Ultrasonic-Assisted Extraction of Phenolic Compounds from Lonicera similis Flowers at Three Harvest Periods: Comparison of Composition, Characterization, and Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
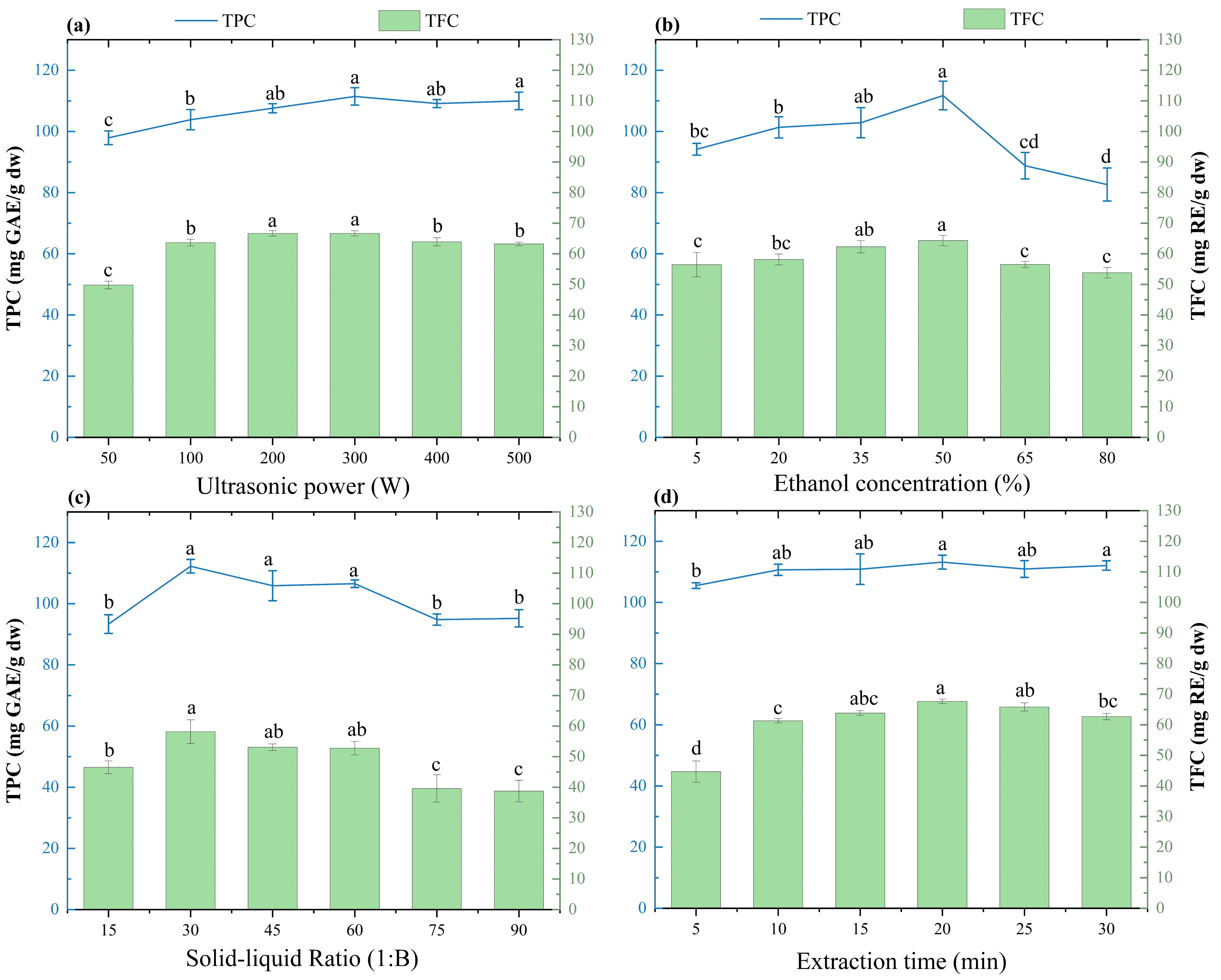
2.1. Single-Factor Experiments
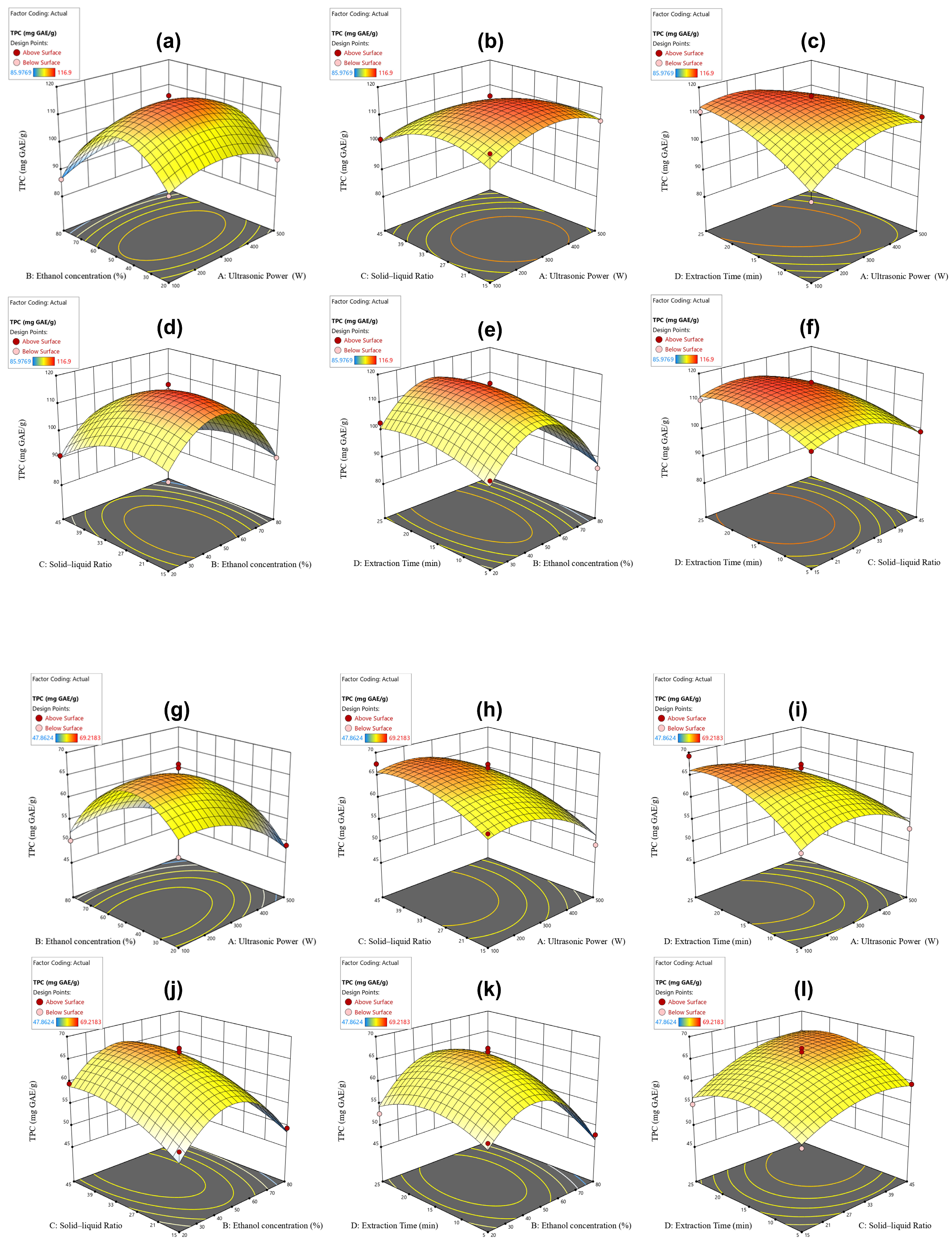
2.2. Optimization Extraction
2.3. Model Validation
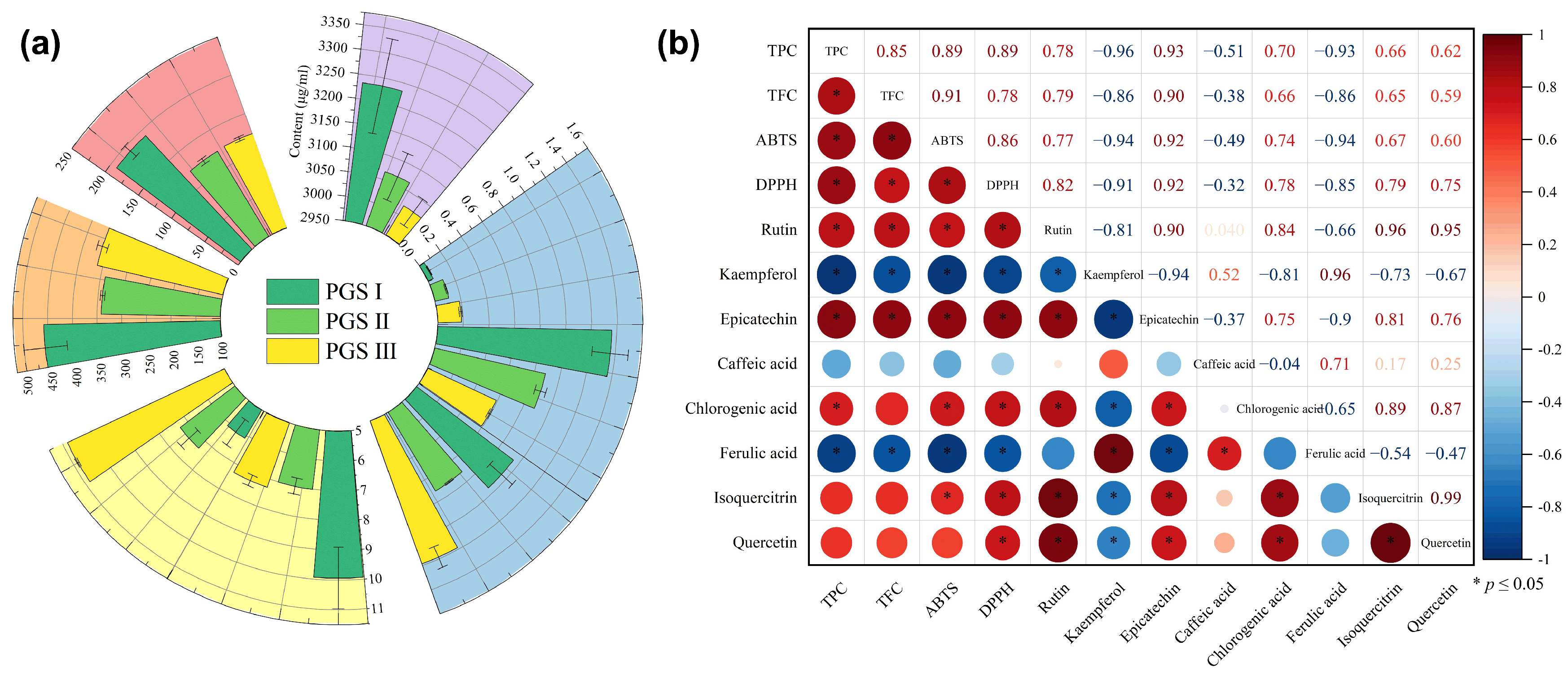
2.4. Composition Analysis of Phenolic Compounds
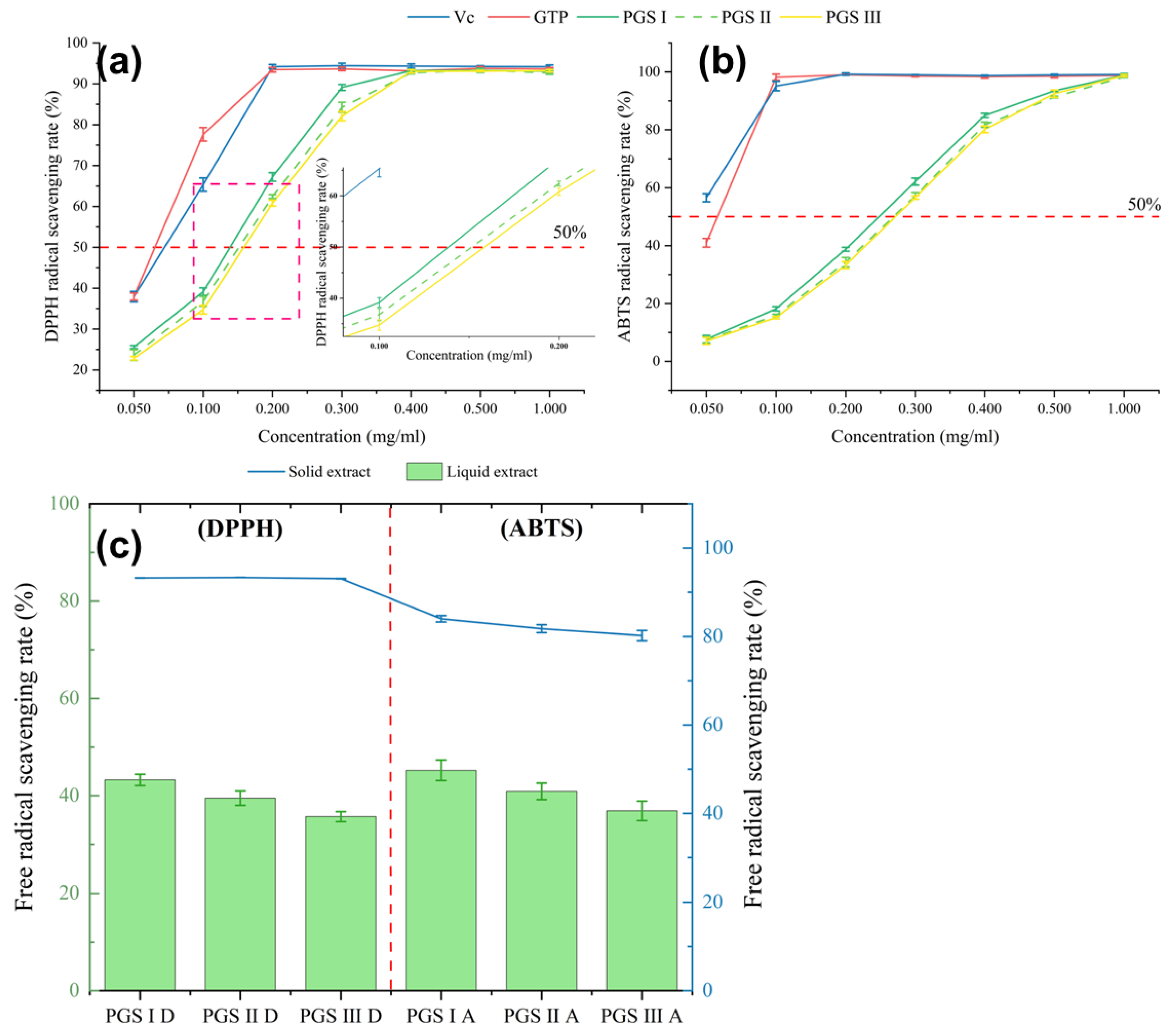
2.5. Antioxidant Capacity of Extracts
2.6. FTIR Analysis
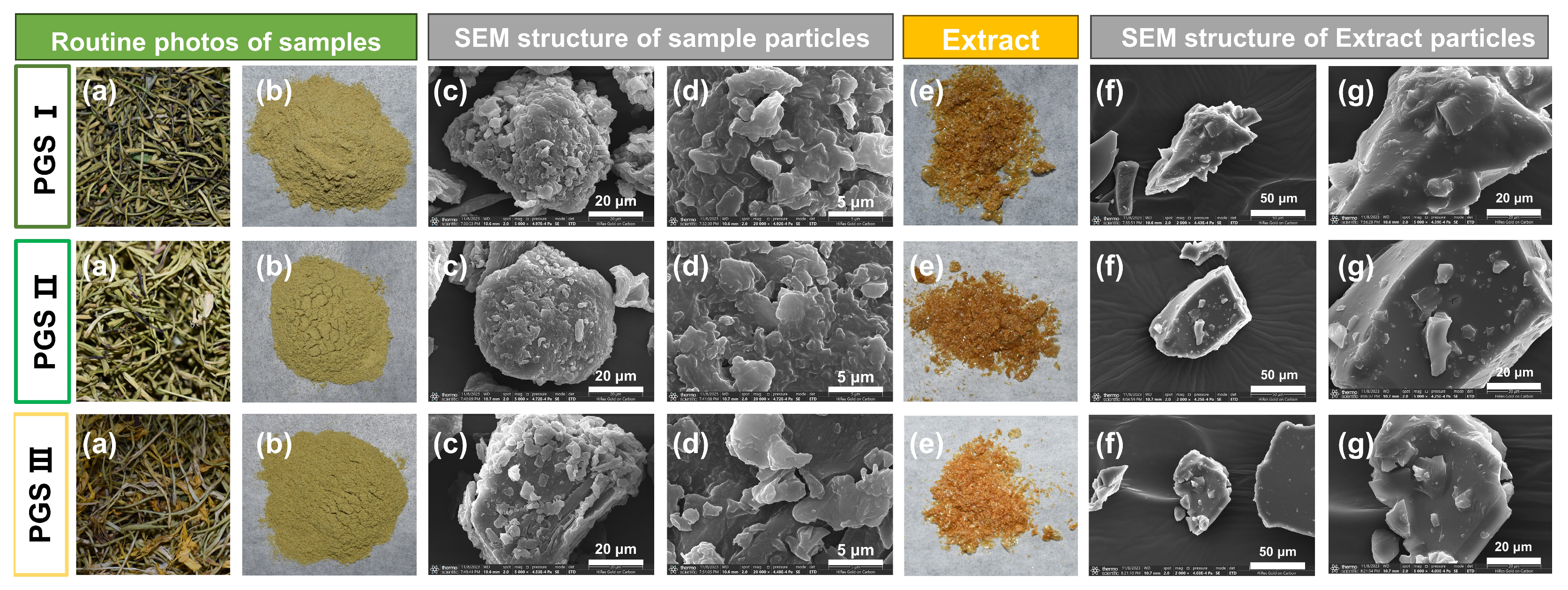
2.7. SEM Analysis
3. Materials and Methods
3.1. Flower Collection and Chemicals
3.2. Single-Factor Experiments
3.3. Optimizing LSF-PC Extraction Based on the Box–Behnken Design (BBD)
3.4. Determination of LSF-PC
3.5. Preparation of LSF Extracts
3.6. HPLC Analysis
3.7. Determination of Antioxidant Activity
3.7.1. DPPH Radical Scavenging Capacity
3.7.2. ABTS Radical Scavenging Activity
3.8. Analysis of Fourier Transform Infrared Spectroscopy (FT-IR)
3.9. Analysis of Scanning Electron Microscope (SEM)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Z.; Li, J.; Yang, R.; Fang, L.; Zhang, Y. A Review: The Triterpenoid Saponins and Biological Activities of Lonicera Linn. Molecules 2020, 25, 3773. [Google Scholar] [CrossRef]
- Hu, S.; Dong, G.; Chen, X.; Huang, L.; Yang, X.; Tong, W.; Bai, L. ITS Sequence-Based Identification and Utilization Evaluation of “Nanjiang” (Lonicera similis Hemsl.), a Local Cultivar in Sichuan, China. Genet. Resour. Crop Evol. 2012, 59, 547–555. [Google Scholar] [CrossRef]
- Wei, X.-M.; Hu, Y.; Wei, K.-H.; Wu, Q.-H.; Huang, Y.; Wei, F. Characterization of the Complete Chloroplast Genome of Lonicera similis (Caprifoliaceae). Mitochondrial DNA Part B 2021, 6, 3067–3069. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.-H.; He, Y.-L.; Peng, C.; Guo, L.; Xiong, L. Triterpenoid Saponins from the Buds of Lonicera similis. Nat. Prod. Res. 2018, 32, 2282–2290. [Google Scholar] [CrossRef]
- Fan, Z.; Li, L.; Bai, X.; Zhang, H.; Liu, Q.; Zhang, H.; Fu, Y.; Moyo, R. Extraction Optimization, Antioxidant Activity, and Tyrosinase Inhibitory Capacity of Polyphenols from Lonicera japonica. Food Sci. Nutr. 2019, 7, 1786–1794. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Q.; Yi, Y.; Xiao, H.; Putra, D.F.; Ke, K.; Zhang, Q.; Li, P. Antiviral Activities of Lonicera japonica Thunb. Components against Grouper Iridovirus in Vitro and in Vivo. Aquaculture 2020, 519, 734882. [Google Scholar] [CrossRef]
- Park, C.; Lee, W.S.; Han, M.; Song, K.S.; Hong, S.; Nagappan, A.; Kim, G.; Kim, G.S.; Jung, J.; Ryu, C.H.; et al. Lonicera japonica Thunb. Induces Caspase-dependent Apoptosis through Death Receptors and Suppression of AKT in U937 Human Leukemic Cells. Phytother. Res. 2018, 32, 504–513. [Google Scholar] [CrossRef]
