Maclura cochinchinensis (Lour.) Corner Heartwood Extracts Containing Resveratrol and Oxyresveratrol Inhibit Melanogenesis in B16F10 Melanoma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yields and Screening for Anti-Tyrosinase Activities
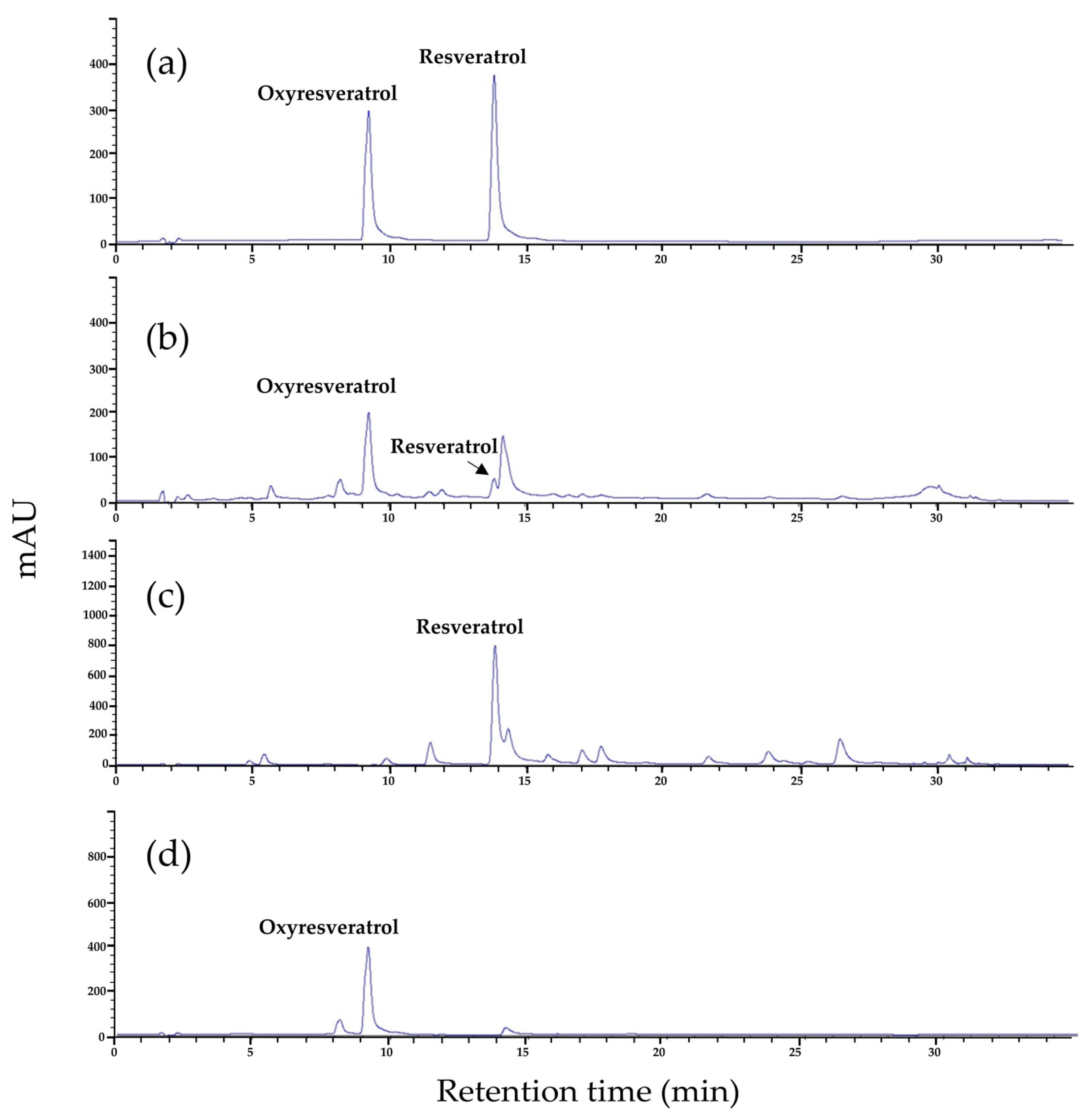
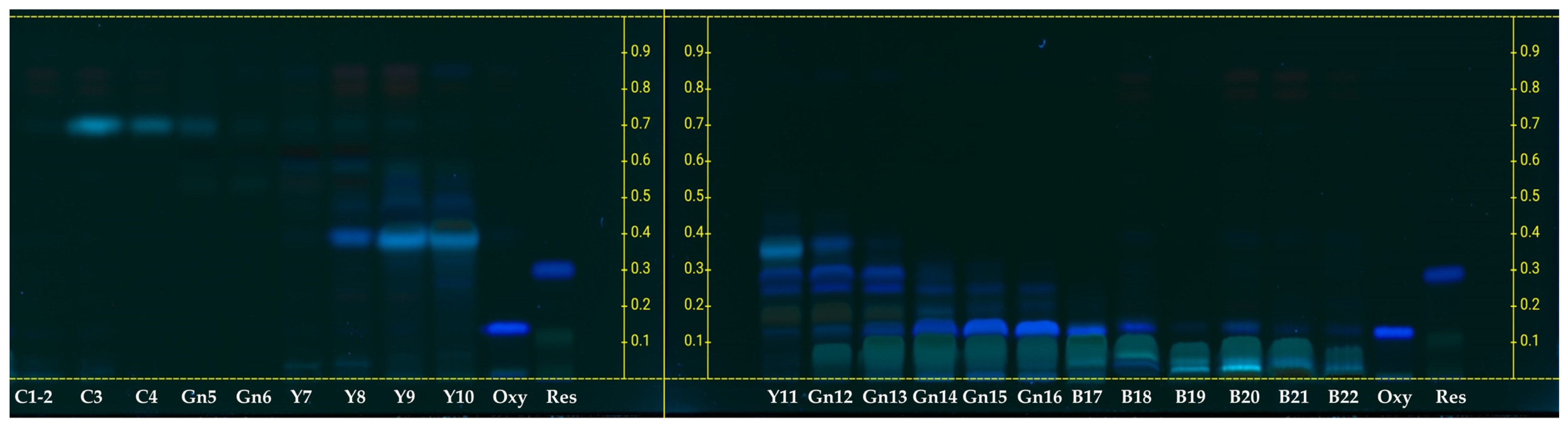
2.2. Stepwise Column Chromatography Fractionation and Resveratrol and Oxyresveratrol Contents
2.3. Anti-Mushroom Tyrosinase Activity
2.4. Cell Viabilities and Melanogenesis
2.5. Antioxidant Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Standard Solutions
3.3. Plant Material, Extraction, and Isolation
3.4. Mushroom Tyrosinase Inhibition Assay
- ∆AC = absorbance of the control at T10 min − absorbance of the control at T0 min
- ∆AB = absorbance of the blank at T10 min − absorbance of the blank at T0 min
- ∆AS = absorbance of the extract at T10 min − absorbance of the extract at T0 min.
