Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations
Abstract
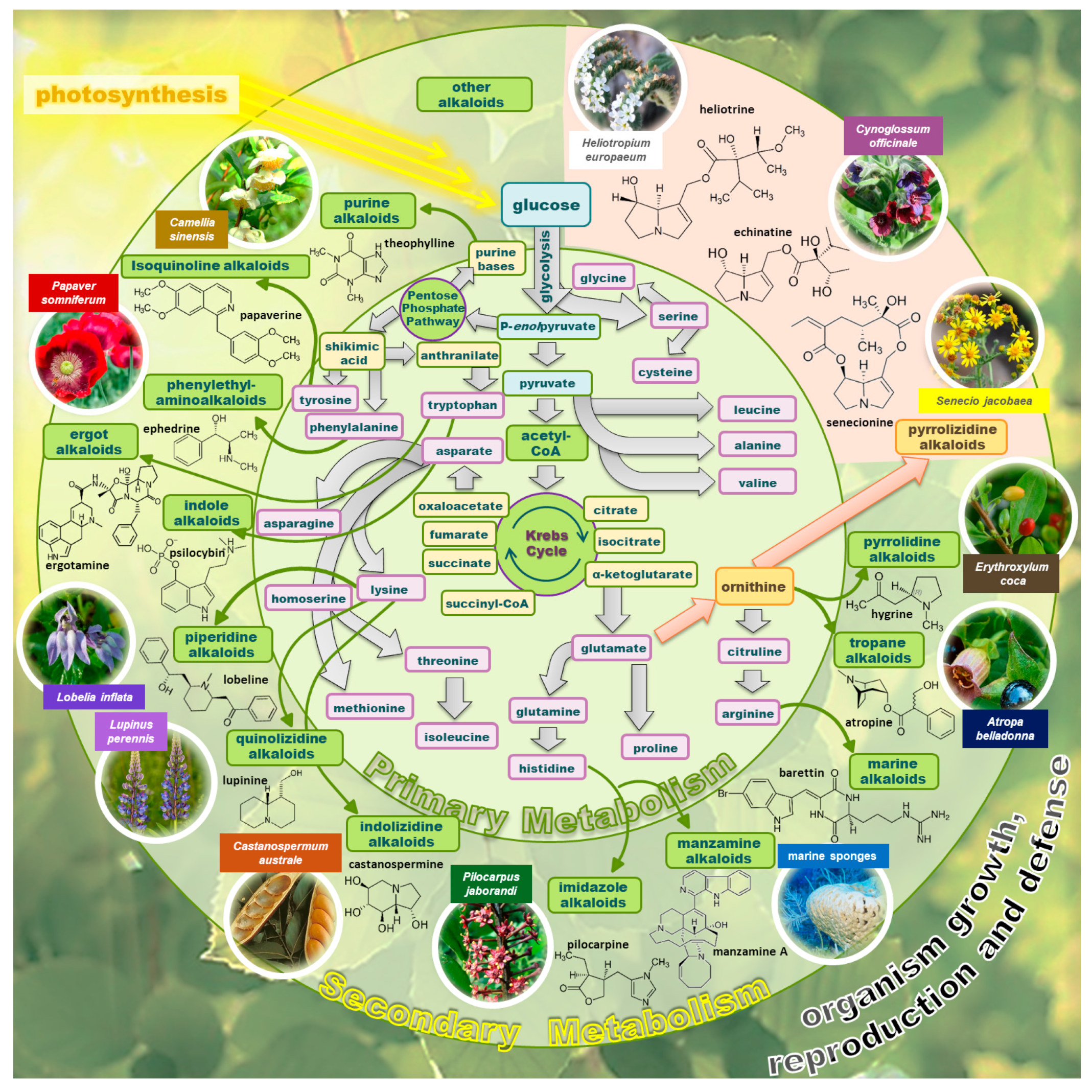
1. Introduction
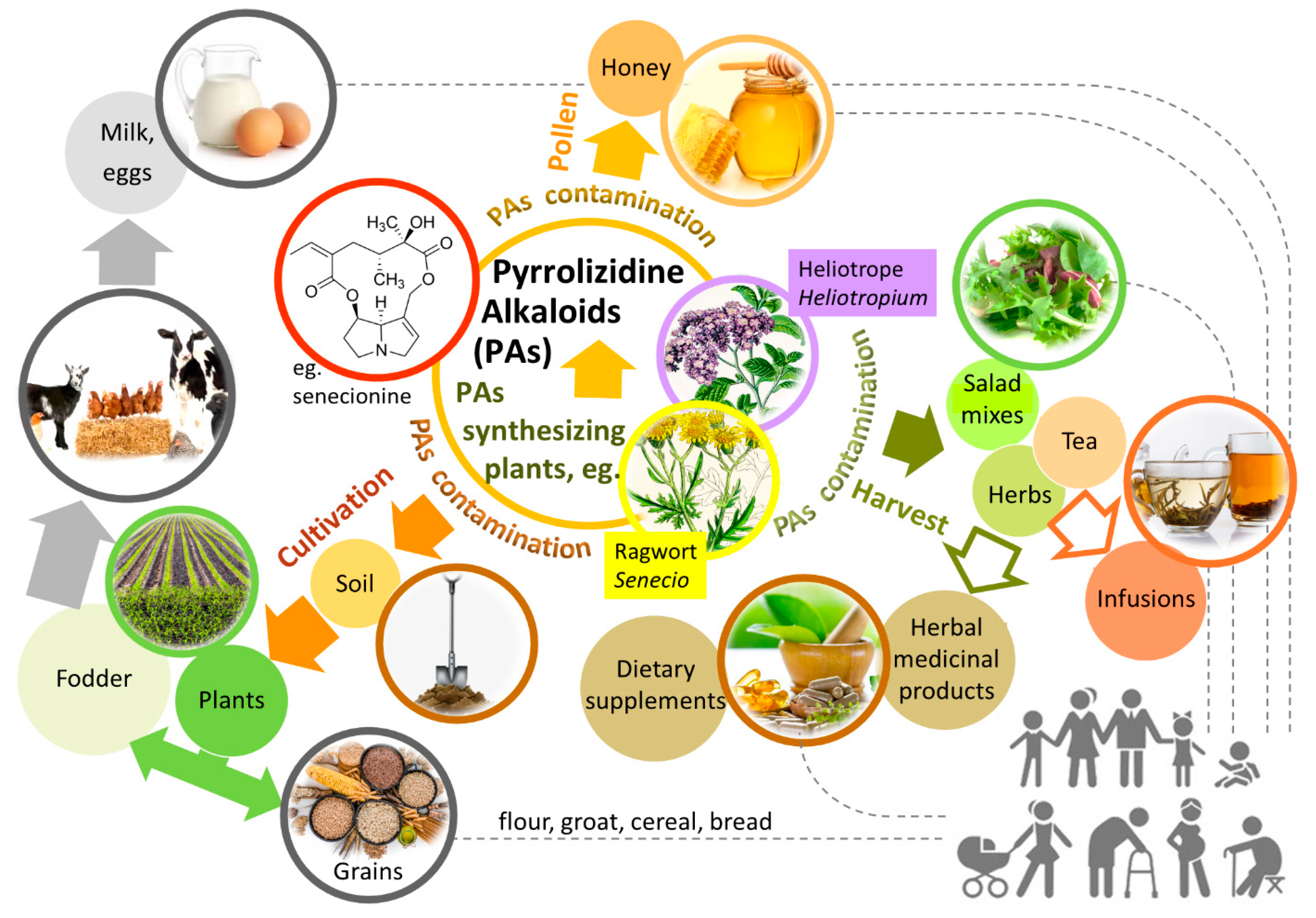
2. Natural Occurrence of PAs, Health Risks, and Possible Routes of Human Intoxication
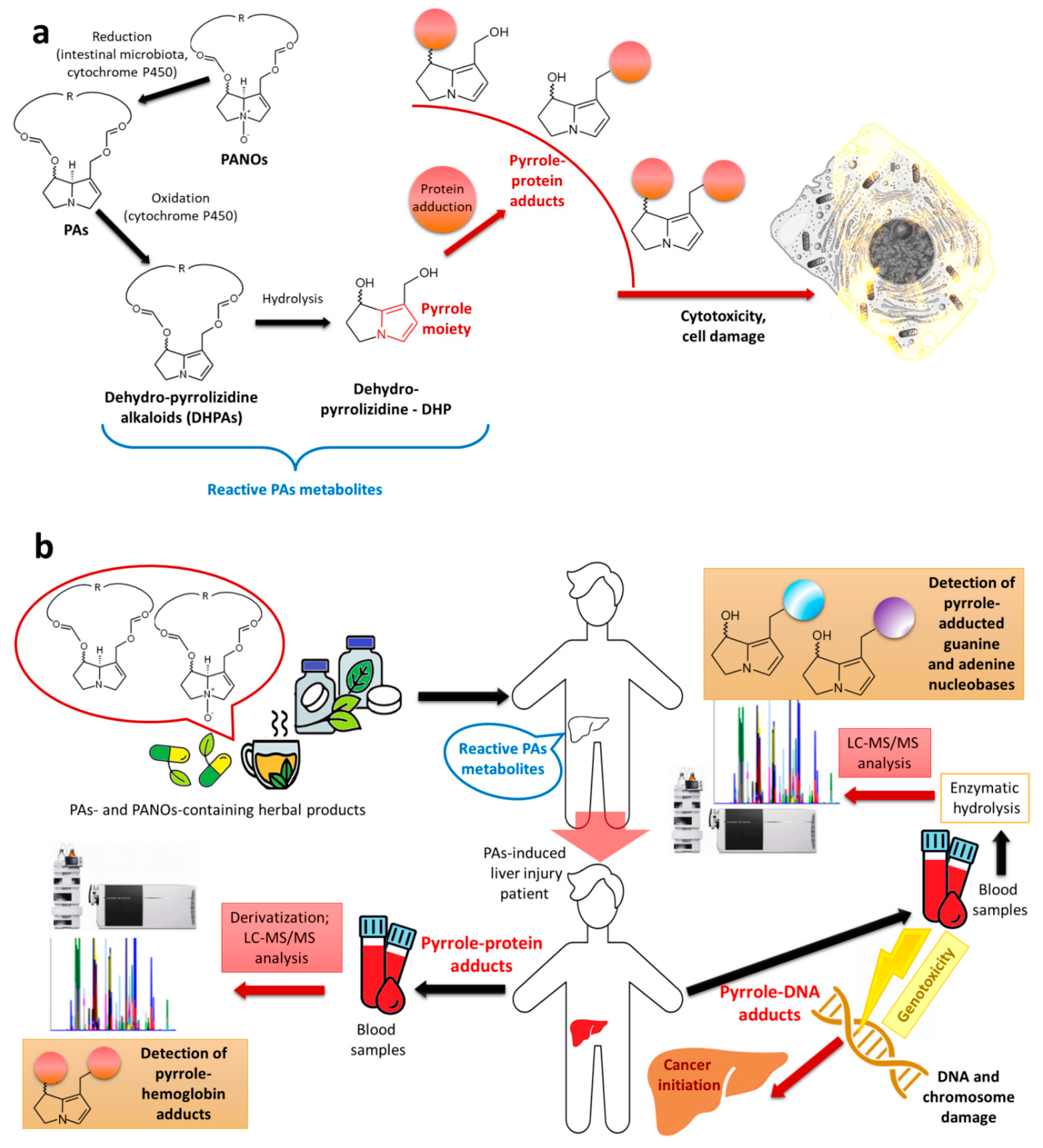
3. Chemistry of PAs
4. Toxicity of PAs and Regulation Limits
5. Methods for PA Determination
5.1. Sample Preparation for the PAs Containing Materials
5.2. LC-and GC-Methods for PAs Analysis
5.3. Electrochemical Methods for PAs Analysis
5.4. LC-MS and GC-MS Methods for PAs Analysis
6. Safety Ensuring, Prevention, and Market Control
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarker, S. Pharmacognosy in modern pharmacy curricula. Pharmacogn. Mag. 2012, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Supplement 2: Pyrrolizidine Alkaloids; WHO Food Additives Series 71-S2; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 1–405. [Google Scholar]
- FAO; WHO. Code of Practice for Weed Control to Prevent and Reduce Pyrrolizidine Alkaloid Contamination in Food and Feed CAC/RCP 74-2014; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- COT—Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. COT Statement on Pyrrolizidine Alkaloids in Food. 2008. Available online: https://cot.food.gov.uk/sites/default/files/cot/cotstatementpa200806.pdf (accessed on 27 April 2024).
- ANZFA—Australia New Zealand Food Authority. Pyrrolizidine Alkaloids in Food. 2001. Available online: https://www.foodstandards.gov.au/sites/default/files/publications/Documents/TR2.pdf (accessed on 27 April 2024).
- Centre for Food Safety Food and Environmental Hygiene Department; The Government of the Hong Kong Special Administrative Region. Pyrrolizidine Alkaloids in Food. 2017. Available online: https://www.cfs.gov.hk/sc_chi/programme/programme_rafs/files/PA_Executive_Report_c.pdf (accessed on 27 April 2024).
