Recent Advances in Regulating Ceramic Monolithic Catalyst Structure for Preferential Oxidation of CO in H2
Abstract
1. Introduction
2. Active Site Regulation of Ceramic Monolithic Catalysts
2.1. Precious Metal-Based Ceramic Monolithic Catalysts
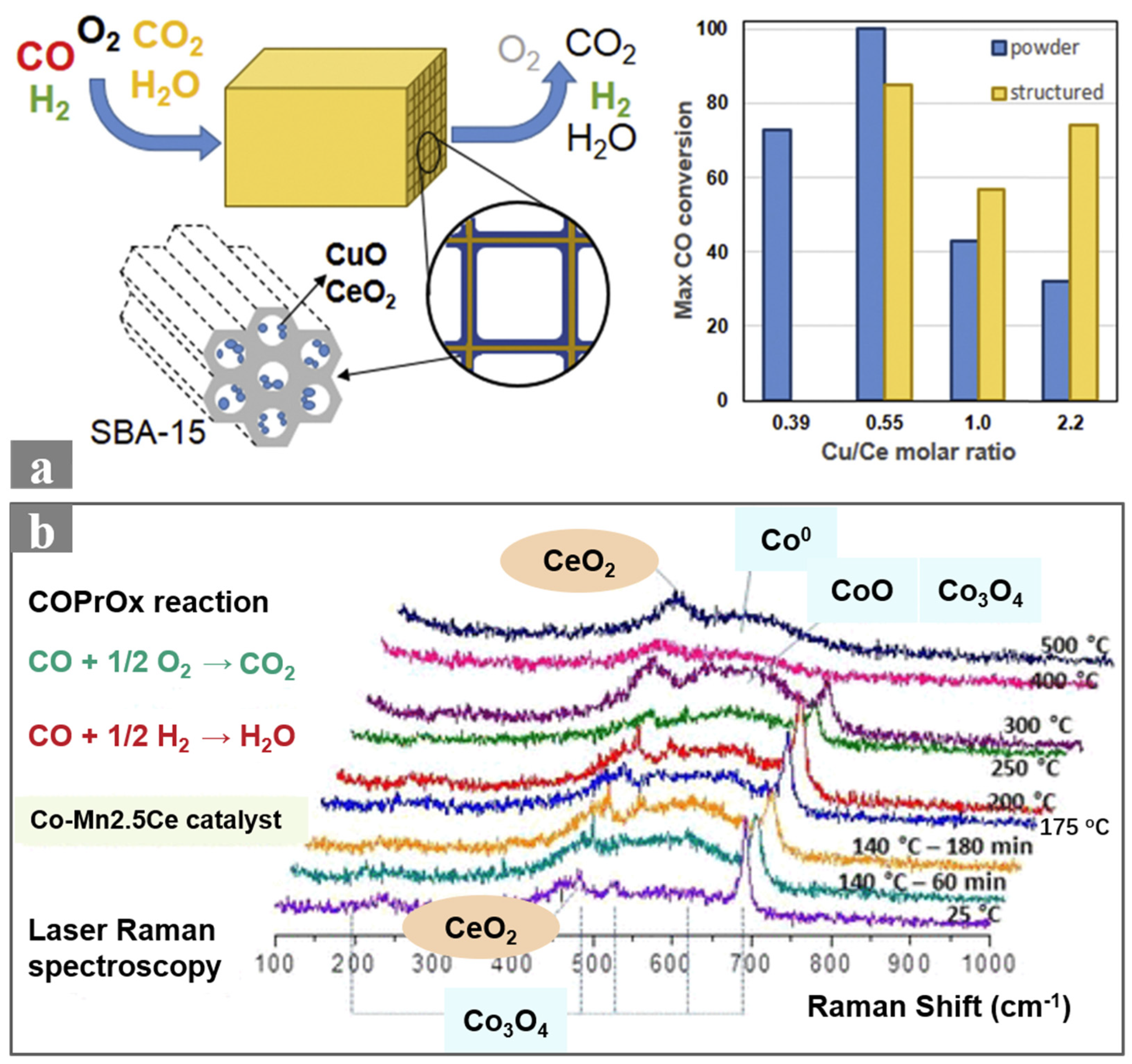
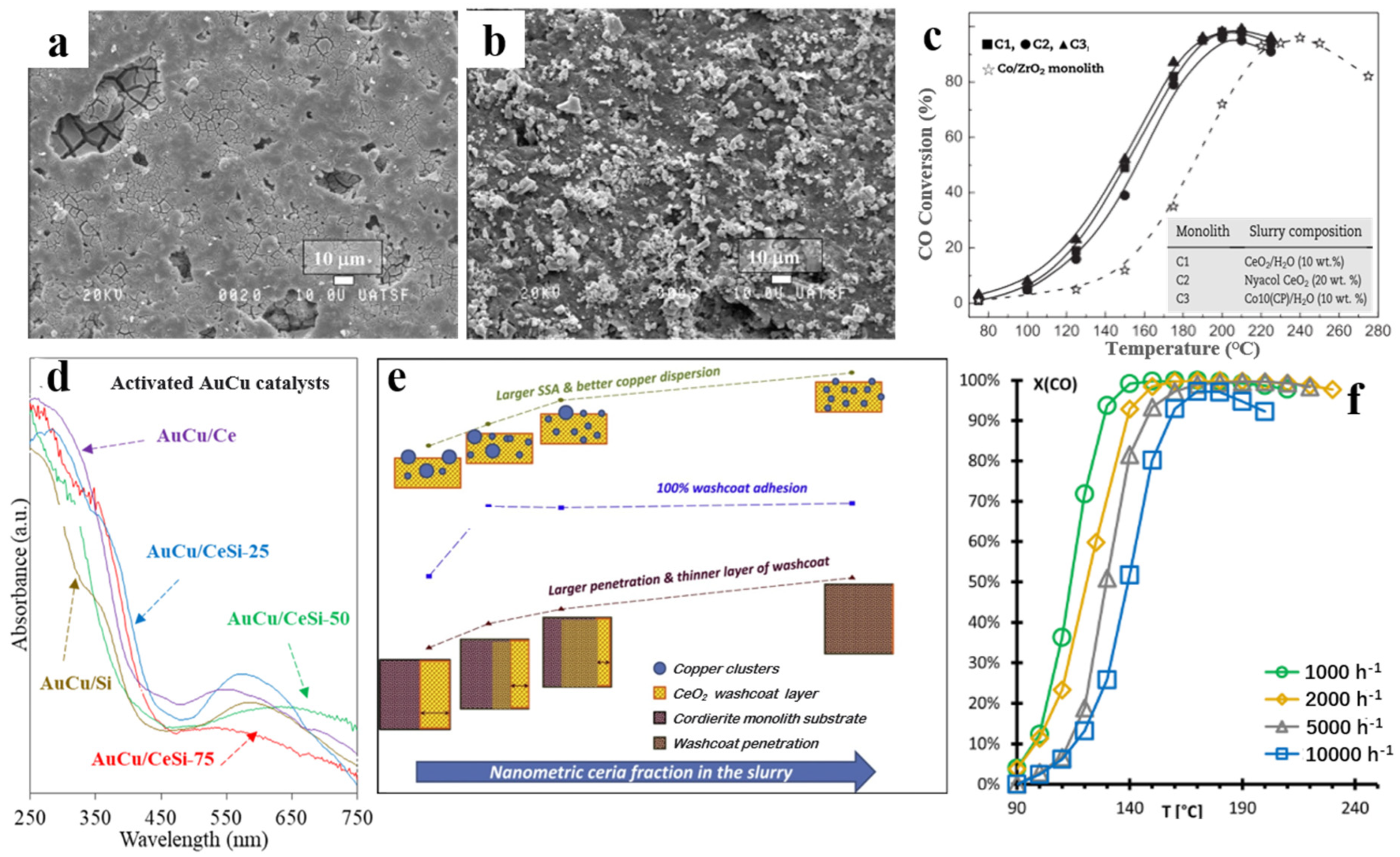
2.2. Non-Precious Metal-Based Ceramic Monolithic Catalysts
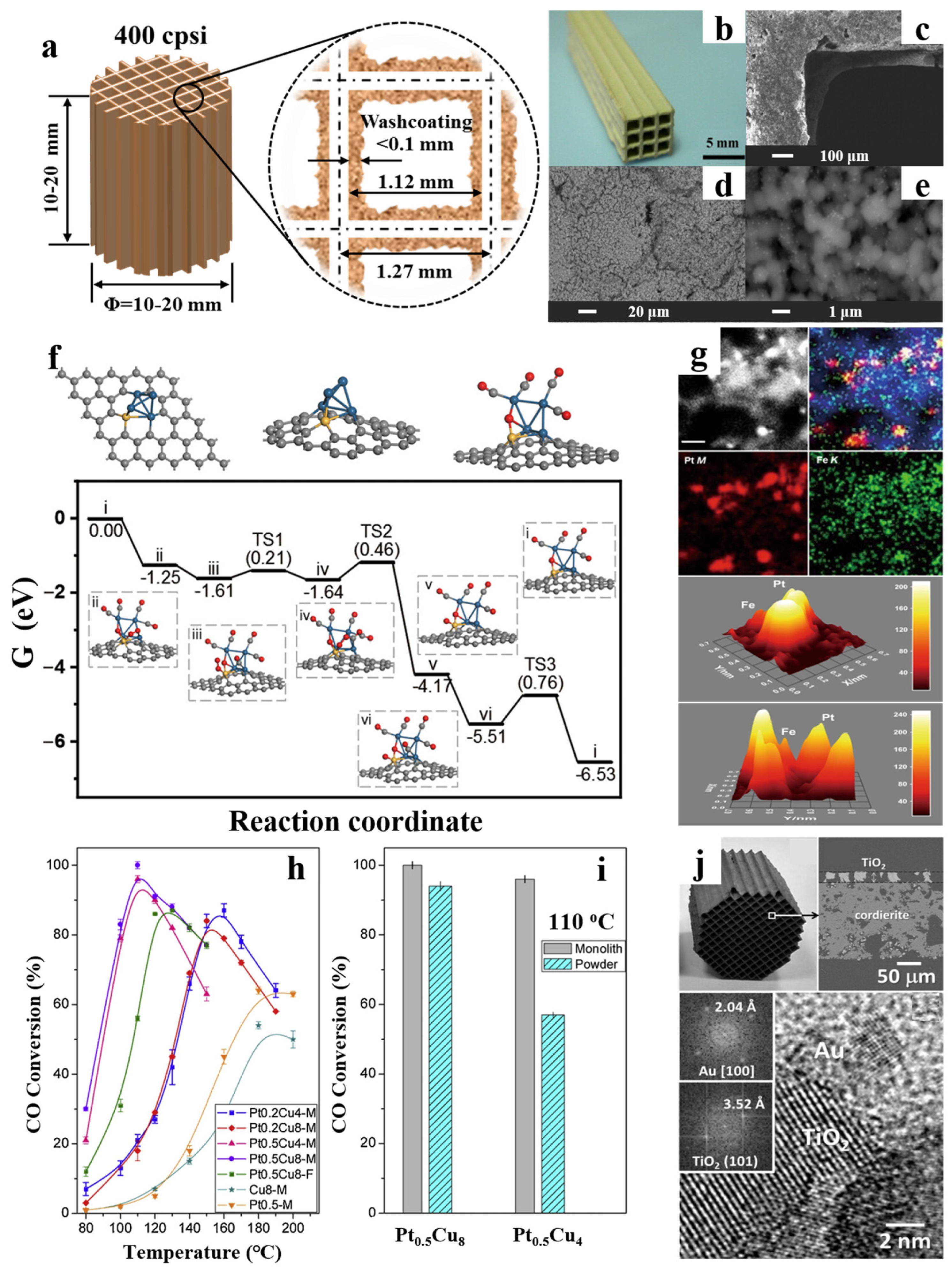
3. Monolith Structure Regulation of Ceramic Monolithic Catalysts
4. Coating Strategies of Ceramic Monolithic Catalysts
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Baroutaji, A.; Arjunan, A.; Robinson, J.; Wilberforce, T.; Abdelkareem, M.A.; Olabi, A.G. PEMFC Poly-Generation Systems: Developments, Merits, and Challenges. Sustainability 2021, 13, 11696. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, Y.; Shi, Z.; Yang, Y.; Zhao, T.; Jiang, Z.; Liu, C.; Xing, W.; Ge, J. The decisive role of adsorbed OH* in low-potential CO electro-oxidation on single-atom catalytic sites. Carbon Energy 2023, 5, 310–321. [Google Scholar] [CrossRef]
- Liu, H.M.; Li, D.Z.; Guo, J.W.; Li, Y.Q.; Liu, A.D.; Bai, Y.T.; He, D.H. Recent advances on catalysts for preferential oxidation of CO. Nano Res. 2023, 16, 4399–4410. [Google Scholar] [CrossRef]
- Rosso, I.; Galletti, C.; Saracco, G.; Garrone, E.; Specchia, V. Development of A zeolites-supported noble-metal catalysts for CO preferential oxidation: H2 gas purification for fuel cell. Appl. Catal. B Environ. 2004, 48, 195–203. [Google Scholar] [CrossRef]
- Malwadkar, S.; Bera, P.; Satyanarayana, C.V.V. Influence of cobalt on performance of Cu-CeO2 catalysts for preferential oxidation of CO. J. Rare Earths 2020, 38, 941–950. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Monoliths in Heterogeneous Catalysis. Catal. Rev.-Sci. Eng. 1994, 36, 179–270. [Google Scholar] [CrossRef]
- Roy, S.; Bauer, T.; Al-Dahhan, M.; Lehner, P.; Turek, T. Monoliths as multiphase reactors: A review. AIChE J. 2004, 50, 2918–2938. [Google Scholar] [CrossRef]
- Giroux, T.; Hwang, S.; Liu, Y.; Ruettinger, W.; Shore, L. Monolithic structures as alternatives to particulate catalysts for the reforming of hydrocarbons for hydrogen generation. Appl. Catal. B Env. 2005, 56, 95–110. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Kreutzer, M.T.; Nijhuis, T.A.; Kapteijn, F. Monolithic Catalysts and Reactors. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2011; Volume 2, pp. 249–327. [Google Scholar]