3.5. Cell Culture
3.6. Cell Viability and Total Melanin Content Assay
3.7. Stepwise Column Chromatography Fractionation and Separation of Resveratrol and Oxyresveratrol
3.8. High-Performance Thin-Layer Chromatography (HPTLC) Analysis
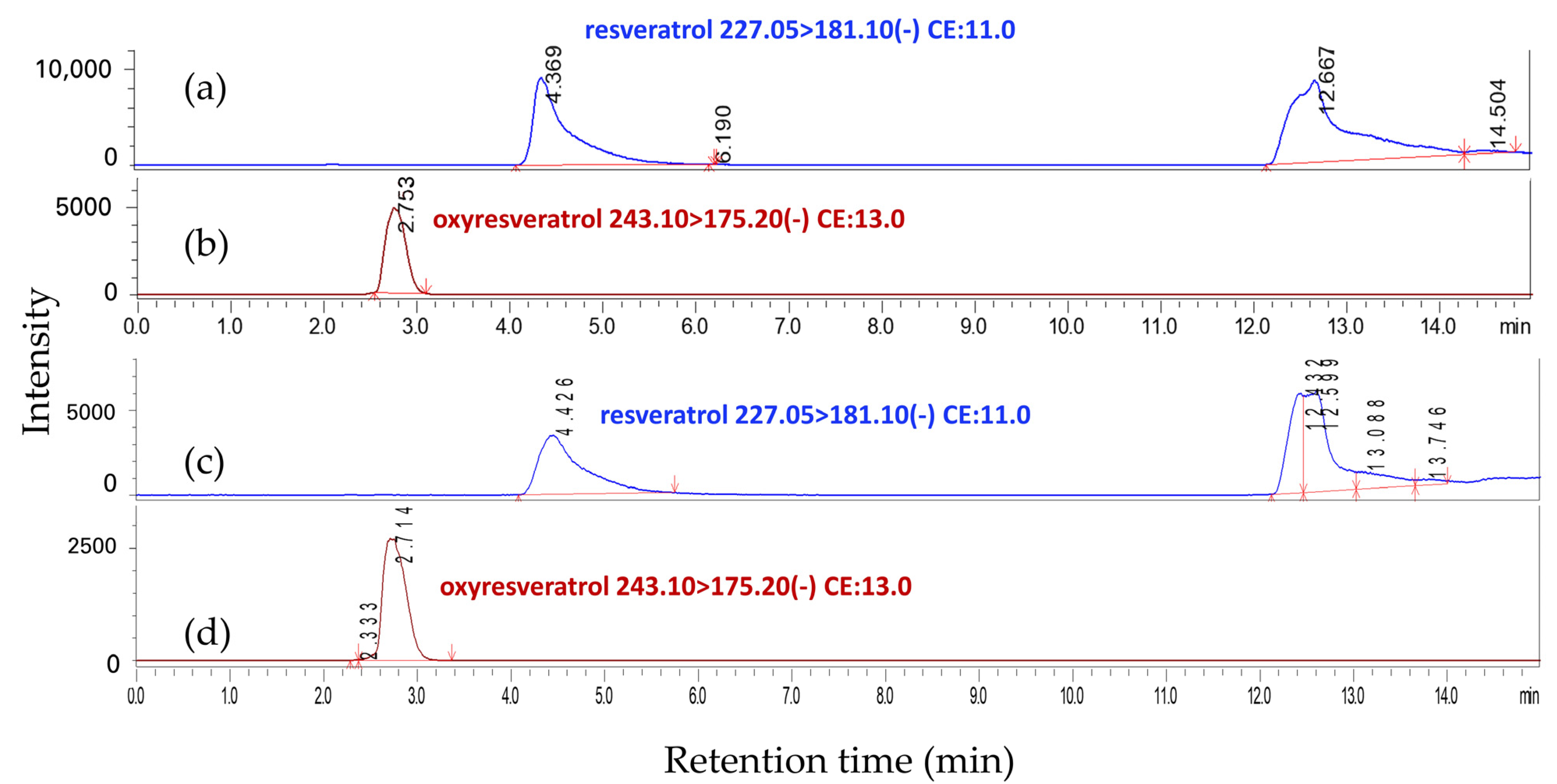
3.9. LC-MS/MS and HPLC Analyses
3.10. DPPH Free Radical Scavenging Activity
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Z.-Y.; Xu, K.; Wang, X.; Wen, Y.-T.; Wang, L.-J.; Huang, D.-Q.; Chen, X.-X.; Chai, W.-M. Punicalagin as a novel tyrosinase and melanin inhibitor: Inhibitory activity and mechanism. LWT 2022, 161, 113318. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, T.I.; Ma, J.-Y. Synergistic effects of novel herbal decoctions from Panax ginseng and Morus alba on tyrosinase activity and melanogenesis in vitro. Heliyon 2022, 8, e08866. [Google Scholar] [CrossRef]
- Shehzadi, S.A.; Saeed, A.; Perveen, F.; Channar, P.A.; Arshad, I.; Abbas, Q.; Kalsoom, S.; Yousaf, S.; Simpson, J. Identification of two novel thiazolidin-2-imines as tyrosinase inhibitors: Synthesis, crystal structure, molecular docking and DFT studies. Heliyon 2022, 8, e10098. [Google Scholar] [CrossRef]
- Bang, E.; Noh, S.G.; Ha, S.; Jung, H.J.; Kim, D.H.; Lee, A.K.; Hyun, M.K.; Kang, D.; Lee, S.; Park, C.; et al. Evaluation of the Novel Synthetic Tyrosinase Inhibitor (Z)-3-(3-bromo-4-hydroxybenzylidene)thiochroman-4-one (MHY1498) In Vitro and In Silico. Molecules 2018, 23, 3307. [Google Scholar] [CrossRef] [PubMed]
- Stapelberg, J.; Nqephe, M.; Lambrechts, I.; Crampton, B.; Lall, N. Selected South African plants with tyrosinase enzyme inhibition and their effect on gene expression. S. Afr. J. Bot. 2019, 120, 280–285. [Google Scholar] [CrossRef]
- Shin, M.; Truong, V.-L.; Lee, M.; Kim, D.; Kim, M.S.; Cho, H.; Jung, Y.H.; Yang, J.; Jeong, W.S.; Kim, Y. Investigation of phenyllactic acid as a potent tyrosinase inhibitor produced by probiotics. Curr. Res. Food Sci. 2023, 6, 100413. [Google Scholar] [CrossRef]
- Singh, M.; Griaud, C.; Collins, C.M. An evaluation of the effectiveness of protected areas in Thailand. Ecol. Indic. 2021, 125, 107536. [Google Scholar] [CrossRef]
- Lumlerdkij, N.; Boonrak, R.; Booranasubkajorn, S.; Akarasereenont, P.; Heinrich, M. In vitro protective effects of plants frequently used traditionally in cancer prevention in Thai traditional medicine: An ethnopharmacological study. J. Ethnopharmacol. 2020, 250, 112409. [Google Scholar] [CrossRef]
- Polbuppha, I.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Limtharakul, T.; Cheenpracha, S.; Pyne, S.G.; Laphookhieo, S. Macluracochinones A-E, antimicrobial flavonoids from Maclura cochinchinensis (Lour.) Corner. Phytochemistry 2021, 187, 112773. [Google Scholar] [CrossRef]
