchi, M.; Terashima, T. Amphiphilic Random-Block Copolymer Micelles in Water: Precise and Dynamic Self-Assembly Controlled by Random Copolymer Association. Macromolecules 2022, 55, 178–189. [Google Scholar] [CrossRef]
- Hishida, M.; Kanno, R.; Terashima, T. Hydration State on Poly(ethylene glycol)-Bearing Homopolymers and Random Copolymer Micelles: In Relation to the Thermoresponsive Property and Micellar Structure. Macromolecules 2023, 56, 7587–7596. [Google Scholar] [CrossRef]
- Chen, W.; Liu, P. Dendritic polymer prodrug-based unimolecular micelles for pH-responsive co-delivery of doxorubicin and camptothecin with synergistic controlled drug release effect. Colloids Surf. B Biointerfaces 2024, 238, 113906. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Albekairi, N.A.; Al-Enazy, S.; Ali, S.; Rytting, E. Transport of digoxin-loaded polymeric nanoparticles across BeWo cells, an in vitro model of human placental trophoblast. Ther. Deliv. 2015, 6, 1325–1334. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Ji, J.; Li, L.; Zhai, G. Redox-responsive nanoparticles based on Chondroitin Sulfate and Docetaxel prodrug for tumor targeted delivery of Docetaxel. Carbohydr. Polym. 2021, 255, 117393. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xu, J.; Ni, C. Preparation of redox responsive modified xanthan gum nanoparticles and the drug controlled release. Int. J. Polym. Mater. Polym. 2021, 70, 994–1001. [Google Scholar] [CrossRef]
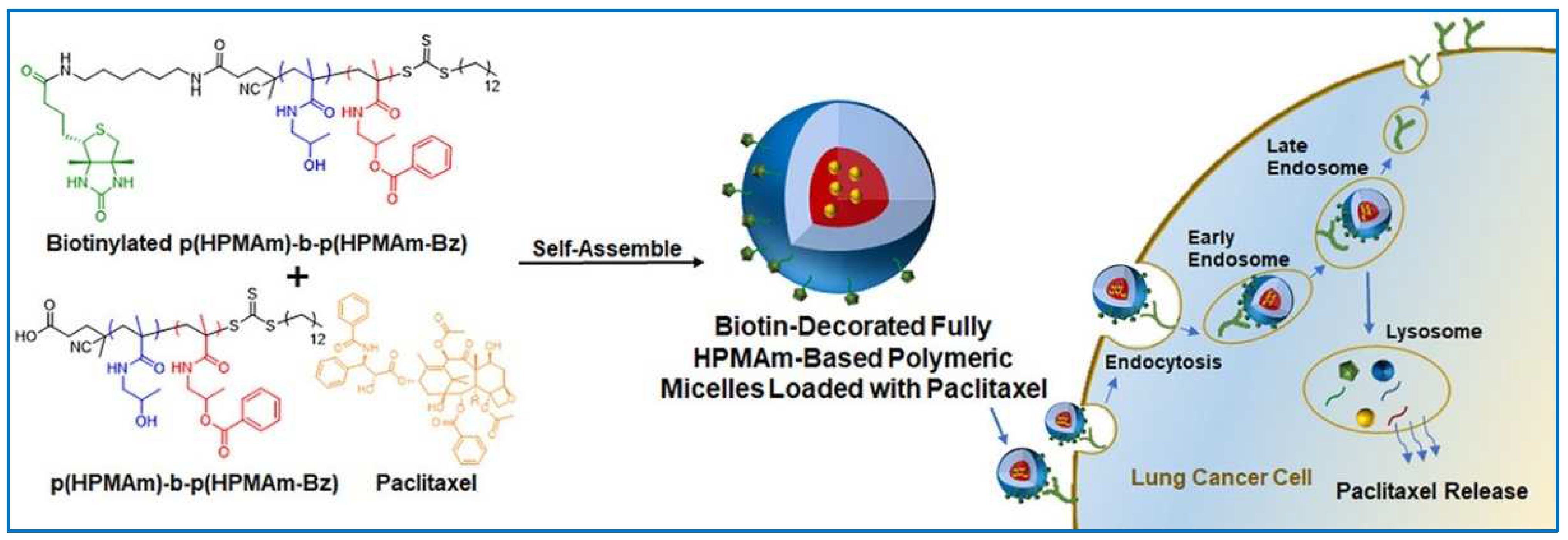
- Wang, Y.; van Steenbergen, M.J.; Beztsinna, N.; Shi, Y.; Lammers, T.; van Nostrum, C.F.; Hennink, W.E. Biotin-decorated all-HPMA polymeric micelles for paclitaxel delivery. J. Control. Release 2020, 328, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer Vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, E.S.; Ko, H.; Son, S.; Kim, S.H.; Lee, C.H.; Na, H.-K.; Shin, J.M.; Park, J.H. Stimuli-Responsive Polymeric Nanomedicine for Enhanced Cancer Immunotherapy. Chem. Mater. 2024, 36, 1088–1112. [Google Scholar] [CrossRef]
- Shu, L.; Gong, Y.; Lin, M.; Sun, J.; Chen, X. Advanced coacervation-driven nanoscale polymeric assemblies for biomedical applications. Appl. Phys. Rev. 2024, 11, 3490. [Google Scholar] [CrossRef]
- Shen, H.; Eisenberg, A. Control of Architecture in Block-Copolymer Vesicles. Angew. Chem. Int. Ed. 2000, 39, 3310–3312. [Google Scholar] [CrossRef]
