Electrochemical Investigation of the Stability of Poly-Phosphocholinated Liposomes
Abstract
1. Introduction
- (1)
- Voltammogram that is the same as that obtained on the bare electrode. This is when the liposomes do not form any kind of lipid layer on the surface, do not hamper the electron transfer between the hydrophilic electroactive species and the electrode, as well as do not change the effective area for electron transfer of the electrode.
- (2)
- Similar voltammograms as those obtained on bare electrodes will also be obtained when the liposomes are physically adsorbed on the electrode surface but do not fuse and spread and do not form lipid layer regions on the electrode surface. In this case, the effective area of the electrode will not be altered or altered to a small extent, bearing in mind the spherical shape of the liposomes and the small contact area between them and the surface.
- (3)
- Voltammogram with oxidation and reduction peaks that are at the same potentials but the peak currents are smaller [18,24]. This happens if the electrodes are partially covered with a lipid film or if a lipid film with defects (or a kind of pores) is formed [18]. In this case, the hydrophilic electroactive species reach the electrode surface in the uncovered regions (or through the pores). Because the electron transfer is not hampered in these areas, the peak potentials are not displaced, and the voltammogram has the typical shape of those of diffusion-limited reactions. However, because the effective area of the electrode is now smaller, the peak currents are smaller.
- (4)
