Study on the Anti-Inflammatory Mechanism of Coumarins in Peucedanum decursivum Based on Spatial Metabolomics Combined with Network Pharmacology
Abstract
1. Introduction
2. Results and Discussion
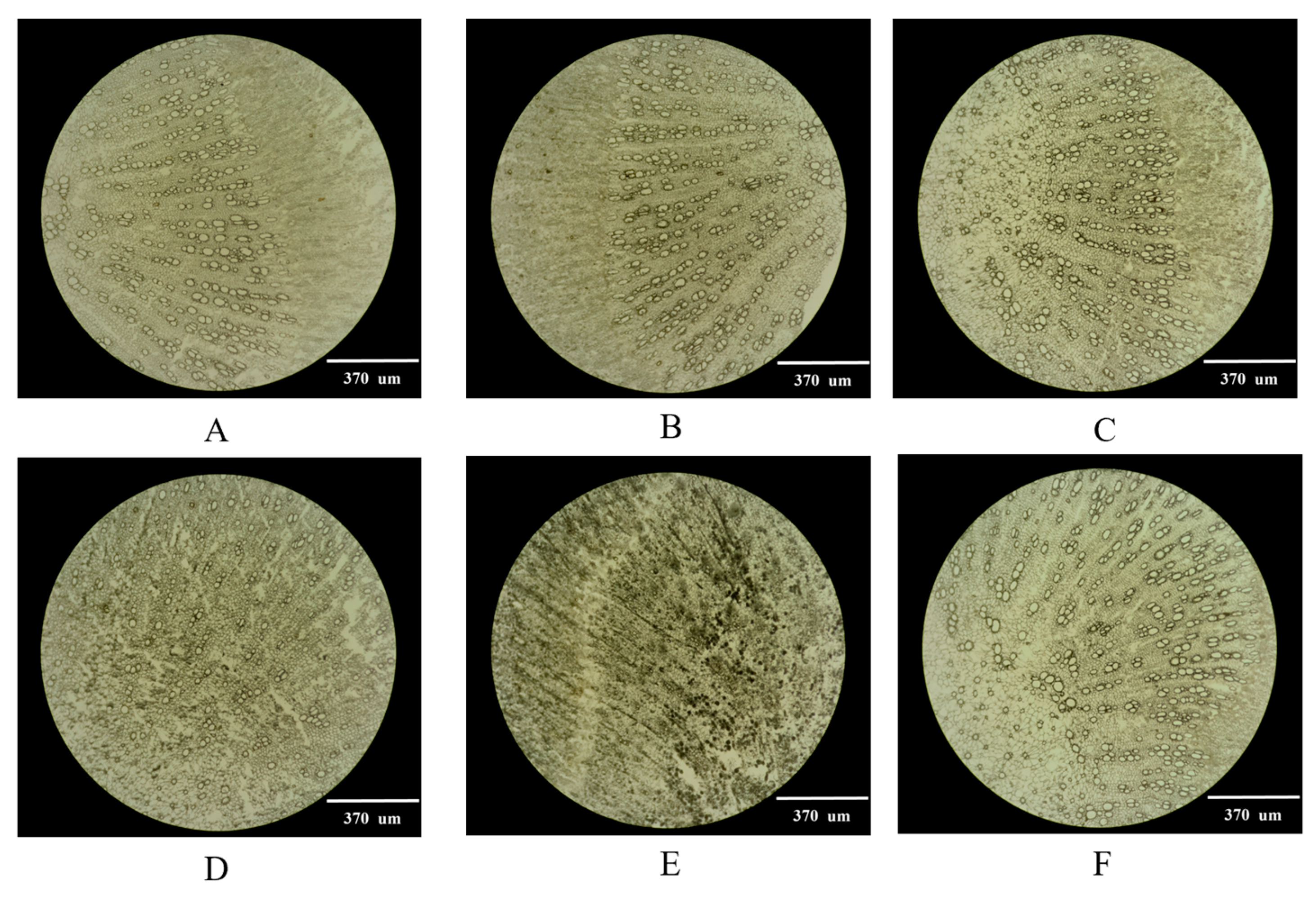
2.1. Selection of the Optimal Thickness of Frozen Sections
2.2. Fluorescence Imaging of the Distribution of Coumarins in the Root of P. decursivum
2.3. Selection of the Matrix
2.4. Distribution Characteristics of Coumarins in the Root of P. decursivum
2.5. Network Pharmacology Research
2.5.1. Active Ingredient and Disease Target Prediction
2.5.2. Protein–Protein Interaction (PPI) Network Analysis
2.5.3. Gene Ontology (GO) Enrichment Analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
2.5.4. Molecular Docking
3. Materials and Methods
3.1. Materials and Reagents
3.2. Fluorescence Imaging Technology for Tissue Distribution of Coumarins in the Root of P. decursivum
3.2.1. Selection of Frozen Section Thickness
3.2.2. Localization and Observation of Coumarins
3.3. Study on the Spatial Distribution of Coumarins in the Root of P. decursivum Using MALDI-TOF-MSI
3.3.1. Preparation of Standard Solutions and Screening of Matrices
3.3.2. Spraying of the Substrate
3.3.3. Sample Preparation
3.3.4. MALDI-TOF-MSI
3.4. Network Pharmacology
3.4.1. Screening of Active Ingredients
3.4.2. Target Prediction
3.4.3. Construction of a PPI Network Diagram and Screening of the Core Targets
3.4.4. GO Enrichment Analysis and KEGG Pathway Analysis
3.4.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shan, F.; Hao, J.D.; Huang, L.Q. Discussion on efficacy of traditional Chinese medicine “Zi-hua Qianhu” in Chinese Pharmacopoeia (2010 Edition). Chin. J. Chin. Mater. Med. 2015, 40, 2464–2469. [Google Scholar]
- Zhou, Y. Identification of Radix Peucedani and its adulterant Radix Peucedani Terebinthaceum. China Pharm. 2008, 14, 62–63. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1, pp. 352–353.
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Jayakumar, T.; Huang, C.J.; Yen, T.L.; Hsia, C.W.; Sheu, J.R.; Bhavan, P.S.; Huang, W.C.; Hsieh, C.Y.; Hsia, C.H. Activation of Nrf2 by esculetin mitigates inflammatory responses through suppression of NF-κB signaling sascade in RAW 264.7 cells. Molecules 2022, 27, 5143. [Google Scholar] [CrossRef]
- Min, J.S.; Jin, Y.-H.; Kwon, S. Auraptene has antiviral activity against human coronavirus OC43 in MRC-5 cells. Nutrients 2023, 15, 2960. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, J.; Yu, L.L.; Wang, C.L.; Yang, Y.J.; Rong, X.; Xu, K.; Chu, M.P. Antifungal activity of coumarin against candida albicans is related to apoptosis. Front. Cell. Infect. Microbiol. 2018, 8, 445. [Google Scholar] [CrossRef]