- Elmahboub, Y.S.M.; Elkordy, A.A. Polymeric nanoparticles: A promising strategy for treatment of Alzheimer’s disease. J. Taibah Univ. Med. Sci. 2024, 19, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Singh, R.; Tonk, M. Polymersomes as Next Generation Nanocarriers for Drug Delivery: Recent Advances, Patents, Synthesis and Characterization. Curr. Nanosci. 2024, 20, 753–768. [Google Scholar] [CrossRef]
- Du, J.; O’Reilly, R.K. Advances and challenges in smart and functional polymer vesicles. Soft Matter 2009, 5, 3544–3561. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuis, R.P.; Rutjes, F.P.J.T.; van Hest, J.C.M. Polymeric vesicles in biomedical applications. Polym. Chem. 2011, 2, 1234. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surf. B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.-P.; Song, R.-X.; Wang, T.; Sun, M.-J.; Liu, Y.; Chen, X.-G. Inducing sustained release and improving oral bioavailability of curcumin via chitosan derivatives-coated liposomes. Int. J. Biol. Macromol. 2018, 120, 702–710. [Google Scholar] [CrossRef]
- Cerritelli, S.; Velluto, D.; Hubbell, J.A. PEG-SS-PPS: Reduction-Sensitive Disulfide Block Copolymer Vesicles for Intracellular Drug Delivery. Biomacromolecules 2007, 8, 1966–1972. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Huang, X.; Pan, J.; Wang, Y.; Zhou, S. Development of a pH-responsive polymersome inducing endoplasmic reticulum stress and autophagy blockade. Sci. Adv. 2020, 6, eabb8725. [Google Scholar] [CrossRef]
- Ahmed, F.; Pakunlu, R.I.; Brannan, A.; Bates, F.; Minko, T.; Discher, D.E. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J. Control. Release 2006, 116, 150–158. [Google Scholar] [CrossRef]
- Charoensit, P.; Pompimon, W.; Khorana, N.; Sungthongjeen, S. Effect of amide linkage of PEG-lipid conjugates on the stability and cytotoxic activity of goniodiol loaded in PEGylated liposomes. J. Drug Deliv. Sci. Technol. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Kolter, M.; Wittmann, M.; Köll-Weber, M.; Süss, R. The suitability of liposomes for the delivery of hydrophobic drugs—A case study with curcumin. Eur. J. Pharm. Biopharm. 2019, 140, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, L.; Cai, L.; Zhang, X.; Zheng, H.; Deng, C.; Duan, X.; Zhao, X.; Wei, Y.; Chen, L. Preparation, characterization, pharmacokinetics, and bioactivity of honokiol-in-hydroxypropyl-β-cyclodextrin-in-liposome. J. Pharm. Sci. 2011, 100, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Carbone, C.; Ennas, G.; Puglisi, G.; Fadda, A.M.; Manconi, M. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid. Interface Sci. 2016, 461, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, T.; Wei, S.; Zhou, C.; Lan, Y.; Cao, A.; Yang, J.; Wang, W. Mucus adhesion- and penetration-enhanced liposomes for paclitaxel oral delivery. Int. J. Pharm. 2018, 537, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Yadollahi, M. Bio-nanocomposite Polymer Hydrogels Containing Nanoparticles for Drug Delivery: A Review. Regen. Eng. Transl. Med. 2021, 7, 129–146. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Review on Magnetic Natural Polymer Constructed Hydrogels as Vehicles for Drug Delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef]
- Raeisi, A.; Farjadian, F. Commercial hydrogel product for drug delivery based on route of administration. Front. Chem. 2024, 12, 1336171. [Google Scholar] [CrossRef]
- Shahid, N.; Erum, A.; Hanif, S.; Malik, S.N.; Tulain, R.U.; Syed, A.M. Nanocomposite Hydrogels-A Promising Approach towards Enhanced Bioavailability and Controlled Drug Delivery. Curr. Pharm. Des. 2024, 30, 48–62. [Google Scholar] [CrossRef]
- Garg, A.; Agrawal, R.; Singh Chauhan, C.; Deshmukh, R. In-situ gel: A smart carrier for drug delivery. Int. J. Pharm. 2024, 652, 123819. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Qiao, T.; Wang, Y.-Q.; Cui, Y.-L.; Wang, Q.-S. Hydrogen bond-enhanced nanogel delivery system for potential intranasal therapy of Parkinson’s disease. Mater. Design 2022, 219, 110741. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, W.; Shen, S.; Wei, L. Chitosan derivative-based mussel-inspired hydrogels as the dressings and drug delivery systems in wound healing. Cellulose 2021, 28, 11429–11450. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Wang, B.; Wei, L. Chitosan derivative-based mussel-inspired hydrogels used as dressings for infectious wound healing. Eur. Polym. J. 2023, 196, 112315. [Google Scholar] [CrossRef]
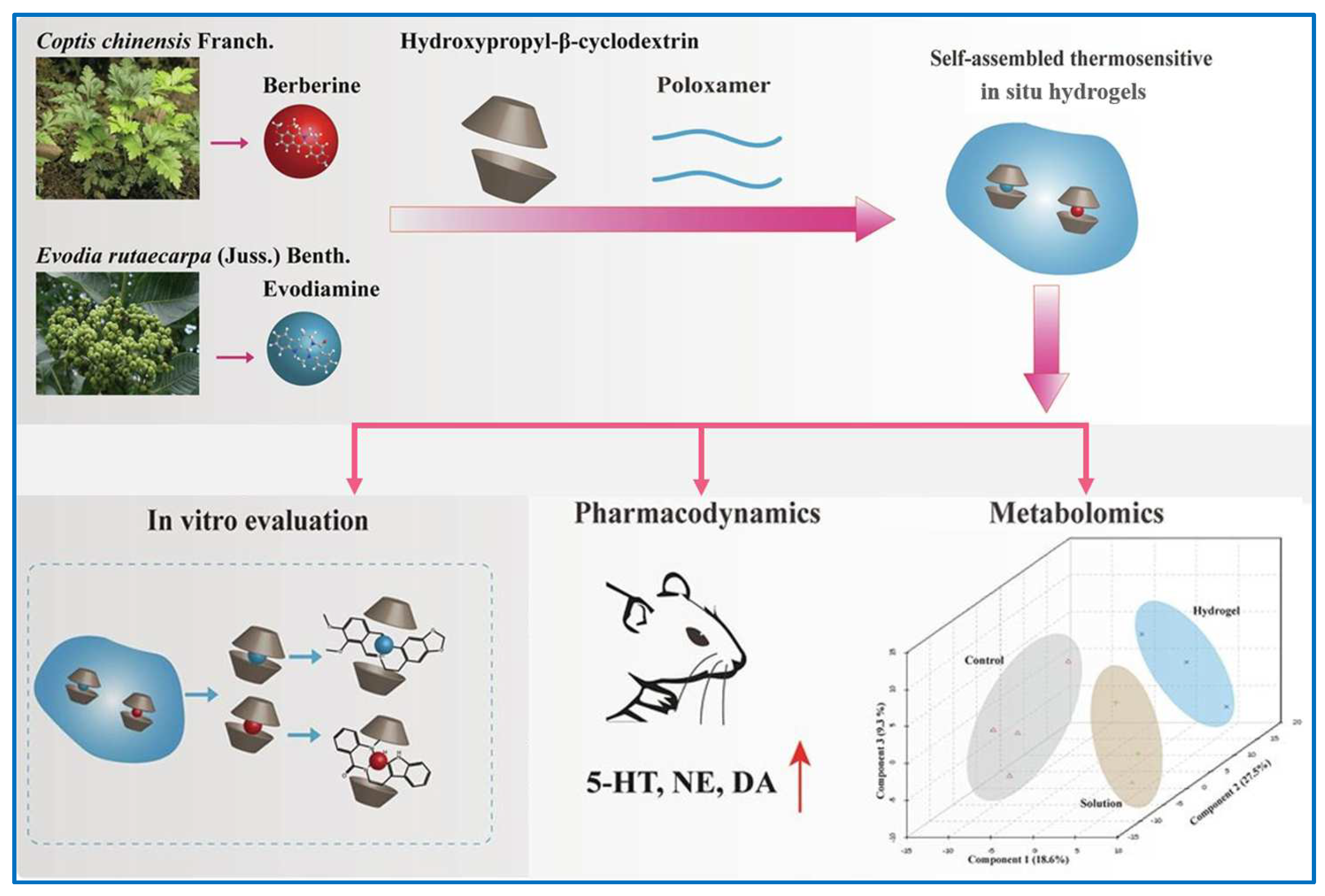
- Wang, Q.S.; Li, K.; Gao, L.N.; Zhang, Y.; Lin, K.M.; Cui, Y.L. Intranasal delivery of berberine via in situ thermoresponsive hydrogels with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 2020, 8, 2853–2865. [Google Scholar] [CrossRef]