- Ge, L.; Wan, H.; Tang, S.; Chen, H.; Li, J.; Zhang, K.; Zhou, B.; Fei, J.; Wu, S.; Zeng, X. Novel Caffeoylquinic Acid Derivatives from Lonicera japonica Thunb. Flower Buds Exert Pronounced Anti-HBV Activities. RSC Adv. 2018, 8, 35374–35385. [Google Scholar] [CrossRef]
- Guo, W.; Wang, L.; Gao, Y.; Zhao, B.; Wang, D.; Duan, W.; Yu, Z. Isolation of Isochlorogenic Acid Isomers in Flower Buds of Lonicera japonica by High-Speed Counter-Current Chromatography and Preparative High Performance Liquid Chromatography. J. Chromatogr. B 2015, 981–982, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Xie, Q.; Jiang, Y.; Xiao, L.; Wan, H.; Zhou, B.; Wu, S.; Tian, J.; Zeng, X. Genus Lonicera: New Drug Discovery from Traditional Usage to Modern Chemical and Pharmacological Research. Phytomedicine 2022, 96, 153889. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kang, S.C. In Vitro Control of Food-Borne and Food Spoilage Bacteria by Essential Oil and Ethanol Extracts of Lonicera japonica Thunb. Food Chem. 2009, 116, 670–675. [Google Scholar] [CrossRef]
- Yang, B.; Zhong, Z.; Wang, T.; Ou, Y.; Tian, J.; Komatsu, S.; Zhang, L. Integrative Omics of Lonicera japonica Thunb. Flower Development Unravels Molecular Changes Regulating Secondary Metabolites. J. Proteom. 2019, 208, 103470. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Boughalleb, F.; Maaloul, S.; Zaidi, S.; Bakhshandeh, E.; Abdellaoui, R. The Effect of Seasonality on the Phytochemical Composition of Two Limonium Species Naturally Growing in a Mediterranean Arid-Salt Marsh: Harvesting Time Optimization by Modeling Approach. Sci. Hortic. 2023, 309, 111616. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Bai, M.; He, H.; Liang, G.; Wu, H. Correlation between the Dynamic Accumulation of the Main Effective Components and Their Associated Regulatory Enzyme Activities at Different Growth Stages in Lonicera japonica Thunb. Ind. Crops Prod. 2017, 96, 16–22. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Ni, H.; Huang, T.; Yang, B. GRAS Transcription Factors Mediate Flowering through Signaling Pathways of Gibberellin and Circadian Rhythm in Lonicera japonica Thunb. Plant Gene 2021, 28, 100340. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ni, H.; Mustafa, G.; Yang, Y.; Wang, Q.; Fu, H.; Zhang, L.; Yang, B. Use Chou’s 5-Steps Rule to Identify Protein Post-Translational Modification and Its Linkage to Secondary Metabolism during the Floral Development of Lonicera japonica Thunb. Plant Physiol. Biochem. 2021, 167, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Fan, H.; Yao, F.; Cao, X.; Liu, M.; Sun, J.; Liu, Y. Transcriptomics and Targeted Metabolomics Profilings for Elucidation of Pigmentation in Lonicera japonica Flowers at Different Developmental Stages. Ind. Crops Prod. 2020, 145, 111981. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Yan, Y.; Ao, M.; Wu, G.; Yu, L. Influence of Flowering Stage of Lonicera japonica Thunb. on Variation in Volatiles and Chlorogenic Acid. J. Sci. Food Agric. 2009, 89, 953–957. [Google Scholar] [CrossRef]