- PAFF Committee. Summary Report of the Standing Committee on Plants, Animals, Food and Feed Held in Brussels on 1 July 2014. 2014. Available online: https://food.ec.europa.eu/system/files/2016-10/cs_contaminants_catalogue_mycotoxins_monitoring_recommendations_en.pdf (accessed on 27 April 2024).
- European Food Safety Authority; EFSA Panel on Contaminants in the Food Chain (CONTAM). Opinion of the Panel on contaminants in the food chain [CONTAM] related to pyrrolizidine alkaloids as undesirable substances in animal feed. EFSA J. 2007, 5, 447. [Google Scholar] [CrossRef]
- European Food Safety Authority; EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products. Public Statement on Contamination of Herbal Medicinal Products/Traditional Herbal Medicinal Products with Pyrrolizidine Alkaloids; Committee on Herbal Medicinal Products: Amsterdam, The Netherlands, 2016. [Google Scholar]
- European Food Safety Authority (EFSA). Dietary exposure assessment to pyrrolizidine alkaloids in the European population. EFSA J. 2016, 14, e04572. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, e04908. [Google Scholar] [CrossRef] [PubMed]
- PAFF Committee. Summary Report of The Standing Committee on Plants, Animals, Food and Feed Held in Brussels on 17 September 2018. 2018. Available online: https://food.ec.europa.eu/system/files/2018-10/reg-com_toxic_20180917_sum.pdf (accessed on 28 April 2024).
- European Commission. Commission Regulation (EU) 2020/2040 of 11 December 2020 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Pyrrolizidine Alkaloids in Certain Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union 2020, 63, 1–5. Available online: https://eur-lex.europa.eu/eli/reg/2020/2040/oj (accessed on 28 April 2024).
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- CCCF—Codex Committee on Contaminants in Foods. The 15th Session of the Codex Committee on Contaminants in Foods. 2022. Available online: https://www.fao.org/fao-who-codexalimentarius/meetings/detail/en/?meeting=CCCF&session=15 (accessed on 27 April 2024).
- CCCF—Codex Committee on Contaminants in Foods. Request for Comments on the Recommendations for Follow-Up Risk Management Actions on Pyrrolizidine Alkaloids CL, 2.0.2.3./.4.0.-C.F. 2023. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FCircular%252520Letters%252FCL%2525202023-40%252Fcl23_40e.pdf (accessed on 27 April 2024).
- Peloso, M.; Minkoumba Sonfack, G.; Paduano, S.; De Martino, M.; De Santis, B.; Caprai, E. Pyrrolizidine Alkaloids in Food on the Italian Market. Molecules 2023, 28, 5346. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Pereira, D.; Valentão, P.; Andrade, P. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ruan, W.; Vrieling, K. Current Knowledge and Perspectives of Pyrrolizidine Alkaloids in Pharmacological Applications: A Mini-Review. Molecules 2021, 26, 1970. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloid chemistry. In Alkaloids; Elsevier: Amsterdam, The Netherlands, 2015; pp. 99–193. [Google Scholar]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- Mroczek, T.; Glowniak, K. Pyrrolizidine Alkaloids. In Natural Products in the New Millennium: Prospects and Industrial Application; Springer: Dordrecht, The Netherlands, 2002; pp. 1–46. [Google Scholar]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and Analyzing Pyrrolizidine Alkaloids in Medicinal Plants: A Review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Al-Subaie, S.F.; Alowaifeer, A.M.; Mohamed, M.E. Pyrrolizidine Alkaloid Extraction and Analysis: Recent Updates. Foods 2022, 11, 3873. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.J.; Peijnenburg, A.; Pfuhler, S.; Rietjens, I.M.C.M.; Rutz, L.; Steinhoff, B.; et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment—A review. Food Chem. Toxicol. 