- Xu, D.; Qiu, C.; Wang, Y.; Qiao, T.; Cui, Y.L. Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int. J. Pharm. 2021, 603, 120667. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Wang, Z.; Yang, X.; Yu, X.; Lu, Y.; Deng, L.; Cui, W. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 2019, 11, 81. [Google Scholar] [CrossRef]
- Cheng, R.; Yan, Y.; Liu, H.; Chen, H.; Pan, G.; Deng, L.; Cui, W. Mechanically enhanced lipo-hydrogel with controlled release of multi-type drugs for bone regeneration. Appl. Mater. Today 2018, 12, 294–308. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Buyana, B.; Aderibigbe, B.A. Polymer-Drug Conjugate, a Potential Therapeutic to Combat Breast and Lung Cancer. Pharmaceutics 2020, 12, 2345. [Google Scholar] [CrossRef]
- Li, C.; Wallace, S. Polymer-drug conjugates: Recent development in clinical oncology. Adv. Drug Del. Rev. 2008, 60, 886–898. [Google Scholar] [CrossRef]
- Jain, V.K.; Jain, K.; Popli, H. Conjugates of amphotericin B to resolve challenges associated with its delivery. Expert. Opin Drug Del. 2024, 21, 187–210. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, Y.; Dheer, D.; Shankar, R. Stimuli responsiveness of recent biomacromolecular systems (concept to market): A review. Int. J. Biol. Macromol. 2024, 261, 129901. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zhu, L. Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate. J. Control. Release 2015, 212, 94–102. [Google Scholar] [CrossRef]
- Liu, Y.; Khan, A.R.; Du, X.; Zhai, Y.; Tan, H.; Zhai, G. Progress in the polymer-paclitaxel conjugate. J. Drug Deliv. Sci. Technol. 2019, 54, 4320. [Google Scholar] [CrossRef]
- Wang, H.; Nie, C.; Luo, M.; Bai, Q.; Yao, Z.; Lv, H.; Chen, B.; Wang, J.; Xu, W.; Wang, S.; et al. Novel GSH-responsive prodrugs derived from indole-chalcone and camptothecin trigger apoptosis and autophagy in colon cancer. Bioorg. Chem. 2024, 143, 107056. [Google Scholar] [CrossRef]
- Elvira, C.; Gallardo, A.; Roman, J.; Cifuentes, A. Covalent Polymer-Drug Conjugates. Biomacromolecules 2005, 10, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Javia, A.; Vanza, J.; Bardoliwala, D.; Ghosh, S.; Misra, L.A.; Patel, M.; Thakkar, H. Polymer-drug conjugates: Design principles, emerging synthetic strategies and clinical overview. Int. J. Pharm. 2022, 623, 121863. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, X.; Zhang, H.; Sun, L.; Pan, D.; Gong, Q.; Gu, Z.; Luo, K. Enzyme-sensitive biodegradable and multifunctional polymeric conjugate as theranostic nanomedicine. Appl. Mater. Today 2018, 11, 207–218. [Google Scholar] [CrossRef]
