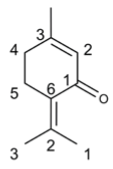
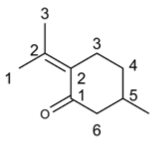
Abstract
Viruses pose a significant threat to human health, causing widespread diseases and impacting the global economy. Perilla frutescens, a traditional medicine and food homologous plant, is well known for its antiviral properties. This systematic review examines the antiviral potential of Perilla frutescens, including its antiviral activity, chemical structure and pharmacological parameters. Utilizing bioinformatics analysis, we revealed the correlation between Perilla frutescens and antiviral activity, identified overlaps between Perilla frutescens target genes and virus-related genes, and explored related signaling pathways. Moreover, a classified summary of the active components of Perilla frutescens, focusing on compounds associated with antiviral activity, provides important clues for optimizing the antiviral drug development of Perilla frutescens. Our findings indicate that Perilla frutescens showed a strong antiviral effect, and its active ingredients can effectively inhibit the replication and spread of a variety of viruses in this review. The antiviral mechanisms of Perilla frutescens may involve several pathways, including enhanced immune function, modulation of inflammatory responses, and inhibition of key enzyme activities such as viral replicase. These results underscore the potential antiviral application of Perilla frutescens as a natural plant and provide important implications for the development of new antiviral drugs.
1. Introduction
The role of viral infection in human diseases is significant, and ensuring the prevention of viral infection is a crucial aspect in safeguarding public health. Certain infectious diseases demonstrate extensive spread and high infectivity, profoundly impacting the global economy and politics. Furthermore, some viruses can induce chronic infectious conditions such as human immunodeficiency virus (HIV) [1,2], hepatitis B virus (HBV) [3,4], and hepatitis C virus (HCV) [5,6]. These conditions progress gradually and chronically, leading to reduced labor capacity in patients and decreased life expectancy. Consequently, these viruses profoundly affect both the quality of life for patients as well as economic aspects. The association between certain tumors and viruses is well-established, such as Epstein Barr virus with nasopharyngeal carcinoma [7,8], human papillomavirus with cervical cancer [9,10,11], and human herpesvirus type 8 (HHV-8) with Kaposi’s sarcoma [12,13]. Viruses exhibit a high mutation rate and continuously generate new variants, posing a significant threat to human health.
The antiviral potential of numerous natural compounds has been demonstrated in various studies, revealing the ability of numerous plant extracts and secondary metabolites to effectively inhibit viral replication and transmission [14]. The mechanisms of antiviral action are diverse, encompassing interference with viral entry into host cells, inhibition of viral gene expression, disruption of viral assembly, and augmentation of the host immune response [15]. For instance, flavonoids primarily inhibit viral protease activity to prevent viral replication [16]. On the other hand, terpenoids mainly interfere with the fusion of viruses and host cell membranes to impede virus entry into host cells [17]. Additionally, certain polyphenolic compounds directly hinder the cytopathic effect [18]. These findings establish a crucial scientific foundation for the development of novel antiviral medications.
The annual herb Perilla frutescens (L.) Britt., belonging to the Labiatae family, exhibits medicinal and culinary properties in traditional Chinese medicine (TCM) [19]. Its dried stems, leaves, and seeds have been utilized as medicinal materials. Perilla frutescens has demonstrated pharmacological activities, including anti-fungal [20], antiviral [21], anti-cancer [22,23], hypoglycemic, and heart-protective effects [24,25]. In this paper, the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://old.tcmsp-e.com/tcmsp.php, accessed on 23 July 2023) were used to sort all reported monomer components of Perilla frutescens by oral bioavailability (OB). The term “oral bioavailability” (OB) refers to the extent and rate at which a drug is absorbed into the systemic circulation. It serves as a crucial parameter for objectively assessing both the oral bioavailability and intrinsic quality of a drug, while also serving as a pivotal criterion for determining its potential as a therapeutic agent. A higher OB value indicates an increased likelihood of clinical development for the compound [26]. Then, the names of monomer components of Perilla frutescens exhibiting OB values exceeding 20% were searched in PubMed and Web of Science databases, using keywords such as “antiviral”. More than 200 recently published papers were reviewed and discussed. This paper focuses on reviewing the antiviral activities of these primary components and their derivatives. The antiviral mechanisms are described in terms of the chemical structure, pharmacological parameters, and bioavailability of these components against viruses.
This review classification summarizes the antiviral abilities of monomeric components of Perilla frutescens with OB greater than 20% against various viruses and their mechanism of action. Furthermore, exploring potential synergistic effects by combining these drugs could pave the way for developing more effective antiviral strategies. In summary, Perilla frutescens shows promising potential as an antiviral drug candidate, highlighting the need for further preclinical studies and clinical trials.
4. Discussion
Viruses can give rise to a range of diseases, including COVID-19 [194], hepatitis B [195], AIDS [196], influenza [197], and others. Certain viral infections have the potential to cause local and even global disruptions, posing substantial risks to public health.
The annual herb Perilla frutescens (L.) Britt., belonging to the Labiatae family, with a long-standing history in China, possesses an abundance of medicinal benefits [198]. Among numerous traditional Chinese medicine prescriptions, Perilla frutescens stands out due to its distinct antiviral efficacy and garners high comments from physicians throughout various dynasties. In the realm of Chinese traditional medicine, Perilla frutescens is often combined with other herbal materials to treat medical conditions such as colds, coughs, asthma, and other viral diseases. For instance, classical Chinese medicine formulas like “Guizhi Soup” in Zhang Zhongjing’s “Typhoid Theory” feature Perilla frutescens as the principal herb for treating fever and headache caused by external wind-cold pathogens. Furthermore, Perilla frutescens can also be incorporated with other herbal ingredients to formulate remedies like Scattered Leaves of Perilla frutescens or Scattered Stems of Perilla that enhance its antiviral properties while promoting surface releasing and dispelling coldness.
