Manniosides G-J, New Ursane- and Lupane-Type Saponins from Schefflera mannii (Hook.f.) Harms
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. The Antibacterial Activity
3.5. The Antioxidant Activity
- Abs (DPPH) = Absorbance of a methanolic solution of DPPH
- Abs (trial) = Absorbance of a methanolic solution of DPPH + sample
- Abs (sample) = Absorbance of a methanolic solution of sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.; Nguyen, V.B.; Dong, J.; Wang, Y.; Park, J.Y.; Lee, S.-C.; Yang, T.-J. Evolution of the Araliaceae family inferred from complete chloroplast genomes and 45S nrDNAs of 10 Panax-related species. Sci. Rep. 2017, 7, 4917. [Google Scholar] [CrossRef]
- Wang, Y.; Khan, F.-A.; Siddiqui, M.; Aamer, M.; Lu, C.; Atta-Ur-Rahman; Wahab, A.-T.; Choudhary, M.I. The genus Schefflera: A review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 279, 113675. [Google Scholar] [CrossRef]
- Ponou, B.K.; Tanaka, C.; Teponno, R.B.; Tapondjou, A.L.; Miyamoto, T. Manniosides B-F, five new triterpenoid saponins from the leaves of Schefflera mannii (Hook.f.) Harms. Carbohydr. Res. 2021, 502, 108279. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Mitaine-Offer, T.A.-C.; Miyamoto, T.; Lerche, H.; Mirjolet, J.-F.; Guilbaud, N.; Lacaille-Dubois, M.-A. Triterpene saponins from Schefflera abyssinica. Biochem. Syst. Ecol. 2006, 34, 887–889. [Google Scholar] [CrossRef]
- Melek, F.R.; Miyase, T.; Khalik, S.M.A.; El-Gindic, M.R. Triterpenoid saponins from Schefflera arboricola. Phytochemistry 2003, 63, 401–407. [Google Scholar] [CrossRef]
- Adam, G.; Lischewski, M.; Phiet, H.V.; Preiss, A.; Schmidt, J.; Sung, T.V. 3α-Hydroxy-lup-20(29)-ene-23,28-dioic acid from Schefflera octophylla. Phytochemistry 1982, 21, 1385–1387. [Google Scholar] [CrossRef]
