The TRPA1 Ion Channel Mediates Oxidative Stress-Related Migraine Pathogenesis
Abstract
1. Introduction
2. Oxidative Stress in Migraine
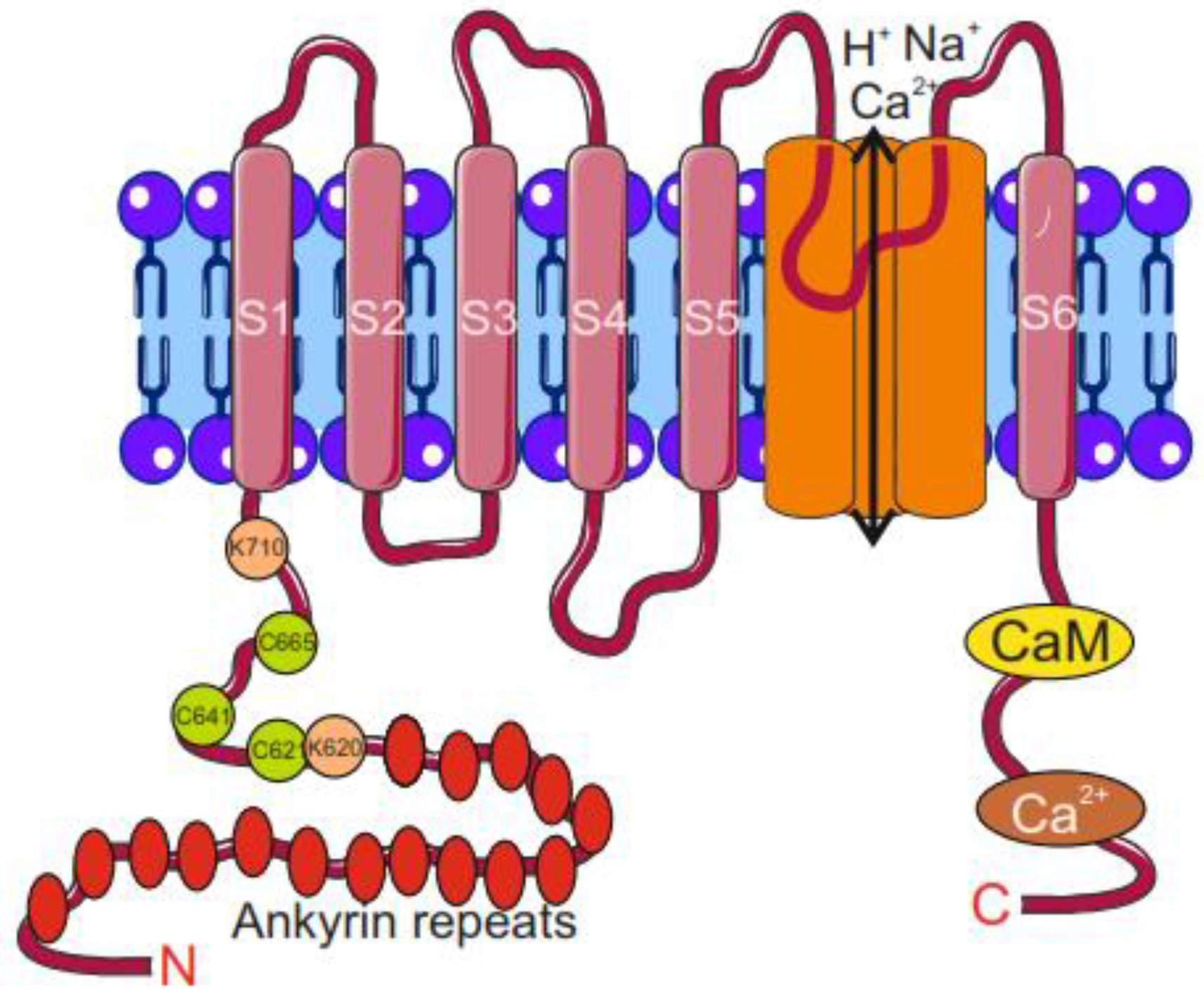
3. The TRPA1 Ion Channel: Its Gene, Structure, and Migraine-Related Activities
4. TRPA1 and Oxidative Stress
5. Role of TRPA1 in Oxidative Stress in Migraine
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bentivegna, E.; Galastri, S.; Onan, D.; Martelletti, P. Unmet Needs in the Acute Treatment of Migraine. Adv. Ther. 2024, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br. J. Clin. Pharmacol. 2015, 80, 193–199. [Google Scholar] [CrossRef]
- Harriott, A.M.; Strother, L.C.; Vila-Pueyo, M.; Holland, P.R. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J. Headache Pain 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Vongseenin, S.; Ha-ji-a-sa, N.; Thanprasertsuk, S.; Bongsebandhu-phubhakdi, S. Deciphering migraine pain mechanisms through electrophysiological insights of trigeminal ganglion neurons. Sci. Rep. 2023, 13, 14449. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. J. Neurosci. 2010, 30, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Levy, D.; Kainz, V.; Noseda, R.; Jakubowski, M.; Burstein, R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2011, 69, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Viana, F. Transduction and Encoding of Noxious Stimuli. In Encyclopedia of Pain; Schmidt, R.F., Willis, W.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 2515–2528. [Google Scholar]
- McEntire, D.M.; Kirkpatrick, D.R.; Dueck, N.P.; Kerfeld, M.J.; Smith, T.A.; Nelson, T.J.; Reisbig, M.D.; Agrawal, D.K. Pain transduction: A pharmacologic perspective. Expert Rev. Clin. Pharmacol. 2016, 9, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef]
- Raciti, L.; Raciti, G.; Militi, D.; Casella, C.; Calabrò, R. Chronic Migraine: A Narrative Review on the Use of Botulinum Toxin with Clinical Indications and Future Directions. J. Integr. Neurosci. 2022, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Tang, C. Implications of Transient Receptor Potential Cation Channels in Migraine Pathophysiology. Neurosci. Bull. 2021, 37, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.A. Channelopathy and Carbohydrates: Bad Mix for Migraines. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Dux, M.; Sántha, P.; Jancsó, G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J. Physiol. 2003, 552, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; Hargreaves, K.M.; Akopian, A.N. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 2009, 29, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Migraine triggers and oxidative stress: A narrative review and synthesis. Headache 2016, 56, 12–35. [Google Scholar] [CrossRef]
- Dussor, G.; Yan, J.; Xie, J.Y.; Ossipov, M.H.; Dodick, D.W.; Porreca, F. Targeting TRP channels for novel migraine therapeutics. ACS Chem. Neurosci. 2014, 5, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; Fusi, C.; Trevisan, G.; Geppetti, P. The TRPA1 channel in migraine mechanism and treatment. Br. J. Pharmacol. 2014, 171, 2552–2567. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Liu, W.; Hua, R.; Xu, H. Association between oxidative balance score and self-reported severe headache or migraine based on NHANES 1999 to 2004 data: A cross-sectional study. Heliyon 2024, 10, e27426. [Google Scholar] [CrossRef] [PubMed]