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Avila, P.; Montes, M.; Miró, E.E. Monolithic reactors for environmental applications: A review on preparation technologies. Chem. Eng. J. 2005, 109, 11–36. [Google Scholar] [CrossRef]
- Mitra, B.; Kunzru, D. Washcoating of Different Zeolites on Cordierite Monoliths. J. Am. Ceram. Soc. 2007, 91, 64–70. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Liu, Y.; Ruettinger, W.; Ilinich, O.; Shore, L.; Giroux, T. Precious Metal Catalysts Supported on Ceramic and Metal Monolithic Structures for the Hydrogen Economy. Catal. Rev.-Sci. Eng. 2007, 49, 141–196. [Google Scholar] [CrossRef]
- Martins, R. Materials as activator of future global science and technology challenges. Prog. Nat. Sci. Mater. Int. 2021, 31, 785–791. [Google Scholar] [CrossRef]
- Fu, K.; Su, Y.; Zheng, Y.; Han, R.; Liu, Q. Novel monolithic catalysts for VOCs removal: A review on preparation, carrier and energy supply. Chemosphere 2022, 308, 136256–1136268. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Li, Z.; Gui, R.; Wang, Q. Research progress of monolithic catalysts for VOCs oxidation. Chin. Sci. Bull. 2024, 60, 2186–2201. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, Z.; Wang, S. Selective CO oxidation with real methanol reformate over monolithic Pt group catalysts: PEMFC applications. Int. J. Hydrogen Energy 2006, 31, 924–933. [Google Scholar] [CrossRef]
- Neri, G.; Rizzo, G.; Corigliano, F.; Arrigo, I.; Caprì, M.; De Luca, L.; Modafferi, V.; Donato, A. A novel Pt/zeolite-based honeycomb catalyst for selective CO oxidation in a H2-rich mixture. Catal. Today 2009, 147, S210–S214. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, X.; Chen, Y.; Cai, X.; Gao, Z.; Peng, M.; Huang, F.; Xiao, D.; Wen, X.; Wang, N.; et al. Fully-exposed Pt-Fe cluster for efficient preferential oxidation of CO towards hydrogen purification. Nat. Commun. 2022, 13, 6798–6807. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.E.; Sollier, B.M.; Lacoste, A.M.; Miró, E.E.; Boix, A.V. Hydrogen purification for fuel cells through CO preferential oxidation using PtCu/Al2O3 structured catalysts. J. Environ. Chem. Eng. 2019, 7, 103376–103386. [Google Scholar] [CrossRef]
