Efficient Transformation of Water Vapor into Hydrogen by Dielectric Barrier Discharge Loaded with Bamboo Carbon Bed Structured by Fibrous Material
Abstract
1. Introduction
2. Results and Discussion
2.1. The Characterization of the Packed Materials in QC-DBD
2.1.1. Morphology
2.1.2. BET Results
2.1.3. XPS Characterization
2.1.4. FT-IR Analysis
2.1.5. Optical Emission Spectroscopy
2.2. Experimental Results and Analysis
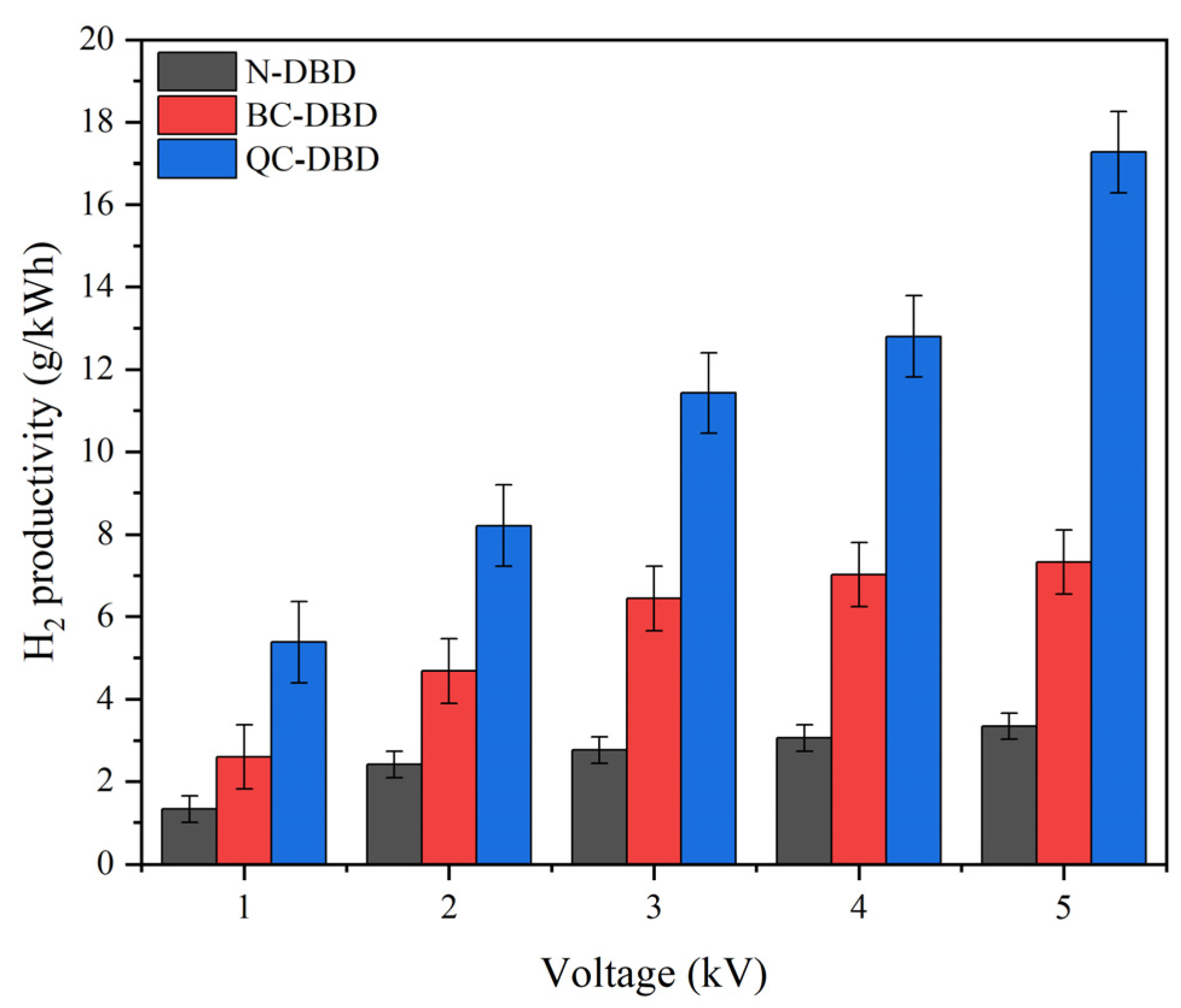
2.2.1. Effects of Discharge Voltage on H2 Productivity in Different DBD Reactors
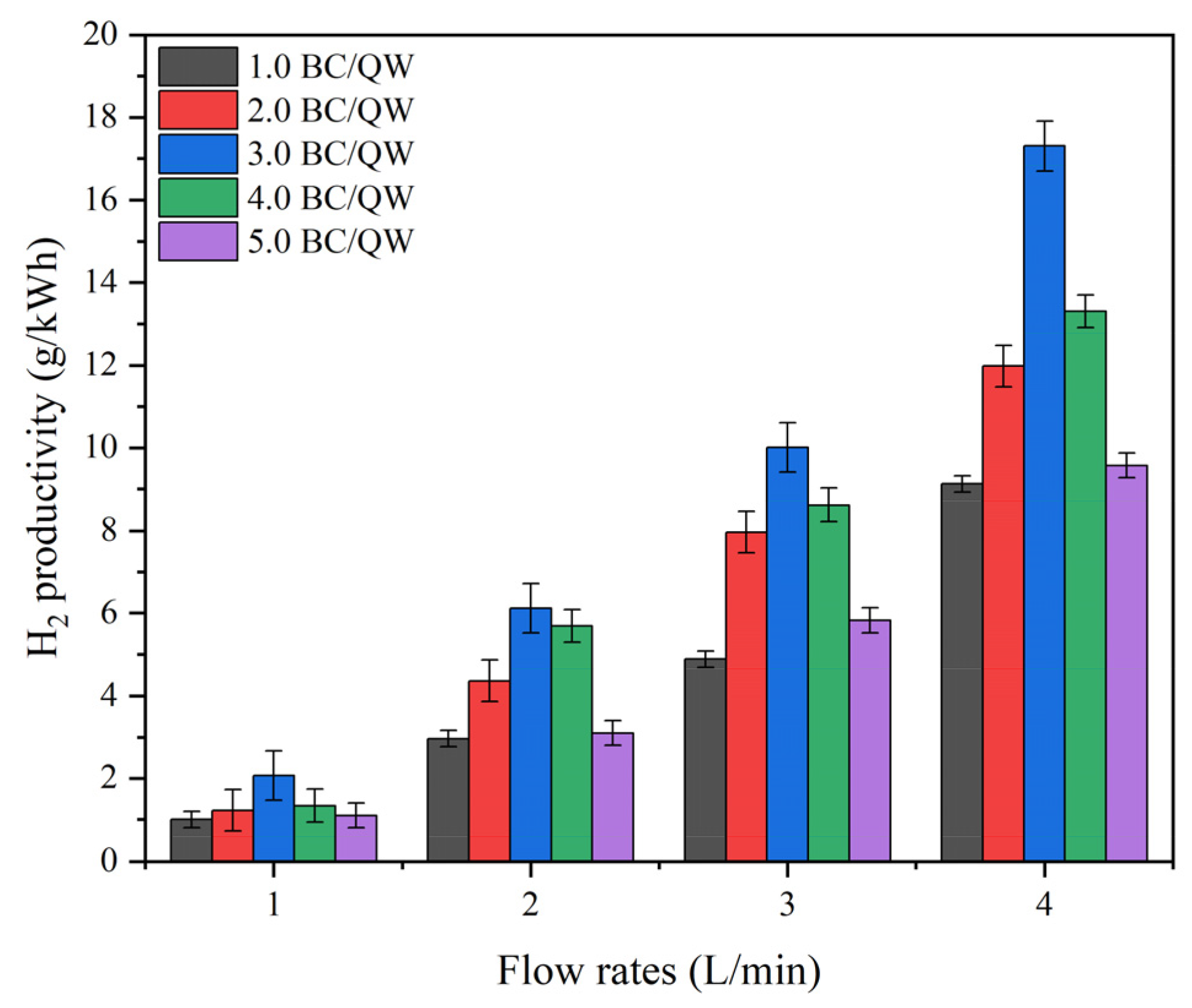
2.2.2. Effects of the BC: QW Quality Ratios on H2 Productivity in QC-DBD
2.2.3. Effects of Relative Humidity on H2 Productivity in N-DBD and QC-DBD
2.2.4. Production of CO, CO2 and CH4 in QC-DBD
3. Experimental Section
3.1. Packed Materials Preparation and Characterization
3.2. Experimental Setup
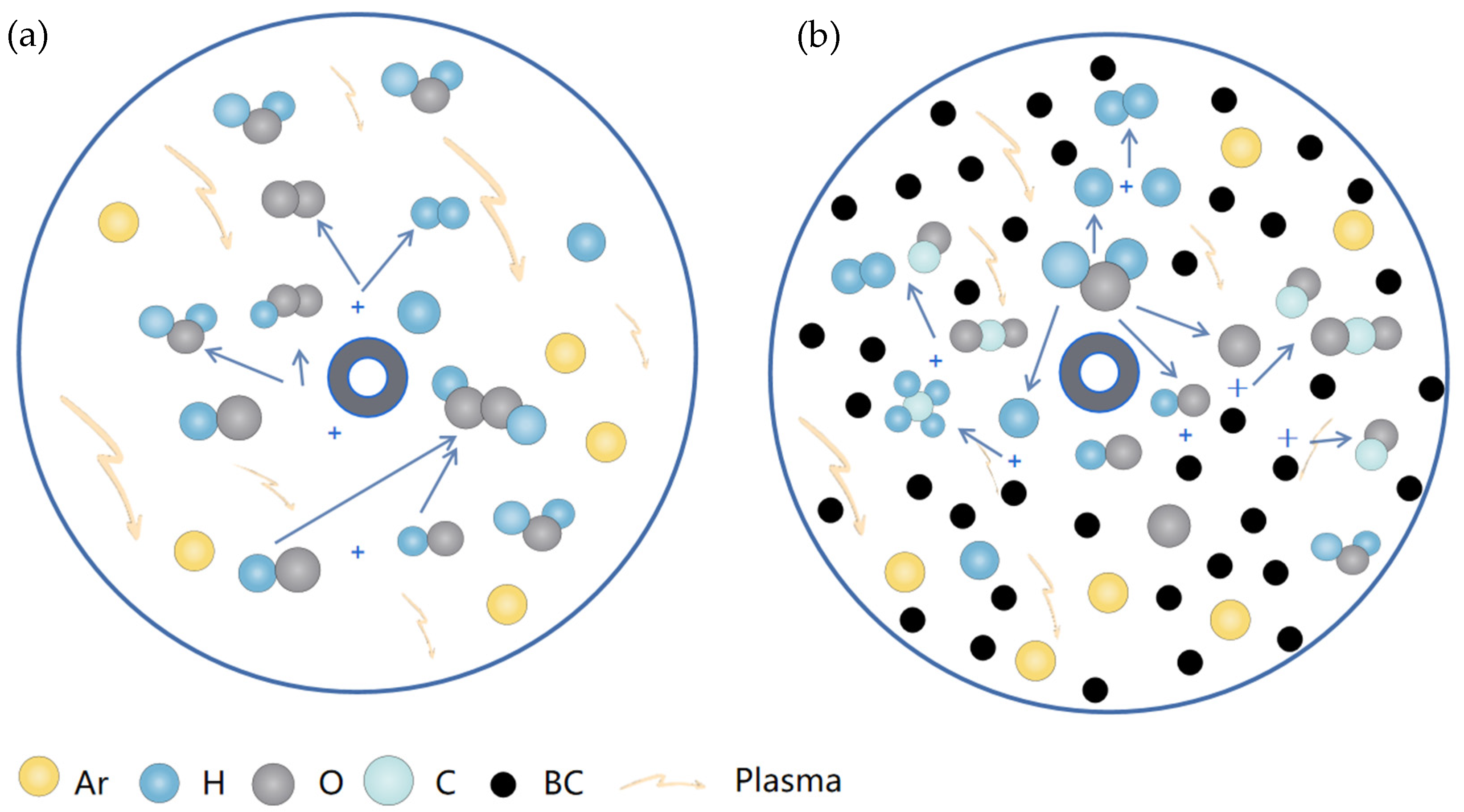
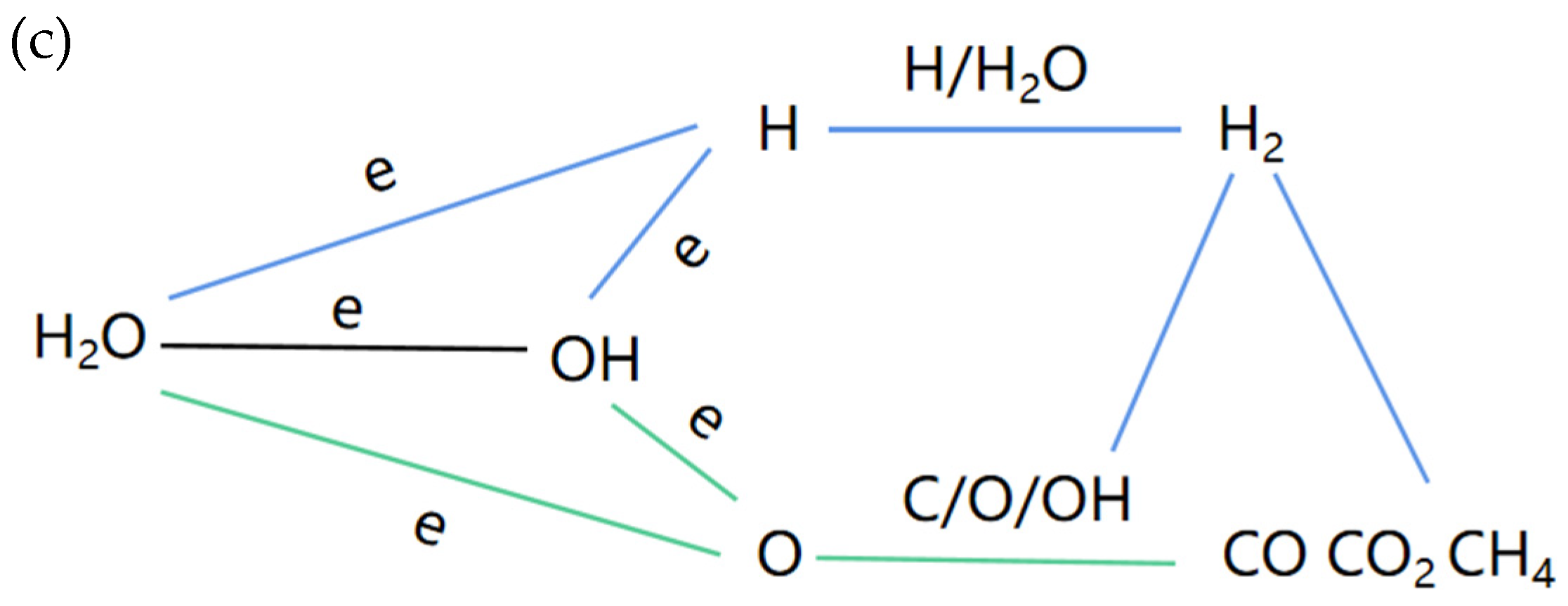
4. Reaction Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusuf, M.; Farooqi, A.S.; Ying, Y.X.; Keong, L.K.; Alam, M.A.; Hellgardt, K.; Abdullah, B. Syngas Production Employing Nickel on Alumina-Magnesia Supported Catalyst via Dry Methane Reforming. Materialwissenschaft Und Werkstofftechnik 2021, 52, 1090–1100. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The Hydrogen Economy—Vision or Reality? Int. J. Hydrogen Energy 2015, 40, 7903–7919. [Google Scholar] [CrossRef]