2020, 136, 111107. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-S.; Qiu, J.; Mu, X.-Y.; Qian, Y.-Z.; Chen, L. Levels, Toxic Effects, and Risk Assessment of Pyrrolizidine Alkaloids in Foods: A Review. Foods 2024, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Jayawickreme, K.; Świstak, D.; Ozimek, E.; Reszczyńska, E.; Rysiak, A.; Makuch-Kocka, A.; Hanaka, A. Pyrrolizidine Alkaloids—Pros and Cons for Pharmaceutical and Medical Applications. Int. J. Mol. Sci. 2023, 24, 16972. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Madilo, F.K.; Effah-Manu, L. Pyrrolizidine alkaloid contamination of food in Africa: A review of current trends and implications. Heliyon 2024, 10, e24055. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Stevens, K. Pyrrolizidine alkaloids: Occurrence, biology, and chemical synthesis. Nat. Prod. Rep. 2017, 34, 62–89. [Google Scholar] [CrossRef] [PubMed]
- Crews, C. Methods for Analysis of Pyrrolizidine Alkaloids. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1049–1068. [Google Scholar]
- Crews, C.; Berthiller, F.; Krska, R. Update on analytical methods for toxic pyrrolizidine alkaloids. Anal. Bioanal. Chem. 2010, 396, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Dzuman, Z.; Jonatova, P.; Stranska-Zachariasova, M.; Prusova, N.; Brabenec, O.; Novakova, A.; Fenclova, M.; Hajslova, J. Development of a new LC-MS method for accurate and sensitive determination of 33 pyrrolizidine and 21 tropane alkaloids in plant-based food matrices. Anal. Bioanal. Chem. 2020, 412, 7155–7167. [Google Scholar] [CrossRef]
- Valese, A.C.; Daguer, H.; Muller, C.M.O.; Molognoni, L.; Da Luz, C.F.P.; De Barcellos Falkenberg, D.; Gonzaga, L.V.; Brugnerotto, P.; Gorniak, S.L.; Barreto, F.; et al. Quantification of pyrrolizidine alkaloids in Senecio brasiliensis, beehive pollen, and honey by LC-MS/MS. J. Environ. Sci. Health Part B 2021, 56, 685–694. [Google Scholar] [CrossRef]
- Bandini, T.B.; Spisso, B.F. Development and validation of an LC-HRMS method for the determination of pyrrolizidine alkaloids and quinolones in honey employing a simple alkaline sample dilution. J. Food Meas. Charact. 2021, 15, 4758–4770. [Google Scholar] [CrossRef]
- Ko, K.Y.; Jeong, S.H.; Choi, E.Y.; Lee, K.; Hong, Y.; Kang, I.; Cho, S.; Lee, C. A LC–ESI–MS/MS analysis procedure coupled with solid phase extraction and MeOH extraction method for determination of pyrrolizidine alkaloids in Tussilago farfara and Lithospermi erythrorhzion. Appl. Biol. Chem. 2021, 64, 53. [Google Scholar] [CrossRef]
- Jiao, W.; Zhu, L.; Shen, T.; Wang, L.; Li, Q.X.; Wang, C.; Wu, X.; Chen, H.; Hua, R. Simultaneous determination of 15 pyrrolizidine alkaloids and their N-oxides in weeds, soil, fresh tea leaves, and tea: Exploring the pollution source of pyrrolizidine alkaloids in tea. Food Chem. 2024, 434, 137305. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K. LC–MS: A Rapid Technique for Understanding the Plant Metabolite Analysis. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–479. [Google Scholar]
- Ganzera, M.; Sturm, S. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. J. Pharm. Biomed. Anal. 2018, 147, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.; Siewert, B.; Toff, S.; Drewe, J. UPLC TOF MS for sensitive quantification of naturally occurring pyrrolizidine alkaloids in Petasites hybridus extract (Ze 339). J. Chromatogr. B 2015, 997, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Xu, J.; Liu, Y.; Xie, Y.; Xiong, A.; Wang, Z.; Yang, L. Mass spectrometric analysis strategies for pyrrolizidine alkaloids. Food Chem. 2024, 445, 138748. [Google Scholar] [CrossRef] [PubMed]