- Sato, V.H.; Chewchinda, S.; Parichatikanond, W.; Vongsak, B. In vitro and in vivo evidence of hypouricemic and anti-inflammatory activities of Maclura cochinchinensis (Lour.) Corner heartwood extract. J. Tradit. Complement. Med. 2020, 10, 85–94. [Google Scholar] [CrossRef]
- Van Chien, T.; Anh, N.T.; Thanh, N.T.; Thao, T.T.P.; Van Loc, T.; Van Sung, T. Two new prenylated isoflavones from Maclura cochinchinensis collected in Hoa Binh province Vietnam. Nat. Prod. Res. 2019, 33, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lakornwong, W.; Kanokmedhakul, K.; Masranoi, J.; Tontapha, S.; Yahuafai, J.; Laphookhieo, S.; Suthiphasilp, V.; Kanokmedhakul, S. Cytotoxic and antibacterial xanthones from the roots of Maclura cochinchinensis. Nat. Prod. Res. 2022, 36, 6021–6030. [Google Scholar] [CrossRef] [PubMed]
- Chewchinda, S.; Leakaya, N.; Sato, H.; Sato, V.H. Antidiabetic effects of Maclura cochinchinensis (Lour.) corner heartwood extract. J. Tradit. Complement. Med. 2021, 11, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Chewchinda, S.; Kongkiatpaiboon, S. A validated HPTLC method for quantitative analysis of morin in Maclura cochinchinensis heartwood. Chin. Herb. Med. 2020, 12, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Kongkiatpaiboon, S.; Tungsukruthai, P.; Sriyakool, K.; Pansuksan, K.; Tunsirikongkon, A.; Pandith, H. Determination of morin in Maclura cochinchinensis heartwood by HPLC. J. Chromatogr. Sci. 2017, 55, 346–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rueankham, L.; Panyajai, P.; Saiai, A.; Rungrojsakul, M.; Tima, S.; Chiampanichayakul, S.; Yeerong, K.; Somwongin, S.; Chaiyana, W.; Dejkriengkraikul, P.; et al. Biological activities of extracts and compounds from Thai Kae-Lae (Maclura cochinchinensis (Lour.) Corner). BMC Complement. Med. Ther. 2023, 23, 191. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.-C.; Lee, E.H.; Lee, J.W.; Yang, S.-H.; Kim, J.-C. Preventive Effect of M. cochinchinensis on Melanogenesis via Tyrosinase Activity Inhibition and p-PKC Signaling in Melan-A Cell. Nutrients 2021, 13, 3894. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Jaisamut, S.; Gonsap, K.; Parmontree, P. Optimization of Maclura cochinchinensis Extract as a Cosmeceutical Component for Antioxidant and Anti-Tyrosinase Activities. Key Eng. Mater. 2020, 859, 188–193. [Google Scholar] [CrossRef]