- Samsatli, S.; Staffell, I.; Samsatli, N.J. Optimal Design and Operation of Integrated Wind-Hydrogen-Electricity Networks for Decarbonising the Domestic Transport Sector in Great Britain. Int. J. Hydrogen Energy 2016, 41, 447–475. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sust. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Service, R.F. MATERIALS SCIENCE New Electrolyzer Splits Water on the Cheap. Science 2020, 367, 1181. [Google Scholar] [CrossRef] [PubMed]
- Fakeeha, A.H.; Fakeeha, R.; El Hassan, N.; Al-Zahrani, S.A.; Al-Awadi, A.S.; Frusteri, L.; Bayahia, H.; Alharth, A.I.; Al-Fatesh, A.S.; Kumar, R. Holmium Promoted Yttria-Zirconia Supported Ni Catalyst for H2 Production via Dry Reforming of Methane. Int. J. Hydrogen Energy 2022, 47, 38242–38257. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Puangpetch, T.; Ramakul, P.; Serivalsatit, K.; Hunsom, M. Light-Assisted Synthesis of Au/TiO2 Nanoparticles for H2 Production by Photocatalytic Water Splitting. Int. J. Hydrogen Energy 2022, 47, 23570–23582. [Google Scholar] [CrossRef]
- Bozieva, A.M.; Khasimov, M.K.; Voloshin, R.A.; Sinetova, M.A.; Kupriyanova, E.V.; Zharmukhamedov, S.K.; Dunikov, D.O.; Tsygankov, A.A.; Tomo, T.; Allakhverdiev, S.I. New Cyanobacterial Strains for Biohydrogen Production. Int. J. Hydrogen Energy 2023, 48, 7569–7581. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Enhanced Sewage Sludge Disintegration and Hydrogen Production by Ionizing Radiation Pretreatment in the Presence of Fe2+. ACS Sustain. Chem. Eng. 2019, 7, 15548–15557. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Arham Khan, M.; Ibrahim, H.; Ekeoma, B.C.; Kamyab, H.; Rahman, M.M.; Nadda, A.K.; Chelliapan, S. A State-of-The-Art Review on the Latest Trends in Hydrogen Production, Storage, and Transportation Techniques. Fuel 2023, 340, 127574. [Google Scholar] [CrossRef]
- Aricò, A.S.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer Electrolyte Membrane Water Electrolysis: Status of Technologies and Potential Applications in Combination with Renewable Power Sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Gong, Y. Perspective of Hydrogen Energy and Recent Progress in Electrocatalytic Water Splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Ramakrishna, S.U.B.; Krishna, S.V.; Srilatha, K.; Devi, B.R.; Himabindu, V. Synthesis of Titanium (IV) Oxide Composite Membrane for Hydrogen Production through Alkaline Water Electrolysis. S. Afr. J. Chem. Eng. 2018, 25, 54–61. [Google Scholar] [CrossRef]
- Hammer, T.; Kappes, T.; Baldauf, M. Plasma Catalytic Hybrid Processes: Gas Discharge Initiation and Plasma Activation of Catalytic Processes. Catal. Today 2004, 89, 5–14. [Google Scholar] [CrossRef]