- Just, M.J.; Recio, M.C.; Giner, R.M.; Cuéllar, M.J.; Măñez, S.; Billa, A.R.; Ríos, J.L. Anti–inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta Med. 1998, 64, 404–407. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Tabata, K.; Nakamura, Y.; Suzuki, T. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. J. Ethnopharmacol. 2006, 107, 249–253. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Staden, V.J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Jeminez-Arellanes, A.; Meckes, M.; Torres, J.; LunaHerrera, J. Antimycobacterial triterpenoids from Lantana hispida (Verbenaceae). J. Ethnopharmacol. 2007, 111, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.M.O.; Amoros, M.; Girre, L. Mechanism of antiviral activity of triterpenoid saponins. Phytother. Res. 1999, 13, 323–328. [Google Scholar] [CrossRef]
- Quéré, L.; Wenger, T.; Schramm, H.J. Triterpenes as potential dimerization inhibitors of HIV-1 protease. Biochem. Biophys. Res. Commun. 1996, 227, 484–488. [Google Scholar] [CrossRef]
- Cheng, T.C.; Lu, J.F.; Wang, J.S.; Lin, L.-J.; Kuo, H.-I.; Chen, B.-H. Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC–3 by flavonoids and saponins prepared from Gynostemma pentaphyllum. J. Agric. Food Chem. 2011, 59, 11319–11329. [Google Scholar] [CrossRef]
- Nzowa, K.L.; Barboni, L.; Teponno, R.B.; Ricciutelli, M.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Tapondjou, A.L. Rheediinosides A and B, two antiproliferative and antioxidant triterpene saponins from Entada rheedii. Phytochemistry 2010, 71, 254–261. [Google Scholar] [CrossRef]
- Eom, H.J.; Kang, H.R.; Kim, H.K.; Jung, E.B.; Park, H.B.; Kang, K.S.; Kim, K.H. Bioactivity-guided isolation of antioxidant triterpenoids from Betula platyphylla var. japonica bark. Bioorg. Chem. 2016, 66, 97–101. [Google Scholar] [CrossRef]
- Yang, H.; Jeong, E.J.; Kim, J.; Sung, S.H.; Kim, Y.C. Antiproliferative triterpenes from the leaves and twigs of Juglans sinensis on HSC-T6 cells. J. Nat. Prod. 2011, 74, 751–756. [Google Scholar] [CrossRef]