- Fu, M.; He, Z.; Zhao, Y.; Yang, J.; Mao, L. Antioxidant Properties and Involved Compounds of Daylily Flowers in Relation to Maturity. Food Chem. 2009, 114, 1192–1197. [Google Scholar] [CrossRef]
- Liu, J.-C.; Jiao, Z.-G.; Yang, W.-B.; Zhang, C.-L.; Liu, H.; Lv, Z.-Z. Variation in Phenolics, Flavanoids, Antioxidant and Tyrosinase Inhibitory Activity of Peach Blossoms at Different Developmental Stages. Molecules 2015, 20, 20460–20472. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Qian, W.-Z.; Yi, L.; Ye, Y.-L.; Gu, T.; Gao, S.; Cao, G.-X. Nutrient Composition and Antioxidant Activity of Cercis chinensis Flower in Response to Different Development Stages. Horticulturae 2023, 9, 961. [Google Scholar] [CrossRef]
- Lee, J.; Kang, Y.-R.; Kim, Y.J.; Chang, Y.H. Effect of High Pressure and Treatment Time on Nutraceuticals and Antioxidant Properties of Lonicera japonica Thunb. Innov. Food Sci. Emerg. Technol. 2019, 54, 243–251. [Google Scholar] [CrossRef]
- Duda, S.C.; Mărghitaş, L.A.; Dezmirean, D.; Duda, M.; Mărgăoan, R.; Bobiş, O. Changes in Major Bioactive Compounds with Antioxidant Activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of Harvest Time and Plant Species. Ind. Crops Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Mabizela, G.S.; Muller, M.; De Beer, D.; Van Der Rijst, M.; Slabbert, M.M.; Joubert, E.; Bester, C. Effect of Genotype and Harvest Season on Quality Characteristics of Cyclopia subternata: Phenolic Content and Sensory Profile. South Afr. J. Bot. 2020, 132, 491–501. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the Environment on the Secondary Metabolic Profile of Tithonia diversifolia: A Model for Environmental Metabolomics of Plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, B.; Huang, W.; Amrouche, A.T.; Maurizio, B.; Simal-Gandara, J.; Tundis, R.; Xiao, J.; Zou, L.; Lu, B. Edible Flowers as Functional Raw Materials: A Review on Anti-Aging Properties. Trends Food Sci. Technol. 2020, 106, 30–47. [Google Scholar] [CrossRef]
- Pires, E.D.O.; Di Gioia, F.; Rouphael, Y.; García-Caparrós, P.; Tzortzakis, N.; Ferreira, I.C.F.R.; Barros, L.; Petropoulos, S.A.; Caleja, C. Edible Flowers as an Emerging Horticultural Product: A Review on Sensorial Properties, Mineral and Aroma Profile. Trends Food Sci. Technol. 2023, 137, 31–54. [Google Scholar] [CrossRef]
- Gai, Z.; Hu, S.; Gong, G.; Zhao, J. Recent Advances in Understanding Dietary Polyphenols Protecting against Hypertension. Trends Food Sci. Technol. 2023, 138, 685–696. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant Properties in Vitro and Total Phenolic Contents in Methanol Extracts from Medicinal Plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Santos, L.G.; Martins, V.G. Optimization of the Green Extraction of Polyphenols from the Edible Flower Clitoria ternatea by High-Power Ultrasound: A Comparative Study with Conventional Extraction Techniques. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100458. [Google Scholar] [CrossRef]