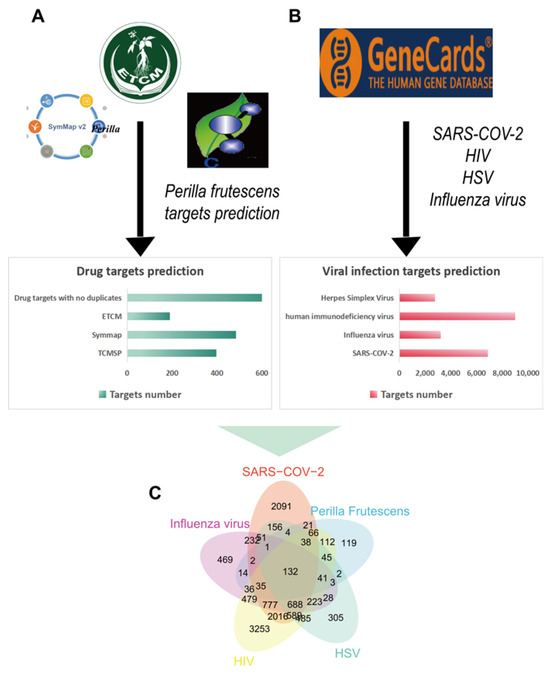
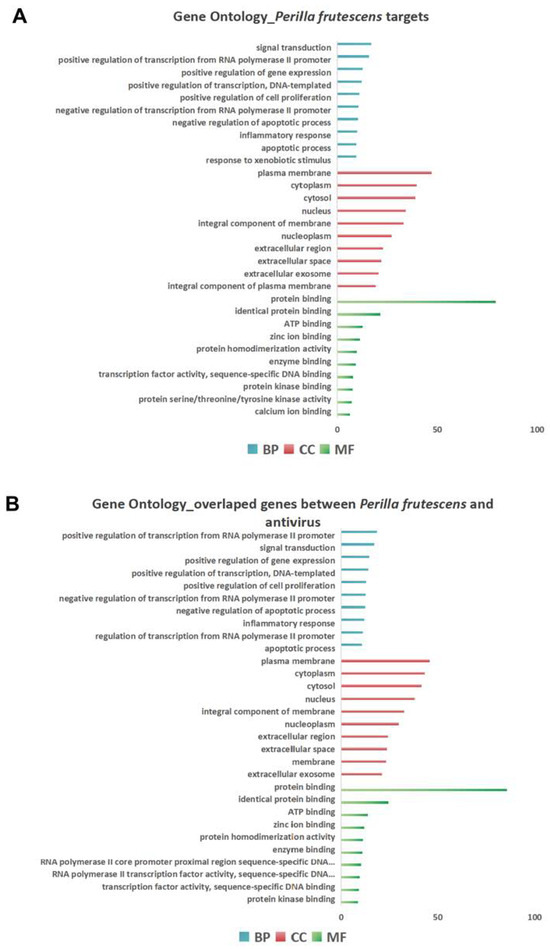
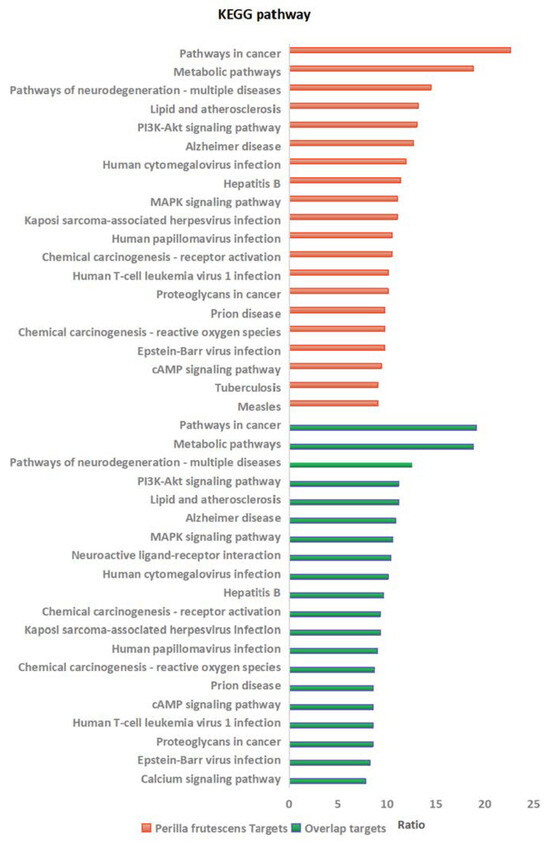
This study utilized bioinformatics analysis methods for the first time to identify target genes associated with perilla from multiple databases. The analysis revealed a significant overlap between target genes of Perilla frutescens and the genes associated with various viral infections (such as HSV, SARS-CoV-2, influenza virus, and HIV), indicating the substantial antiviral potential of Perilla frutescens. GO enrichment analysis and KEGG pathway enrichment analysis conducted using the DAVID platform demonstrated that Perilla frutescens primarily participates in biological processes including signal transduction, transcriptional regulation, negative regulation of apoptosis, and inflammatory responses. The results of GO enrichment analysis indicated significant predicted enrichment of target genes of Perilla frutescens in biological processes, molecular functions, and cellular components mainly involving regulatory functions and response mechanisms. These analyses provide theoretical support for the antiviral activity of Perilla frutescens and serve as a reference for further research on its pharmacological effects and the development of related drugs.
From the literature review, it is evident that Perilla frutescens contains key active components such as phenolic compounds and terpenes, which exhibit potent antiviral potential through diverse mechanisms of action against viruses. Among these compounds, certain ones inhibit virus attachment and entry into cells. For instance, thymol prevented HIV-1 entry into target cells by altering the cholesterol content of the viral membrane [44]. Additionally, some compounds interfere with the late stages of virus release. Perillyl alcohol, for example, inhibits the release of infectious HSV-1 particles during maturation in Vero cells [91]. Other compounds like β-sitosterol exert their antiviral activity by directly inactivating viral particles [168]. Furthermore, certain compounds indirectly exert their antiviral effects through immune system regulation. For example, treatment with different doses of Methyl caffeate increased the expression of IL-2, IL-4, IFN-g, soluble Fas in HIV-infected mice [67]. The anti-influenza virus mechanism of pulegone is related to its regulation of IFN-α, IFN-β and IL-2 [46]. Moreover, some compounds demonstrate antiviral activity through antioxidant properties or by inhibiting viral replication protein synthesis or inflammatory response pathways. These compounds exhibit strong antiviral activity without cytotoxicity under tested conditions. In vivo mouse models have also confirmed that these compounds have therapeutic effects on virus-infected mice. Furthermore, combinations of certain compounds show synergistic antiviral effects. For instance, Eugenol combined with acyclovir synergistically inhibited herpes virus replication in vitro [56], while the combination of germacone and oseltamivir demonstrated an additive effect in suppressing influenza virus infection both in vitro and in vivo [101]; these findings provided new insights for developing more effective strategies for antiviral therapy and drug combinations.
However, previous studies have primarily focused on modeling virus infection in vitro using cell lines, with only a limited number of recent studies validating these findings through in vivo experiments in mice. Nevertheless, there is a significant lack of clinical trial data to substantiate the therapeutic effects on humans. Therefore, further clinical studies are necessary to evaluate the safety, efficacy, and potential clinical applications of Perilla frutescens as an antiviral agent. Additionally, some active ingredients in Perilla frutescens have relatively low bioavailability and do not strictly adhere to Lipinski’s rules [199]. These physicochemical properties may affect drug absorption, distribution, and metabolism thereby impacting their antiviral effects in vivo. Furthermore, although Perilla frutescens has demonstrated antiviral activity against a wide range of viruses, it is important to note that different viruses possess unique replication mechanisms and infection routes, leading to the development of diverse diseases. Therefore, it is imperative to conduct an in-depth investigation into the antiviral mechanism of the components derived from Perilla frutescens. In addition to the summarized antiviral components above, there are several active components present within Perilla frutescens that require further investigation. Future studies should aim at gaining insight into the mechanism of action for different viral infection models using Perilla frutescens to gain a more comprehensive understanding of its antiviral activity providing effective strategies for treating virus-related diseases.
In conclusion, Perilla frutescens has shown remarkable potential as a potent antiviral agent. Its efficacy in combating viral infections extends beyond humans and encompasses other species as well. Consequently, Perilla frutescens holds significant application prospects in the field of antiviral therapy. Given this, it is crucial to further research and develop Perilla frutescens and its primary constituents to enhance its antiviral capabilities. Moreover, efforts should be made to mitigate the adverse effects of viral infections on public health by deriving effective prevention strategies from these natural drugs such as Perilla frutescens.