- Wen, Z.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E.; et al. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: Synthesis, structure-activity relationships, and X-ray crystallographic studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.-L.; Wang, T.; Qian, S.-H. Chemical constituents of the aerial parts of Lycopus lucidus var. hirtus. Chem. Nat. Compd. 2014, 50, 253–255. [Google Scholar] [CrossRef]
- Ye, W.-C.; Ji, N.-N.; Zhao, S.-X.; Liu, J.-H.; Ye, T.; McKervey, M.A.; Stevenson, P. Triterpenoids from Pulsatilla chinensis. Phytochemistry 1996, 42, 799–802. [Google Scholar]
- Cai, X.F.; Lee, I.S.; Shen, G.; Dat, N.T.; Lee, J.J.; Kim, Y.H. Triterpenoids from Acanthopanax koreanum root and their inhibitory activities on NFAT transcription. Arch. Pharm. Res. 2004, 27, 825–828. [Google Scholar] [CrossRef]
- Fourie, T.G.; Matthee, E.; Snyckers, O.F. A pentacyclic triterpene acid, with anti-ulcer properties from Cussonia natalensis. Phytochemisty 1989, 28, 2851–2852. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.-H.; Feng, X.; Zou, Y.-X.; Diao, Z.; Chuc, Z.-Y. Microbial transformation of hederagenin by Cunninghamella echinulate, Mucor subtilissimus, and Pseudomonas oleovorans. J. Asian Nat. Prod. Res. 2016, 6, 712–718. [Google Scholar] [CrossRef]
- Dais, P.; Plessel, R.; Williamson, K.; Hatzakis, E. Complete 1H and 13C NMR assignment and NMR determination of pentacyclic triterpenic acids. Anal. Methods 2017, 9, 949–957. [Google Scholar] [CrossRef]
- Silva, A.T.M.; Magalhães, C.G.; Duarte, L.P.; da Nova Mussel, W.; Ruiz, A.L.T.G.; Shiozawa, L.; de Carvalho, J.E.; Trindade, I.C.; Filho, S.A.V. Lupeol and its esters: NMR, powder XRD data and in vitro evaluation of cancer cell growth. Braz. J. Pharm. Sci. 2017, 53, 251–261. [Google Scholar] [CrossRef]
- Teponno, R.B.; Tanaka, C.; Jie, B.; Tapondjou, L.A.; Miyamoto, T. Trifasciatosides A–J, steroidal saponins from Sansevieria trifasciata. Chem. Pharm. Bull. 2016, 64, 1347–1355. [Google Scholar] [CrossRef]