- Yasar, B.; Kutlu, G.; Tornuk, F. Edible Flowers as Sources of Bioactive Compounds: Determination of Phenolic Extraction Conditions. Int. J. Gastron. Food Sci. 2022, 30, 100618. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Aydar, A.Y. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes; IntechOpen: London, UK, 2018; ISBN 978-953-51-3878-5. [Google Scholar]
- Li, A.-N.; Li, S.; Li, Y.; Xu, D.-P.; Li, H.-B. Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Osmanthus fragrans Flower. Molecules 2016, 21, 218. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Zhai, J.-W.; Cui, Q.; Liu, J.-Z.; Luo, M.; Fu, Y.-J.; Zu, Y.-G. Ultra-Turrax Based Ultrasound-Assisted Extraction of Five Organic Acids from Honeysuckle (Lonicera japonica Thunb.) and Optimization of Extraction Process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Nivetha, C.V. Optimization of Ultrasound-Assisted Extraction of Natural Pigments from Bougainvillea glabra Flowers. Ind. Crops Prod. 2015, 63, 182–189. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Limonium sinuatum: Optimization and Comparison with Conventional Methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Adjé, F.; Lozano, Y.F.; Lozano, P.; Adima, A.; Chemat, F.; Gaydou, E.M. Optimization of Anthocyanin, Flavonol and Phenolic Acid Extractions from Delonix regia Tree Flowers Using Ultrasound-Assisted Water Extraction. Ind. Crops Prod. 2010, 32, 439–444. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of Ultrasonic-Assisted Extraction of Flavonoids, Polysaccharides, and Eleutherosides from Acanthopanax Senticosus Using Response Surface Methodology in Development of Health Wine. LWT 2022, 165, 113725. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Rakotondramasy-Rabesiaka, L.; Havet, J.-L.; Porte, C.; Fauduet, H. Solid–Liquid Extraction of Protopine from Fumaria officinalis L.—Experimental Study and Process Optimization. Sep. Purif. Technol. 2008, 59, 253–261. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.; Li, W.; Chen, J.; Wang, D.; Zhu, L. Optimum Extraction Process of Polyphenols from the Bark of Phyllanthus emblica L. Based on the Response Surface Methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Ložienė, K.; Venskutonis, P.R.; Šipailienė, A.; Labokas, J. Radical Scavenging and Antibacterial Properties of the Extracts from Different Thymus pulegioides L. Chemotypes. Food Chem. 2007, 103, 546–559. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of Ultrasound-Assisted Extraction of Polyphenolic Compounds from Pomegranate Peel Using Response Surface Methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L.; Karabegović, I.T. Optimization of Microwave-Assisted Extraction of Total Polyphenolic Compounds from Chokeberries by Response Surface Methodology and Artificial Neural Network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Liao, J.; Xue, H.; Li, J. Extraction of Phenolics and Anthocyanins from Purple Eggplant Peels by Multi-Frequency Ultrasound: Effects of Different Extraction Factors and Optimization Using Uniform Design. Ultrason. Sonochem. 2022, 90, 106174. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Extraction Kinetic Modelling of Total Polyphenols and Total Anthocyanins from Saffron Floral Bio-residues: Comparison of Extraction Methods. Food Chem. 2018, 258, 137–143. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, X.; Liu, C.; Sun, Y.; Lin, Z.; Liu, H. Extraction Characteristics and Optimal Parameters of Anthocyanin from Blueberry Powder under Microwave-Assisted Extraction Conditions. Sep. Purif. Technol. 2013, 104, 17–25. [Google Scholar] [CrossRef]
- Turhan, I.; Bialka, K.L.; Demirci, A.; Karhan, M. Ethanol Production from Carob Extract by Using Saccharomyces cerevisiae. Bioresour. Technol. 2010, 101, 5290–5296. [Google Scholar] [CrossRef]
- Schmitzer, V.; Mikulic-Petkovsek, M.; Stampar, F. Sepal Phenolic Profile during Helleborus niger Flower Development. J. Plant Physiol. 2013, 170, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.J.; Park, C.H.; Park, Y.E.; Hyeon, H.; Kim, J.K.; Lee, S.Y.; Park, S.U. Metabolic Profiling and Antioxidant Activity during Flower Development in Agastache rugosa. Physiol. Mol. Biol. Plants 2021, 27, 445–455. [Google Scholar] [CrossRef]
- Amira, E.A.; Behija, S.E.; Beligh, M.; Lamia, L.; Manel, I.; Mohamed, H.; Lotfi, A. Effects of the Ripening Stage on Phenolic Profile, Phytochemical Composition and Antioxidant Activity of Date Palm Fruit. J. Agric. Food Chem. 2012, 60, 10896–10902. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dabbou, S.; Dhibi, M.; Hammami, M. The Efficacy of Phenolics Compounds with Different Polarities as Antioxidants from Olive Leaves Depending on Seasonal Variations. Ind. Crops Prod. 2012, 38, 146–152. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From in Vitro Results to in Vivo Evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, Y.; Meng, J.; Yin, J.; Li, H. Analysis of Physicochemical and Antioxidant Properties of Malus Spp. Petals Reveals Factors Involved in Flower Color Change and Market Value. Sci. Hortic. 2023, 310, 111688. [Google Scholar] [CrossRef]
- Kanani, M.; Chamani, E.; Shokouhian, A.A.; Torabi-Giglou, M. Plant Secondary Metabolism and Flower Color Changes in Damask Rose at Different Flowering Development Stages. Acta Physiol. Plant. 2021, 43, 55. [Google Scholar] [CrossRef]