- Varne, M. Evaluation of Optimum Conditions for Hydrogen Generation in Argon-Water Vapor Dielectric Barrier Discharge. Int. J. Hydrogen Energy 2016, 41, 22769–22774. [Google Scholar] [CrossRef]
- Rehman, F.; Abdul Majeed, W.S.; Zimmerman, W.B. Hydrogen Production from Water Vapor Plasmolysis Using DBD-Corona Hybrid Reactor. Energy Fuels 2013, 27, 2748–2761. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Faisal, A.; Hafeez, A.; Javed, F.; Mustafa, M.; Rehman, F. Hydrogen Production through Water Vapors Using Optimized Corona-DBD Hybrid Plasma Micro-Reactor. Fuel 2023, 331, 125838. [Google Scholar] [CrossRef]
- Dey, G.R.; Das, T.N. Yields of Hydrogen and Hydrogen Peroxide from Argon–Water Vapor in Dielectric Barrier Discharge. Plasma Chem Plasma Process 2016, 36, 523–534. [Google Scholar] [CrossRef]
- Gholami, Z.; Luo, G.; Gholami, F.; Yang, F. Recent Advances in Selective Catalytic Reduction of NOx by Carbon Monoxide for Flue Gas Cleaning Process: A Review. Catal. Rev.-Sci. Eng. 2021, 63, 68–119. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Obermajer, J.; Dvorák, B. Catalysts for the water-gas shift reaction. Chem. Listy 2002, 96, 685–692. [Google Scholar]
- Zhang, F. Promotion of Microwave Discharge over Carbon Catalysts for CO2 Reforming of CH4 to Syngas. Fuel 2023, 331, 125914. [Google Scholar] [CrossRef]