- Moreno, M.S.; López, E.; Adrover, M.E.; Divins, N.J.; Llorca, J. CO-PrOx over nano-Au/TiO2: Monolithic catalyst performance and empirical kinetic model fitting. Int. J. Hydrogen Energy 2016, 41, 22043–22054. [Google Scholar] [CrossRef]
- Korotkikh, O.; Farrauto, R. Selective catalytic oxidation of CO in H2: Fuel cell applications. Catal. Today 2000, 62, 249–254. [Google Scholar] [CrossRef]
- Roberts, G.W.; Chin, P.; Sun, X.; Spivey, J.J. Preferential oxidation of carbon monoxide with Pt/Fe monolithic catalysts: Interactions between external transport and the reverse water-gas-shift reaction. Appl. Catal. B Environ. 2003, 46, 601–611. [Google Scholar] [CrossRef]
- Zhang, Q.; Shore, L.; Farrauto, R.J. Selective CO oxidation over a commercial PROX monolith catalyst for hydrogen fuel cell applications. Int. J. Hydrogen Energy 2012, 37, 10874–10880. [Google Scholar] [CrossRef]
- Maeda, N.; Matsushima, T.; Uchida, H.; Yamashita, H.; Watanabe, M. Performance of Pt-Fe/mordenite monolithic catalysts for preferential oxidation of carbon monoxide in a reformate gas for PEFCs. Appl. Catal. A Gen. 2008, 341, 93–97. [Google Scholar] [CrossRef]
- Maeda, N.; Matsushima, T.; Kotobuki, M.; Miyao, T.; Uchida, H.; Yamashita, H.; Watanabe, M. H2O-tolerant monolithic catalysts for preferential oxidation of carbon monoxide in the presence of hydrogen. Appl. Catal. A Gen. 2009, 370, 50–53. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chen, Y.-Y.; Su, C.-C.; Hsu, C.-F. The cleanup of CO in hydrogen for PEMFC applications using Pt, Ru, Co, and Fe in PROX reaction. J. Power Sources 2007, 174, 294–301. [Google Scholar] [CrossRef]
- Lacoste, A.M.; Tiscornia, I.S.; Boix, A.V. CO preferential oxidation on cordierite monoliths coated with CuO-CeO2/SBA-15 catalysts. Further insights into the physico-chemical aspects of the catalytic behavior. Int. J. Hydrogen Energy 2018, 43, 14238–14251. [Google Scholar] [CrossRef]