- Golfakhrabadi, F.; Abdollahi, M.; Ardakani, M.R.S.; Saeidnia, S.; Akbarzadeh, T.; Ahmadabadi, A.N.; Ebrahimi, A.; Yousefbeyk, F.; Hassanzadeh, A.; Khanavi, M. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm. Biol. 2014, 52, 1335–1340. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, M.H.; Wang, F.; Chen, P.Y.; Ke, X.G.; Yu, B.; Yang, Y.F.; You, P.T.; Wu, H.Z. Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sci. 2021, 270, 119105. [Google Scholar] [CrossRef]
- Wang, P.P.; Fan, Z.J.; Wei, W.P.; Yang, C.S.; Wang, Y.; Shen, X.; Yan, X.; Zhou, Z.H. Biosynthesis of the plant coumarin osthole by engineered saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 2455–2462. [Google Scholar] [CrossRef]
- Jin, Z.X.; Liao, X.Y.; Da, W.W.; Zhao, Y.J.; Li, X.F.; Tang, D.Z. Osthole enhances the bone mass of senile osteoporosis and stimulates the expression of osteoprotegerin by activating β-catenin signaling. Stem. Cell. Res. Ther. 2021, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, J.H.; Lim, C. Neuroprotective effect of Angelica gigas root in mouse of ischemic brain injury through MAPK signaling pathway regulation. Chin. Med. 2020, 15, 101. [Google Scholar] [CrossRef]
- Mcdonnell, L.A.; Römpp, A.; Balluff, B.; Heeren, R.M.; Albar, J.P.; Andrén, P.E.; Corthals, G.L.; Walch, A.; Stoeckli, M. Discussion point: Reporting guidelines for mass spectrometry imaging. Anal. Bioanal. Chem. 2015, 407, 2035–2045. [Google Scholar] [CrossRef]
- Parrot, D.; Papazian, S.; Foil, D.; Deniz, T. Imaging the unimaginable: Desorption electrospray ionization-imaging mass spectrometry (DESI-IMS) in natural product research. Planta. Med. 2018, 84, 584–593. [Google Scholar] [CrossRef]
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular imaging of biological Samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef]
- Weaver, E.M.; Hummon, A.B. Imaging mass spectrometry: From tissue sections to cell cultures. Adv. Drug Deliv. Rev. 2013, 65, 1039–1055. [Google Scholar] [CrossRef]
- Bjarnholt, N.; Li, B.; D’Alvise, J.; Janfelt, C. Mass spectrometry imaging of plant metabolites-principles and possibilities. Nat. Prod. Rep. 2014, 31, 818–837. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Nie, H.G. Advances in mass spectrometry imaging technology. Anal. Instrum. Cent. 2018, 5, 1–10. [Google Scholar]
- Kuo, T.H.; Huang, H.C.; Hsu, C.C. Mass spectrometry imaging guided molecular networking to expedite discovery and structural analysis of agarwood natural products. Anal Chim Acta. 2019, 1080, 95–103. [Google Scholar] [CrossRef]
- Mi, Y.L.; Sun, W.; Li, M.L.; Zhao, H.Y.; Bian, B.L.; Zhou, Y.Y. Application of MALDI-mass spectrometry imaging in spatial distribution of secondary metabolites in medicinal plants—A case study of Lepdium meyenii root. Chin. J. Chin. Mater. Med. 2022, 45, 596–601. [Google Scholar]