- Tábuas, B.; Cruz Barros, S.; Diogo, C.; Cavaleiro, C.; Sanches Silva, A. Pyrrolizidine Alkaloids in Foods, Herbal Drugs, and Food Supplements: Chemistry, Metabolism, Toxicological Significance, Analytical Methods, Occurrence, and Challenges for Future. Toxins 2024, 16, 79. [Google Scholar] [CrossRef]
- Sharma, B.; Bhatia, R.; Ganti, S.S.; Rangra, N.K. Recent Trends in the Detection of Alkaloids through Analytical, Bioanalytical, and Electrochemical Techniques: Analytical Techniques Used in Detection of Alkaloids. Curr. Pharm. Anal. 2024, 20, 241–263. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant Alkaloids: Structures and Bioactive Properties. In Plant-Derived Bioactives; Springer Singapore: Singapore, 2020; pp. 85–117. [Google Scholar]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Edgar, J.A.; Colegate, S.M.; Gardner, D.R.; Schoch, T.K.; Coulombe, R.A.; Molyneux, R.J. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 1999, 8, 95–116. [Google Scholar] [PubMed]
- Koleva, I.I.; Van Beek, T.A.; Soffers, A.E.M.F.; Dusemund, B.; Rietjens, I.M.C.M. Alkaloids in the human food chain—Natural occurrence and possible adverse effects. Mol. Nutr. Food Res. 2012, 56, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.J.; Sánchez, P.L.; These, A.; Preiss-Weigert, A.; Castellari, M. Occurrence of Pyrrolizidine Alkaloids in food. EFSA Support. Publ. 2015, 12, 859E. [Google Scholar] [CrossRef]
- Prakash, A.S.; Pereira, T.N.; Reilly, P.E.B.; Seawright, A.A. Pyrrolizidine alkaloids in human diet. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1999, 443, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Sieradzki, Z.; Kwiatek, K. Determination of Pyrrolizidine Alkaloids in Honey with Sensitive Gas Chromatography-Mass Spectrometry Method. Food Anal. Methods 2018, 11, 1345–1355. [Google Scholar] [CrossRef]
- Wiedenfeld, H. Pyrrolizidine Alkaloids. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1170–1174. [Google Scholar]
- Ober, D.; Hartmann, T. Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 14777–14782. [Google Scholar] [CrossRef]
- Wink, M. Quinolizidine and Pyrrolizidine Alkaloid Chemical Ecology—A Mini-Review on Their Similarities and Differences. J. Chem. Ecol. 2019, 45, 109–115. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and carcinogenesis studies of riddelliine (CAS No. 23246-96-0) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2003, 508, 1–280. [Google Scholar]
- European Commission. COMMISSION REGULATION (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 66, 103–157. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 28 April 2024).
- Yang, M.; Ma, J.; Ruan, J.; Ye, Y.; Fu, P.P.-C.; Lin, G. Intestinal and hepatic biotransformation of pyrrolizidine alkaloid N-oxides to toxic pyrrolizidine alkaloids. Arch. Toxicol. 2019, 93, 2197–2209. [Google Scholar] [CrossRef]
- Wang, Z.; Han, H.; Wang, C.; Zheng, Q.; Chen, H.; Zhang, X.; Hou, R. Hepatotoxicity of Pyrrolizidine Alkaloid Compound Intermedine: Comparison with Other Pyrrolizidine Alkaloids and Its Toxicological Mechanism. Toxins 2021, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.W.; Wang, Y.-P.; Yan, J.; Yang, Y.-C.; Beger, R.D.; Williams, L.D.; Doerge, D.R.; Fu, P.P. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol. Lett. 2003, 145, 239–247. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, W.; Ma, J.; Xia, Q.; Song, Z.; Zhu, L.; Zhang, C.; Liu, J.; Ye, Y.; Fu, P.P.; et al. Blood Pyrrole–DNA Adducts Define the Early Tumorigenic Risk in Patients with Pyrrolizidine Alkaloid-Induced Liver Injury. Environ. Sci. Technol. Lett. 2021, 8, 551–557. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Ma, J.; Lin, G. Metabolism-mediated cytotoxicity and genotoxicity of pyrrolizidine alkaloids. Arch. Toxicol. 2021, 95, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Yang, M.; Fu, P.; Ye, Y.; Lin, G. Metabolic Activation of Pyrrolizidine Alkaloids: Insights into the Structural and Enzymatic Basis. Chem. Res. Toxicol. 2014, 27, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Frei, H.; Lüthy, J.; Brauchli, J.; Zweifel, U.; Würgler, F.E.; Schlatter, C. Structure/activity relationships of the genotoxic potencies of sixteen pyrrolizidine alkaloids assayed for the induction of somatic mutation and recombination in wing cells of Drosophila melanogaster. Chem. Biol. Interact. 1992, 83, 1–22. [Google Scholar] [CrossRef]
- Schoental, R.; Head, M.A.; Peacock, P.R. Senecio Alkaloids: Primary Liver Tumours in Rats as a Result of Treatment with (1) A Mixture of Alkaloids from S. Jacobaea Lin.; (2) Retrorsine; (3) Isatidine. Br. J. Cancer 1954, 8, 458–465. [Google Scholar] [CrossRef]
- Edgar, J.A.; Molyneux, R.J.; Colegate, S.M. Pyrrolizidine Alkaloids: Potential Role in the Etiology of Cancers, Pulmonary Hypertension, Congenital Anomalies, and Liver Disease. Chem. Res. Toxicol. 2015, 28, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Hadi, N.S.A.; Bankoglu, E.E.; Stopper, H. Genotoxicity of pyrrolizidine alkaloids in metabolically inactive human cervical cancer HeLa cells co-cultured with human hepatoma HepG2 cells. Arch. Toxicol. 2023, 97, 295–306. [Google Scholar] [CrossRef]
- Cheeke, P.R. Toxicity and Metabolism of Pyrrolizidine Alkaloids. J. Anim. Sci. 1988, 66, 2343. [Google Scholar] [CrossRef]
- He, X.; Xia, Q.; Shi, Q.; Fu, P.P. Effects of glutathione and cysteine on pyrrolizidine alkaloid-induced hepatotoxicity and DNA adduct formation in rat primary hepatocytes. J. Environ. Sci. Health Part C 2020, 38, 109–123. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, Y.; Ren, Z.; Wang, Z.; Wang, S.; Xiong, J.; Zhang, H.; Jiang, H. Toxic Prediction of Pyrrolizidine Alkaloids and Structure-Dependent Induction of Apoptosis in HepaRG Cells. Oxid. Med. Cell Longev. 2021, 2021, 8822304. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, B. Pyrrolizidine alkaloid contamination in herbal medicinal products: Limits and occurrence. Food Chem. Toxicol. 2019, 130, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, J.; Ruan, J.; Zhang, C.; Ye, Y.; Pi-Cheng Fu, P.; Lin, G. Absorption difference between hepatotoxic pyrrolizidine alkaloids and their N-oxides—Mechanism and its potential toxic impact. J. Ethnopharmacol. 2020, 249, 112421. [Google Scholar] [CrossRef] [PubMed]
- Chapter 2.8.26. Contaminant pyrrolizidine alkaloids. In European Pharmacopoeia, 11th ed.; European Medicines Agency: Amsterdam, The Netherlands, 2023.
- European Commission. Commission Implementing Regulation (EU) 2023/2783 of 14 December 2023 Laying Down the Methods of Sampling and Analysis for the Control of the Levels of Plant Toxins in Food and Repealing Regulation (EU) 2015/705. Off. J. Eur. Union 2023, 1–13. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202302783 (accessed on 28 April 2024).
- EURL EU Reference Laboratory WU&, R. Guidance Document on Performance Criteria for Methods of Analysis for Mycotoxins and Plant Toxins in Food and Feed (Draft Version). 2024. Available online: https://www.wur.nl/en/show/eurlmp-guidance-document-performance-criteria-draft-17.05.2024.htm (accessed on 27 June 2024).
- ECA Academy (ECA). USP Adopts New Chapter on Pyrrolizidine Alkaloids. 2023. Available online: https://www.gmp-compliance.org/gmp-news/usp-adopts-new-chapter-on-pyrrolizidine-alkaloids (accessed on 28 April 2024).