- Gao, F.; Jin, X.; Wang, G.; Sun, L.; Tan, Y.; Zhang, R.; Zhao, W.; Hou, J.; Zhang, R. Removal of NO by Carbon-Based Catalytic Reduction Bed Loaded with Mn Induced by Dielectric Barrier Discharge at Low Temperature. Environ. Eng. Res. 2023, 28, 210500. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Singh, B.; Murad, L.; Laffir, F.; Dickinson, C.; Dempsey, E. Pt Based Nanocomposites (Mono/Bi/Tri-Metallic) Decorated Using Different Carbon Supports for Methanol Electro-Oxidation in Acidic and Basic Media. Nanoscale 2011, 3, 3334–3349. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Wagner, C.D.; Zatko, D.A.; Raymond, R.H. Use of the Oxygen KLL Auger Lines in Identification of Surface Chemical States by Electron Spectroscopy for Chemical Analysis. Anal. Chem. 1980, 52, 1445–1451. [Google Scholar] [CrossRef]
- Ramesh Kumar, C.; Rambabu, N.; Maheria, K.C.; Dalai, A.K.; Lingaiah, N. Iron Exchanged Tungstophosphoric Acid Supported on Activated Carbon Derived from Pinecone Biomass: Evaluation of Catalysts Efficiency for Liquid Phase Benzylation of Anisole with Benzyl Alcohol. Appl. Catal. A Gen. 2014, 485, 74–83. [Google Scholar] [CrossRef]
- Tryba, B.; Toyoda, M.; Morawski, A.W.; Inagaki, M. Modification of Carbon-Coated TiO2 by Iron to Increase Adsorptivity and Photoactivity for Phenol. Chemosphere 2005, 60, 477–484. [Google Scholar] [CrossRef]