- Voltammogram with peaks that are smaller and displaced to greater peak overpotentials [19]. This happens when the electrode is covered with intact lipid film with no defects at all or with pinhole defects [25]. In this case, the hydrophilic electroactive species cannot reach the electrode surface, and the electron transfer, if any, can happen only via the tunneling effect through the lipid phase. This leads to a significant decrease in the rate constants, and the voltammogram has the typical shape obtained from kinetically limited reactions. This situation can occur with either films with lipids physically adsorbed on the surface [19] or films with lipids chemically adsorbed on the surface [25].
2. Results and Discussion
2.1. Gold Electrodes
2.2. Carbon Electrodes
3. Materials and Methods
3.1. Preparation of Liposomes
3.2. Characterization of the Liposomes—Sizing by Dynamic Light Scattering (DLS) and Zeta Potential Measurements
3.3. Electrodes
3.4. Electrochemical Measurements
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramot, Y.; Dolkart, O.; Steiner, M.; Jahn, S.; Goldberg, R.; Cacical, O.; Lavie, Y.; Ezov, N.; Agar, G.; Nyska, A. Preclinical In Vivo Safety of Poly-Phosphorylated Superlubrication Vectors for the Treatment of Osteoarthritis. Toxicol. Pathol. 2022, 50, 787–792. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, Y.; Tao, Y.; Lin, W.; Wang, P. Intra-Articular Drug Delivery for Osteoarthritis Treatment. Pharmaceutics 2021, 13, 2166. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.; Schroeder, A.; Silbert, G.; Turjeman, K.; Barenholz, Y.; Klein, J. Boundary lubricants with exceptionally low friction coefficients based on 2D close-packed phosphatidylcholine liposomes. Adv. Mater. 2011, 23, 3517–3521. [Google Scholar] [CrossRef] [PubMed]