- Li, B.; Neumann, E.K.; Ge, J.Y.; Gao, W.; Yang, H.; Li, P.; Sweedler, J.V. Interrogation of spatial metabolome of Ginkgo biloba with high-resolution matrix-assisted laser desorption/ionization and laser desorption/ionization mass spectrometry imaging. Plant Cell Environ. 2018, 41, 2693–2703. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, H.S.; Sun, G.W.; Song, Z.; Xu, F.J.; Ma, M.Y.; Zhang, S.C. Visual analysis of spatial distribution of Panax notoginseng saponins using matrix assisted laser desorption mass spectrometry imaging. Chin. J. Anal. Chem. 2020, 48, 881–888. [Google Scholar]
- Nei, L.X.; Huang, L.Y.; Wang, X.P.; Lv, L.F.; Yang, X.X.; Jia, X.F.; Kang, S.A.; Wao, L.W.; Dai, Z.; Ma, S.C. Desorption electrospray ionization mass spectrometry imaging illustrates the quality characters of Isatidis Radix. Front. Plant Sci. 2022, 13, 897528. [Google Scholar]
- Yang, Y.; Yang, Y.; Qiu, H.; Ju, Z.C.; Shi, Y.C.; Wang, Z.T.; Yang, L. Localization of constituents for determining the age and parts of ginseng through ultraperfomance liquid chromatography quadrupole/time of flight-mass spectrometry combined with desorption electrospray ionization mass spectrometry imaging. J. Pharm. Biomed. Anal. 2021, 193, 113722. [Google Scholar] [CrossRef] [PubMed]
- Honeker, L.K.; Hildebrand, G.A.; Fudyma, J.D.; Daber, E.; Hoyt, D.; Flowers, S.E.; Gil-Loaiza, J.; Kübert, A.; Bamberger, I.; Anderton, C.R.; et al. Elucidating drought-tolerance mechanisms in plant roots through 1H NMR metabolomics in parallel with MALDI-MS, and nanoSIMS imaging techniques. Environ. Sci. Technol. 2022, 56, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J. Traditional medicine a culture in the balance. Nature 2007, 448, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.S.; Zhu, X.H. Application progress of network pharmacology in traditional Chinese medicine. J. Tradit. Chin. Med. 2021, 29, 98–102. [Google Scholar]
- Jiang, H.Y.; Gao, H.Y.; Li, J.; Zhou, T.Y.; Wang, S.T.; Yang, J.B.; Hao, R.R.; Pang, F.; Wei, F.; Liu, Z.G.; et al. Integrated spatially resolved metabolomics and network toxicology to investigate the hepatotoxicity mechanisms of component D of Polygonum multiflorum Thunb. J. Ethnopharm. 2022, 298, 115630. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Li, B.; Malitsky, S.; Rogachev, I.; Aharoni, A.; Kaftan, F.; Svatoš, A.; Franceschi, A. Sample preparation for mass spectrometry imaging of plant tissues: A review. Front Plant Sci. 2016, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhang, L.; Xiang, Y.H.; Ye, N.S.; Liu, K.H. Systematic study of tissue section thickness for MALDI MS profiling and imaging. Analyst 2023, 148, 888–897. [Google Scholar] [CrossRef]