Author Contributions
Conducted the study: Y.L.; manuscript draft: J.C. (Jing Chen); manuscript revision: Y.Z., J.C. (Jie Cheng), S.P. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_4035) to J.C. and the National Natural Science Foundation of China 82101630 to Y.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
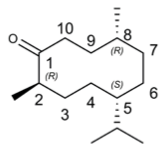
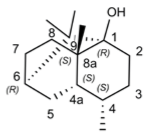
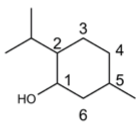
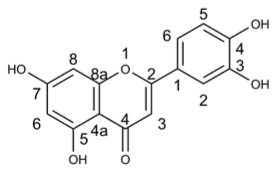
We express my gratitude to Mengzhu Xue from Shanghai Jiao Tong University School of Medicine for providing an exceptional course on Professional Chemical Structural Formula Writing.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Gallo, R.C. HIV/AIDS Research for the Future. Cell Host Microbe 2020, 27, 499–501. [Google Scholar] [CrossRef]
- Van Heuvel, Y.; Schatz, S.; Rosengarten, J.F.; Stitz, J. Infectious RNA: Human Immunodeficiency Virus (HIV) Biology, Therapeutic Intervention, and the Quest for a Vaccine. Toxins 2022, 14, 138. [Google Scholar] [CrossRef]
- Shih, C.; Yang, C.C.; Choijilsuren, G.; Chang, C.H.; Liou, A.T. Hepatitis B Virus. Trends Microbiol. 2018, 26, 386–387. [Google Scholar] [CrossRef]
- Tsai, K.N.; Kuo, C.F.; Ou, J.J. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol. 2018, 26, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M. Hepatitis C Virus: 30 Years after Its Discovery. Cold Spring Harb. Perspect. Med. 2019, 9, a037069. [Google Scholar] [CrossRef]
- Lee, J.; Ou, J.J. Hepatitis C virus and intracellular antiviral response. Curr. Opin. Virol. 2022, 52, 244–249. [Google Scholar] [CrossRef]
- Vasudevan, H.N.; Yom, S.S. Nasopharyngeal Carcinoma and Its Association with Epstein-Barr Virus. Hematol. Oncol. Clin. N. Am. 2021, 35, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, Y.; Huang, Y.; Ye, J.; Xie, S.; He, Q.; Chen, Y.; Lin, Y.; Liang, R.; Wei, J.; et al. Nasopharyngeal Carcinoma Progression: Accumulating Genomic Instability and Persistent Epstein-Barr Virus Infection. Curr. Oncol. 2022, 29, 6035–6052. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2021, 88, 187–197. [Google Scholar] [CrossRef]
- Yuan, Y.; Cai, X.; Shen, F.; Ma, F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021, 497, 243–254. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- Dupin, N. Update on oncogenesis and therapy for Kaposi sarcoma. Curr. Opin. Oncol. 2020, 32, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.; Dai, L.; Wang, S.; Qin, Z. Kaposi’s sarcoma-associated herpesvirus and extracellular vesicles. J. Med. Virol. 2021, 93, 3294–3299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, S.; Bai, Y.; Guo, J.; Kai, G.; Huang, X.; Jia, X. Promising natural products against SARS-CoV-2: Structure, function, and clinical trials. Phytother. Res. PTR 2022, 36, 3833–3858. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Q.; Zhang, W.; Lai, Y.; Long, H.; Huang, H.; Zhan, S.; Liu, X.; Lai, J.; Zhang, Z.; et al. Inhibitory effects of Patchouli alcohol on the early lifecycle stages of influenza A virus. Front. Microbiol. 2022, 13, 938868. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dong, S.; Chen, H.; Guo, M.; Sun, Z.; Luo, H. Perilla frutescens: A traditional medicine and food homologous plant. Chin. Herb. Med. 2023, 15, 369–375. [Google Scholar] [CrossRef]
- Chen, L.; Qu, S.; Yang, K.; Liu, M.; Li, Y.X.; Keller, N.P.; Zeng, X.; Tian, J. Perillaldehyde: A promising antifungal agent to treat oropharyngeal candidiasis. Biochem. Pharmacol. 2020, 180, 114201. [Google Scholar] [CrossRef]
- Tang, W.F.; Tsai, H.P.; Chang, Y.H.; Chang, T.Y.; Hsieh, C.F.; Lin, C.Y.; Lin, G.H.; Chen, Y.L.; Jheng, J.R.; Liu, P.C.; et al. Perilla (Perilla frutescens) leaf extract inhibits SARS-CoV-2 via direct virus inactivation. Biomed. J. 2021, 44, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Ju, J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr. Res. Pract. 2015, 9, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kang, S.J.; Rhee, W.J. Perilla-Leaf-Derived Extracellular Vesicles Selectively Inhibit Breast Cancer Cell Proliferation and Invasion. Int. J. Mol. Sci. 2023, 24, 15633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.W.; Zhang, L. Perilla frutescens Leaf Extract and Fractions: Polyphenol Composition, Antioxidant, Enzymes (α-Glucosidase, Acetylcholinesterase, and Tyrosinase) Inhibitory, Anticancer, and Antidiabetic Activities. Foods 2021, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Korotkich, I.; Senikiene, Z.; Simoniene, G.; Lazauskas, R.; Laukeviciene, A.; Kevelaitis, E. Inotropic and lusitropic effects of Perilla frutescens (L.) Britton extract on the rabbit myocardium. Medicina 2006, 42, 406–412. [Google Scholar] [PubMed]
- Ren, Y.; Nie, L.; Zhu, S.; Zhang, X. Nanovesicles-Mediated Drug Delivery for Oral Bioavailability Enhancement. Int. J. Nanomed. 2022, 17, 4861–4877. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.31–31.30.33. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Cox, P.B.; Njardarson, J.T. Phenols in Pharmaceuticals: Analysis of a Recurring Motif. J. Med. Chem. 2022, 65, 7044–7072. [Google Scholar] [CrossRef]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Righi, N.; Boumerfeg, S.; Deghima, A.; Fernandes, P.A.R.; Coelho, E.; Baali, F.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Phenolic profile, safety assessment, and anti-inflammatory activity of Salvia verbenaca L. J. Ethnopharmacol. 2021, 272, 113940. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Ascrizzi, R.; Chiboub, W.; Cheikh Mhamed, A.; ElAyeb, A.; Skhiri, F.; Tounsi Saidani, M.; Mastouri, M.; Flamini, G. Volatiles, phenolic compounds, antioxidant and antibacterial properties of kohlrabi leaves. Nat. Prod. Res. 2022, 36, 3143–3148. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Armenta, F.J.; Leyva, J.M.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Cruz-Valenzuela, M.R.; Esqueda, M.; Gutierrez, A.; Nazzaro, F.; Fratianni, F.; Gaitán-Hernández, R.; et al. Phenolic compounds of Phellinus spp. with antibacterial and antiviral activities. Braz. J. Microbiol. 2022, 53, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. 2019, 13, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Zrig, A. The Effect of Phytocompounds of Medicinal Plants on Coronavirus (2019-NCOV) Infection. Pharm. Chem. J. 2022, 55, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Arshad, L.; Septama, A.W.; Haque, M.A.; Mohamed-Hussein, Z.A.; Govender, N.T. Antiviral effects of phytochemicals against severe acute respiratory syndrome coronavirus 2 and their mechanisms of action: A review. Phytother. Res. PTR 2023, 37, 1036–1056. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives-Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Gabbai-Armelin, P.R.; Sales, L.S.; Ferrisse, T.M.; De Oliveira, A.B.; De Oliveira, J.R.; Giro, E.M.A.; Brighenti, F.L. A systematic review and meta-analysis of the effect of thymol as an anti-inflammatory and wound healing agent: A review of thymol effect on inflammation and wound healing: A review of thymol effect on inflammation and wound healing. Phytother. Res. PTR 2022, 36, 3415–3443. [Google Scholar] [CrossRef]