- Tchegnitegni, B.T.; Teponno, R.B.; Jenett-Siems, K.; Melzig, M.F.; Miyamoto, T.; Tapondjou, L.A. A dihydrochalcone derivative and further steroidal saponins from Sansevieria trifasciata Prain. Z. Naturforschung C 2017, 72, 477–482. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Mitaine-Offer, A.C.; Miyamoto, T.; Khan, I.; Delaude, C. Three new triterpene saponins from two species of Carpolobia. J. Nat. Prod. 2002, 65, 553–557. [Google Scholar] [CrossRef]
- Sung, T.V.; Adam, G. A sulphated triterpenoid saponin from Schefflera octophylla. Phytochemistry 1991, 3, 2717–2720. [Google Scholar] [CrossRef]
- Wanas, A.S.; Fouad, M.A.; Kamel, M.S.; Matsunami, K.; Otsuka, H. Bioactive triterpene saponins from the leaves of Schefflera elegantissima. Nat. Prod. Commun. 2013, 8, 765–769. [Google Scholar]
- Phat, N.T.; Hoa, L.T.V.; Tri, M.D.; Dung, L.T.; Minh, P.N.; Da, B.T. Two new oleanane-type triterpene saponins from the leaves of Schefflera sessiliflora. Phytochem. Lett. 2015, 11, 102–105. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, D.; Khan, F.A.; Zhang, C.L.; Liu, Y.F.; Chen, R.Y.; Choudhary, M.I.; Yu, D.Q. Chemical constituents from Schefflera leucantha R.Vig. (Araliaceae). Biochem. Syst. Ecol. 2020, 91, 104076. [Google Scholar] [CrossRef]
- Maeda, C.; Ohtani, K.; Kasai, R.; Yamasaki, K.; Nguyen, M.D.; Nguyen, T.N.; Nguyen, K.Q. Oleanane and ursane glycosides from Schefflera octophylla. Phytochemistry 1994, 37, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Duan, Y.-H.; Li, M.-M.; Tang, W.; Wu, X.; Wang, G.C.; Ye, W.-C.; Zhou, G.-X.; Li, Y.-L. Triterpenoid saponins from the stem barks of Schefflera heptaphylla. Planta Med. 2013, 79, 1348–1355. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Gallo, M.B.C.; Sarachine, M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009, 3, 46–66. [Google Scholar]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003, 65, 472–474. [Google Scholar]