- Shi, L.; Song, X.-B.; Wang, Y.; Wang, K.-T.; Liu, P.; Pang, B.; Wei, F.-C. Docetaxel-conjugated monomethoxy-poly(ethylene glycol)-b-poly(lactide) (mPEG-PLA) polymeric micelles to enhance the therapeutic efficacy in oral squamous cell carcinoma. RSC Adv. 2016, 6, 42819–42826. [Google Scholar] [CrossRef]
- Kumar, A.; Lale, S.V.; Aji Alex, M.R.; Choudhary, V.; Koul, V. Folic acid and trastuzumab conjugated redox responsive random multiblock copolymeric nanocarriers for breast cancer therapy: In-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2017, 149, 369–378. [Google Scholar] [CrossRef]
- Hong, W.; Guo, F.; Yu, N.; Ying, S.; Lou, B.; Wu, J.; Gao, Y.; Ji, X.; Wang, H.; Li, A.; et al. A Novel Folic Acid Receptor-Targeted Drug Delivery System Based on Curcumin-Loaded beta-Cyclodextrin Nanoparticles for Cancer Treatment. Drug Des. Devel. Ther. 2021, 15, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Mosiane, K.S.; Nweke, E.E.; Balogun, M.; Fru, P.N. Polyethyleneglycol-Betulinic Acid (PEG-BA) Polymer-Drug Conjugate Induces Apoptosis and Antioxidation in a Biological Model of Pancreatic Cancer. Polymers 2023, 15, 3456. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Q.; Li, L.; Chen, S.; Zhao, Y.; Hu, Y.; Wang, L.; Lan, X.; Zhong, L.; Lu, D. Gastrodin modified polyurethane conduit promotes nerve repair via optimizing Schwann cells function. Bioact. Mater. 2022, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, J.; Wan, J.; Li, Z. Disulfide based prodrugs for cancer therapy. RSC Adv. 2020, 10, 24397–24409. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Jamal Tabatabaei Rezaei, S.; Nedaei, K.; Ramazani, A.; Ramazani, A. PEGylated Redox/pH Dual-Responsive Dendritic Prodrugs Based on Boltorn® H40 for Tumor Triggered Paclitaxel Delivery. ChemistrySelect 2023, 8, e202204246. [Google Scholar] [CrossRef]
- Cuervo, N.Z.; Grandvaux, N. Redox proteomics and structural analyses provide insightful implications for additional non-catalytic thiol-disulfide motifs in PDIs. Redox Biol. 2023, 59, 102583. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Wang, H.; Li, Z.; Chen, J.; Zhang, Z.; Zeng, H.; Yu, X.; Yang, X.; Yang, X.; et al. Hydroxyethyl starch-folic acid conjugates stabilized theranostic nanoparticles for cancer therapy. J. Control. Release 2023, 353, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pillow, T.H.; Ma, Y.; Cruz-Chuh, J.d.; Kozak, K.R.; Sadowsky, J.D.; Lewis Phillips, G.D.; Guo, J.; Darwish, M.; Fan, P.; et al. Linker Immolation Determines Cell Killing Activity of Disulfide-Linked Pyrrolobenzodiazepine Antibody–Drug Conjugates. ACS Med. Chem. Lett. 2016, 7, 988–993. [Google Scholar] [CrossRef]
- Bach, R.D.; Dmitrenko, O.; Thorpe, C. Mechanism of Thiolate-Disulfide Interchange Reactions in Biochemistry. J. Org. Chem. 2008, 73, 12–21. [Google Scholar] [CrossRef]
- Dordevic, S.; Medel, M.; Hillaert, J.; Masia, E.; Conejos-Sanchez, I.; Vicent, M.J. Critical Design Strategies Supporting Optimized Drug Release from Polymer-Drug Conjugates. Small 2024, 20, e2303157. [Google Scholar] [CrossRef] [PubMed]
- Sindhi, K.; Kanugo, A. Recent Developments in Nanotechnology and Immunotherapy for the Diagnosis and Treatment of Pancreatic Cancer. Curr. Pharm. Biotechnol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Wang, A.; Yang, T.; Ke, H.; Tang, Y.; Wu, H.; Chen, H. Harness arsenic in medicine: Current status of arsenicals and recent advances in drug delivery. Expert Opin. Drug Deli. 2024. ahead of print. [Google Scholar] [CrossRef]


| Nanocarriers | Materials | TCMs | Advantages | Reference |
|---|---|---|---|---|
| PMs | Carboxymethyl chitosan-rhein | PTX | A drug loading capacity of 35.46 ± 1.07%; improved absorption of PTX in the intestine with negligible intestinal villi injury | [88] |
| PEGylated PLGA polymer (Resomer® RGPd50105 and RGPd5055) | Digoxin | Cross BeWo b30 cell monolayers easily; high encapsulation efficiency and sustained release; increased the permeability of digoxin | [93] | |
| Chondroitin sulfate; | DTX | High permeability and cytotoxicity of Cys-DTX prodrug, targeting transportation of encapsulated redox-responsive Cys-DTX prodrug; improved permeability in tumor tissues, enhanced cytotoxicity, and decreased side effects | [94] | |
| Cystamine; Xanthan gum | Resveratrol | Good redox responsiveness; biocompatible; controlled in vitro drug release similar to the internal environment of tumor cells | [95] | |
| Biotin modified p(HPMAm)-b-p(HPMAm-Bz) | PTX | Controlled structure of polymers and PM size; high efficiency in A549 lung cancer cells overexpressing and pretty low internalization; stronger cytotoxicity in A549 cells | [96] | |