- Saud, S.; Nguyen, D.; Kim, S.-G.; Matyakubov, N.; Nguyen, V.T.; Mok, Y.S. Influence of Background Gas for Plasma-Assisted Catalytic Removal of Ethylene in a Modified Dielectric Barrier Discharge-Reactor. ACS Agric. Sci. Technol 2022, 2, 113–122. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Azzolini, J.A.; Stechel, E.B.; Ayers, K.E.; Valdez, T. Review-Engineering Challenges in Green Hydrogen Production Systems. J. Electrochem. Soc. 2022, 169, 054503. [Google Scholar] [CrossRef]
- Qiu, F.; Han, Z.; Peterson, J.J.; Odoi, M.Y.; Sowers, K.L.; Krauss, T.D. Photocatalytic Hydrogen Generation by CdSe/CdS Nanoparticles. Nano Lett. 2016, 16, 5347–5352. [Google Scholar] [CrossRef]
- Burlica, R.; Shih, K.-Y.; Locke, B.R. Formation of H2 and H2O2 in a Water-Spray Gliding Arc Nonthermal Plasma Reactor. Ind. Eng. Chem. Res. 2010, 49, 6342–6349. [Google Scholar] [CrossRef]
- Kirkpatrick, M.J.; Locke, B.R. Hydrogen, Oxygen, and Hydrogen Peroxide Formation in Aqueous Phase Pulsed Corona Electrical Discharge. Ind. Eng. Chem. Res. 2005, 44, 4243–4248. [Google Scholar] [CrossRef]
- Hrycak, B.; Czylkowski, D.; Miotk, R.; Dors, M.; Jasinski, M.; Mizeraczyk, J. Hydrogen Production from Ethanol in Nitrogen Microwave Plasma at Atmospheric Pressure. Open Chem. 2015, 13, 317–324. [Google Scholar] [CrossRef]
- Sarmiento, B.; Brey, J.J.; Viera, I.G.; González-Elipe, A.R.; Cotrino, J.; Rico, V.J. Hydrogen Production by Reforming of Hydrocarbons and Alcohols in a Dielectric Barrier Discharge. J. Power Sources 2007, 169, 140–143. [Google Scholar] [CrossRef]