- Mbaveng, T.A.; Sandjo, P.L.; Tankeo, B.T.; Ndifor, R.A.; Pantaleon, A.; Ngadjui, T.B.; Kuete, V. Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes resistant Gram-negative phenotypes. SpringerPlus 2015, 4, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; Dos Santos, T.C.; Coube, C.S.; Leitao, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
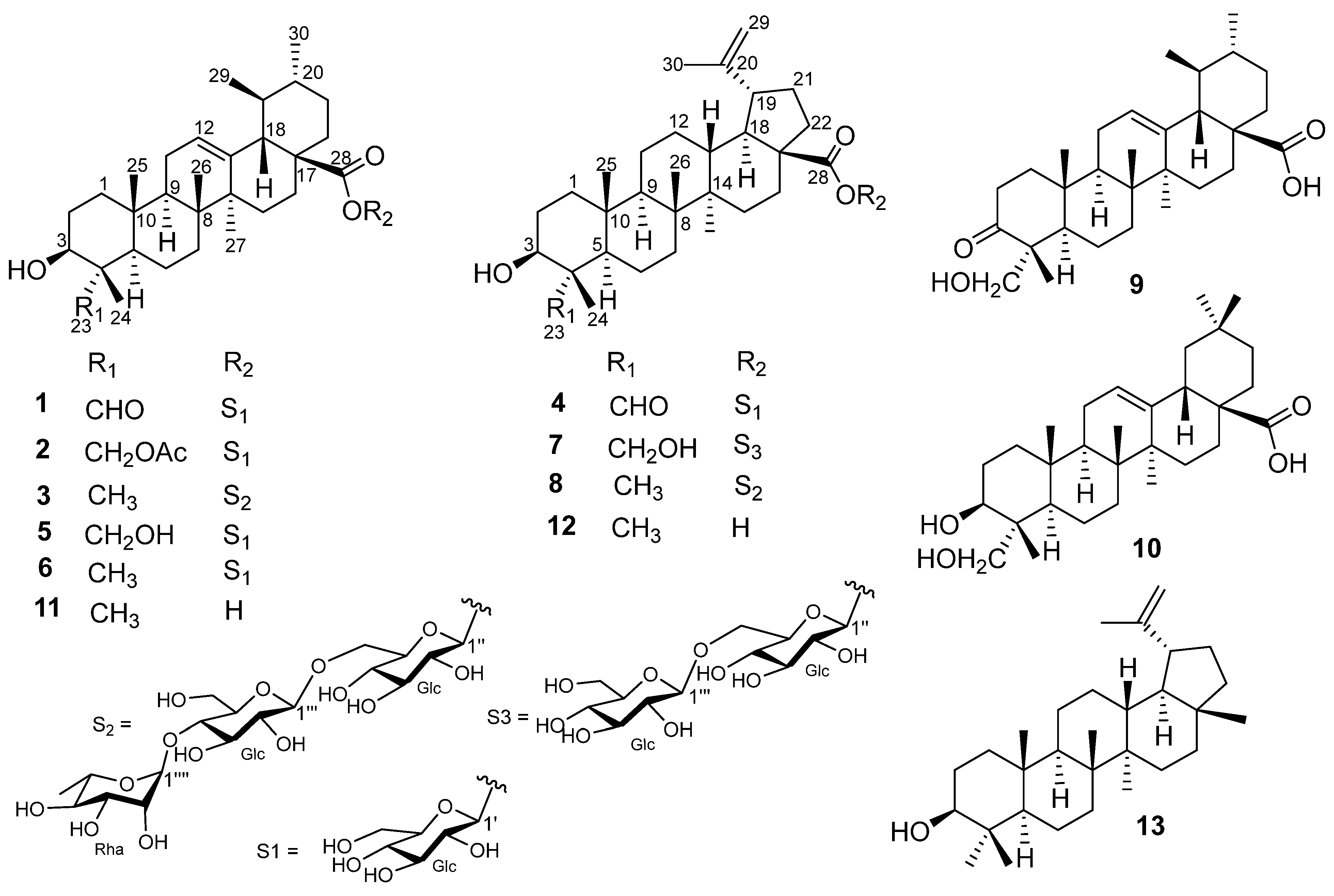
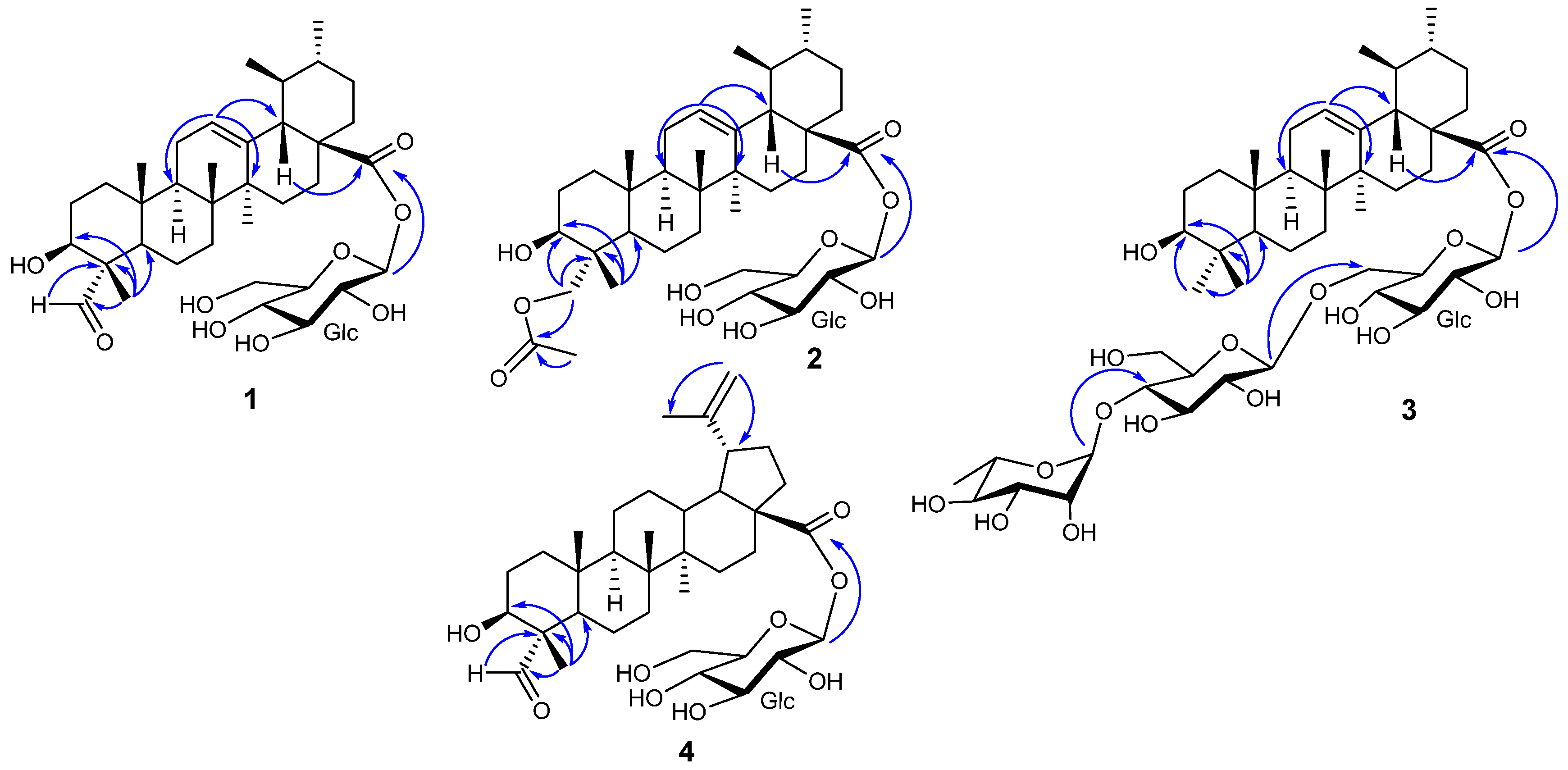
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | |
| 1 | 38.2 | 1.64 (o), 1.04 (m) | 38.3 | 1.70 (o), 1.02 (o) | 38.6 | 1.67 (m), 1.01 (o) | 38.2 | 1.67 (o), 0.93 (o) |
| 2 | 25.5 | 1.60 (o) | 25.9 | 1.65 (o) | 26.5 | 1.57 (o) | 25.8 | 1.57 (o), 0.99 (o) |
| 3 | 71.4 | 3.67 (o) | 71.3 | 3.55 (dd, 11.4, 5.1) | 78.1 | 3.16 (m) | 71.4 | 3.64 (m) |
| 4 | 55.4 | 41.4 | 38.5 | 55.5 | ||||
| 5 | 47.3 | 1.22 (o) | 47.5 | 1.13 (o) | 55.3 | 0.76 (o) | 47.4 | 1.19 (o) |
| 6 | 20.4 | 0.78 (m) | 17.8 | 1.40 (o) | 18.2 | 1.56 (m) | 20.6 | 0.72 (m), 1.36 (o) |
| 7 | 32.2 | 1.45 (o),1.17 (m) | 32.6 | 1.33 (m), 1.48 (m) | 32.9 | 1.34 (o), 1.51 (o) | 33.4 | 1.36 (o), 1.20 (o) |
| 8 | 39.9 | 39.8 | 39.6 | 42.2 | ||||
| 9 | 47.5 | 1.58 (o) | 47.8 | 1.57 (o) | 47.6 | 1.56 (o) | 50.5 | 1.36 (o) |
| 10 | 35.5 | 36.4 | 36.7 | 38.2 | ||||
| 11 | 23.0 | 1.86 (m) | 23.1 | 1.96 (o) | 23.1 | 1.95 (o) | 20.7 | 1.35 (o) |
| 12 | 125.6 | 5.16 (t, 3.8) | 125.8 | 5.27 (brt, 3.8) | 125.9 | 5.25 (m) | 25.2 | 1.64 (m), 0.99 (m) |
| 13 | 138.0 | 137.8 | 137.9 | 38.1 | 2.20 (o) | |||
| 14 | 41.9 | 41.8 | 41.7 | 41.1 | ||||
| 15 | 28.0 | 1.94 (m) 1.08 (m) | 27.9 | 1.95 (o) 1.09 (o) | 27.5 | 1.93 (o) 1.07 (o) | 29.5 | 1.45(dd, 13.0, 3.3), 1.02 (o) |
| 16 | 23.6 | 1.87 (m), 1.64 (o) | 23.9 | 2.08 (o), 1.77 (m) | 25.2 | 1.75 (o) | 31.4 | 2.25 (o), 1.36 (o) |
| 17 | 47.7 | 47.9 | 48.2 | 56.6 | ||||
| 18 | 52.9 | 2.14 (d, 11.3) | 52.9 | 2.25 (d, 11.3) | 52.8 | 2.25 (m) | 49.2 | 1.57 (o) |
| 19 | 38.9 | 1.30 (o) | 30.0 | 1.41 (o) | 39.1 | 1.41 (m) | 47.1 | 2.91 (td, 11.0, 4.6) |
| 20 | 38.8 | 0.88 (m) | 38.8 | 0.99 (o) | 39.0 | 0.98 (o) | 150.4 | |