- Dekkiche, F.; Corneci, M.C.; Trunfio-Sfarghiu, A.M.; Munteanu, B.; Berthier, Y.; Kaabar, W.; Rieu, J.P. Stability and tribological performances of fluid phospholipid bilayers: Effect of buffer and ions. Colloids Surf. B Biointerfaces 2010, 80, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gaisinskaya-Kipnis, A.; Kampf, N.; Klein, J. Origins of hydration lubrication. Nat. Commun. 2015, 6, 6060. [Google Scholar] [CrossRef] [PubMed]
- Raviv, U.; Klein, J. Fluidity of Bound Hydration Layers. Science 2002, 297, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Goldberg, R.; Klein, J. Poly-phosphocholination of liposomes leads to highly-extended retention time in mice joints. J. Mater. Chem. B 2022, 10, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef]
- Ishihara, K.; Iwasaki, Y. Reduced Protein Adsorption on Novel Phospholipid Polymers. J. Biomater. Appl. 1998, 13, 111–127. [Google Scholar] [CrossRef]
- Ramot, Y.; Kronfeld, N.; Steiner, M.; Lee, E.D.; Goldberg, R.; Jahn, S. Abraham Nyska Biocompatible Solutions: Evaluating the Safety of Repeated Intra-Articular Injections of pMPCylated Liposomes for Knee Osteoarthritis Therapy in Rat Models. Toxicol. Pathol. 2024; Acceped. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Stelzle, M.; Weissmüller, G.; Sackmann, E. On the application of supported bilayers as receptive layers for biosensors with electrical detection. J. Phys. Chem. 1993, 97, 2974–2981. [Google Scholar] [CrossRef]
- Miller, C.; Cuendet, P.; Grätzel, M. K+ sensitive bilayer supporting electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1990, 278, 175–192. [Google Scholar] [CrossRef]
- Sugawara, M.; Kojima, K.; Sazawa, H.; Umezawa, Y. Ion-channel sensors. Anal. Chem. 1987, 59, 2842–2846. [Google Scholar] [CrossRef]