- Yang, K.-R.; Yu, H.-C.; Huang, C.-Y.; Kuo, J.-M.; Chang, C.; Shieh, C.-J.; Kuo, C.-H. Bioprocessed Production of Resveratrol-Enriched Rice Wine: Simultaneous Rice Wine Fermentation, Extraction, and Transformation of Piceid to Resveratrol from Polygonum cuspidatum Roots. Foods 2019, 8, 258. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, B.-Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. Production of Resveratrol by Piceid Deglycosylation Using Cellulase. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Lin, J.A.; Kuo, C.H.; Chen, B.Y.; Li, Y.; Liu, Y.C.; Chen, J.H.; Shieh, C.J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Iswandana, R.; Olisia, S.; Adriyani, M.; Jufri, M.; Mun’im, A. Application of Tween 80 and Tween 20 for microwave-assisted extraction of oxyresveratrol from mulberry (Morus alba L.) twigs. J. Appl. Pharm. Sci. 2020, 10, 93–100. [Google Scholar] [CrossRef]
- Jiratanakittiwat, K.; Satirapipathkul, C.; Charnvanich, D. The Influences of Extraction on the Quantity of Oxyresveratrol from Artocarpus lakoocha Roxb. Int. J. Biosci. Biochem. Bioinform. 2020, 10, 110–116. [Google Scholar] [CrossRef][Green Version]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and Analysis of Chemical Compositions of Natural Products and Plants. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.; Tian, Y.; Xie, K.; Li, D. Effect of heat reflux extraction on the structure and composition of a high-volatile bituminous coal. Appl. Therm. Eng. 2016, 109, 560–568. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-formulation studies of resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]
- Tongped, C.; Thonkham, P.; Trongsiriwat, N. The solubility profile of oxyresveratrol. Mater. Today Proc. 2023, 77, 1064–1067. [Google Scholar] [CrossRef]
- Berthon, B. Resveratrol: An original mechanism on tyrosinase inhibition. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef]
- Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the potency of flavonoids on mushroom tyrosinase inhibitory activity and melanin synthesis in melanocytes. Molecules 2018, 23, 1403. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yun, J.; Lee, C.-K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and Hydroxystilbene Compounds: Inhibitory Effect on Tyrosinase and Mechanism of Action. J. Biol. Chem. 2002, 277, 16340–16344. [Google Scholar] [CrossRef] [PubMed]
- Rodboon, T.; Palipoch, S.; Okada, S.; Charoenchon, N.; Nakornpakdee, Y.; Suwannalert, P. Oxyresveratrol inhibits cellular tyrosinase-related oxidative stress-induced melanogenesis in B16 melanoma cells. J. Appl. Pharm. Sci. 2020, 10, 008–013. [Google Scholar] [CrossRef]
- Lin, C.B.; Babiarz, L.; Liebel, F.; Kizoulis, M.; Gendimenico, G.J.; Seiberg, M.; Roydon Price, E.; Fisher, D.E. Modulation of Microphthalmia-associated Transcription Factor Gene Expression Alters Skin Pigmentation. J. Investig. Dermatol. 2002, 119, 1330–1340. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.Y.; Lin, Y.F.; Hu, H.Y.; Liao, H.F. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid.-Based Complement. Altern. Med. Ecam 2013, 2013, 632121. [Google Scholar] [CrossRef]