- Barbato, P.S.; Landi, G.; Lisi, L.; Di Benedetto, A. CFD Simulations of Copper-Ceria Based Microreactor for COPROX. Int. J. Chem. React. Eng. 2016, 14, 1301–1313. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Gamboa, N.K.; González-Marcos, M.P.; Gutiérrez-Ortiz, M.A. CuO/CeO2 washcoated ceramic monoliths for CO-PROX reaction. Chem. Eng. J. 2011, 171, 224–231. [Google Scholar] [CrossRef]
- Tiscornia, I.S.; Lacoste, A.M.; Gómez, L.E.; Boix, A.V. CuO-CeO2/SiO2 coating on ceramic monolith: Effect of the nature of the catalyst support on CO preferential oxidation in a H2-rich stream. Int. J. Hydrogen Energy 2020, 45, 6636–6650. [Google Scholar] [CrossRef]
- Gómez, L.E.; Múnera, J.F.; Boix, A.V. In Situ Raman Spectroscopic Study of Species in Co-MnCeOx Catalysts under COPrOx Reaction Conditions. Ind. Eng. Chem. Res. 2021, 60, 18640–18650. [Google Scholar] [CrossRef]
- Gómez, L.E.; Tiscornia, I.S.; Boix, A.V.; Miró, E.E. Co/ZrO2 catalysts coated on cordierite monoliths for CO preferential oxidation. Appl. Catal. A Gen. 2011, 401, 124–133. [Google Scholar] [CrossRef]
- Gómez, L.E.; Boix, A.V.; Miró, E.E. Co/ZrO2, Co/CeO2 and MnCoCe structured catalysts for COPrOx. Catal. Today 2013, 216, 246–253. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Landi, G.; Lisi, L. CO reactive adsorption at low temperature over CuO/CeO2 structured catalytic monolith. Int. J. Hydrogen Energy 2017, 42, 12262–12275. [Google Scholar] [CrossRef]
- Landi, G.; Di Benedetto, A.; Lisi, L. Two-Stage Strategy for CO Removal from H2-Rich Streams over (Nano-) CuO/CeO2 Structured Catalyst at Low Temperature. Appl. Sci. 2018, 8, 789. [Google Scholar] [CrossRef]
- Xin, Q.; Hua, Z.; Fu, Y.; Yang, Y.; Liu, S.; Song, H.; Yu, X.; Xiao, L.; Zheng, C.; Gao, X. Investigation on optimal active layer thickness and pore size in dual-layer NH3-SCR monolith for low SO2 oxidation by numerical simulation. Fuel 2020, 279, 118420. [Google Scholar] [CrossRef]
- Delrieux, T.; Sharma, S.; Maurer, F.; Dolcet, P.; Lausch, M.; Zimina, A.; Cárdenas, C.; Lott, P.; Casapu, M.; Sheppard, T.L.; et al. A laboratory scale fast feedback characterization loop for optimizing coated catalysts for emission control. React. Chem. Eng. 2024. [Google Scholar] [CrossRef]
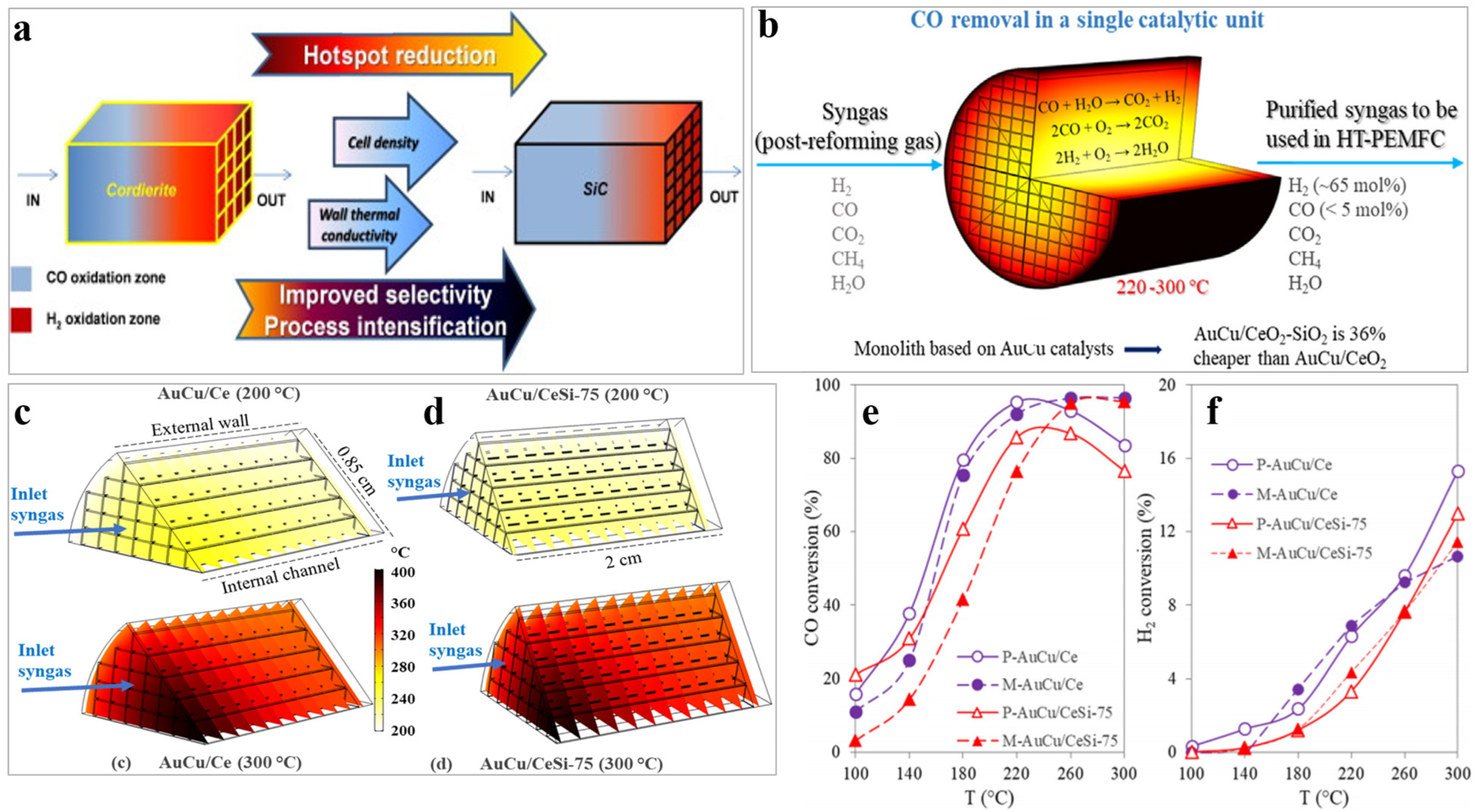
- Zhang, W.; Lin, Y.; Zhang, Y.; Li, T.; Li, J.; Chen, Z.; Norinaga, K. Regulation of temperature distribution in fixed bed reactor for CO2 methanation through “CHESS” monolith structure catalyst. Appl. Therm. Eng. 2024, 236, 121826–121841. [Google Scholar] [CrossRef]