- Shatillo, A.; Koroleva, K.; Giniatullina, R.; Naumenko, N.; Slastnikova, A.A.; Aliev, R.R.; Bart, G.; Atalay, M.; Gu, C.; Khazipov, R.; et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience 2013, 253, 341–349. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, D.; Grubb, B.D.; Wang, M. ROS/TRPA1/CGRP signaling mediates cortical spreading depression. J. Headache Pain 2019, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. The Migraine Attack as a Homeostatic, Neuroprotective Response to Brain Oxidative Stress: Preliminary Evidence for a Theory. Headache 2018, 58, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Harnessing migraines for neural regeneration. Neural Regen. Res. 2018, 13, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Brain Energy Deficit as a Source of Oxidative Stress in Migraine: A Molecular Basis for Migraine Susceptibility. Neurochem. Res. 2021, 46, 1913–1932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Yue, G.; Zhao, Y. Energy metabolism disturbance in migraine: From a mitochondrial point of view. Front. Physiol. 2023, 14, 1133528. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, S.; Schwedt, T.J. Magnetic resonance spectroscopy studies in migraine. Neurobiol. Pain 2022, 12, 100102. [Google Scholar] [CrossRef] [PubMed]
- Grech, O.; Mollan, S.P.; Wakerley, B.R.; Fulton, D.; Lavery, G.G.; Sinclair, A.J. The Role of Metabolism in Migraine Pathophysiology and Susceptibility. Life 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Reyngoudt, H.; Paemeleire, K.; Descamps, B.; De Deene, Y.; Achten, E. 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia 2011, 31, 1243–1253. [Google Scholar] [CrossRef]
- Sparaco, M.; Feleppa, M.; Lipton, R.B.; Rapoport, A.M.; Bigal, M.E. Mitochondrial dysfunction and migraine: Evidence and hypotheses. Cephalalgia 2006, 26, 361–372. [Google Scholar] [CrossRef]
- Lodi, R.; Iotti, S.; Cortelli, P.; Pierangeli, G.; Cevoli, S.; Clementi, V.; Soriani, S.; Montagna, P.; Barbiroli, B. Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res. Bull. 2001, 54, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Reyngoudt, H.; Achten, E.; Paemeleire, K. Magnetic resonance spectroscopy in migraine: What have we learned so far? Cephalalgia 2012, 32, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.M.; Kalita, J.; Misra, U.K. A study of oxidative stress in migraine with special reference to prophylactic therapy. Int. J. Neurosci. 2018, 128, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, Y.; Dasdemir, S.; Gencer, M.; Bireller, E.S.; Ozkok, E.; Aydin, M.; Cakmakoglu, B. DNA repair gene variants in migraine. Genet. Test. Mol. Biomarkers 2014, 18, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Jablkowska, A.; Pawlowska, E.; Blasiak, J. DNA Damage and Repair in Migraine: Oxidative Stress and Beyond. Neuroscientist 2022, 29, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.G.; Park, S.P. Vitamin D Deficiency and Its Correlates in Migraine Patients. Ann. Indian Acad. Neurol. 2020, 23, 233–235. [Google Scholar] [CrossRef]
- Song, T.J.; Chu, M.K.; Sohn, J.H.; Ahn, H.Y.; Lee, S.H.; Cho, S.J. Effect of Vitamin D Deficiency on the Frequency of Headaches in Migraine. J. Clin. Neurol. 2018, 14, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yu, X.; Wu, L.; Zheng, H.; Zhong, X.; Xie, Y.; Wu, W. Vitamin B(6) and folate intake are associated with lower risk of severe headache or migraine in adults: An analysis based on NHANES 1999–2004. Nutr. Res. 2024, 121, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations. Oxid. Med. Cell. Longev. 2019, 2019, 7286737. [Google Scholar] [CrossRef]