- Vogt, T.; Pollak, P.; Tarlyn, N.; Taylor, L.P. Pollination- or Wound-Induced Kaempferol Accumulation in Petunia stigmas Enhances Seed Production. Plant Cell 1994, 6, 11–23. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In Vivo Antioxidant Activity of Phenolic Compounds: Facts and Gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- dos Santos Grasel, F.; Ferrão, M.F.; Wolf, C.R. Development of Methodology for Identification the Nature of the Polyphenolic Extracts by FTIR Associated with Multivariate Analysis. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical Screening and Determination of Phenolics and Flavonoids in Dillenia pentagyna Using UV–Vis and FTIR Spectroscopy. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef] [PubMed]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Unsalan, O.; Erdogdu, Y.; Gulluoglu, M.T. FT-raman and FT-IR Spectral and Quantum Chemical Studies on Some Flavonoid Derivatives: Baicalein and Naringenin. J. Raman Spectrosc. 2009, 40, 562–570. [Google Scholar] [CrossRef]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Spruce Wood Bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of Chlorogenic Acid with β-Cyclodextrins: Inclusion Effects, Antioxidative Properties and Potential Application in Grape Juice. Food Hydrocoll. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Popescu, C.; Jones, D.; Kržišnik, D.; Humar, M. Determination of the Effectiveness of a Combined Thermal/Chemical Wood Modification by the Use of FT–IR Spectroscopy and Chemometric Methods. J. Mol. Struct. 2020, 1200, 127133. [Google Scholar] [CrossRef]
- Tsou, C.-H.; Zeng, R.; Wan, N.; Reyes De Guzman, M.; Hu, X.-F.; Yang, T.; Gao, C.; Wei, X.; Yi, J.; Lan, L.; et al. Biological Oyster Shell Waste Enhances Polyphenylene Sulfide Composites and Endows Them with Antibacterial Properties. Chin. J. Chem. Eng. 2023, 57, 118–131. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Huang, W.; Tian, F.; Wang, H.; Wu, S.; Jin, W.; Shen, W.; Hu, Z.; Cai, Q.; Liu, G. Comparative Assessment of Extraction, Composition, and in Vitro Antioxidative Properties of Wheat Bran Polyphenols. LWT 2023, 180, 114706. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Chen, L.; Shao, W.; Chen, Y.; Fan, X.; Liu, Y.; Tang, C.; Ding, S.; Xu, X.; et al. Tea Polyphenols Coated Sodium Alginate-Gelatin 3D Edible Scaffold for Cultured Meat. Food Res. Int. 2023, 173, 113267. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Q.; Hu, T.-G.; Xu, Y.-J.; Wu, J.-J.; Song, X.-L.; Yu, Y.-S. Interaction Mechanism of Carotenoids and Polyphenols in Mango Peels. Food Res. Int. 2023, 173, 113303. [Google Scholar] [CrossRef] [PubMed]