- Andersen, J.A.; Christensen, J.M.; Østberg, M.; Bogaerts, A.; Jensen, A.D. Plasma-Catalytic Ammonia Decomposition Using a Packed-Bed Dielectric Barrier Discharge Reactor. Int. J. Hydrogen Energy 2022, 47, 32081–32091. [Google Scholar] [CrossRef]
- Xi, J.; Zhou, E.; Liu, Y.; Gao, W.; Ying, J.; Chen, Z.; Gao, C. Wood-Based Straightway Channel Structure for High Performance Microwave Absorption. Carbon 2017, 124, 492–498. [Google Scholar] [CrossRef]
- Zhao, H.; Yeow Seow, J.Z.; Cheng, Y.; Xu, Z.J.; Ji, G. Green Synthesis of Hierarchically Porous Carbons with Tunable Dielectric Response for Microwave Absorption. Ceram. Int. 2020, 46, 15447–15455. [Google Scholar] [CrossRef]
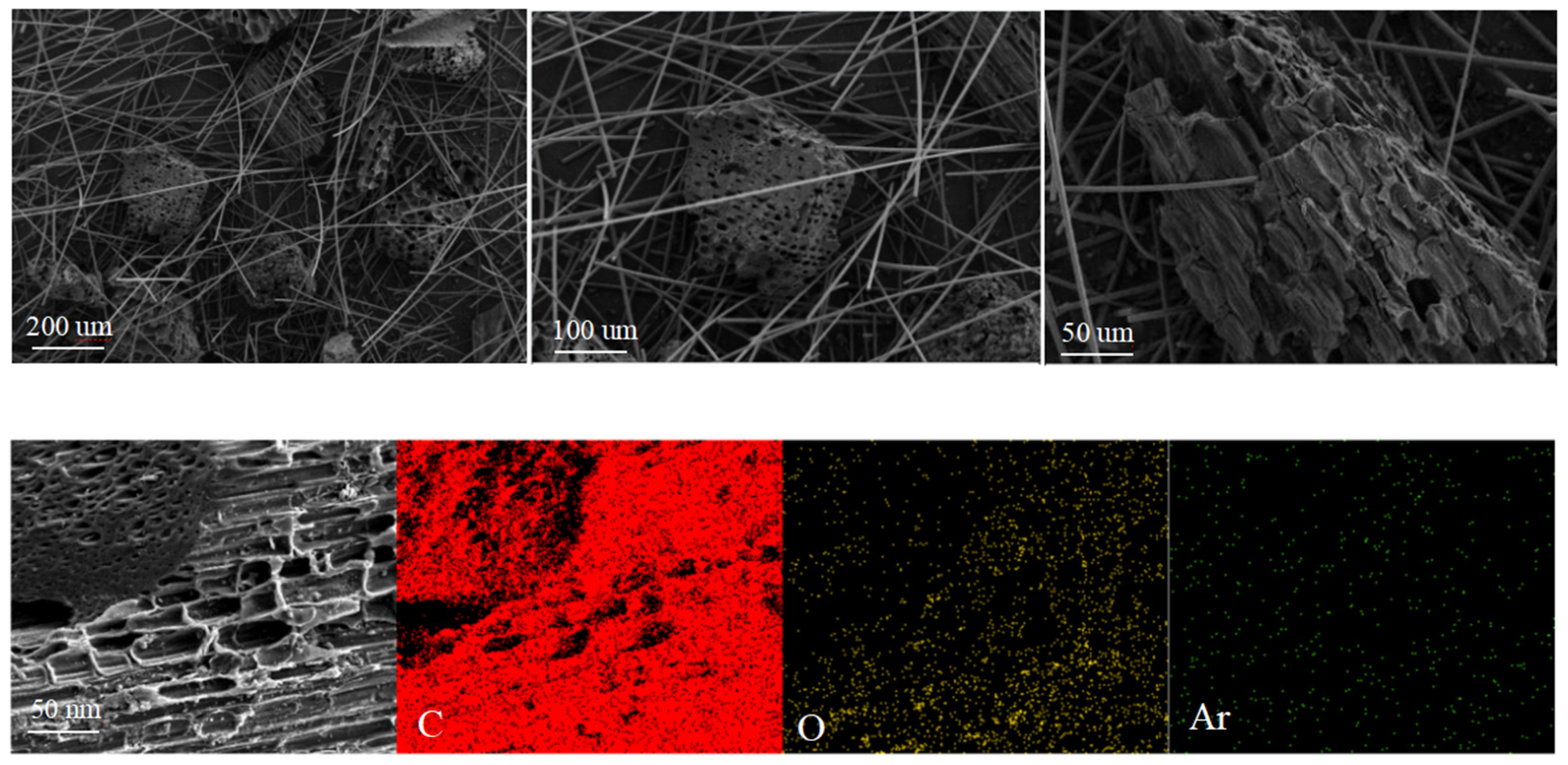

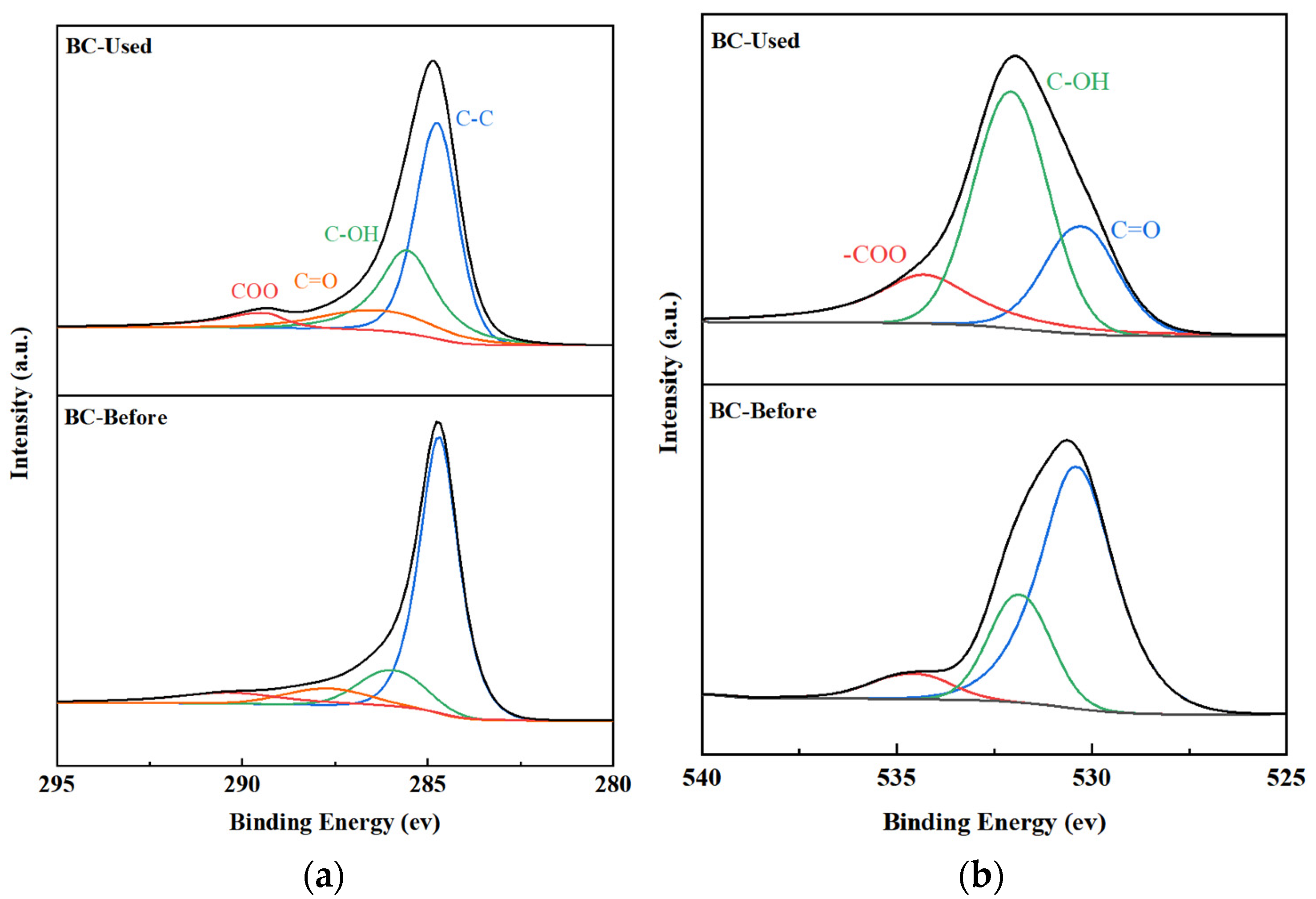

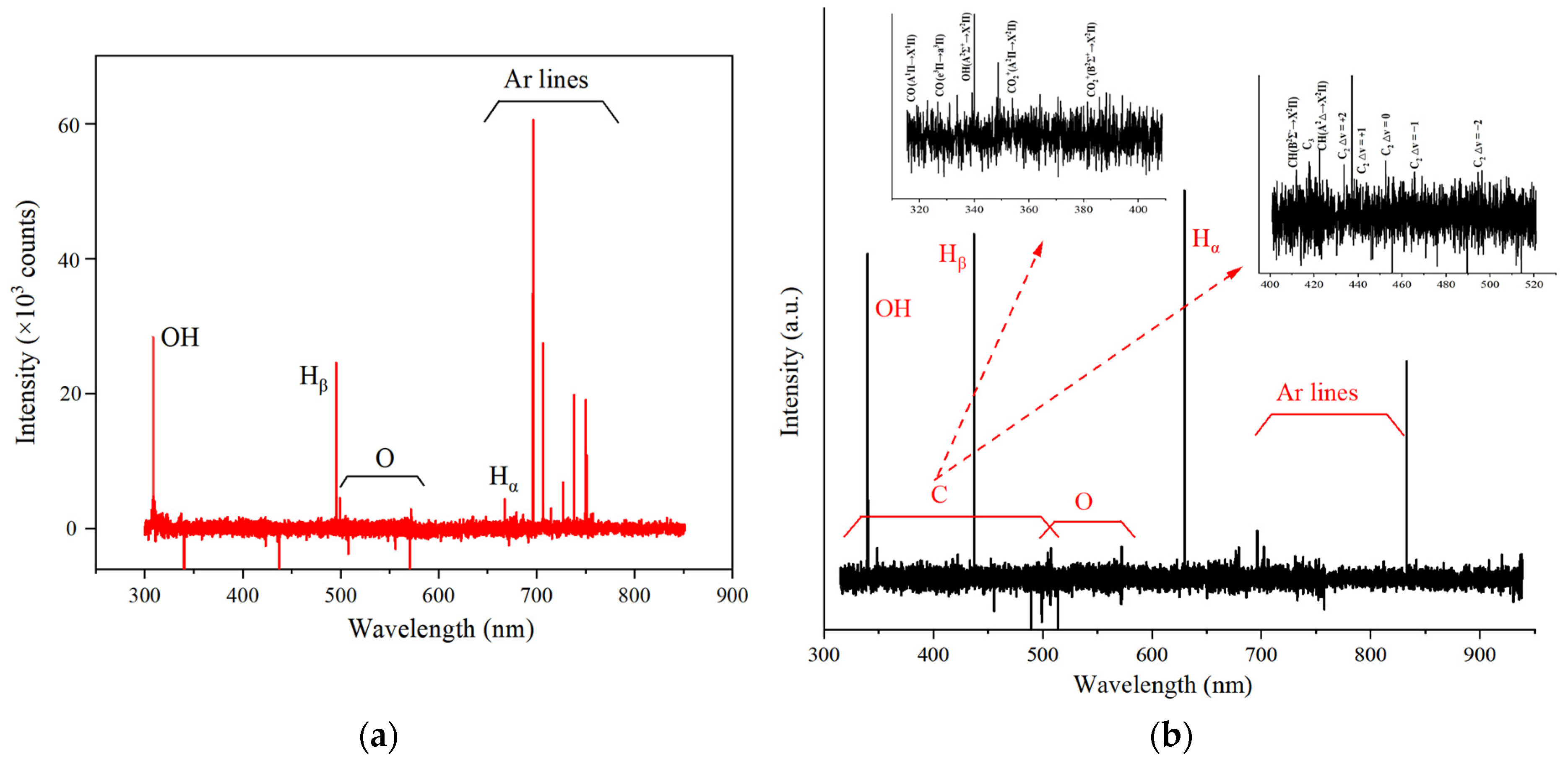



| BC | SBET (m2/g) | dp (nm) | Vp (cm3/g) | O 1s (%) | C 1s (%) |
|---|---|---|---|---|---|
| Before | 160.70 | 2.06 | 0.08 | 11.4 | 88.6 |
| Used | 206.79 | 2.09 | 0.11 | 27.9 | 72.1 |
| Production Method | Hydrogen Production Gas | Energy Yield, g(H2)/kWh | Reference |
|---|---|---|---|
| Electrolysis | H2O | >20 | [33] |
| Photocatalysis | H2O | 0.01 | [34] |
| AC gliding arc | H2O | 1.3 | [35] |
| Corona | H2O | 2.0 | [36] |
| Microwave (2.45 MHz) | C2H5OH + H2O | 14.8 | [37] |
| DBD | CH3OH + H2O | 3.3 | [38] |
| NH3 | 4.1 | [39] | |
| H2O | 5.5 | [16] | |