- Klenov, O.P.; Chumakova, N.A.; Pokrovskaya, S.A.; Noskov, A.S. Modeling of Heat Transfer in a Porous Monolith Catalyst with Square Channels. Ind. Eng. Chem. Res. 2016, 55, 3879–3889. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Landi, G.; Lisi, L. Structuring CuO/CeO2 Catalyst as Option to Improve Performance Towards CO-PROX. Top. Catal. 2016, 59, 1371–1382. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Landi, G.; Lisi, L. CuO/CeO2 based monoliths for CO preferential oxidation in H2-rich streams. Chem. Eng. J. 2015, 279, 983–993. [Google Scholar] [CrossRef]
- Cifuentes, B.; Cifuentes, A.; Bustamante, F.; Soler, L.; Llorca, J.; Cobo, M. Monoliths washcoated with AuCu catalysts for CO removal in an ethanol fuel processor: Effect of CeO2-SiO2 dual support on the catalytic performance and reactor cost. Int. J. Hydrogen Energy 2021, 46, 2166–2181. [Google Scholar] [CrossRef]
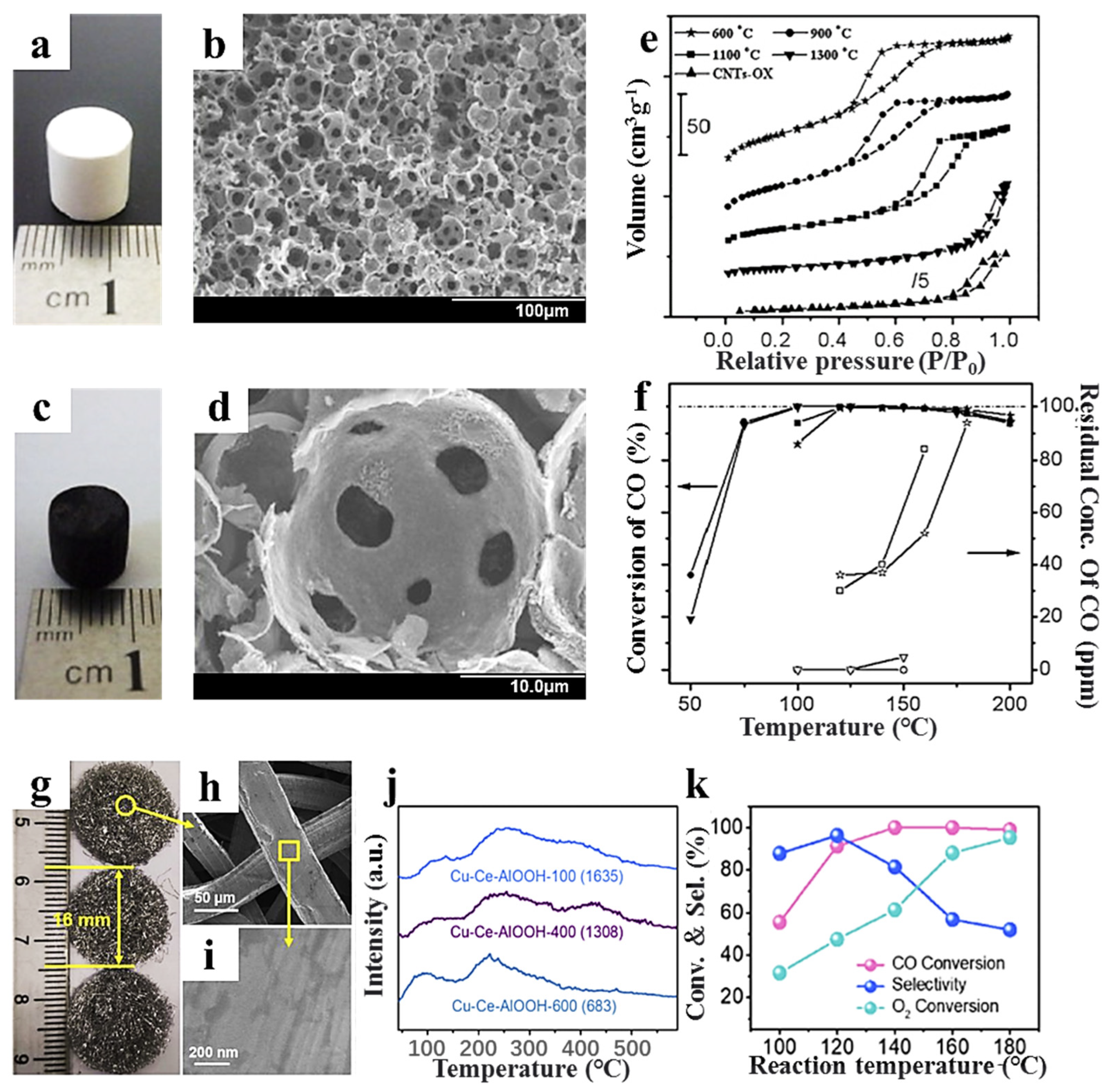
- Lu, S.; Liu, Y. Preparation of meso-macroporous carbon nanotube-alumina composite monoliths and their application to the preferential oxidation of CO in hydrogen-rich gases. Appl. Catal. B Environ. 2012, 111–112, 492–501. [Google Scholar] [CrossRef]
- Lu, S.; Liu, Y.; Wang, Y. Meso-macro-porous monolithic Pt-Ni/Al2O3 catalysts used for miniaturizing preferential carbon monoxide oxidation reactor. Chem. Commun. 2010, 46, 634–636. [Google Scholar] [CrossRef]
- Gu, C.; Miao, J.; Liu, Y.; Wang, Y. Meso-macroporous monolithic CuO-CeO2/γ-Al2O3 catalysts and their catalytic performance for preferential oxidation of CO. J. Mater. Sci. 2010, 45, 5660–5668. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.Y.; Liang, H.; Liu, Y. Macroporous Monolithic Pt/γ-Al2O3 and K–Pt/γ-Al2O3 Catalysts Used for Preferential Oxidation of CO. Catal. Lett. 2008, 127, 339–347. [Google Scholar] [CrossRef]
- Miguel-García, I.; Navlani-García, M.; García-Aguilar, J.; Berenguer-Murcia, Á.; Lozano-Castelló, D.; Cazorla-Amorós, D. Capillary microreactors based on hierarchical SiO2 monoliths incorporating noble metal nanoparticles for the Preferential Oxidation of CO. Chem. Eng. J. 2015, 275, 71–78. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, Y.; Xu, X.; Si, J.; Sun, W.; Zhao, G.; Liu, Y.; Lu, Y. High jolt-resistance monolithic CuO-CeO2/AlOOH/Al-fiber catalyst for CO-PROX: Influence of AlOOH/Al-fiber calcination on Cu-Ce interaction. Int. J. Hydrogen Energy 2022, 47, 13030–13043. [Google Scholar] [CrossRef]