- Iscimen, E.M.; Dursun Capar, T.; McClements, D.J.; Yalcin, H.; Hayta, M. Ultrasound-Assisted Preparation of Faba Bean Protein Isolate-Vitis vinifera L. Polyphenol Extract Conjugates: Structural and Functional Characterization. Food Biosci. 2023, 55, 103041. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Zhang, S.; Wang, Y.; Li, Y.; Qi, B. Improving the Biological Activity and Emulsification Ability of Soybean Meal Hydrolysate via Non-Covalent Interactions with Polyphenols. LWT 2023, 182, 114869. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Qian, W.; Hu, Y.; Lin, X.; Yu, D.; Jia, S.; Ye, Y.; Mao, Y.; Yi, L.; Gao, S. Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale. Agronomy 2023, 13, 1328. [Google Scholar] [CrossRef]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant Mediated Extraction of Total Phenolic Contents (TPC) and Antioxidants from Fruits Juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chuang, Y.-C.; Hsu, H.-W. The FLavonoid, Carotenoid and Pectin Content in Peels of Citrus Cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- de Moraes Barros, H.R.; de Castro Ferreira, T.A.P.; Genovese, M.I. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, D.; Liu, S. Effects of Drying Temperature on the Flavonoid, Phenolic Acid and Antioxidative Capacities of the Methanol Extract of Citrus Fruit (Citrus sinensis (L.) Osbeck) Peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]

| Source | df | TPC | TFC | ||||
|---|---|---|---|---|---|---|---|
| SS | F-Value | p-Value | SS | F-Value | p-Value | ||
| Model | 14 | 2223.96 | 15.39 | <0.0001 *** | 950.827 | 9.068 | 0.0002 ** |
| A | 1 | 0.3729 | 0.0361 | 0.8524 | 188.76 | 25.2 | 0.0003 *** |
| B | 1 | 198.52 | 19.23 | 0.0009 ** | 50.9 | 6.8 | 0.0229 * |
| C | 1 | 201.59 | 19.53 | 0.0008 ** | 121.11 | 16.17 | 0.0017 ** |
| D | 1 | 78.3 | 7.58 | 0.0175 * | 38.49 | 5.14 | 0.0427 * |
| AB | 1 | 5.33 | 0.5158 | 0.4864 | 10.15 | 1.36 | 0.2669 |
| AC | 1 | 13.21 | 1.28 | 0.2801 | 0.0104 | 0.0014 | 0.9709 |
| AD | 1 | 102.32 | 9.91 | 0.0084 ** | 27.6 | 3.69 | 0.6420 |
| BC | 1 | 3.2 | 0.3098 | 0.588 | 1.7 | 0.2274 | 0.0790 |
| BD | 1 | 0.1198 | 0.0116 | 0.916 | 10.81 | 1.44 | 0.2527 |
| CD | 1 | 0.1956 | 0.0189 | 0.8928 | 5.8 | 0.7739 | 0.3963 |
| A2 | 1 | 191.79 | 18.58 | 0.001 ** | 86.61 | 11.57 | 0.0053 ** |
| B2 | 1 | 1586.41 | 153.65 | <0.0001 *** | 487.36 | 65.07 | <0.0001 *** |
| C2 | 1 | 204.67 | 19.82 | 0.0008 ** | 39.97 | 5.34 | 0.0395 * |
| D2 | 1 | 71.45 | 6.92 | 0.0219 * | 71.65 | 9.57 | 0.0093 ** |
| Residual | 12 | 123.89 | 89.872 | ||||
| Lack of Fit | 10 | 116.46 | 3.13 | 0.2660 | 72.761 | 0.8504 | 0.6522 |
| Pure Error | 2 | 7.43 | 17.111 | ||||
| C. Total | 26 | 2347.85 | 1040.70 | ||||
| Std.Dev. | 3.21 | 2.7367 | |||||
| Mean | 100.19 | 56.491 | |||||
| C.V.% | 3.21 | 4.84 | |||||
| Adeq Precision | 12.8242 | 9.5148 | |||||
| R2 | 0.9472 | 0.9136 | |||||
| Adjusted R² | 0.8857 | 0.8129 | |||||
| AICc | 191.40 | 182.73 | |||||
| Ultrasound Power | Ethanol Content | SLR | Extraction Time | TPC Yield (mg GAE/g) | TFC Yield (mg RE/g) | |
|---|---|---|---|---|---|---|
| PGSI | 205.9 W | 46.4% | 1:31.7 | 20.1 min | 117.22 ± 0.55 a | 68.48 ± 2.01 a |
| PGSII | 205.9 W | 46.4% | 1:31.7 | 20.1 min | 112.73 ± 1.68 b | 63.20 ± 1.01 b |