| DBD coupled BC | H2O | 17.3 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Sun, R.; Tan, Y.; Pei, C.; Shu, R.; Song, L.; Zhang, R.; Ouyang, C.; Xia, M.; Hou, J.; et al. Efficient Transformation of Water Vapor into Hydrogen by Dielectric Barrier Discharge Loaded with Bamboo Carbon Bed Structured by Fibrous Material. Molecules 2024, 29, 3273. https://doi.org/10.3390/molecules29143273
Xu H, Sun R, Tan Y, Pei C, Shu R, Song L, Zhang R, Ouyang C, Xia M, Hou J, et al. Efficient Transformation of Water Vapor into Hydrogen by Dielectric Barrier Discharge Loaded with Bamboo Carbon Bed Structured by Fibrous Material. Molecules. 2024; 29(14):3273. https://doi.org/10.3390/molecules29143273
Chicago/Turabian StyleXu, Hui, Ran Sun, Yujie Tan, Chenxiao Pei, Ruchen Shu, Lijie Song, Ruina Zhang, Chuang Ouyang, Min Xia, Jianyuan Hou, and et al. 2024. "Efficient Transformation of Water Vapor into Hydrogen by Dielectric Barrier Discharge Loaded with Bamboo Carbon Bed Structured by Fibrous Material" Molecules 29, no. 14: 3273. https://doi.org/10.3390/molecules29143273
APA StyleXu, H., Sun, R., Tan, Y., Pei, C., Shu, R., Song, L., Zhang, R., Ouyang, C., Xia, M., Hou, J., Zhang, X., Yuan, Y., & Zhang, R. (2024). Efficient Transformation of Water Vapor into Hydrogen by Dielectric Barrier Discharge Loaded with Bamboo Carbon Bed Structured by Fibrous Material. Molecules, 29(14), 3273. https://doi.org/10.3390/molecules29143273