| Hyaluronate-chitosan-liposomes | Quercetin | High stability and skin permeation; controlled release behavior | [107] | |
| Carboxymethyl chitosan and chitosan (TMC)-coated liposomes | Curcumin | High stability and safety; favorable gastric acid tolerance; satisfactory biocompatibility and oral absolute bioavailability | [108] | |
| Polymer vesicles | PEG-b-PPS | Hydroxychloroquine; tunicamycin | Simultaneously inducing endoplasmic reticulum stress and autophagic flux blockade; inhibiting tumor metastasis | [110] |
| PEG-PLA/PEG-PBD hybrid vesicles | PTX | Thick hydrophobic membrane and an aqueous lumen to efficiently carry both hydrophobic and hydrophilic drugs; higher maximum tolerated dose; controlled drug release; two-fold higher cell death in tumors than free drug | [111] | |
| Chitosan and liposomes | Quercetin | Impressive encapsulation efficiency; pH-dependent release of quercetin | [115] | |
| PEGylated liposomes | Goniodiol | Reduced leakage and degradation of goniodiol; enhanced stability and cytotoxicity | [112] | |
| Phospholipid dioleoylphosphatidylcholine and liposomes | Curcumin | Pronounced solubility and stability of loaded curcumin; rapid release; high in vivo efficacy | [113] | |
| Chitosan-thioglycolic acid-Pluronic F127 | PTX | Well-designed formulation; controlled-release profile; enhanced GI mucosa uptake and intestine drug absorption | [116] | |
| Polymer hydrogels | Catechol-modified chitosan | Panax notoginseng or flavones | Enhanced bonding strength and drug loading efficiency; porous structure; sustained cumulative release rates; high wound healing rate | [123,124] |
| Hydroxylpropyl-β-cyclodextrin and poloxamers P407 and P188 | BBR and EVO | High drug loading efficiency and total cumulative release rate; thermosensitive; higher intranasal administration | [125,126] | |
| SH-PEG, Ag+ and liposomes | Cholesterol | Injectable hydrogel; enhanced antibacterial, bone growth, and self-healing properties | [127] | |
| Methacrylate-modified gelatin and liposomes | PTX | Enhanced the mechanical strength and controlled drugs’ release; enabling complex bone healing | [128] | |
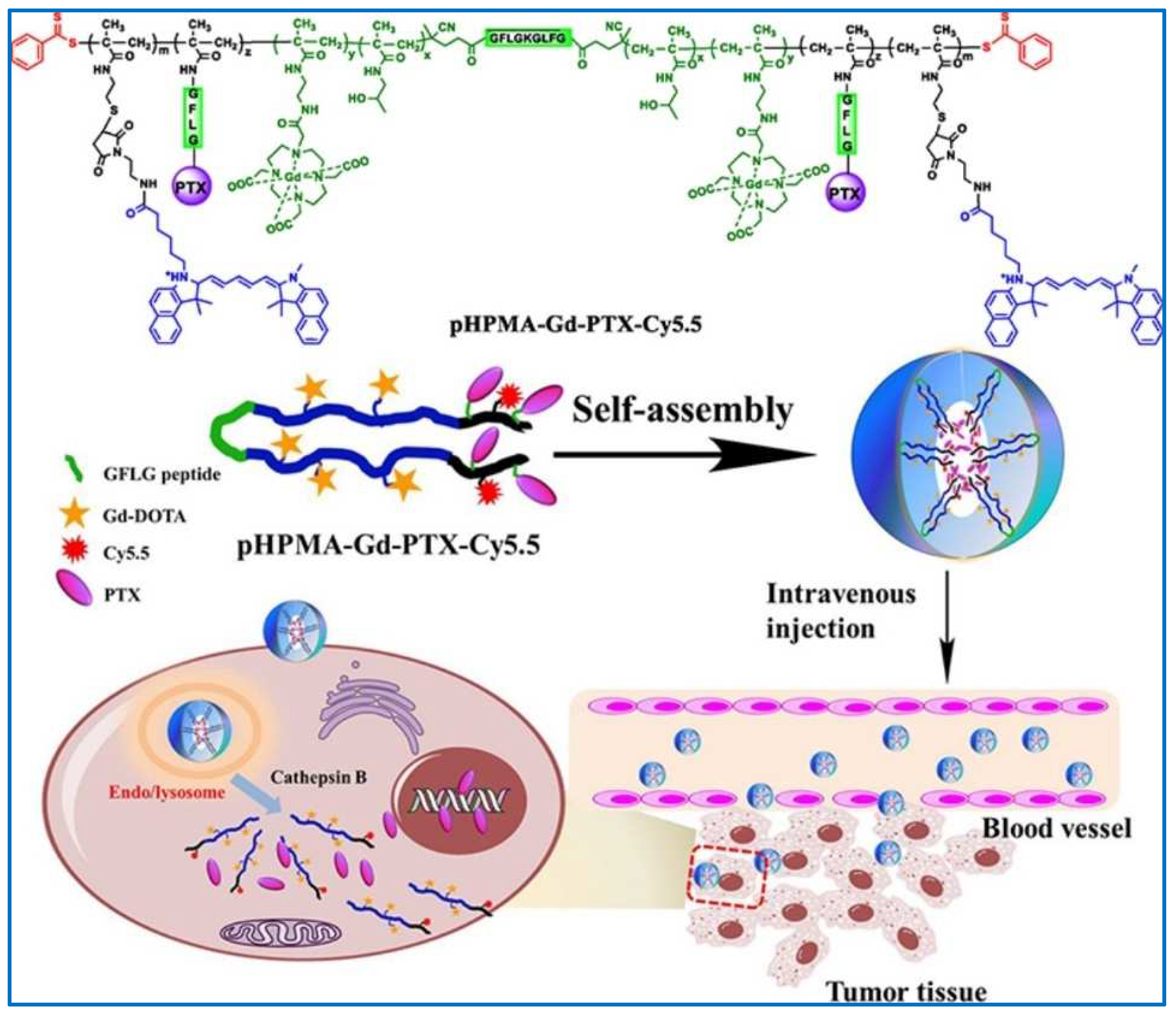
| PDCs | pHPMA-Gd-PTX-Cy5.5 | PTX | Enhanced imaging capacity of the theranostic nanomedicine; residence time significantly prolonged; increased accumulation at the tumor site; inhibited proliferation and induced apoptosis of the 4T1 murine breast cancer cells | [138] |