- Moncelli, M.R.; Herrero, R.; Becucci, L.; Guidelli, R. Kinetics of electron and proton transfer to ubiquinone-10 and from ubiquinol-10 in a self-assembled phosphatidylcholine monolayer. Biochim. Biophys. Acta-Bioenerg. 1998, 1364, 373–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, H.; Park, J.S.; Shim, Y.B. Redox reaction of benzoquinone on a lipid coated glassy carbon electrode. J. Electroanal. Chem. 1997, 438, 113–119. [Google Scholar] [CrossRef]
- Du, L.; Liu, X.; Huang, W.; Wang, E. A study on the interaction between ibuprofen and bilayer lipid membrane. Electrochim. Acta 2006, 51, 5754–5760. [Google Scholar] [CrossRef]
- Kochev, V.; Karabaliev, M. Wetting films of lipids in the development of sensitive interfaces. An electrochemical approach. Adv. Colloid Interface Sci. 2004, 107, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Montis, C.; Salvatore, A.; Valle, F.; Paolini, L.; Carlà, F.; Bergese, P.; Berti, D. Biogenic supported lipid bilayers as a tool to investigate nano-bio interfaces. J. Colloid Interface Sci. 2020, 570, 340–349. [Google Scholar] [CrossRef]
- Hellberg, D.; Scholz, F.; Schubert, F.; Lovrić, M.; Omanović, D.; Hernández, V.A.; Thede, R. Kinetics of Liposome Adhesion on a Mercury Electrode. J. Phys. Chem. B 2005, 109, 14715–14726. [Google Scholar] [CrossRef] [PubMed]
- Agmo Hernández, V.; Scholz, F. The lipid composition determines the kinetics of adhesion and spreading of liposomes on mercury electrodes. Bioelectrochemistry 2008, 74, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-S.; Ottova, A.; Tien, H.T.; Sheu, F.-S. Nanostructured platinum-lipid bilayer composite as biosensor. Bioelectrochemistry 2003, 59, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; Jiang, D.; Cui, X.; Gu, D.; Tong, R.; Zhong, B. Studies of structural disorder of self-assembled thiol monolayers on gold by cyclic voltammetry and ac impedance. J. Electroanal. Chem. 1999, 464, 61–67. [Google Scholar] [CrossRef]