- Peng, L.; Chen, H.G.; Zhou, X. Mass spectrometry imaging technology and its application in medicinal plants research. Chin. J. Chin. Mater. Med. 2020, 45, 1023–1033. [Google Scholar]
- Pei, X.L.; Liu, X.N.; Du, J.L.; Gong, C.; Xu, X. MALDI-MS imaging of lipids in corn using a flexible ultrasonic spraying device as matrix deposition method. Int. J. Mass Spectrom. 2020, 455, 116373. [Google Scholar] [CrossRef]
- Taira, S.; Kiriake-Yoshinaga, A.; Shikano, H.; Ikeda, R.; Kobayashi, S.; Yoshinaga, K. Localization analysis of essential oils in perilla herb (Perilla frutescens var. crispa) using derivatized mass spectrometry imaging. Food Sci. Nutr. 2021, 9, 2779–2784. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Ma, Y.H.; Zhang, Y. Research progress on chemical constituents and pharmacological effects of Angelicae Dahuricae Radix. Food Drug 2020, 22, 509–514. [Google Scholar]
- Marumoto, S.; Miyazawa, M. beta-secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother. Res. 2010, 24, 510–513. [Google Scholar] [CrossRef]
- Wu, Q.H.; Jiang, L.; Yan, Y.H.; Yan, Q.; Zhu, X.L.; Zhang, J.X.; Huang, C.F.; Zhou, T.; Ren, C.X.; Wen, F.Y.; et al. Geographical distribution-based differentiation of cultivated Angelica dahurica, exploring the relationship between the secretory tract and the quality. Sci. Rep. 2023, 13, 21733. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Paudel, S.B.; Hyun, C.; Lee, G.; Choi, H.-I.; Ryoo, G.-H.; Kil, Y.-S.; Nam, J.-W.; Jung, C.-H.; Kim, B.-R.; et al. Comparative analysis of coumarin profiles in different parts of Peucedanum japonicum and their aldo-keto reductase inhibitory activities. Molecules 2022, 27, 7391. [Google Scholar] [CrossRef] [PubMed]
- Emad, A.M.; Rasheed, D.M.; El-Kased, R.F.; El-Kersh, D.M. Antioxidant, antimicrobial activities and characterization of polyphenol-enriched extract of Egyptian celery (Apium graveolens L., Apiaceae) aerial parts via UPLC/ESI/TOF-MS. Molecules 2022, 27, 698. [Google Scholar] [CrossRef]
- Wei, C.C.; Guan, W.J.; Hu, D.D.; Zhou, J.; Li, X. Chemical constituents from Heracleum scabridum. J. Chin. Med. Mater. 2017, 40, 1105–1108. [Google Scholar]
- Wu, R.R.; Wu, X.D.; Wu, J.J.; Liu, G.R.; Chen, X.Y.; Wang, Z.; Dong, Z.K.; Tan, N.H. A novel micellar system of hybrid deep eutectic solvents for extracting coumarins from herbal medicines: As a case of Suhuang antitussive capsule. Microchem. J. 2022, 183, 107883. [Google Scholar] [CrossRef]
- Wang, B.; Liu, A.H.; Zhou, A.; Menga, M.; Li, Q.L. Simultaneous analysis of coumarin derivatives in extracts of Radix Angelicae pubescentis (Duhuo) by HPLC-DAD-ESI-MSN technique. Anal. Methods 2014, 6, 7996–8002. [Google Scholar] [CrossRef]