- Ferreira, K.S.; Dhillon, H.; Velly, A.M. The role of a potential biomarker in patients with migraine: Review and new insights. Expert Rev. Neurother. 2021, 21, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharmacol. 2014, 167, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Takahashi, N.; Chen, C.; Pauli, J.; Kuroki, C.; Kaminosono, J.; Kashiwadani, H.; Kanmura, Y.; Mori, Y.; Ou, S.; et al. Article 576209 T (2020) Transient Receptor Potential Ankyrin 1 Mediates Hypoxic Responses in Mice. Front. Physiol. 2020, 11, 576209. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, R. A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 2008, 4, 372–379. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wei, H.; Yang, Z.; Luo, R.; Gao, Y.; Zhang, W.; Liu, X.; Sun, L. Molecular architecture and gating mechanisms of the Drosophila TRPA1 channel. Cell Discov. 2023, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.S.J.; Gaudet, R. How the TRPA1 receptor transmits painful stimuli: Inner workings revealed by electron cryomicroscopy. BioEssays 2015, 37, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Sub-Cell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Du, Q.; Gu, J.; Wu, J.; Liu, Q.; Li, Z.; Zhang, T.; Xu, J.; Xie, R. Research Progress on TRPA1 in Diseases. J. Membr. Biol. 2023, 256, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rajagopal, S. A Comprehensive Review on the Regulatory Action of TRP Channels: A Potential Therapeutic Target for Nociceptive Pain. Neurosci. Insights 2023, 18, 26331055231220340. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Chapman, H.; Jalava, N.; Korjamo, T.; Saarnilehto, M.; Lindstedt, K.; Pertovaara, A. TRPA1: A transducer and amplifier of pain and inflammation. Basic Clin. Pharmacol. Toxicol. 2014, 114, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Körtési, T.; Vécsei, L. TRP Channels: Recent Development in Translational Research and Potential Therapeutic Targets in Migraine. Int. J. Mol. Sci. 2022, 24, 700. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Dou, B.; Zhang, Y.; Chen, Z.; Li, Y.; Fan, Z.; Ma, Y.; Du, S.; Wang, J.; Xu, Z.; et al. Inflammation-the role of TRPA1 channel. Front. Physiol. 2023, 14, 1093925. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Tassorelli, C.; Zanaboni, A.M.; Tonsi, G.; Francesconi, O.; Nativi, C.; Greco, R. The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: Evaluation in an animal model. J. Headache Pain 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Al-Karagholi, M.A.; Kalatharan, V.; Fagerberg, P.S.; Amin, F.M. The vascular role of CGRP: A systematic review of human studies. Front. Neurol. 2023, 14, 1204734. [Google Scholar] [CrossRef] [PubMed]
- Levy, D. CGRP signalling in migraine: Time to look downstream? Brain 2023, 146, 4796–4798. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Goadsby, P.J. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Investig. Drugs 2001, 10, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009, 104, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.N.; Gonzales, A.L.; Pires, P.W.; Bruhl, A.; Leo, M.D.; Li, W.; Oulidi, A.; Boop, F.A.; Feng, Y.; Jaggar, J.H.; et al. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal. 2015, 8, ra2. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Azubuine, J.; Schmeer, C. A systematic literature review on the role of glial cells in the pathomechanisms of migraine. Front. Mol. Neurosci. 2023, 16, 1219574. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Li Puma, S.; Landini, L.; Portelli, F.; Innocenti, A.; de Araujo, D.S.M.; Janal, M.N.; Patacchini, R.; Bunnett, N.W.; Geppetti, P.; et al. Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J. Clin. Investig. 2019, 129, 5424–5441. [Google Scholar] [CrossRef] [PubMed]