- Nadi, A.; Shiravi, A.A.; Mohammadi, Z.; Aslani, A.; Zeinalian, M. Thymus vulgaris, a natural pharmacy against COVID-19: A molecular review. J. Herb. Med. 2023, 38, 100635. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell. Mol. Biol. 2017, 63, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Mediouni, S.; Jablonski, J.A.; Tsuda, S.; Barsamian, A.; Kessing, C.; Richard, A.; Biswas, A.; Toledo, F.; Andrade, V.M.; Even, Y.; et al. Oregano Oil and Its Principal Component, Carvacrol, Inhibit HIV-1 Fusion into Target Cells. J. Virol. 2020, 94, 101128. [Google Scholar] [CrossRef]
- Wu, Q.F.; Wang, W.; Dai, X.Y.; Wang, Z.Y.; Shen, Z.H.; Ying, H.Z.; Yu, C.H. Chemical compositions and anti-influenza activities of essential oils from Mosla dianthera. J. Ethnopharmacol. 2012, 139, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Jaiswal, A.; Singh, R.K. In Silico study on spice-derived antiviral phytochemicals against SARS-CoV-2 TMPRSS2 target. J. Biomol. Struct. Dyn. 2022, 40, 11874–11884. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Aznar, R. Evaluation of Natural Compounds of Plant Origin for Inactivation of Enteric Viruses. Food Environ. Virol. 2015, 7, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kubiça, T.F.; Alves, S.H.; Weiblen, R.; Lovato, L.T. In Vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Braz. J. Microbiol. 2014, 45, 209–214. [Google Scholar] [CrossRef][Green Version]
- Taglienti, A.; Donati, L.; Ferretti, L.; Tomassoli, L.; Sapienza, F.; Sabatino, M.; Di Massimo, G.; Fiorentino, S.; Vecchiarelli, V.; Nota, P.; et al. In Vivo Antiphytoviral Activity of Essential Oils and Hydrosols From Origanum vulgare, Thymus vulgaris, and Rosmarinus officinalis to Control Zucchini Yellow Mosaic Virus and Tomato Leaf Curl New Delhi Virus in Cucurbita pepo L. Front. Microbiol. 2022, 13, 840893. [Google Scholar] [CrossRef]
- Troszok, A.; Roszko, M. Thyme essential oil inhibits intracellular replication of CyHV-3 and inactivates extracellular virus. An InVitro study. J. Fish Dis. 2023, 46, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol-A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, D.; Yu, B.; He, J.; Mao, X.; Huang, Z.; Yan, H.; Wu, A.; Luo, Y.; Zheng, P.; et al. Eugenol Alleviates TGEV-Induced Intestinal Injury via Suppressing ROS/NLRP3/GSDMD-Dependent Pyroptosis. J. Agric. Food Chem. 2023, 71, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.P.; Zhao, X.F.; Zeng, J.; Wan, Q.Y.; Yang, J.C.; Li, W.Z.; Chen, X.X.; Wang, G.F.; Li, K.S. Drug screening for autophagy inhibitors based on the dissociation of Beclin1-Bcl2 complex using BiFC technique and mechanism of eugenol on anti-influenza A virus activity. PLoS ONE 2013, 8, e61026. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement. Altern. Med. Ecam 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Benencia, F.; Courrèges, M.C. In Vitro and In Vivo activity of eugenol on human herpesvirus. Phytother. Res. PTR 2000, 14, 495–500. [Google Scholar] [CrossRef]
- Chandra Manivannan, A.; Malaisamy, A.; Eswaran, M.; Meyyazhagan, A.; Arumugam, V.A.; Rengasamy, K.R.R.; Balasubramanian, B.; Liu, W.C. Evaluation of Clove Phytochemicals as Potential Antiviral Drug Candidates Targeting SARS-CoV-2 Main Protease: Computational Docking, Molecular Dynamics Simulation, and Pharmacokinetic Profiling. Front. Mol. Biosci. 2022, 9, 918101. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Paramasivam, E.; Palanisamy, A.; Ragavendran, S.; Thangavel, S.N. Molecular insights on bioactive compounds against COVID-19: A Network pharmacological and computational study. Curr. Comput. Aided Drug Des. 2022, 18, 425–439. [Google Scholar] [CrossRef]
- Kaushik, S.; Kaushik, S.; Dar, L.; Yadav, J.P. Eugenol isolated from supercritical fluid extract of Ocimum sanctum: A potent inhibitor of DENV-2. AMB Express 2023, 13, 105. [Google Scholar] [CrossRef]
- Sun, W.J.; Lv, W.J.; Li, L.N.; Yin, G.; Hang, X.; Xue, Y.; Chen, J.; Shi, Z. Eugenol confers resistance to Tomato yellow leaf curl virus (TYLCV) by regulating the expression of SlPer1 in tomato plants. New Biotechnol. 2016, 33, 345–354. [Google Scholar] [CrossRef]
- Tsai, W.A.; Weng, S.H.; Chen, M.C.; Lin, J.S.; Tsai, W.S. Priming of Plant Resistance to Heat Stress and Tomato Yellow Leaf Curl Thailand Virus With Plant-Derived Materials. Front. Plant Sci. 2019, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, B.; Ding, Y.; Meng, J.; Hu, J.; Zhou, X.; Liu, L.; Wu, Z.; Yang, S. Insights into a class of natural eugenol and its optimized derivatives as potential tobacco mosaic virus helicase inhibitors by structure-based virtual screening. Int. J. Biol. Macromol. 2023, 248, 125892. [Google Scholar] [CrossRef]
- Mustafa, G.; Majid, M.; Ghaffar, A.; Yameen, M.; Samad, H.A.; Mahrosh, H.S. Screening and molecular docking of selected phytochemicals against NS5B polymerase of hepatitis c virus. Pak. J. Pharm. Sci. 2020, 33, 2317–2322. [Google Scholar]
- Lane, T.; Anantpadma, M.; Freundlich, J.S.; Davey, R.A.; Madrid, P.B.; Ekins, S. The Natural Product Eugenol Is an Inhibitor of the Ebola Virus In Vitro. Pharm. Res. 2019, 36, 104. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Ding, X.R.; Chen, S.H.; Yang, J.; Wang, X.J.; Jia, G.L.; Chen, H.S.; Bo, X.C.; Wang, S.Q. Protocatechuic aldehyde inhibits hepatitis B virus replication both In Vitro and In Vivo. Antivir. Res. 2007, 74, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.H.; Deng, J.S.; Jiang, W.P.; Chen, C.C.; Chou, Y.N.; Lin, J.G.; Huang, G.J. Study on the potential of Sanghuangporus sanghuang and its components as COVID-19 spike protein receptor binding domain inhibitors. Biomed. Pharmacother. 2022, 153, 113434. [Google Scholar] [CrossRef]
- Ho, C.C.; Lin, S.S.; Chou, M.Y.; Chen, F.L.; Hu, C.C.; Chen, C.S.; Lu, G.Y.; Yang, C.C. Effects of CAPE-like compounds on HIV replication In Vitro and modulation of cytokines In Vivo. J. Antimicrob. Chemother. 2005, 56, 372–379. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Peng, C.W.; Su, Z.Q.; Huang, H.T.; Liu, X.H.; Zhan, S.F.; Huang, X.F. A Practical Strategy for Exploring the Pharmacological Mechanism of Luteolin Against COVID-19/Asthma Comorbidity: Findings of System Pharmacology and Bioinformatics Analysis. Front. Immunol. 2021, 12, 769011. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.; Doerksen, R.J. Topological polar surface area: A useful descriptor in 2D-QSAR. Curr. Med. Chem. 2009, 16, 21–41. [Google Scholar] [CrossRef]