- Chen, F.Y.; Zou, Y.; Chen, J.; Huang, W.M.; Bian, Y.T.; Luo, Y.M. Studies on chemical constituents of Chloranthus fortunei. Chin. Tradit. Herb. Drugs. 2020, 51, 1485–1490. [Google Scholar]
- Niu, Y.; Wang, L.; Huang, X.J.; Zhang, X.Q.; Yin, Z.Q.; Ye, W.C. Chemical constituents of Citrus grandis ‘Tomentosa’. J. Jinan Univ. Med. Ed. 2012, 33, 501–505+515. [Google Scholar]
- Wei, W.; Yang, X.W.; Zhou, Y.Y. Chemical constituents from n-butanol soluble parts of roots of Angelica dahurica cv. Hangbaizhi. Mod. Chin. Med. 2017, 19, 630–634. [Google Scholar]
- Deng, G.G.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Wei, W.; Chen, T.L. Chemical constituents from lipophilic parts in roots of Angelica dahurica var. formosana cv. Chuanbaizhi. Chin. J. Chin. Mater. Med. 2015, 40, 2148–2156. [Google Scholar]
- Kang, H.; Lim, Y.; Yeo, W.S. Optimized MALDI-TOF mass analysis conditions for natural small molecules. Bull. Korean Chem. Soc. 2020, 41, 84–87. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.K.; Xiao, X.R.; Zhao, Q.; Huang, J.F.; Zhu, W.F.; Li, F. Metabolic profiling of coumarins by the combination of UPLC-MS-based metabolomics and multiple mass defect filter. Xenobiotica 2020, 50, 1076–1089. [Google Scholar] [CrossRef]
- Sun, X.C.; Zhang, C.M.; Li, J.N.; Feng, J.L.; Zhou, H.G.; Fu, S.; Chen, W.Q. Chemical constituents from Peucedanum decursivum. Chin. Tradit. Herb. Drugs. 2013, 44, 2044–2047. [Google Scholar]
- Yrjönen, T.; Vuorela, H.; Kauppila, T.J. Direct analysis of Peucedanum palustre samples by desorption atmospheric pressure photoionization-mass spectrometry. Phytochem. Lett. 2017, 20, 49–53. [Google Scholar] [CrossRef][Green Version]
- Li, B. Analysis of Chemical Constituents of Angelica dahurica and Study on Quality Standards for Urena lobata; Beijing University of Traditional Chinese Medicine: Beijing, China, 2014. [Google Scholar]
- Wan, M.Q.; Liu, X.Y.; Gao, H.; Wang, T.X.; Yang, Y.F.; Jia, L.Y.; Yang, X.W.; Zhang, Y.B. Systematic analysis of the metabolites of Angelicae pubescentis Radix by UPLC-Q-TOF-MS combined with metabonomics approaches after oral administration to rats. J. Pharm. Biomed. Anal. 2020, 188, 113445. [Google Scholar] [CrossRef]
- Luo, L.J.; Liu, X.; Jin, X.X.; Liu, Y.K.; Ma, J.; Zhang, S.; Zhang, D.M.; Chen, X.G.; Sheng, L.; Li, Y. Simultaneous determination of skimmin, apiosylskimmin, 7-hydroxycoumarin and 7-hydroxycoumarin glucuronide in rat plasma by liquid chromatography–Orbitrap mass spectrometry and its application to pharmacokinetics. Biomed. Chromatogr. 2022, 36, e5223. [Google Scholar] [CrossRef]
- Fiorito, S.; Palumbo, L.; Epifano, F.; Fraternale, D.; Collevecchio, C.; Genovese, S. Modulation of the biosynthesis of oxyprenylated coumarins in calli from Ferulago campestris elicited by ferulic acid. Biomass Conv. Bioref. 2022, 9, 1–7. [Google Scholar] [CrossRef]