- Wu, D.; Kong, S.; Zhang, H.; Li, Y. Mechanical stability of monolithic catalysts: Factors affecting washcoat adhesion and cohesion during preparation. AIChE J. 2014, 60, 2765–2773. [Google Scholar] [CrossRef]
- Gómez, L.E.; Tiscornia, I.S.; Boix, A.V.; Miró, E.E. CO preferential oxidation on cordierite monoliths coated with Co/CeO2 catalysts. Int. J. Hydrogen Energy 2012, 37, 14812–14819. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Benedetto, A.; Lisi, L. Optimization of the preparation method of CuO/CeO2 structured catalytic monolith for CO preferential oxidation in H2-rich streams. Appl. Catal. B Environ. 2016, 181, 727–737. [Google Scholar] [CrossRef]
- Meissner, J.; Ahrens, L.; Pasel, J.; Schwedt, A.; Wohlrab, S.; Mayer, J.; Peters, R. An improved preparation method for a CuO/CeO2-coated monolith for the CO-PrOx reaction. Sci. Rep. 2023, 13, 9345–9355. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; He, J.; Jiang, W.; She, X.; Song, Y.; Ji, H.; Xu, H.; Li, H. Ionic liquids assisted construction of efficient ceramic-based catalyst by direct ink writing 3D printing for ultra-deep oxidative desulfurization of diesel. Ceram. Int. 2024, 50, 10990–11002. [Google Scholar] [CrossRef]
- Davo-Quinonero, A.; Sorolla-Rosario, D.; Bailon-Garcia, E.; Lozano-Castello, D.; Bueno-Lopez, A. Improved asymmetrical honeycomb monolith catalyst prepared using a 3D printed template. J. Hazard. Mater. 2019, 368, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, N.; Mukherjee, M.; Basavaraj, M.G. Porous Ceramics Prepared from 3D Printed Pickering Emulsions as Gold Nanoparticle Supports for Reduction Reactions. ACS Appl. Nano Mater. 2023, 6, 21201–21215. [Google Scholar] [CrossRef]
- Tubio, C.R.; Malatini, C.; Barrio, V.L.; Masaguer, C.F.; Amorín, M.; Nabgan, W.; Taboada, P.; Guitián, F.; Gil, A.; Coelho, A. 3D printing of a palladium-alumina cermet monolithic catalyst: Catalytic evaluation in microwave-assisted cross-coupling reactions. Mater. Today Chem. 2023, 27, 101355–101366. [Google Scholar] [CrossRef]