| PGSIII | 205.9 W | 46.4% | 1:31.7 | 20.1 min | 107.33 ± 1.39 c | 59.66 ± 1.87 c |
| Phenols Compounds | Formula | Retention Time (min) | PGS I Content | PGS II Content | PGS III Content | ||
|---|---|---|---|---|---|---|---|
| PGS I | PGS II | PGS III | |||||
| Rutin (mg/g) | C27H30O16 | 15.988 | 15.995 | 15.978 | 6.13 ± 0.31 a | 3.91 ± 0.12 b | 3.95 ± 0.09 b |
| Kaempferol (μg/g) | C15H10O6 | 20.454 | 20.473 | 20.471 | 1.42 ± 0.15 c | 3.45 ± 0.19 b | 5.91 ± 0.385 a |
| Epicatechin (μg/g) | C15H14O6 | 19.460 | 19.487 | 19.478 | 43.50 ± 4.50 a | 28.30 ± 1.42 b | 18.90 ± 0.37 c |
| Caffeic acid (μg/g) | C9H8O4 | 6.837 | 6.820 | 6.842 | 30.30 ± 3.73 b | 22.90 ± 0.25 c | 37.90 ± 2.80 a |
| Chlorogenic acid (mg/g) | C16H18O9 | 14.590 | 14.589 | 14.622 | 103.40 ± 3.13 a | 98.10 ± 1.66 b | 96.60 ± 1.05 c |
| Ferulic acid (mg/g) | C10H10O4 | 20.786 | 20.791 | 20.797 | 0.195 ± 0.02 c | 0.23 ± 0.01 b | 0.35 ± 0.001 a |
| Isoquercitrin (mg/g) | C21H20O12 | 8.297 | 8.270 | 8.251 | 0.32 ± 0.03 a | 0.22 ± 0.01 b | 0.23 ± 0.01 b |
| Quercetin (mg/g) | C15H10O7 | 12.912 | 12.860 | 12.832 | 14.60 ± 1.42 a | 10.90 ± 0.14 c | 11.60 ± 0.34 b |
| Experiment Factor | Ultrasonic Power (W) | Ethanol Concentration (%) | SLR | Extraction Time (min) |
|---|---|---|---|---|
| SLR | 100 | 20 | 15–90 | 5 |
| Ethanol concentration | 100 | 5, 20, 35, 50, 65, 80 | 30 | 5 |
| Ultrasonic power | 100, 200, 300, 400, 500 | 20 | 30 | 5 |
| Extraction time | 100 | 20 | 30 | 5, 10, 15, 20, 25, 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Qian, W.; Fan, S.; Yang, Y.; Liao, H.; Zhuang, G.; Gao, S. Ultrasonic-Assisted Extraction of Phenolic Compounds from Lonicera similis Flowers at Three Harvest Periods: Comparison of Composition, Characterization, and Antioxidant Activity. Molecules 2024, 29, 3280. https://doi.org/10.3390/molecules29143280
Hu Y, Qian W, Fan S, Yang Y, Liao H, Zhuang G, Gao S. Ultrasonic-Assisted Extraction of Phenolic Compounds from Lonicera similis Flowers at Three Harvest Periods: Comparison of Composition, Characterization, and Antioxidant Activity. Molecules. 2024; 29(14):3280. https://doi.org/10.3390/molecules29143280
Chicago/Turabian StyleHu, Yunyi, Wenzhang Qian, Shaojun Fan, Yao Yang, Hai Liao, Guoqing Zhuang, and Shun Gao. 2024. "Ultrasonic-Assisted Extraction of Phenolic Compounds from Lonicera similis Flowers at Three Harvest Periods: Comparison of Composition, Characterization, and Antioxidant Activity" Molecules 29, no. 14: 3280. https://doi.org/10.3390/molecules29143280
APA StyleHu, Y., Qian, W., Fan, S., Yang, Y., Liao, H., Zhuang, G., & Gao, S. (2024). Ultrasonic-Assisted Extraction of Phenolic Compounds from Lonicera similis Flowers at Three Harvest Periods: Comparison of Composition, Characterization, and Antioxidant Activity. Molecules, 29(14), 3280. https://doi.org/10.3390/molecules29143280