- Rashid, M. Design, synthesis and ADMET prediction of bis-benzimidazole as anticancer agent. Bioorganic Chem. 2020, 96, 103576. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Tiwari, R.K.; Ansari, I.A. Revisiting the Antiviral Efficacy of Terpenoids: Plausible Adjunct Therapeutics for Novel SARS-CoV-2? Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Z.; Chen, J.; Zheng, Y.; Limsila, B.; Lu, M.; Gao, T.; Yang, Q.; Fu, C.; Liao, W. Terpenoids from Curcumae Rhizoma: Their anticancer effects and clinical uses on combination and versus drug therapies. Biomed. Pharmacother. 2021, 138, 111350. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Runthala, A.; Rajput, V.S.; Chandrasai, P.D.; Tripathi, A.; Phulara, S.C. Computational and Synthetic Biology Approaches for the Biosynthesis of Antiviral and Anticancer Terpenoids from Bacillus subtilis. Med. Chem. 2022, 18, 307–322. [Google Scholar] [CrossRef]
- Hu, Y.J.; Chen, M.L.; Liang, D. Lignans and terpenoids from Gaultheria leucocarpa var. yunnanensis and their anti-inflammatory and antioxidant activities. Fitoterapia 2022, 162, 105293. [Google Scholar]
- Tu, P.C.; Liang, Y.C.; Huang, G.J.; Lin, M.K.; Kao, M.C.; Lu, T.L.; Sung, P.J.; Kuo, Y.H. Cytotoxic and Anti-inflammatory Terpenoids from the Whole Plant of Vaccinium emarginatum. Planta Med. 2020, 86, 1313–1322. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P.; Hernández, F. Antidiabetic, Anticholinesterase and Antioxidant Activity vs. Terpenoids and Phenolic Compounds in Selected New Cultivars and Hybrids of Artichoke Cynara scolymus L. Molecules 2019, 24, 1222. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef]
- Yamaguchi, T. Antibacterial effect of the combination of terpenoids. Arch. Microbiol. 2022, 204, 520. [Google Scholar] [CrossRef]
- Zacchino, S.A.; Butassi, E.; Liberto, M.D.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomed. Int. J. Phytother. Phytopharm. 2017, 37, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Li, Y.; Zhou, J.C.; Lu, J.H.; Zhu, R.X.; Qiao, Y.N.; Zhang, J.Z.; Zong, Y.; Wang, X.; Jin, X.Y.; et al. Terpenoids from the Chinese liverwort Odontoschisma grosseverrucosum and their antifungal virulence activity. Phytochemistry 2020, 174, 112341. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.B.; Xiao, Y.H.; Zhang, Q.Y.; Zhou, M.; Liao, S.G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Kuzminac, I.Z.; Savić, M.P.; Ajduković, J.J.; Nikolić, A.R. Steroid and Triterpenoid Compounds with Antiparasitic Properties. Curr. Top. Med. Chem. 2023, 23, 791–815. [Google Scholar] [PubMed]
- Mukherjee, N.; Mukherjee, S.; Saini, P.; Roy, P.; Babu, S.P. Phenolics and Terpenoids; the Promising New Search for Anthelmintics: A Critical Review. Mini Rev. Med. Chem. 2016, 16, 1415–1441. [Google Scholar] [CrossRef]
- Cho, H.Y.; Wang, W.; Jhaveri, N.; Torres, S.; Tseng, J.; Leong, M.N.; Lee, D.J.; Goldkorn, A.; Xu, T.; Petasis, N.A.; et al. Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol. Cancer Ther. 2012, 11, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Amaral, R.G.; Dória, G.A.; Fonseca, C.S.; da Silva, T.K.; Albuquerque Júnior, R.L.; Thomazzi, S.M.; do Nascimento, L.G.; Carvalho, A.A.; de Sousa, D.P. In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol. Int. J. Mol. Sci. 2016, 17, 32. [Google Scholar] [CrossRef]
- Rezende, A.A.; Santos, R.S.; Andrade, L.N.; Amaral, R.G.; Pereira, M.M.; Bani, C.; Chen, M.; Priefer, R.; da Silva, C.F.; de Albuquerque Júnior, R.L.C.; et al. Anti-Tumor Efficiency of Perillylalcohol/β-Cyclodextrin Inclusion Complexes in a Sarcoma S180-Induced Mice Model. Pharmaceutics 2021, 13, 245. [Google Scholar] [CrossRef]
- Mello, C.P.; Quirico-Santos, T.; Amorim, L.F.; Silva, V.G.; Fragel, L.M.; Bloom, D.C.; Paixão, I.P. Perillyl alcohol and perillic acid exert efficient action upon HSV-1 maturation and release of infective virus. Antivir. Ther. 2020, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beik, A.; Najafipour, H.; Joukar, S.; Rajabi, S.; Iranpour, M.; Kordestani, Z. Perillyl alcohol suppresses monocrotaline-induced pulmonary arterial hypertension in rats via anti-remodeling, anti-oxidant, and anti-inflammatory effects. Clin. Exp. Hypertens. 2021, 43, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.D.A.; Ortega, A.C.; González Maldonado, L.A.; Castro, R.D.; Ávila-Campos, M.J.; Rossa, C.; Aquino, S.G. Perillyl alcohol has antibacterial effects and reduces ROS production in macrophages. J. Appl. Oral Sci. Rev. FOB 2020, 28, e20190519. [Google Scholar] [CrossRef] [PubMed]
- Le-Trilling, V.T.K.; Mennerich, D.; Schuler, C.; Sakson, R.; Lill, J.K.; Kasarla, S.S.; Kopczynski, D.; Loroch, S.; Flores-Martinez, Y.; Katschinski, B.; et al. Identification of herbal teas and their compounds eliciting antiviral activity against SARS-CoV-2 In Vitro. BMC Biol. 2022, 20, 264. [Google Scholar] [CrossRef]
- Malhi, M.; Norris, M.J.; Duan, W.; Moraes, T.J.; Maynes, J.T. Statin-mediated disruption of Rho GTPase prenylation and activity inhibits respiratory syncytial virus infection. Commun. Biol. 2021, 4, 1239. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Huang, Y.; Wang, Y.; Chen, S.; Liu, C.; Li, Y.; Feng, J. Germacrone, A Novel and Safe Anticancer Agent from Genus Curcuma: A Review of its Mechanism. Anti-Cancer Agents Med. Chem. 2023, 23, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Bai, X.; Cui, T.; Zhou, H.; Chen, Y.; Xie, J.; Shi, Q.; Wang, H.; Zhang, G. In Vitro Antiviral Activity of Germacrone Against Porcine Reproductive and Respiratory Syndrome Virus. Curr. Microbiol. 2016, 73, 317–323. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Kanwal, N.; Hussain, G.; Shah, M.A.; Sarfraz, I.; Ishfaq, R.; Batool, R.; Rukhsar, F.; Adem, Ş. Germacrone: A Potent Secondary Metabolite with Therapeutic Potential in Metabolic Diseases, Cancer and Viral Infections. Curr. Drug Metab. 2020, 21, 1079–1090. [Google Scholar] [CrossRef]
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai Curcuma Species: Antioxidant and Bioactive Compounds. Foods 2020, 9, 1219. [Google Scholar] [CrossRef]
- Oriola, A.O.; Oyedeji, A.O. Essential Oils and Their Compounds as Potential Anti-Influenza Agents. Molecules 2022, 27, 7797. [Google Scholar] [CrossRef]
- Liao, Q.; Qian, Z.; Liu, R.; An, L.; Chen, X. Germacrone inhibits early stages of influenza virus infection. Antivir. Res. 2013, 100, 578–588. [Google Scholar] [CrossRef]
- Li, L.; Xie, Q.; Bian, G.; Zhang, B.; Wang, M.; Wang, Y.; Chen, Z.; Li, Y. Anti-H1N1 viral activity of three main active ingredients from zedoary oil. Fitoterapia 2020, 142, 104489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, Y.; Jiao, Y.; Hou, L.; Shi, Y.; Gu, T.; Zhou, P.; Shi, Z.; Xu, L.; Wang, C. In Vitro antiviral activity of germacrone against porcine parvovirus. Arch. Virol. 2015, 160, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Zu, S.; Sun, X.; Liu, C.; Liu, D.; Zhang, X.; Tian, J.; Qu, L. In Vitro antiviral effect of germacrone on feline calicivirus. Arch. Virol. 2016, 161, 1559–1567. [Google Scholar] [CrossRef]
- He, W.; Zhai, X.; Su, J.; Ye, R.; Zheng, Y.; Su, S. Antiviral Activity of Germacrone against Pseudorabies Virus In Vitro. Pathogens 2019, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Wang, S.; Liu, W.; Hao, C.; Wang, W. Inhibition effects of patchouli alcohol against influenza a virus through targeting cellular PI3K/Akt and ERK/MAPK signaling pathways. Virol. J. 2019, 16, 163. [Google Scholar] [CrossRef]