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol. Ther. 2014, 22, 35–40. [Google Scholar] [CrossRef]
- Na, J.I.; Shin, J.W.; Choi, H.R.; Kwon, S.H.; Park, K.C. Resveratrol as a Multifunctional Topical Hypopigmenting Agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef] [PubMed]
- Park, K.T.; Kim, J.K.; Hwang, D.; Yoo, Y.; Lim, Y.H. Inhibitory effect of mulberroside A and its derivatives on melanogenesis induced by ultraviolet B irradiation. Food Chem. Toxicol. 2011, 49, 3038–3045. [Google Scholar] [CrossRef]
- Sainz-Hernández, J.C.; Rueda-Puente, E.O.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; González-Ocampo, H.A.; López-Corona, B.E. Biological Application of the Allopathic Characteristics of the Genus Maclura: A Review. Plants 2023, 12, 3480. [Google Scholar] [CrossRef]
- Shin, S.; Ko, J.; Kim, M.; Song, N.; Park, K. Morin induces melanogenesis via activation of MAPK signaling pathways in B16F10 mouse melanoma cells. Molecules 2021, 26, 2150. [Google Scholar] [CrossRef]
- Jin, J.-H.; Jiang, Y.-Y.; Wang, Y.; Meng, Z.-W.; Li, D.-H.; Zhang, L.; Wang, H.; Zhang, Y.-J. Oxyresveratrol-induced Activation of Nrf2/HO-1 Signaling Pathway Enhances Ability of Resveratrol to Inhibit UVB-induced Melanin. Int. J. Dermatol. Venereol. 2021, 4, 152–162. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y. Resveratrol alleviates LPS-induced injury in human keratinocyte cell line HaCaT by up-regulation of miR-17. Biochem. Biophys. Res. Commun. 2018, 501, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kang, J.H.; Seo, J.O.; Baek, S.H.; Moh, S.H.; Chae, J.K.; Park, Y.U.; Ko, Y.T.; Kim, S.Y. Anti-Melanogenic Potentials of Nanoparticles from Calli of Resveratrol-Enriched Rice against UVB-Induced Hyperpigmentation in Guinea Pig Skin. Biomol. Ther. 2016, 24, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From In Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef]
- Promden, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Structure and antioxidant activity relationships of isoflavonoids from Dalbergia parviflora. Molecules 2014, 19, 2226–2237. [Google Scholar] [CrossRef]
| No. | Solvents Used in Serial Exhaustive Extraction * | Extraction Yield | Tyrosinase Inhibitory Activity | ||
|---|---|---|---|---|---|
| mg ** | % | % *** | IC50 (µg/mL) **** | ||
| 1 | Hexane (H0) | 0 | 0 | ND | ND |
| Ethyl acetate (H1) | 240 | 2.4 | 69.98 | 0.52 ± 0.04 | |
| Methanol (H2) | 900 | 9.0 | 83.64 | 0.28 ± 0.11 | |