- Hu, M.Y.; Fan, Y.X.; Xu, R.W.; Sun, Y.J.; Dong, C.M.; Feng, W.S.; Chen, H. Study on the chemical constituents of Lycium chinense var. potaninii and α-glucosidase inhibitory activity. J. Chin. Med. Mater. 2024, 3, 624–628. [Google Scholar]
- Liu, Y.J. The Pharmacognostical Study on Peucedanum praeruptorum Dunn. and Peucedanum decursivum (Miq.) Maxim; Hunan University of Chinese Medicine: Changsha, China, 2013. [Google Scholar]
- Robe, K.; Conéjéro, G.; Gao, F.; Lefebvre-Legendre, L.; Sylvestre-Gonon, E.; Rofidal, V.; Hem, S.; Rouhier, N.; Barberon, M.; Hecker, A.; et al. Coumarin accumulation and trafficking in Arabidopsis thaliana: A complex and dynamic process. N. Phytol. 2020, 229, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ji, D.T.; Gao, H. Spatial distribution of coumarins in Angelica pubescens fresh roots by MALDI-MSI. Chin. Tradit. Herb. Drugs. 2023, 54, 3438–3445. [Google Scholar]
- Wang, Q.D.; Zhao, C.L.; Wang, X.T.; Cheng, Y.J.; Han, L.; Long, X.Y.; Yu, B.; Liu, J.; Li, G.J. Research progress on distribution, extraction and analysis, and biological activity of natural citrus coumarins. Food Ferment. Ind. 2024, 50, 343–355. [Google Scholar]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.P.; Li, Y.B.Q.; Song, C.; Manzoor, M.A.; Dai, J.; Yin, Q.H.; Zhang, Y.M.; Han, B.X. Analysis of coumarin content and key enzyme genes expression involved in coumarin biosynthesis from Peucedanum praeruptorum Dunn at different stages. Acta Physiol. Plant. 2023, 45, 141. [Google Scholar] [CrossRef]
- Li, F.; Yousif, M.; Huang, R.Y.; Qiao, Y.L.; Hu, Y.C. Network pharmacology- and molecular docking-based analyses of the antihypertensive mechanism of Ilex kudingcha. Front. Endocrinol. 2023, 14, 1216086. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, Y.Y.; Gao, H.; Li, Z.Y.; Qiu, D.Y.; Hu, G.Z. In situ analysis of volatile oil in Angelica sinensis roots by fluorescence imaging combined with mass spectrometry imaging. Talanta 2023, 255, 124253. [Google Scholar] [CrossRef]
- Ni, X.Y.; Ni, X.D.; Liu, F.F.; Liao, S.N.; Wu, S.P.; Qin, D.; Cao, J.J.; Zhang, Q.; Lai, Q.X.; Fu, P. Research progress in the application of coumarin and its derivatives. Res. Zhuang Yao Ethn. Med. 2023, 1, 302–303. [Google Scholar]
- Du, H.T.; Wang, L.; Ding, J.; Du, Y.X.; Wang, P. Application status and challenges of molecular docking in development of traditional Chinese medicine. Chin. J. Chin. Mater. Med. 2024, 49, 671–680. [Google Scholar]
- Tu, W.Q.; Liang, Q.E.; Xie, P.C.; Xie, T.; Lin, L.Q.; Chen, L.G. Mechanism of Xiaoji Pills in treatment of atherosclerosis in apoE-/- mice based on TNF-α/IKKB/NF-κB signaling pathway. Chin. Tradit. Herb. Drugs. 2022, 53, 5074–5084. [Google Scholar]