- He, H.; Xie, X.; Zhang, J.; Mo, L.; Kang, X.; Zhang, Y.; Wang, L.; Hu, N.; Xie, L.; Peng, C.; et al. Patchouli alcohol ameliorates depression-like behaviors through inhibiting NLRP3-mediated neuroinflammation in male stress-exposed mice. J. Affect. Disord. 2023, 326, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, S.Y.; Lou, H. Patchouli alcohol protects against myocardial ischaemia-reperfusion injury by regulating the Notch1/Hes1 pathway. Pharm. Biol. 2022, 60, 949–957. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Q.; Tan, X.; Zhao, Q.; Wu, J.; Liao, H.; Ai, W.; Liu, Y.; Lai, Z.; Fu, L. Patchouli alcohol ameliorates acute liver injury via inhibiting oxidative stress and gut-origin LPS leakage in rats. Int. Immunopharmacol. 2021, 98, 107897. [Google Scholar] [CrossRef]
- Hu, G.Y.; Peng, C.; Xie, X.F.; Xiong, L.; Zhang, S.Y.; Cao, X.Y. Patchouli alcohol isolated from Pogostemon cablin mediates endothelium-independent vasorelaxation by blockade of Ca2+ channels in rat isolated thoracic aorta. J. Ethnopharmacol. 2018, 220, 188–196. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J.; Smolensky, D.; Lee, S.H. Potential benefits of patchouli alcohol in prevention of human diseases: A mechanistic review. Int. Immunopharmacol. 2020, 89, 107056. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Guo, Y.L.; Chen, Y.R.; Zhang, L.Y.; Wang, Z.C.; Zhang, T.; Wang, B. A potential drug combination of omeprazole and patchouli alcohol significantly normalizes oxidative stress and inflammatory responses against gastric ulcer in ethanol-induced rat model. Int. Immunopharmacol. 2020, 85, 106660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, M.; Song, M.; Wang, J.; Cai, J.; Lin, C.; Li, Y.; Jin, X.; Shen, C.; Chen, Z.; et al. Patchouli alcohol activates PXR and suppresses the NF-κB-mediated intestinal inflammatory. J. Ethnopharmacol. 2020, 248, 112302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Zhang, J.; Wang, R.; Cheng, B.; Kalambhe, D.; Wang, Y.; Gu, Z.; Chen, D.; Wang, B.; et al. Lactoferrin-mediated macrophage targeting delivery and patchouli alcohol-based therapeutic strategy for inflammatory bowel diseases. Acta Pharm. Sinica. B 2020, 10, 1966–1976. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, J.; Gao, P.; Xia, Y. Patchouli alcohol inhibits GPBAR1-mediated cell proliferation, apoptosis, migration, and invasion in prostate cancer. Transl. Androl. Urol. 2022, 11, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.S.; Lee, S.H. Preventive Activity of Patchouli Alcohol Against Colorectal Cancer and Diabetes. J. Med. Food 2023, 26, 255–261. [Google Scholar] [CrossRef]
- Kiyohara, H.; Ichino, C.; Kawamura, Y.; Nagai, T.; Sato, N.; Yamada, H. Patchouli alcohol: In Vitro direct anti-influenza virus sesquiterpene in Pogostemon cablin Benth. J. Nat. Med. 2012, 66, 55–61. [Google Scholar] [CrossRef]
- Wu, X.L.; Ju, D.H.; Chen, J.; Yu, B.; Liu, K.L.; He, J.X.; Dai, C.Q.; Wu, S.; Chang, Z.; Wang, Y.P.; et al. Immunologic mechanism of Patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLH signal pathway In Vitro. Curr. Microbiol. 2013, 67, 431–436. [Google Scholar] [CrossRef]
- Wu, H.; Li, B.; Wang, X.; Jin, M.; Wang, G. Inhibitory effect and possible mechanism of action of patchouli alcohol against influenza A (H2N2) virus. Molecules 2011, 16, 6489–6501. [Google Scholar] [CrossRef]
- Zrieq, R.; Ahmad, I.; Snoussi, M.; Noumi, E.; Iriti, M.; Algahtani, F.D.; Patel, H.; Saeed, M.; Tasleem, M.; Sulaiman, S.; et al. Tomatidine and Patchouli Alcohol as Inhibitors of SARS-CoV-2 Enzymes (3CLpro, PLpro and NSP15) by Molecular Docking and Molecular Dynamics Simulations. Int. J. Mol. Sci. 2021, 22, 10693. [Google Scholar] [CrossRef]
- Oliveira, F.; Silva, E.; Matias, A.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C. Menthol-based deep eutectic systems as antimicrobial and anti-inflammatory agents for wound healing. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 182, 106368. [Google Scholar] [CrossRef] [PubMed]
- Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Oliveira de Souza, E.; Hussni, C.A.; Martinez, E.R.M.; Nóbrega, R.H.; Pellizzon, C.H. The Use of Menthol in Skin Wound Healing-Anti-Inflammatory Potential, Antioxidant Defense System Stimulation and Increased Epithelialization. Pharmaceutics 2021, 13, 1902. [Google Scholar] [CrossRef] [PubMed]
- Hazime, N.; Belguesmia, Y.; Barras, A.; Amiche, M.; Boukherroub, R.; Drider, D. Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles-Effects of Menthol and Lactic Acid on Its Potentialization. Antibiotics 2022, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.R.; Hamid, S.M.; Andres, A.M.; Saadaeijahromi, H.; Piplani, H.; Germano, J.F.; Song, Y.; Sawaged, S.; Feuer, R.; Pandol, S.J.; et al. Antiviral Effects of Menthol on Coxsackievirus B. Viruses 2020, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Primo, V.; Rovera, M.; Zanon, S.; Oliva, M.; Demo, M.; Daghero, J.; Sabini, L. Determination of the antibacterial and antiviral activity of the essential oil from Minthostachys verticillata (Griseb.) Epling. Rev. Argent. Microbiol. 2001, 33, 113–117. [Google Scholar] [PubMed]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Li, S.; Cai, X.; Fu, L.; Shao, Y.; Zhu, Y. Antiviral Activity of Luteolin against Pseudorabies Virus In Vitro and In Vivo. Animals 2023, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Wang, W.J.; Xu, C.; Xie, Y.J.; Wang, X.R.; Zhang, Y.Z.; Huang, J.M.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight Into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef] [PubMed]
- Shawan, M.; Halder, S.K.; Hasan, M.A. Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: An In Silico molecular modeling approach in battling the COVID-19 outbreak. Bull. Natl. Res. Cent. 2021, 45, 27. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. -Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Bahun, M.; Jukić, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovičar, K.; Bratkovič, T.; Podlipnik, Č.; Poklar Ulrih, N. Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef]
- Versace, V.; Ortelli, P.; Dezi, S.; Ferrazzoli, D.; Alibardi, A.; Bonini, I.; Engl, M.; Maestri, R.; Assogna, M.; Ajello, V.; et al. Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2023, 145, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Y.; Song, Z.; Chang, H.; Kuang, Q.; Zheng, Z.; Wang, H.; Zhang, G. Luteolin restricts ASFV replication by regulating the NF-κB/STAT3/ATF6 signaling pathway. Vet. Microbiol. 2022, 273, 109527. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Wang, Z.; Song, X.; Ren, Z.; Wang, X.; Wang, Y.; Zheng, K. Luteolin inhibits herpes simplex virus 1 infection by activating cyclic guanosine monophosphate-adenosine monophosphate synthase-mediated antiviral innate immunity. Phytomed. Int. J. Phytother. Phytopharm. 2023, 120, 155020. [Google Scholar] [CrossRef]