- Heyse, S.; Stora, T.; Schmid, E.; Lakey, J.H.; Vogel, H. Emerging Techniques for Investigating Molecular Interactions at Lipid Membranes; Elsevier: Amsterdam, The Netherlands, 1998; Volume 1376, pp. 319–338. [Google Scholar]
- Tacheva, B.; Georgieva, R.; Karabaliev, M. Interactions of the spin-labeled chloroethylnitrosourea SLCNUgly with electrode-supported lipid films. Electrochim. Acta 2016, 192, 439–447. [Google Scholar] [CrossRef]
- Karabaliev, M.; Kochev, V. The potential of manganese in construction of electrodes modified with thin liquid films of lipids. J. Electroanal. Chem. 2004, 571, 73–80. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Sheepwash, E.; Brosseau, C.L.; Li, H.; Pettinger, B.; Gruler, H.; Lipkowski, J. AFM studies of solid-supported lipid bilayers formed at a Au(111) electrode surface using vesicle fusion and a combination of Langmuir-Blodgett and Langmuir-Schaefer techniques. Langmuir 2008, 24, 10313–10323. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Tai, C.Y.; Zen, J.M. A Simple Method to Tune Up Screen-Printed Carbon Electrodes Applicable to the Design of Disposable Electrochemical Sensors. Electroanalysis 2013, 25, 2539–2546. [Google Scholar] [CrossRef]
- Erb, R.A. The wettability of gold. J. Phys. Chem. 1968, 72, 2412–2417. [Google Scholar] [CrossRef]
- Gruber, H.J.; Schindler, H. External surface and lamellarity of lipid vesicles: A practice-oriented set of assay methods. Biochim. Biophys. Acta-Biomembr. 1994, 1189, 212–224. [Google Scholar] [CrossRef]
- Hernández, V.A.; Scholz, F. The Electrochemistry of Liposomes. Isr. J. Chem. 2008, 48, 169–184. [Google Scholar] [CrossRef]