| Water (H3) | 640 | 6.4 | 60.65 | 0.95 ± 0.12 | |
| 2 | Ethyl acetate (A0) | 350 | 3.5 | 74.31 | 0.71 ± 0.18 |
| Methanol (A1) | 940 | 9.4 | 82.50 | 0.12 ± 0.01 | |
| Water (A2) | 300 | 3.0 | 61.81 | 2.36 ± 0.18 | |
| 3 | Methanol (M0) | 1460 | 14.6 | 80.61 | 0.21 ± 0.01 |
| Water (M1) | 180 | 1.8 | 27.11 | 6.90 ± 1.03 | |
| 4 | Water (W0) | 860 | 8.6 | 69.32 | 0.67 ± 0.03 |
| Methanol (W1) | 660 | 6.6 | 78.53 | 0.19 ± 0.09 | |
| Ethyl acetate (W2) | 200 | 2.0 | 79.30 | 0.38 ± 0.08 | |
| Hexane (W3) | 240 | 2.4 | ND | ND | |
| Oxyresveratrol | - | - | 98.48 | 0.020 ± 0.004 | |
| Kojic acid | - | - | 18.81 | 13.85 ± 1.52 | |
| No. | Fractions Collected | Eluent Ratio (%) | Elution Volume (mL) | ||
|---|---|---|---|---|---|
| Hexane | Ethyl Acetate | Methanol | |||
| 1 | FA | 100 | - | - | 800 |
| 80 | 20 | - | 1600 | ||
| 60 | 40 | - | 2400 | ||
| - | 100 | - | 1600 | ||
| 2 | FM | - | - | 100 | 1000 |
| No. | Fractions Collected * | Eluent Ratio (%) | Elution Volume (mL) | ||
|---|---|---|---|---|---|
| Hexane | Ethyl Acetate | Methanol | |||
| 1 | C1–2 | 100 | - | - | 1400 |
| 2 | C3–Y10 | 80 | 20 | - | 2100 |
| 3 | Y11–Gn13 | 60 | 40 | - | 2100 |
| 4 | Gn14 | 55 | 45 | - | 300 |
| 5 | Gn15 | 50 | 50 | - | 300 |
| 6 | Gn16–B18 | 40 | 60 | - | 700 |
| 7 | B19–B20 | 20 | 80 | - | 700 |
| 8 | B21 | - | 100 | - | 700 |
| 9 | B22 | - | - | 100 | 1400 |
| Fraction | Yield (g) | Resveratrol | Oxyresveratrol | DPPH Scavenging Activity (%) *,** | Anti-Mushroom Tyrosinase, IC50 (µg/mL) | Melanogenesis in B16F10 (%) *,***,† | ||
|---|---|---|---|---|---|---|---|---|
| Conc. (mg/g) | Total (mg) | Conc. (mg/g) | Total (mg) | |||||
| Methanolic crude extract | 74.46 | 4.32 | 321.67 | 33.62 | 2503.35 | 41.76 ± 3.64 | 0.30 ± 0.02 | 50.62 ± 1.26 |
| FA | 48.46 | 6.45 | 312.57 | 50.39 | 2441.90 | 33.63 ± 9.78 | 0.25 ± 0.01 | 45.59 ± 2.02 |
| FM | 13.68 | 0.13 | 1.78 | 3.82 | 52.26 | 34.12 ± 2.55 | NF | 105.65 ± 7.66 |
| C1–2 | 0.23 | ND | ND | NF | NF | NF | NF | 112.09 ± 6.67 |
| C3 | 0.11 | ND | ND | NF | NF | NF | NF | 109.02 ± 8.13 |
| C4 | 0.174 | ND | ND | NF | NF | NF | NF | 105.00 ± 6.07 |
| Gn5 | 0.13 | ND | ND | NF | NF | NF | NF | 106.53 ± 7.42 |
| Gn6 | 0.09 | ND | ND | NF | NF | NF | NF | 94.71 ± 2.31 |
| Y7 | 0.15 | 0.04 | 0.006 | NF | NF | NF | NF | 103.43 ± 7.98 |
| Y8 | 0.22 | 0.05 | 0.011 | NF | NF | NF | NF | 33.50 ± 2.33 |
| Y9 | 0.31 | 0.07 | 0.02 | NF | NF | 29.37 ± 2.16 | NF | 34.80 ± 3.24 |
| Y10 | 0.22 | 0.22 | 0.05 | NF | NF | 46.76 ± 5.12 | 0.27 ± 0.02 | 38.32 ± 2.69 |
| Y11 | 1.21 | 94.90 | 114.83 | NF | NF | 44.21 ± 6.34 | 0.21 ± 0.03 | 29.45 ± 1.54 |
| Gn12 | 0.35 | 110.21 | 38.57 | 0.16 | 0.06 | 46.22 ± 5.97 | 0.13 ± 0.01 | 30.63 ± 4.65 |