- Murali, K.S.; Sivasubramanian, S.; Vincent, S.; Murugan, S.B.; Giridaran, B.; Dinesh, S.; Gunasekaran, P.; Krishnasamy, K.; Sathishkumar, R. Anti-chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac. J. Trop. Med. 2015, 8, 352–358. [Google Scholar] [CrossRef]
- Fan, W.; Qian, S.; Qian, P.; Li, X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016, 220, 112–116. [Google Scholar] [CrossRef]
- Fu, L.; Li, S.; Men, X.; Cai, X.; Wang, Z.; Xu, Y.; Ren, Z.; Shao, Y.; Zhu, Y. Optimizing the Extraction and Enrichment of Luteolin from Patrinia villosa and Its Anti-Pseudorabies Virus Activity. Molecules 2023, 28, 5005. [Google Scholar] [CrossRef]
- Xu, L.; Su, W.; Jin, J.; Chen, J.; Li, X.; Zhang, X.; Sun, M.; Sun, S.; Fan, P.; An, D.; et al. Identification of luteolin as enterovirus 71 and coxsackievirus A16 inhibitors through reporter viruses and cell viability-based screening. Viruses 2014, 6, 2778–2795. [Google Scholar] [CrossRef]
- Theerawatanasirikul, S.; Thangthamniyom, N.; Kuo, C.J.; Semkum, P.; Phecharat, N.; Chankeeree, P.; Lekcharoensuk, P. Natural Phytochemicals, Luteolin and Isoginkgetin, Inhibit 3C Protease and Infection of FMDV, In Silico and In Vitro. Viruses 2021, 13, 2118. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Miao, J.; Shao, Q.; Gao, Y.; Hong, L. Apigenin suppresses influenza A virus-induced RIG-I activation and viral replication. J. Med. Virol. 2020, 92, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.G.; Lee, J.; Hong, S.H.; Seo, Y.J. Apigenin’s Therapeutic Potential Against Viral Infection. Front. Biosci. 2023, 28, 237. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. PTR 2020, 34, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Gupta, M.; Sarwat, M.; Siddique, H.R. Apigenin in cancer prevention and therapy: A systematic review and meta-analysis of animal models. Crit. Rev. Oncol. Hematol. 2022, 176, 103751. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and In Vitro and In Vivo studies: A review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef]
- Cicek, M.; Unsal, V.; Doganer, A.; Demir, M. Investigation of oxidant/antioxidant and anti-inflammatory effects of apigenin on apoptosis in sepsis-induced rat lung. J. Biochem. Mol. Toxicol. 2021, 35, e22743. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Khandelwal, N.; Chander, Y.; Kumar, R.; Riyesh, T.; Dedar, R.K.; Kumar, M.; Gulati, B.R.; Sharma, S.; Tripathi, B.N.; Barua, S.; et al. Antiviral activity of Apigenin against buffalopox: Novel mechanistic insights and drug-resistance considerations. Antivir. Res. 2020, 181, 104870. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin restricts FMDV infection and inhibits viral IRES driven translational activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Fang, C.Y.; Cheng, Y.J.; Hsu, H.Y.; Chou, S.P.; Huang, S.Y.; Tsai, C.H.; Chen, J.Y. Inhibition of Epstein-Barr virus reactivation by the flavonoid apigenin. J. Biomed. Sci. 2017, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Zannella, C.; Giugliano, R.; Chianese, A.; Buonocore, C.; Vitale, G.A.; Sanna, G.; Sarno, F.; Manzin, A.; Nebbioso, A.; Termolino, P.; et al. Antiviral Activity of Vitis vinifera Leaf Extract against SARS-CoV-2 and HSV-1. Viruses 2021, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Qiu, M.; Chen, D.; Zheng, N.; Jin, Y.; Wu, Z. Apigenin inhibits enterovirus 71 replication through suppressing viral IRES activity and modulating cellular JNK pathway. Antivir. Res. 2014, 109, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiao, H.; Lv, Y.; Wang, J.; Chen, X.; Hou, Y.; Tan, R.; Li, E. Apigenin inhibits enterovirus-71 infection by disrupting viral RNA association with trans-acting factors. PLoS ONE 2014, 9, e110429. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ashfaq, U.A.; Ijaz, B.; Riazuddin, S. Anti-hepatitis C virus activity and synergistic effect of Nymphaea alba extracts and bioactive constituents in liver infected cells. Microb. Pathog. 2018, 121, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Fredsgaard, M.; Kaniki, S.E.K.; Antonopoulou, I.; Chaturvedi, T.; Thomsen, M.H. Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review. Molecules 2023, 28, 5312. [Google Scholar] [CrossRef]
- Hengphasatporn, K.; Kaewmalai, B.; Jansongsaeng, S.; Badavath, V.N.; Saelee, T.; Chokmahasarn, T.; Khotavivattana, T.; Shigeta, Y.; Rungrotmongkol, T.; Boonyasuppayakorn, S. Alkyne-Tagged Apigenin, a Chemical Tool to Navigate Potential Targets of Flavonoid Anti-Dengue Leads. Molecules 2021, 26, 6967. [Google Scholar] [CrossRef]
- Acchioni, C.; Acchioni, M.; Mancini, F.; Amendola, A.; Marsili, G.; Tirelli, V.; Gwee, C.P.; Chan, K.W.; Sandini, S.; Bisbocci, M.; et al. A cellular screening platform, stably expressing DENV2 NS5, defines a novel anti-DENV mechanism of action of Apigenin based on STAT2 activation. Virology 2023, 583, 1–13. [Google Scholar] [CrossRef]
- Farhat, A.; Ben Hlima, H.; Khemakhem, B.; Ben Halima, Y.; Michaud, P.; Abdelkafi, S.; Fendri, I. Apigenin analogues as SARS-CoV-2 main protease inhibitors: In-Silico screening approach. Bioengineered 2022, 13, 3350–3361. [Google Scholar] [CrossRef] [PubMed]
- Abdizadeh, R.; Hadizadeh, F.; Abdizadeh, T. Evaluation of apigenin-based biflavonoid derivatives as potential therapeutic agents against viral protease (3CLpro) of SARS-CoV-2 via molecular docking, molecular dynamics and quantum mechanics studies. J. Biomol. Struct. Dyn. 2023, 41, 5915–5945. [Google Scholar] [CrossRef] [PubMed]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant Sterols: Diversity, Biosynthesis, and Physiological Functions. Biochem. Biokhimiia 2016, 81, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Hisham Shady, N.; Youssif, K.A.; Sayed, A.M.; Belbahri, L.; Oszako, T.; Hassan, H.M.; Abdelmohsen, U.R. Sterols and Triterpenes: Antiviral Potential Supported by In-Silico Analysis. Plants 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, A.; Bhattacharyya, D.; Chauhan, R.S. Computational identification of potential inhibitory compounds in Indian medicinal and aromatic plant species against major pathogenicity determinants of SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 14096–14114. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences. Nat. Prod. Bioprospecting 2020, 10, 297–306. [Google Scholar] [CrossRef]
- Chen, C.; Shen, J.L.; Liang, C.S.; Sun, Z.C.; Jiang, H.F. First Discovery of Beta-Sitosterol as a Novel Antiviral Agent against White Spot Syndrome Virus. Int. J. Mol. Sci. 2022, 23, 10448. [Google Scholar] [CrossRef]
- Toujani, M.M.; Rittà, M.; Civra, A.; Genovese, S.; Epifano, F.; Ghram, A.; Lembo, D.; Donalisio, M. Inhibition of HSV-2 infection by pure compounds from Thymus capitatus extract In Vitro. Phytother. Res. PTR 2018, 32, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Shokry, S.; Hegazy, A.; Abbas, A.M.; Mostafa, I.; Eissa, I.H.; Metwaly, A.M.; Yahya, G.; El-Shazly, A.M.; Aboshanab, K.M.; Mostafa, A. Phytoestrogen β-Sitosterol Exhibits Potent In Vitro Antiviral Activity against Influenza A Viruses. Vaccines 2023, 11, 228. [Google Scholar] [CrossRef]