- Wang, J.; Bian, Y.Y.; Zhang, C.; Kai, G.; Lu, Y.M. Research progress of TNF-α and its receptors in rheumatoid arthritis. Br. J. Pharm. Pract. 2017, 35, 289–293. [Google Scholar]
- Xu, Y. Curcumin Promotes Post-Stroke Depression by Activating the cAMP/PKA Pathway to Inhibit the Inflammatory; Nanhua University: Hengyang, China, 2020. [Google Scholar]









| Standard Compound | Matrix | Ionic Mode | Relative Ionic Intensities (%) |
|---|---|---|---|
| Nodakenin | CHCA | positive | 11,199 |
| negative | 32 | ||
| DHB | positive | 22 | |
| negative | -- | ||
| 9-AA | positive | 21 | |
| negative | 27 | ||
| Imperatorin | CHCA | positive | 223,641 |
| negative | 949 | ||
| DHB | positive | 2064 | |
| negative | 49 | ||
| 9-AA | positive | 521 | |
| negative | 1620 | ||
| Oxypeucedanin | CHCA | positive | 101,031 |
| negative | 3488 | ||
| DHB | positive | 6623 | |
| negative | 174 | ||
| 9-AA | positive | 83 | |
| negative | 36 |
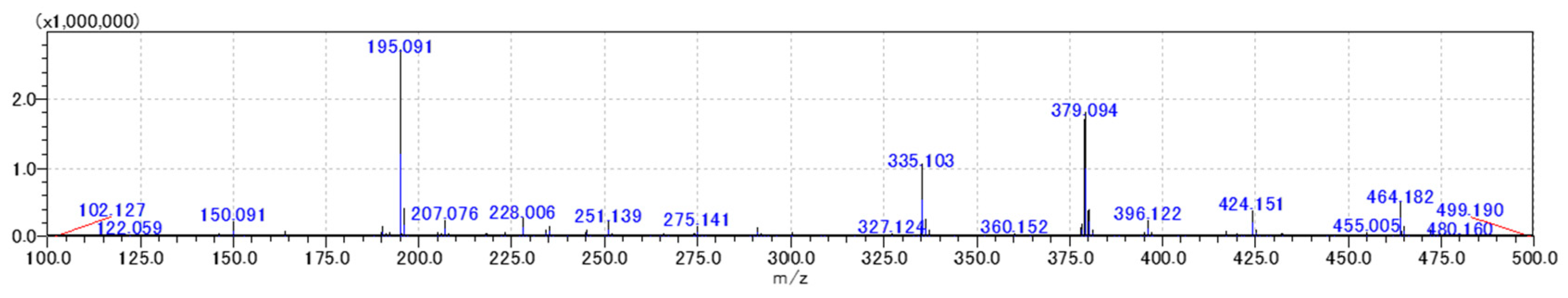
| Compound (or Its Isomer) | Molecular Formula | Adduct Ions | Theoretical (m/z) | Observed (m/z) | Error (ppm) | Ref. |
|---|---|---|---|---|---|---|
| Nodakenin | C20H24O9 | [M + Na]+ | 433.1385 | 433.1383 | −0.46 | [34] |
| Oxypeucedanin | C16H14O5 | [M + H]+ | 289.0987 | 289.0969 | −6.23 | [35] |
| Imperatorin | C16H14O4 | [M + Na]+ | 294.0824 | 294.0847 | 7.82 | [35] |
| Byakangelicol | C17H16O6 | [M + H]+ | 318.1059 | 318.1048 | −3.46 | [35] |
| Phellopterin | C17H16O5 | [M + H]+ | 301.1077 | 301.1055 | −7.31 | [36] |
| Oxypeucedanin hydrate | C16H16O6 | [M + H]+ | 305.1026 | 305.1066 | −6.56 | [37] |
| Isopimpinellin | C13H10O5 | [M + K]+ | 286.0199 | 286.0150 | −17.13 | [38] |
| Praeruptorin B | C24H26O7 | [M]+ | 427.1712 | 427.1724 | 2.81 | [39] |
| Praeruptorin A | C21H22O7 | [M + H]+ | 387.1445 | 387.1453 | 2.07 | [40] |
| Osthenol | C14H14O3 | [M]+ | 232.1010 | 232.0963 | −20.25 | [41] |
| Scopolin | C16H18O9 | [M + Na]+ | 377.0849 | 377.0789 | −15.91 | [42] |
| Isomeranzin | C15H16O4 | [M + Na]+ | 283.0947 | 232.0963 | 7.78 | [43] |
| Byakangelicin | C17H18O7 | [M + K]+ | 374.0723 | 374.0629 | −2.51 | [44] |
| Dehydrogeijerin | C15H14O4 | [M + H]+ | 259.0971 | 259.1008 | 14.28 | [45] |
| Decursin | C19H20O5 | [M + H]+ | 329.1344 | 329.1328 | −4.86 | [46] |
| Scoparone | C11H10O4 | [M + H]+ | 208.0692 | 208.0702 | 4.81 | [47] |
| Herniarin | C10H8O3 | [M + H]+ | 177.0552 | 177.0583 | 17.51 | [38] |
| Columbianetin acetate | C16H16O5 | [M]+ | 290.1040 | 290.1017 | −7.93 | [48] |
| Umbelliprenine | C24H30O3 | [M + K]+ | 405.1832 | 405.1748 | −20.73 | [49] |
| Oxypeucedanin hydrate-3″-ethyl ether | C18H20O6 | [M + Na]+ | 355.1158 | 355.1184 | 7.32 | [50] |
| Angelol A | C20H24O7 | [M + Na]+ | 400.1454 | 400.1477 | 5.75 | [51] |