| Gn13 | 1.78 | 63.85 | 113.65 | 34.86 | 62.05 | 56.66 ± 7.01 | 0.10 ± 0.00 | 47.40 ± 3.21 |
| Gn14 | 0.82 | 1.23 | 1.01 | 195.62 | 160.41 | 77.67 ± 8.82 | 0.05 ± 0.01 | 45.30 ± 5.37 |
| Gn15 | 3.35 | NF | NF | 321.93 | 1078.47 | 84.86 ± 8.97 | 0.05 ± 0.00 | 46.72 ± 6.62 |
| Gn16 | 0.99 | NF | NF | 274.59 | 271.84 | 75.69 ± 6.54 | 0.12 ± 0.02 | 47.00 ± 5.12 |
| B17 | 1.31 | NF | NF | 88.96 | 116.54 | 68.95 ± 7.32 | 0.30 ± 0.02 | 66.73 ± 5.64 |
| B18 | 3.42 | NF | NF | 2.28 | 7.80 | 35.46 ± 2.11 | 0.32 ± 0.06 | 91.81 ± 7.72 |
| B19 | 8.49 | NF | NF | 1.81 | 15.37 | 27.80 ± 1.01 | NF | 90.78 ± 6.13 |
| B20 | 3.07 | NF | NF | NF | NF | 31.35 ± 2.78 | NF | 91.51 ± 8.11 |
| B21 | 4.68 | NF | NF | NF | NF | 31.28 ± 2.24 | NF | 96.10 ± 5.78 |
| B22 | 0.33 | NF | NF | NF | NF | 25.47 ± 1.09 | NF | 93.00 ± 6.54 |
| Resveratrol | 40.74 ± 2.56 | ND | 20.78 ± 1.24 | |||||
| Oxyresveratrol | 83.68 ± 6.13 | 0.04 ± 0.00 | 20.67 ± 1.17 | |||||
| Kojic acid | NF | 2.04 ± 0.10 | 68.45 ± 8.34 | |||||
| No. | Substances | Retention Time (min) | Precursor Ion [M − H]− (m/z) | Product Ion (m/z) | Q1 Pre Bias (V) | Collision Energy (V) | Q3 Pre Bias (V) |
|---|---|---|---|---|---|---|---|
| 1 | Resveratrol | 4.369 | 227.05 | 181.1 | 27.0 | 11.0 | 24.0 |
| 2 | Oxyresveratrol | 2.753 | 243.10 | 175.2 | 28.0 | 13.0 | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promden, W.; Chanvorachote, P.; Viriyabancha, W.; Sintupachee, S.; De-Eknamkul, W. Maclura cochinchinensis (Lour.) Corner Heartwood Extracts Containing Resveratrol and Oxyresveratrol Inhibit Melanogenesis in B16F10 Melanoma Cells. Molecules 2024, 29, 2473. https://doi.org/10.3390/molecules29112473
Promden W, Chanvorachote P, Viriyabancha W, Sintupachee S, De-Eknamkul W. Maclura cochinchinensis (Lour.) Corner Heartwood Extracts Containing Resveratrol and Oxyresveratrol Inhibit Melanogenesis in B16F10 Melanoma Cells. Molecules. 2024; 29(11):2473. https://doi.org/10.3390/molecules29112473
Chicago/Turabian StylePromden, Worrawat, Pithi Chanvorachote, Wittawat Viriyabancha, Siriluk Sintupachee, and Wanchai De-Eknamkul. 2024. "Maclura cochinchinensis (Lour.) Corner Heartwood Extracts Containing Resveratrol and Oxyresveratrol Inhibit Melanogenesis in B16F10 Melanoma Cells" Molecules 29, no. 11: 2473. https://doi.org/10.3390/molecules29112473
APA StylePromden, W., Chanvorachote, P., Viriyabancha, W., Sintupachee, S., & De-Eknamkul, W. (2024). Maclura cochinchinensis (Lour.) Corner Heartwood Extracts Containing Resveratrol and Oxyresveratrol Inhibit Melanogenesis in B16F10 Melanoma Cells. Molecules, 29(11), 2473. https://doi.org/10.3390/molecules29112473