- Wang, R.; Gong, Y.; Wang, P.; He, W.; Song, Y.; Xin, M.; Jiang, Q.; Sha, Y.; Cao, T.; Song, H.; et al. In situ crystal engineering on 3D-printed woodpile scaffolds: A monolith catalyst with highly accessible active sites for enhanced catalytic cracking. J. Mater. Chem. A 2023, 11, 13945–13955. [Google Scholar] [CrossRef]
- Pereira, V.G.F.; Rodrigues, C.P.; Toniolo, F.S. Ni/Al2O3 supported on cordierite monoliths for methane steam reforming: Influence of catalyst coating methodology. Catal. Commun. 2023, 183, 106759–106772. [Google Scholar] [CrossRef]
- Osorio-Zabala; Alejandra, M.; Baquero, E.A.; Daza, C. Dry reforming of methane using cordierite monoliths with immobilized Ni-Ce catalysts. Int. J. Hydrogen Energy 2024, 60, 1157–1169. [Google Scholar] [CrossRef]
| Catalyst | Carrier Type | Preparation Method | Reaction Conditions | T50% (°C) | T100% (°C) | # |
|---|---|---|---|---|---|---|
| Pt/Al2O3 | Cordierite monoliths | Washcoating method | 1% CO, 1% O2, 15% CO2, 20% H2O, 50% H2, N2 balance | 180 | 180–230 (>90%) | [17] |
| 5 wt.% Pt/0.5 wt.% Fe/γ-Al2O3 | Cordierite monoliths | Washcoating method | 42% H2, 9% CO2, 12% H2O, 1% CO, 1%O2, N2 balance | - | 100 (80%) | [23] |
| Pt-Cu-Fe/Al2O3 | Cordierite monoliths | Washcoating method | O2: 4500 ppm, 25% H2O, CO: 3000 ppm | - | 80–100 | [24] |
| Pt/Z-PM | Cordierite monoliths | Washcoating method | 1% CO, 1.5% O2, H2 balance | 75 | / | [18] |
| 1 wt.% Ru/γ-Al2O3 | Cordierite monoliths | Washcoating method | 0.5% CO, 2% O2, 28.5% CO2, 69% H2 | 140 | 150–200 | [27] |
| Au/TiO2 | Cordierite monoliths | Washcoating method | 1.41% CO, 24.33% CO2, H2 balance, O2/CO = 0.4–4.1 | 100 | / | [21] |
| CuO/CeO2 | SiC monoliths | Washcoating method | CO/O2/H2 = 0.5/0.9/50 | 90 | 140–180 | [41] |
| CuO/CeO2 | SiC monoliths | Washcoating method | CO/O2/H2 = 0.5/0.9/50 | 90 | 120–140 | [42] |
| AuCu/CeO2-SiO2 | Cordierite monoliths | Washcoating method | 19.9% H2, 6.3% CO, 5.2%CO2, 5.6% O2, 7.8% H2O, 55.2% N2 | 185 | 260–300 | [43] |
| Pt-Ni | Macro-porous monolithic γ-Al2O3 | Template method and washcoating method | 1% CO, 1% O2, and 50% H2 in N2 balance | 60 | 100–175 | [45] |
| CuO/CeO2 | Macro-porous monolithic γ-Al2O3 | Template method and washcoating method | 1% CO, 1% O2, 50% H2, 15% CO2, 8% H2O in N2 | 90 | 120–160 | [46] |