- Kozai, D.; Ogawa, N.; Mori, Y. Redox regulation of transient receptor potential channels. Antioxid. Redox Signal. 2014, 21, 971–986. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Mori, Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radical Biol. Med. 2020, 146, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Montagna, D. Transient Receptor Potential Ankyrin 1 (TRPA1) Channel as a Sensor of Oxidative Stress in Cancer Cells. Cells 2023, 12, 1261. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003.E7. [Google Scholar] [CrossRef] [PubMed]
- Faris, P.; Rumolo, A.; Pellavio, G.; Tanzi, M.; Vismara, M.; Berra-Romani, R.; Gerbino, A.; Corallo, S.; Pedrazzoli, P.; Laforenza, U.; et al. Transient receptor potential ankyrin 1 (TRPA1) mediates reactive oxygen species-induced Ca(2+) entry, mitochondrial dysfunction, and caspase-3/7 activation in primary cultures of metastatic colorectal carcinoma cells. Cell Death Discov. 2023, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Song, X.; Long, D. Transient receptor potential ankyrin 1 and calcium: Interactions and association with disease (Review). Exp. Ther. Med. 2021, 22, 1462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chang, R.B.; Waters, H.N.; McKemy, D.D.; Liman, E.R. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 2008, 283, 32691–32703. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.D.; Timperley, C.M. TRPA1 and issues relating to animal model selection for extrapolating toxicity data to humans. Hum. Exp. Toxicol. 2019, 39, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Wang, Z.; Zubcevic, L.; Hsu, A.L.; He, Q.; Borgnia, M.J.; Ji, R.-R.; Lee, S.-Y. Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 2020, 105, 882–894.e885. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Hinman, A.; Chuang, H.H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef]
- Takahashi, N.; Kuwaki, T.; Kiyonaka, S.; Numata, T.; Kozai, D.; Mizuno, Y.; Yamamoto, S.; Naito, S.; Knevels, E.; Carmeliet, P.; et al. TRPA1 underlies a sensing mechanism for O2. Nat. Chem. Biol. 2011, 7, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, Y.; Blair, N.T. Benzoquinone reveals a cysteine-dependent desensitization mechanism of TRPA1. Mol. Pharmacol. 2013, 83, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cvetkov, T.L.; Chance, M.R.; Moiseenkova-Bell, V.Y. Identification of in vivo disulfide conformation of TRPA1 ion channel. J. Biol. Chem. 2012, 287, 6169–6176. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Kiselar, J.; Pumroy, R.A.; Han, S.; Moiseenkova-Bell, V.Y. Structural insights into the molecular mechanism of mouse TRPA1 activation and inhibition. J. Gen. Physiol. 2018, 150, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, S.W. Emerging roles of TRPA1 in sensation of oxidative stress and its implications in defense and danger. Arch. Pharm. Res. 2013, 36, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Hatano, N.; Itoh, Y.; Suzuki, H.; Muraki, Y.; Hayashi, H.; Onozaki, K.; Wood, I.C.; Beech, D.J.; Muraki, K. Hypoxia-inducible factor-1α (HIF1α) switches on transient receptor potential ankyrin repeat 1 (TRPA1) gene expression via a hypoxia response element-like motif to modulate cytokine release. J. Biol. Chem. 2012, 287, 31962–31972. [Google Scholar] [CrossRef] [PubMed]