| 21 | 29.5 | 1.41 (o), 1.31 (o) | 30.4 | 1.52 (o), 1.36 (m) | 30.4 | 1.52 (o), 1.35 (o) | 30.1 | 1.83 (o), 1.28 (o) |
| 22 | 36.1 | 1.52 (m), 1.66 (o) | 36.1 | 1.77 (o), 1.66 (o) | 36.3 | 1.75 (o), 1.62 (o) | 36.5 | 1.88 (m), 1.35 (o) |
| 23 | 207.1 | 9.20 (s) | 65.6 | 4.03 (d, 11.3) 3.90 (d, 11.3) | 27.5 | 0.99 (o) | 207.2 | 9.17 (s) |
| 24 | 8.1 | 0.90 (o) | 11.4 | 0.76 (s) | 15.0 | 0.79 (s) | 7.7 | 0.88 (s) |
| 25 | 15.0 | 0.90 (o) | 15.1 | 1.02 (s) | 14.9 | 0.98 (o) | 15.4 | 0.80 (s) |
| 26 | 16.5 | 0.74 (s) | 16.6 | 0.86 (s) | 16.6 | 0.85 (s) | 15.2 | 0.86 (s) |
| 27 | 22.6 | 1.04 (s) | 22.5 | 1.12 (o) | 22.6 | 1.12 (s) | 13.7 | 0.92 (s) |
| 28 | 176.6 | 176.5 | 176.5 | 174.7 | ||||
| 29 | 16.3 | 0.80 (d, 6.5) | 16.3 | 0.92 (d, 6.4) | 16.3 | 0.92 (d, 6.5) | 108.9 | 4.62 (brd, 2.4) 4.50 (brdd, 2.4, 1.4) |
| 30 | 20.3 | 0.86 (d, 5.8) | 20.1 | 0.99 (o) | 20.2 | 0.99 (d, 7.2) | 18.2 | 1.60 (s) |
| 23-COCH3 | 171.4 | |||||||
| 23-COCH3 | 19.4 | 2.07 (s) | ||||||
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | |
| 1′ | 94.3 | 5.24 (d, 8.0) | 94.4 | 5.36 (d, 8.1) | 94.6 | 5.32 (d, 8.1) | 93.8 | 5.39 (d, 8.2) |
| 2′ | 72.5 | 3.21 (o) | 72.5 | 3.32 (o) | 72.5 | 3.35 (o) | 72.7 | 3.21 (o) |
| 3′ | 77.3 | 3.24 (o) | 77.1 | 3.35 (o) | 76.8 | 3.43 (o) | 77.4 | 3.27 (o) |
| 4′ | 69.7 | 3.25 (o) | 69.8 | 3. 37 (o) | 69.7 | 3.42 (0) | 69.7 | 3.27 (o) |
| 5′ | 76.5 | 3.28 (o) | 76.9 | 3.41 (o) | 76.5 | 3.51 (o) | 77.0 | 3.27 (o) |
| 6′ | 61.0 | 3.58 (dd, 11.9, 4.3) 3.68 (o) | 61.9 | 3.58 (dd, 11.9, 2.0) 3.68 (dd, 11.9, 4.3) | 68.5 | 4.10 (dd, 11.8, 2.0) 3.78 (o) | 60.9 | 3.60 (o) 3.73 (m) |
| 1″ | 103.1 | 4.40 (d, 7.9) | ||||||
| 2″ | 73.9 | 3.25 (dd, 9.0, 7.9) | ||||||
| 3″ | 75.4 | 3.31 (o) | ||||||
| 4″ | 78.1 | 3.55 (o) | ||||||
| 5″ | 75.6 | 3.47 (o) | ||||||
| 6″ | 60.5 | 3.82 (o), 3.66 (o) | ||||||
| 1‴ | 101.5 | 4.86 (o) | ||||||
| 2‴ | 71.0 | 3.85 (o) | ||||||
| 3‴ | 70.9 | 3.64 (o) | ||||||
| 4‴ | 72.4 | 3.42 (o) | ||||||
| 5‴ | 69.3 | 3.99 (dd, 9.6, 6.2) | ||||||