- Parvez, M.K.; Alam, P.; Arbab, A.H.; Al-Dosari, M.S.; Alhowiriny, T.A.; Alqasoumi, S.I. Analysis of antioxidative and antiviral biomarkers β-amyrin, β-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2018, 26, 685–693. [Google Scholar] [CrossRef]
- Parvez, M.K.; Tabish Rehman, M.; Alam, P.; Al-Dosari, M.S.; Alqasoumi, S.I.; Alajmi, M.F. Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019, 27, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Agarwal, S.; Patil, P.; Alagarasu, K.; Panda, K.; Prashar, C.; Kakade, M.; Davuluri, K.S.; Cherian, S.; Parashar, D.; et al. Effect of Sauropus androgynus L. Merr. on dengue virus-2: An In Vitro and In Silico study. J. Ethnopharmacol. 2023, 304, 116044. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.X.; Li, J.; Liang, X.L.; Pan, X.P.; Hao, Y.B.; Xie, P.F.; Jiang, H.M.; Yang, Z.F.; Zhong, N.S. β-sitosterol ameliorates influenza A virus-induced proinflammatory response and acute lung injury in mice by disrupting the cross-talk between RIG-I and IFN/STAT signaling. Acta Pharmacol. Sin. 2020, 41, 1178–1196. [Google Scholar] [CrossRef]
- Wang, L.; Guo, J.; Wang, Y.; Zhao, P.; Liu, B.; Zhang, Y.; Xiong, Y.; Chen, Q.; Lin, L.; Li, L.; et al. Anti-inflammatory effects of Chaishi Tuire Granules on influenza A treatment by mediating TRAF6/MAPK14 axis. Front. Med. 2022, 9, 943681. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elghiet, F.; Rushdi, A.; Ibrahim, M.H.; Mahmoud, S.H.; Rabeh, M.A.; Alshehri, S.A.; El Menofy, N.G. Chemical Profile, Antibacterial, Antibiofilm, and Antiviral Activities of Pulicaria crispa Most Potent Fraction: An In Vitro and In Silico Study. Molecules 2023, 28, 4184. [Google Scholar] [CrossRef] [PubMed]
- Aljaafari, M.N.; Alkhoori, M.A.; Hag-Ali, M.; Cheng, W.H.; Lim, S.H.; Loh, J.Y.; Lai, K.S. Contribution of Aldehydes and Their Derivatives to Antimicrobial and Immunomodulatory Activities. Molecules 2022, 27, 3589. [Google Scholar] [CrossRef] [PubMed]
- Hamedani, N.F.; Azad, L.; Shafiee, S.; Noushin, A. Green Synthesis of Thiazole Derivatives using Multi-component Reaction of Aldehydes, Isothiocyanate and Alkyl Bromides: Investigation of Antioxidant and Antimicrobial Activity. Comb. Chem. High Throughput Screen. 2021, 24, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Yan, Z. Antinociceptive and Anti-inflammatory Effect of Corynoline in Different Nociceptive and Inflammatory Experimental Models. Appl. Biochem. Biotechnol. 2022, 194, 4783–4799. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; Bosco, L.; Moschetti, M.; Tinnirello, V.; Pucci, M.; Corleone, V.; Raimondo, S.; Alessandro, R.; Fontana, S. Anti-inflammatory properties of an aldehydes-enriched fraction of grapefruit essential oil. J. Food Sci. 2023, 88, 1172–1187. [Google Scholar] [CrossRef]
- Gampe, C.; Verma, V.A. Curse or Cure? A Perspective on the Developability of Aldehydes as Active Pharmaceutical Ingredients. J. Med. Chem. 2020, 63, 14357–14381. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, S.H.; Lee, J.W.; Ban, J.O.; Lee, S.Y.; Yoo, H.S.; Jung, J.K.; Moon, D.C.; Oh, K.W.; Hong, J.T. 2-hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth through AP-1 inactivation. J. Pharmacol. Sci. 2007, 104, 19–28. [Google Scholar] [CrossRef]
- Zobeiri, M.; Parvizi, F.; Shahpiri, Z.; Heydarpour, F.; Pourfarzam, M.; Memarzadeh, M.R.; Rahimi, R.; Farzaei, M.H. Evaluation of the Effectiveness of Cinnamon Oil Soft Capsule in Patients with Functional Dyspepsia: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Evid. Based Complement. Altern. Med. Ecam 2021, 2021, 6634115. [Google Scholar] [CrossRef]
- Yuan, X.; Han, L.; Fu, P.; Zeng, H.; Lv, C.; Chang, W.; Runyon, R.S.; Ishii, M.; Han, L.; Liu, K.; et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab. Investig. A J. Tech. Methods Pathol. 2018, 98, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.Y.; Zhang, B.S.; Ren, L.N.; Lu, Y.P.; Tang, J.W.; Lv, D.; Yong, L.; Lin, L.T.; Lin, Z.X.; et al. In Vivo antiviral effect of plant essential oils against avian infectious bronchitis virus. BMC Vet. Res. 2022, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Imanishi, N.; Kashiwayama, Y.; Kawano, A.; Terasawa, K.; Shimada, Y.; Ochiai, H. Inhibitory effect of cinnamaldehyde, derived from Cinnamomi cortex, on the growth of influenza A/PR/8 virus In Vitro and In Vivo. Antivir. Res. 2007, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, W.; Xie, Y.H.; Yang, Q.; Li, X.Q.; Liu, X.X.; Wang, S.W. The comparison of α-bromo-4-chlorocinnamaldehyde and cinnamaldehyde on coxsackie virus B3-induced myocarditis and their mechanisms. Int. Immunopharmacol. 2012, 14, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Qiu, L.; Zhao, G.; Xu, J.; Wang, S. Influence of cinnamaldehyde on viral myocarditis in mice. Am. J. Med. Sci. 2010, 340, 114–120. [Google Scholar] [CrossRef]
- Chen, Y.H.; Guo, D.S.; Lu, M.H.; Yue, J.Y.; Liu, Y.; Shang, C.M.; An, D.R.; Zhao, M.M. Inhibitory Effect of Osthole from Cnidium monnieri on Tobacco Mosaic Virus (TMV) Infection in Nicotiana glutinosa. Molecules 2019, 25, 65. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, P.; Wan, S.; Guo, J.; Zheng, X.; Sun, Y.; Fan, K.; Yin, W.; Sun, N.; Li, H. The combined usage of Matrine and Osthole inhibited endoplasmic reticulum apoptosis induced by PCV2. BMC Microbiol. 2020, 20, 303. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, S.; Sun, P.; Khan, A.; Guo, J.; Zheng, X.; Sun, Y.; Fan, K.; Yin, W.; Li, H.; et al. Matrine combined with Osthole inhibited the PERK apoptosis of splenic lymphocytes in PCV2-infected mice model. BMC Vet. Res. 2023, 19, 26. [Google Scholar] [CrossRef]
- Civitelli, L.; Panella, S.; Marcocci, M.E.; De Petris, A.; Garzoli, S.; Pepi, F.; Vavala, E.; Ragno, R.; Nencioni, L.; Palamara, A.T.; et al. In Vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.K.; Chopra, B. Pulegone: An Emerging Oxygenated Cyclic Monoterpene Ketone Scaffold Delineating Synthesis, Chemical Reactivity, and Biological potential. Recent Adv. Anti-Infect. Drug Discov. 2023, 18, 16–28. [Google Scholar]
- Habas, K.; Nganwuchu, C.; Shahzad, F.; Gopalan, R.; Haque, M.; Rahman, S.; Majumder, A.A.; Nasim, T. Resolution of coronavirus disease 2019 (COVID-19). Expert Rev. Anti-Infect. Ther. 2020, 18, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Trépo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Bbosa, N.; Kaleebu, P.; Ssemwanga, D. HIV subtype diversity worldwide. Curr. Opin. HIV AIDS 2019, 14, 153–160. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef]
- Karami, T.K.; Hailu, S.; Feng, S.; Graham, R.; Gukasyan, H.J. Eyes on Lipinski’s Rule of Five: A New “Rule of Thumb” for Physicochemical Design Space of Ophthalmic Drugs. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2022, 38, 43–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).