- Moulton, S.E.; Barisci, J.N.; Bath, A.; Stella, R.; Wallace, G.G. Studies of double layer capacitance and electron transfer at a gold electrode exposed to protein solutions. Electrochim. Acta 2004, 49, 4223–4230. [Google Scholar] [CrossRef]
- Yaguchi, M.; Uchida, T.; Motobayashi, K.; Osawa, M. Speciation of Adsorbed Phosphate at Gold Electrodes: A Combined Surface-Enhanced Infrared Absorption Spectroscopy and DFT Study. J. Phys. Chem. Lett. 2016, 7, 3097–3102. [Google Scholar] [CrossRef] [PubMed]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Kerner, Z.; Pajkossy, T. On the origin of capacitance dispersion of rough electrodes. Electrochim. Acta 2000, 46, 207–211. [Google Scholar] [CrossRef]
- Pajkossy, T. Impedance spectroscopy at interfaces of metals and aqueous solutions—Surface roughness, CPE and related issues. Solid State Ion. 2005, 176, 1997–2003. [Google Scholar] [CrossRef]
- Vladikova, D.; Stoynov, Z. Secondary differential impedance analysis—A tool for recognition of CPE behavior. J. Electroanal. Chem. 2004, 572, 377–387. [Google Scholar] [CrossRef]
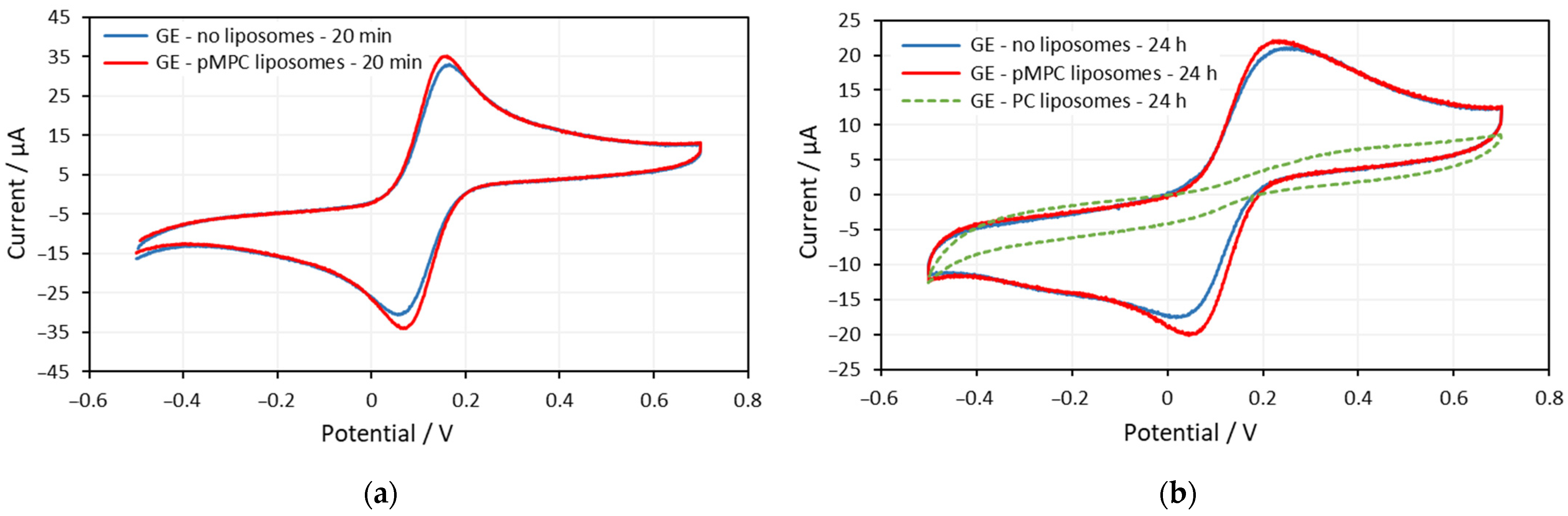
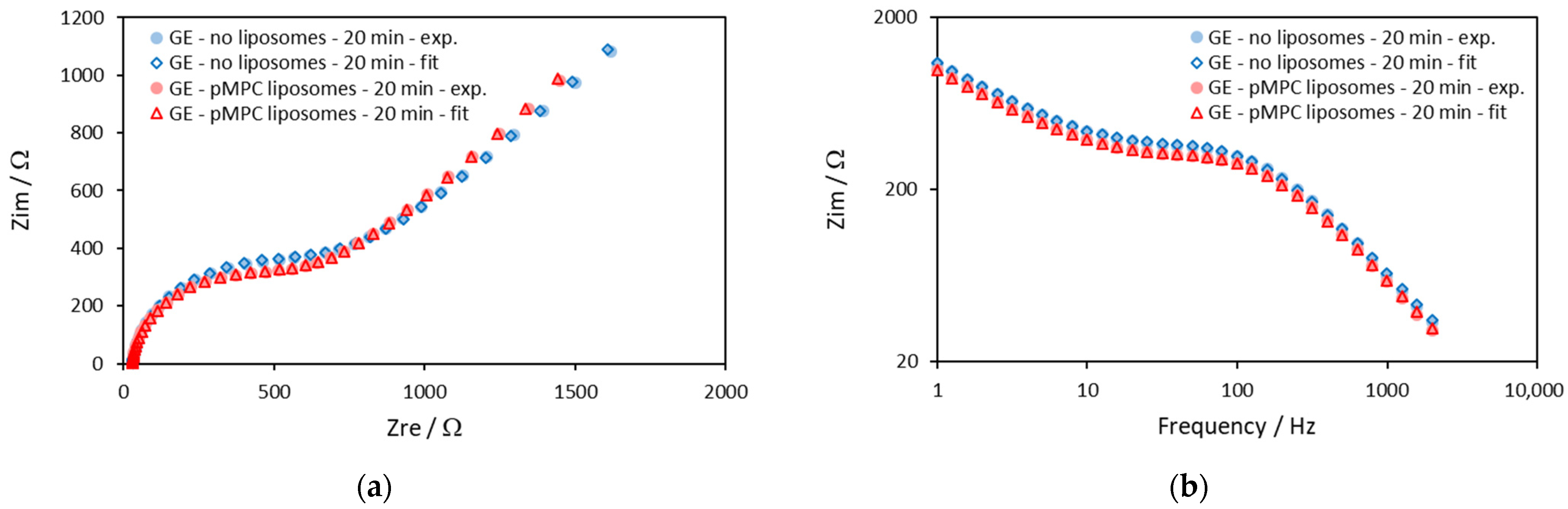
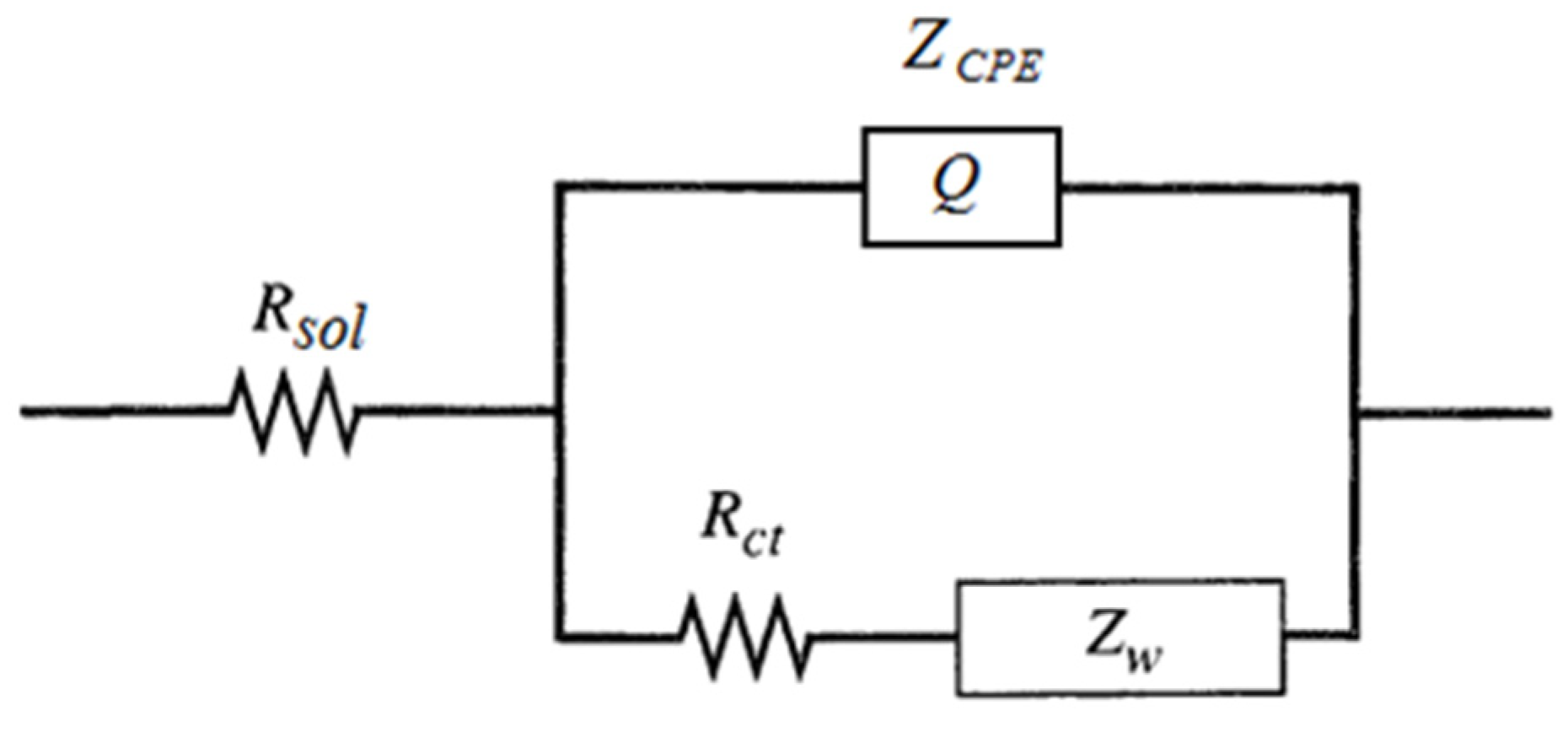

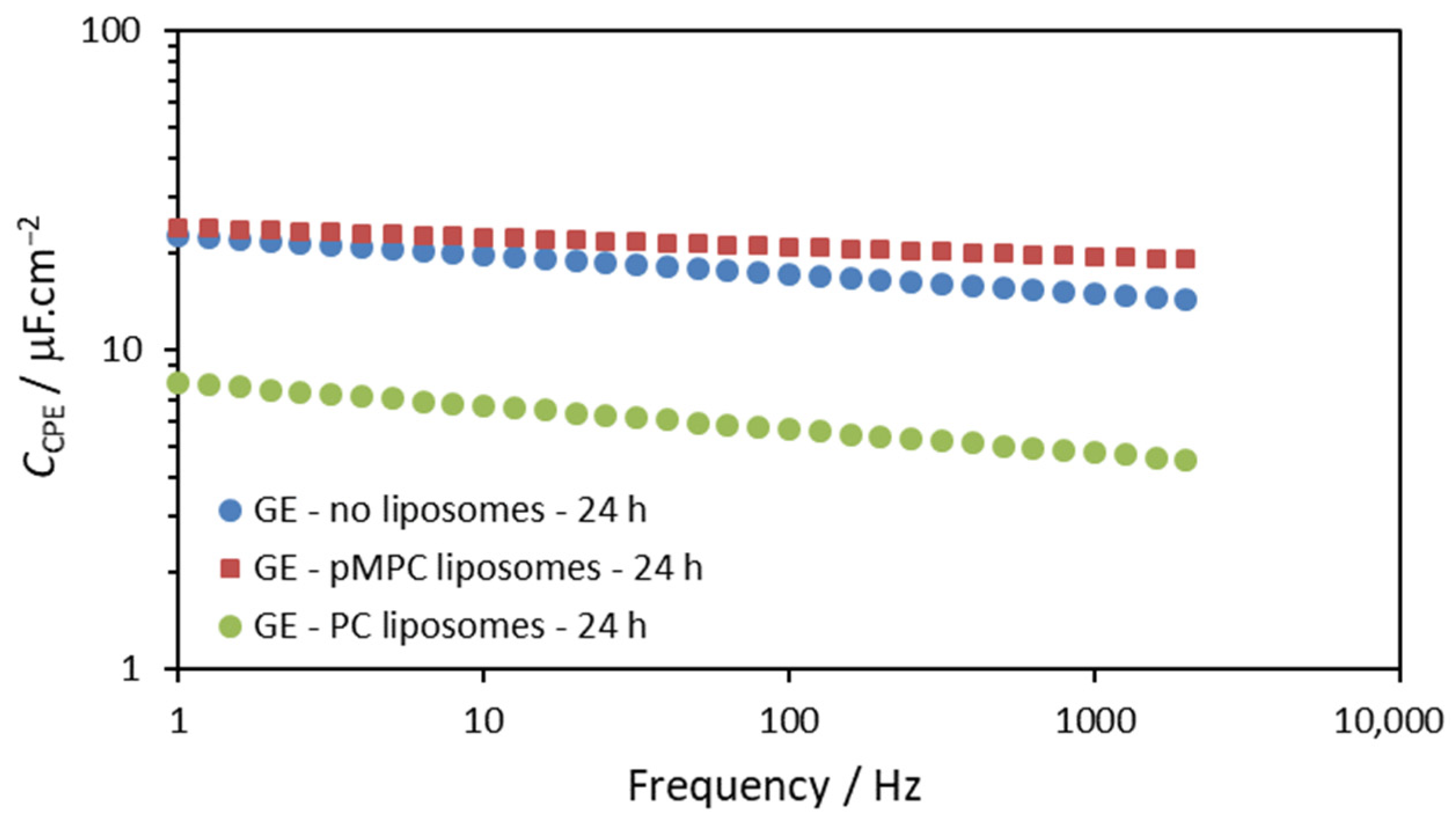




| Gold Electrode—20 min | Rsol/Ω | Q/Ω−1sα | α | Rct/Ω | W/Ω−1s0.5 |
|---|---|---|---|---|---|
| no liposomes | 30.05 | 4.118 × 10−6 | 0.9356 | 598.7 | 2.659 × 10−4 |
| with pMPC liposomes | 29.89 | 3.904 × 10−6 | 0.9532 | 512.7 | 2.924 × 10−5 |
| Gold Electrode | Rsol/Ω | Q/Ω−1sα | α | Rct/Ω | W/Ω−1s0.5 |
|---|---|---|---|---|---|
| no liposomes—0 min | 30.51 | 3.570 × 10−6 | 0.9539 | 117 | 28.3 × 10−5 |
| no liposomes—24 h | 32.66 | 3.237 × 10−6 | 0.9393 | 15,820 | 10.6 × 10−5 |
| with pMPC liposomes—24 h | 35.44 | 3.207 × 10−6 | 0.9705 | 12,190 | 11.4 × 10−5 |