| 6‴ | 16.4 | 1.29 (d, 6.2) | ||||||
| Samples | Minimum Inhibitory Concentrations (MIC, µg/mL) | |||
|---|---|---|---|---|
| SA1026 | SE35984 | EC10536 | KP13882 | |
| MeOH extract | 1024 | nd | 512 | 512 |
| EtOAc fraction | 512 | 128 | 128 | 256 |
| n-BuOH fraction | 1024 | 64 | 256 | 256 |
| 5 | nd | nd | nd | nd |
| 9 | nd | nd | nd | nd |
| 10 | 1024 | nd | nd | nd |
| 11 | nd | nd | nd | nd |
| 12 | nd | nd | nd | nd |
| 13 | nd | nd | nd | nd |
| Doxycycline | 2 | 8 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonga, S.L.K.; Tchegnitegni, B.T.; Siwe-Noundou, X.; Tsopmene, U.J.; Ponou, B.K.; Dzoyem, J.P.; Poka, M.; Demana, P.H.; Tapondjou, L.A.; Beukes, D.R.; et al. Manniosides G-J, New Ursane- and Lupane-Type Saponins from Schefflera mannii (Hook.f.) Harms. Molecules 2024, 29, 3447. https://doi.org/10.3390/molecules29153447
Tonga SLK, Tchegnitegni BT, Siwe-Noundou X, Tsopmene UJ, Ponou BK, Dzoyem JP, Poka M, Demana PH, Tapondjou LA, Beukes DR, et al. Manniosides G-J, New Ursane- and Lupane-Type Saponins from Schefflera mannii (Hook.f.) Harms. Molecules. 2024; 29(15):3447. https://doi.org/10.3390/molecules29153447
Chicago/Turabian StyleTonga, Simionne Lapoupée Kuitcha, Billy Toussie Tchegnitegni, Xavier Siwe-Noundou, Ulrich Joël Tsopmene, Beaudelaire Kemvoufo Ponou, Jean Paul Dzoyem, Madan Poka, Patrick H. Demana, Léon Azefack Tapondjou, Denzil R. Beukes, and et al. 2024. "Manniosides G-J, New Ursane- and Lupane-Type Saponins from Schefflera mannii (Hook.f.) Harms" Molecules 29, no. 15: 3447. https://doi.org/10.3390/molecules29153447
APA StyleTonga, S. L. K., Tchegnitegni, B. T., Siwe-Noundou, X., Tsopmene, U. J., Ponou, B. K., Dzoyem, J. P., Poka, M., Demana, P. H., Tapondjou, L. A., Beukes, D. R., Antunes, E. M., & Teponno, R. B. (2024). Manniosides G-J, New Ursane- and Lupane-Type Saponins from Schefflera mannii (Hook.f.) Harms. Molecules, 29(15), 3447. https://doi.org/10.3390/molecules29153447