- Pandi, A.; Lal, V.; Chakraborty, B.; Kalappan, V.M. Evaluation of Oxidative Stress and Antioxidant Biomarkers in Chronic Cigarette Smokers: A Pilot Study. Cureus 2024, 16, e60629. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.H.; Seng, E.K. The Relationship of Tobacco Use and Migraine: A Narrative Review. Curr. Pain Headache Rep. 2023, 27, 39–47. [Google Scholar] [CrossRef]
- Schürks, M.; Rist, P.M.; Bigal, M.E.; Buring, J.E.; Lipton, R.B.; Kurth, T. Migraine and cardiovascular disease: Systematic review and meta-analysis. BMJ 2009, 339, b3914. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Conklin, D.J.; Haberzettl, P.; Jagatheesan, G.; Kong, M.; Hoyle, G.W. Role of TRPA1 in acute cardiopulmonary toxicity of inhaled acrolein. Toxicol. Appl. Pharmacol. 2017, 324, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Kurhanewicz, N.; McIntosh-Kastrinsky, R.; Tong, H.; Ledbetter, A.; Walsh, L.; Farraj, A.; Hazari, M. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol. Appl. Pharmacol. 2017, 324, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Kichko, T.I.; Kobal, G.; Reeh, P.W. Cigarette smoke has sensory effects through nicotinic and TRPA1 but not TRPV1 receptors on the isolated mouse trachea and larynx. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L812–L820. [Google Scholar] [CrossRef] [PubMed]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Lau, G.S.; Taylor, W.R.; Critchley, J.A. Acute effects of the potent lacrimator o-chlorobenzylidene malononitrile (CS) tear gas. Hum. Exp. Toxicol. 1996, 15, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Marone, I.M.; De Logu, F.; Nassini, R.; De Carvalho Goncalves, M.; Benemei, S.; Ferreira, J.; Jain, P.; Li Puma, S.; Bunnett, N.W.; Geppetti, P.; et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.L.; Olesen, J. Nitric oxide in primary headaches. Curr. Opin. Neurol. 2001, 14, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008, 120, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Williamson, D.J.; Kaube, H.; Goadsby, P.J. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002, 137, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, J.; Bowen, E.J.; Russo, A.F.; Durham, P.L. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur. J. Neurosci. 2006, 23, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Grant, S.; Wadsworth, R.M. Selective Arterial Dilatation by Glyceryl Trinitrate Is Not Associated with Nitric Oxide Formation in vitro. J. Vasc. Res. 2008, 45, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tian, G.F.; Peng, W.; Lou, N.; Lovatt, D.; Hansen, A.J.; Kasischke, K.A.; Nedergaard, M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat. Neurosci. 2007, 10, 754–762. [Google Scholar] [CrossRef]
- Benemei, S.; De Logu, F.; Li Puma, S.; Marone, I.M.; Coppi, E.; Ugolini, F.; Liedtke, W.; Pollastro, F.; Appendino, G.; Geppetti, P.; et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br. J. Pharmacol. 2017, 174, 2897–2911. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Dodick, D.; Goadsby, P.J.; Reuter, U.; Silberstein, S.; Zhang, F.; Gage, J.R.; Cheng, S.; Mikol, D.D.; Lenz, R.A. Erenumab (AMG 334) in episodic migraine: Interim analysis of an ongoing open-label study. Neurology 2017, 89, 1237–1243. [Google Scholar] [CrossRef]
- Benemei, S.; Dussor, G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.J. Anti-Calcitonin Gene-Related Peptide (CGRP) Therapies: Update on a Previous Review After the American Headache Society 60th Scientific Meeting, San Francisco, June 2018. Headache 2018, 58 (Suppl. S3), 276–290. [Google Scholar] [CrossRef] [PubMed]