| Indole-chalcone derivatives | VBL, CPT and PTX | Facile structure design; pronounced anti-proliferative activity; wide drug-resistant variants; lower cytotoxicity to human normal cells; enhanced intracellular uptake | [135] | |
| mPEG-PLA | DTX | Clear spherical shape; sustained release of the drug; time-dependent anticancer effect in the squamous cancer cells; significantly higher cancer cell apoptosis in HSC-3 cancer cells; controlled the tumor progression in HSC-3 cancer cells | [139] | |
| β-Cyclodextrin-polycaprolactone and FA | Curcumin | Well-designed PDC structure; higher curcumin release rate; reduced tumor volume | [141] | |
| Polyethylene glycol-BA (PEG-BA) | BA | Increased NFκB/p65 protein expression; comparable antioxidant potential with ascorbic acid; improved reduction in hydroperoxide levels | [142] | |
| Polyurethane | Gastrodin | Tunable gastrodin content; leveraging the bioactivity of gastrodin; mitigated inflammation; enhanced nerve repair process | [143] | |
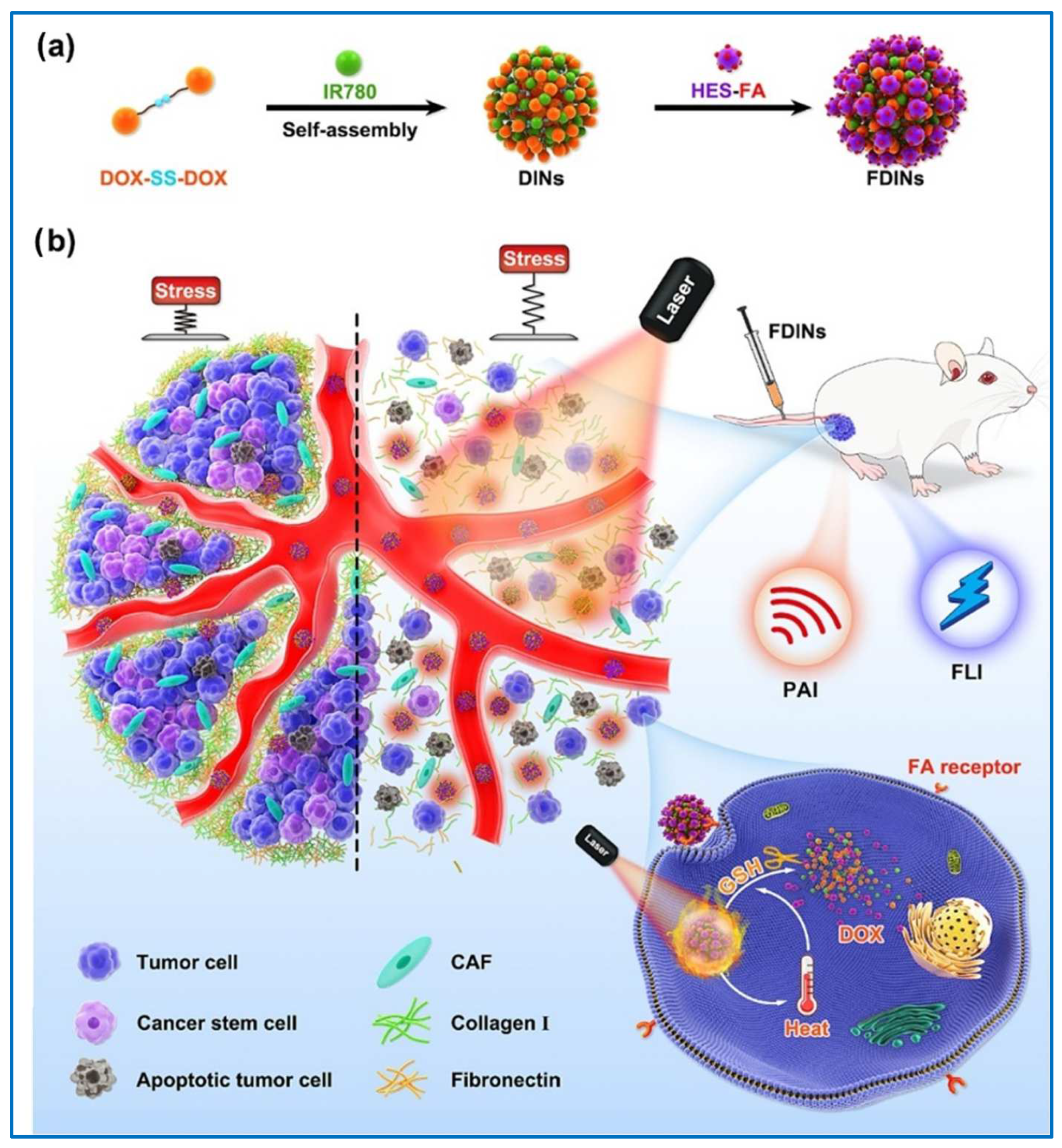
| Hydroxyethyl starch-FA | FA | Redox-sensitive; targets triple-negative breast cancer 4T1 tumor tissues | [147] | |
| PEGylated-PTX and polyester | PTX | Controlled chemical structure; redox-sensitive; controlled drug release rate; excellent stability and safety | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Niu, J.; Xu, L.; Xu, J. Advanced Application of Polymer Nanocarriers in Delivery of Active Ingredients from Traditional Chinese Medicines. Molecules 2024, 29, 3520. https://doi.org/10.3390/molecules29153520
Zhai Z, Niu J, Xu L, Xu J. Advanced Application of Polymer Nanocarriers in Delivery of Active Ingredients from Traditional Chinese Medicines. Molecules. 2024; 29(15):3520. https://doi.org/10.3390/molecules29153520
Chicago/Turabian StyleZhai, Zhiyuan, Jianda Niu, Liguo Xu, and Jinbao Xu. 2024. "Advanced Application of Polymer Nanocarriers in Delivery of Active Ingredients from Traditional Chinese Medicines" Molecules 29, no. 15: 3520. https://doi.org/10.3390/molecules29153520
APA StyleZhai, Z., Niu, J., Xu, L., & Xu, J. (2024). Advanced Application of Polymer Nanocarriers in Delivery of Active Ingredients from Traditional Chinese Medicines. Molecules, 29(15), 3520. https://doi.org/10.3390/molecules29153520