| Pabulenol | C17H16O4 | [M + H]+ | 286.1161 | 286.1147 | −4.89 | [51] |
| Apiosylskimmin | C20H24O12 | [M + H]+ | 459.1414 | 459.1455 | 8.93 | [52] |
| Auraptene | C19H22O3 | [M + K]+ | 338.1239 | 338.1214 | −7.39 | [53] |
| Scopoletin | C10H8O4 | [M + K]+ | 231.0060 | 231.0059 | −0.43 | [54] |
| Angenomalin | C14H12O3 | [M + H]+ | 230.0899 | 230.0894 | −2.17 | [51] |
| (+)-Decursinol | C14H14O4 | [M]+ | 246.0892 | 246.0882 | −4.06 | [45] |
| MOL ID | MOL Name | CAS |
|---|---|---|
| MOL001999 | Scoparone | 120-08-1 |
| MOL013434 | Auraptene | 495-02-3 |
| MOL004653 | Praeruptorin B | 81740-07-0 |
| MOL013079 | Praeruptorin A | 73069-25-7 |
| MOL005800 | Byakangelicol | 26091-79-2 |
| MOL005806 | Oxypeucedanin hydrate | 133164-11-1 |
| MOL003608 | Columbianetin acetate | 23180-65-6 |
| MOL013077 | Decursin | 5928-25-6 |
| MOL004792 | Nodakenin | 495-31-8 |
| MOL001941 | Imperatorin | 482-44-0 |
| MOL002644 | Phellopterin | 2543-94-4 |
| Targets | Binding Energy (kcal/mol) | ||
|---|---|---|---|
| Scoparone | Decursin | Columbianetin Acetate | |
| TNF | −6.4 | −7.8 | −5.8 |
| PTGS2 | −7.3 | −8.6 | −7.5 |
| PRKACA | −5.5 | −7.9 | −6.8 |
| No. | Ingredients | Molecular Weight | Signal Intensity |
|---|---|---|---|
| 1 | Scopaone | 208.0702 | 31,187 |
| 2 | Oxypeucedanin | 289.0969 | 18,697 |
| 3 | Auraptene | 338.1214 | 10,089 |
| 4 | Praeruptorin B | 427.1724 | 9969 |
| 5 | Praeruptorin A | 387.1453 | 8560 |
| 6 | (±)-Decursinol | 246.0882 | 8334 |
| 7 | Byakangelicol | 318.1048 | 7330 |
| 8 | Oxypeucedanin hydrate | 305.1006 | 6887 |
| 9 | Columbianetin acetate | 290.1017 | 6299 |
| 10 | Decursin | 329.1328 | 6033 |
| 11 | Nodakenin | 433.1383 | 5930 |
| 12 | Imperatorin | 294.0847 | 3593 |
| 13 | Phellopterin | 301.1055 | 3269 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Q. Study on the Anti-Inflammatory Mechanism of Coumarins in Peucedanum decursivum Based on Spatial Metabolomics Combined with Network Pharmacology. Molecules 2024, 29, 3346. https://doi.org/10.3390/molecules29143346
Li Z, Li Q. Study on the Anti-Inflammatory Mechanism of Coumarins in Peucedanum decursivum Based on Spatial Metabolomics Combined with Network Pharmacology. Molecules. 2024; 29(14):3346. https://doi.org/10.3390/molecules29143346
Chicago/Turabian StyleLi, Zeyu, and Qian Li. 2024. "Study on the Anti-Inflammatory Mechanism of Coumarins in Peucedanum decursivum Based on Spatial Metabolomics Combined with Network Pharmacology" Molecules 29, no. 14: 3346. https://doi.org/10.3390/molecules29143346
APA StyleLi, Z., & Li, Q. (2024). Study on the Anti-Inflammatory Mechanism of Coumarins in Peucedanum decursivum Based on Spatial Metabolomics Combined with Network Pharmacology. Molecules, 29(14), 3346. https://doi.org/10.3390/molecules29143346