- Geppetti, P.; Veldhuis, N.A.; Lieu, T.; Bunnett, N.W. G Protein-Coupled Receptors: Dynamic Machines for Signaling Pain and Itch. Neuron 2015, 88, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Santos-Otte, P.; Leysen, H.; van Gastel, J.; Hendrickx, J.O.; Martin, B.; Maudsley, S. G Protein-Coupled Receptor Systems and Their Role in Cellular Senescence. Comput. Struct. Biotechnol. J. 2019, 17, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Ashina, S.; Bentivegna, E.; Martelletti, P.; Eikermann-Haerter, K. Structural and Functional Brain Changes in Migraine. Pain Ther. 2021, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Kruit, M.C.; Launer, L.J.; Ferrari, M.D.; van Buchem, M.A. Brain stem and cerebellar hyperintense lesions in migraine. Stroke 2006, 37, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Kruit, M.C.; van Buchem, M.A.; Launer, L.J.; Terwindt, G.M.; Ferrari, M.D. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: The population-based MRI CAMERA study. Cephalalgia 2010, 30, 129–136. [Google Scholar] [CrossRef]
- Ge, R.; Chang, J.; Cao, Y. Headache disorders and relevant sex and socioeconomic patterns in adolescents and young adults across 204 countries and territories: An updated global analysis. J. Headache Pain 2023, 24, 110. [Google Scholar] [CrossRef] [PubMed]
- Persoons, E.; Kerselaers, S.; Voets, T.; Vriens, J.; Held, K. Partial Agonistic Actions of Sex Hormone Steroids on TRPM3 Function. Int. J. Mol. Sci. 2021, 22, 13652. [Google Scholar] [CrossRef]
- Artero-Morales, M.; González-Rodríguez, S.; Ferrer-Montiel, A. TRP Channels as Potential Targets for Sex-Related Differences in Migraine Pain. Front. Mol. Biosci. 2018, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Cabañero, D.; Villalba-Riquelme, E.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. ThermoTRP channels in pain sexual dimorphism: New insights for drug intervention. Pharmacol. Ther. 2022, 240, 108297. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.; Alonso, I.; Barros, J.; Sequeiros, J.; Pereira-Monteiro, J.; Mendonça, D.; Sousa, A. Assessing risk factors for migraine: Differences in gender transmission. PLoS ONE 2012, 7, e50626. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, D.; Ying, C.; Hong, Y. Association between brain structures and migraine: A bidirectional Mendelian randomization study. Front. Neurosci. 2023, 17, 1148458. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fila, M.; Przyslo, L.; Derwich, M.; Sobczuk, P.; Pawlowska, E.; Blasiak, J. The TRPA1 Ion Channel Mediates Oxidative Stress-Related Migraine Pathogenesis. Molecules 2024, 29, 3385. https://doi.org/10.3390/molecules29143385
Fila M, Przyslo L, Derwich M, Sobczuk P, Pawlowska E, Blasiak J. The TRPA1 Ion Channel Mediates Oxidative Stress-Related Migraine Pathogenesis. Molecules. 2024; 29(14):3385. https://doi.org/10.3390/molecules29143385
Chicago/Turabian StyleFila, Michal, Lukasz Przyslo, Marcin Derwich, Piotr Sobczuk, Elzbieta Pawlowska, and Janusz Blasiak. 2024. "The TRPA1 Ion Channel Mediates Oxidative Stress-Related Migraine Pathogenesis" Molecules 29, no. 14: 3385. https://doi.org/10.3390/molecules29143385
APA StyleFila, M., Przyslo, L., Derwich, M., Sobczuk, P., Pawlowska, E., & Blasiak, J. (2024). The TRPA1 Ion Channel Mediates Oxidative Stress-Related Migraine Pathogenesis. Molecules, 29(14), 3385. https://doi.org/10.3390/molecules29143385