| Pt/γ-Al2O3 | Macro-porous monolithic γ-Al2O3 | Template method and washcoating method | 1% CO, 1% O2, 50% H2 in N2 | 180 | 225–275 | [47] |
| K-Pt/γ-Al2O3 | Macro-porous monolithic γ-Al2O3 | Template method and washcoating method | 1% CO, 1% O2, 50% H2 in N2 | 150 | 200–275 | [47] |
| Pd/SiO2 | Novel hierarchical SiO2 monolithic microreactors | Sol-gel method and impregnation method | 2% CO, 2% O22, 30% H2, balance He. | 160 | / | [48] |
| Pt/SiO2 | Novel hierarchical SiO2 monolithic microreactors | Sol-gel method and impregnation method | 2% CO, 2% O2, 30% H, balance He. | 190 | / | [48] |
| CuO-CeO2 | Al-fiber | Steam oxidation method and impregnation method | CO/O2/H2 = 0.5/0.5/49.5 (balance N2) | 100 | 140–180 | [49] |
| Co/CeO2 | Cordierite monoliths | Washcoating method | 1% CO, 1% O2, 40% H2, He balance | 120 | 160 | [51] |
| CuO/CeO2 | Commercial honeycomb monoliths | Washcoating method | CO/O2/H2 = 0.5/0.9/50 (balance N2) | 90 | 140–200 | [52] |
| CuO/CeO2 | Ceramic monolith | Direct coating | 39% H2, 20% CO2, 1% CO, balance: N2 | 110 | 140–210 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Sui, J.; Li, L.; Tuo, Y.; Zhang, W.; Zhong, G.; Zhou, H.; Feng, X. Recent Advances in Regulating Ceramic Monolithic Catalyst Structure for Preferential Oxidation of CO in H2. Molecules 2024, 29, 3481. https://doi.org/10.3390/molecules29153481
Wang Q, Sui J, Li L, Tuo Y, Zhang W, Zhong G, Zhou H, Feng X. Recent Advances in Regulating Ceramic Monolithic Catalyst Structure for Preferential Oxidation of CO in H2. Molecules. 2024; 29(15):3481. https://doi.org/10.3390/molecules29153481
Chicago/Turabian StyleWang, Qing, Jiancai Sui, Linlin Li, Yongxiao Tuo, Wenfa Zhang, Guoyu Zhong, Huanxin Zhou, and Xiang Feng. 2024. "Recent Advances in Regulating Ceramic Monolithic Catalyst Structure for Preferential Oxidation of CO in H2" Molecules 29, no. 15: 3481. https://doi.org/10.3390/molecules29153481
APA StyleWang, Q., Sui, J., Li, L., Tuo, Y., Zhang, W., Zhong, G., Zhou, H., & Feng, X. (2024). Recent Advances in Regulating Ceramic Monolithic Catalyst Structure for Preferential Oxidation of CO in H2. Molecules, 29(15), 3481. https://doi.org/10.3390/molecules29153481