| with PC liposomes—24 h | 32.64 | 1.141 × 10−6 | 0.9272 | 92,740 | 0.53 × 10−5 |
| Carbon Electrode—20 min | Rsol/Ω | Q/Ω−1sα | α | Rct/Ω | W/Ω−1s0.5 |
|---|---|---|---|---|---|
| no liposomes | 157.7 | 1.263 × 10−6 | 0.9705 | 7216 | 2.397 × 10−4 |
| with pMPC liposomes | 171.6 | 1.370 × 10−6 | 0.9653 | 6687 | 2.518 × 10−4 |
| Carbon Electrode | Rsol/Ω | Q/Ω−1sα | α | Rct/Ω | W/Ω−1s0.5 |
|---|---|---|---|---|---|
| no liposomes—0 min | 189.2 | 1.170 × 10−6 | 0.9701 | 10,043 | 2.03 × 10−4 |
| no liposomes—24 h | 198.3 | 3.782 × 10−6 | 0.9658 | 20,920 | 1.14 × 10−4 |
| with pMPC liposomes—24 h | 203.4 | 5.569 × 10−6 | 0.9767 | 6302 | 2.35 × 10−4 |
| with PC liposomes—24 h | 184.8 | 54.77 × 10−6 | 0.8449 | 19,540 | 8.42 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabaliev, M.; Paarvanova, B.; Savova, G.; Tacheva, B.; Jahn, S.; Georgieva, R. Electrochemical Investigation of the Stability of Poly-Phosphocholinated Liposomes. Molecules 2024, 29, 3511. https://doi.org/10.3390/molecules29153511
Karabaliev M, Paarvanova B, Savova G, Tacheva B, Jahn S, Georgieva R. Electrochemical Investigation of the Stability of Poly-Phosphocholinated Liposomes. Molecules. 2024; 29(15):3511. https://doi.org/10.3390/molecules29153511
Chicago/Turabian StyleKarabaliev, Miroslav, Boyana Paarvanova, Gergana Savova, Bilyana Tacheva, Sabrina Jahn, and Radostina Georgieva. 2024. "Electrochemical Investigation of the Stability of Poly-Phosphocholinated Liposomes" Molecules 29, no. 15: 3511. https://doi.org/10.3390/molecules29153511
APA StyleKarabaliev, M., Paarvanova, B., Savova, G., Tacheva, B., Jahn, S., & Georgieva, R. (2024). Electrochemical Investigation of the Stability of Poly-Phosphocholinated Liposomes. Molecules, 29(15), 3511. https://doi.org/10.3390/molecules29153511