- European Tea Committee (ETC). Code of Practice to Prevent and Reduce Pyrrolizidine Alkaloid Contamination in Raw Materials for Tea and Herbal Infusions; European Tea Committee (ETC): Hamburg, Germany, 2018. [Google Scholar]
- Avula, B.; Sagi, S.; Wang, Y.-H.; Zweigenbaum, J.; Wang, M.; Khan, I.A. Characterization and screening of pyrrolizidine alkaloids and N-oxides from botanicals and dietary supplements using UHPLC-high resolution mass spectrometry. Food Chem. 2015, 178, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Wuilloud, J.C.A.; Gratz, S.R.; Gamble, B.M.; Wolnik, K.A. Simultaneous analysis of hepatotoxic pyrrolizidine alkaloids and N-oxides in comfrey root by LC-ion trap mass spectrometry. Analyst 2004, 129, 150. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, N.H.; Kim, N.-C.; Brine, D.R.; Collins, B.J.; Handy, R.W.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Analysis of herbal teas made from the leaves of comfrey (Symphytum officinale): Reduction of N-oxides results in order of magnitude increases in the measurable concentration of pyrrolizidine alkaloids. Public. Health Nutr. 2004, 7, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Adams, Z.S.; Dzikunoo, J.; Asante-Donyinah, D. Uptake and accumulation of pyrrolizidine alkaloids in the tissues of maize (Zea mays L.) plants from the soil of a 4-year-old Chromolaena odorata dominated fallow farmland. Chemosphere 2021, 270, 128669. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.A. Chapter 11 Solid-phase extraction: High throughput techniques. Prog. Pharm. Biomed. Anal. 2003, 5, 361–432. [Google Scholar]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Sierra, I. A Miniaturized QuEChERS Method Combined with Ultrahigh Liquid Chromatography Coupled to Tandem Mass Spectrometry for the Analysis of Pyrrolizidine Alkaloids in Oregano Samples. Foods 2020, 9, 1319. [Google Scholar] [CrossRef]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Miniaturized and modified QuEChERS method with mesostructured silica as clean-up sorbent for pyrrolizidine alkaloids determination in aromatic herbs. Food Chem. 2022, 380, 132189. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Cañongo, C.; Pérez-Hernández, N.; Hernández-Carlos, B.; Cedillo-Portugal, E.; Joseph-Nathan, P.; Burgueño-Tapia, E. Complete 1H NMR assignments of pyrrolizidine alkaloids and a new eudesmanoid from Senecio polypodioides. Magn. Reson. Chem. 2014, 52, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Oplatowska, M.; Elliott, C.T.; Huet, A.-C.; McCarthy, M.; Mulder, P.P.J.; Von Holst, C.; Delahaut, P.; Van Egmond, H.P.; Campbell, K. Development and validation of a rapid multiplex ELISA for pyrrolizidine alkaloids and their N-oxides in honey and feed. Anal. Bioanal. Chem. 2014, 406, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid. Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Gañán, J.; Martínez-García, G.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Nanomaterials-modified electrochemical sensors for sensitive determination of alkaloids: Recent trends in the application to biological, pharmaceutical and agri-food samples. Microchem. J. 2023, 184, 108136. [Google Scholar] [CrossRef]
- Haroon, N.; Stine, K.J. Electrochemical Detection of Hormones Using Nanostructured Electrodes. Coatings 2023, 13, 2040. [Google Scholar] [CrossRef]
- Câmpean, A.; Tertiş, M.; Săndulescu, R. Voltammetric determination of some alkaloids and other compounds in pharmaceuticals and urine using an electrochemically activated glassy carbon electrode. Open Chem. 2011, 9, 688–700. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Guo, L. Simultaneous determination of theophylline and caffeine by large mesoporous carbon/Nafion modified electrode. J. Electroanal. Chem. 2013, 706, 7–12. [Google Scholar] [CrossRef]
- Shu, X.; Bian, F.; Wang, Q.; Qin, X.; Wang, Y. Electrochemical Sensor for Simultaneous Determination of Theophylline and Caffeine Based on a Novel poly(folic acid)/graphene Composite Film Modified Electrode. Int. J. Electrochem. Sci. 2017, 12, 4251–4264. [Google Scholar] [CrossRef]
- Kesavan, S.; Abraham John, S. Fabrication of aminotriazole grafted gold nanoparticles films on glassy carbon electrode and its application towards the simultaneous determination of theophylline and uric acid. Sens. Actuators B Chem. 2014, 205, 352–362. [Google Scholar] [CrossRef]
- Senturk, H.; Eksin, E.; Zeybek, U.; Erdem, A. Detection of Senecionine in Dietary Sources by Single-Use Electrochemical Sensor. Micromachines 2021, 12, 1585. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yi, H.; Wang, G.; Chen, S.; Li, X.; Wu, Q.; Zhang, S.; Deng, K.; He, Y.; Yang, X. Electrochemiluminescence sensor for point-of-care detection of pyrrolizidine alkaloids. Talanta 2022, 249, 123645. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Cristofoli, C.; Gallina, A.; Mutinelli, F. Easy and rapid method for the quantitative determination of pyrrolizidine alkaloids in honey by ultra performance liquid chromatography-mass spectrometry: An evaluation in commercial honey. Food Control 2014, 37, 146–152. [Google Scholar] [CrossRef]
- Mudge, E.M.; Jones, A.M.P.; Brown, P.N. Quantification of pyrrolizidine alkaloids in North American plants and honey by LC-MS: Single laboratory validation. Food Addit.Contam. Part A 2015, 32, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, R.; Carter, J.F. MASS SPECTROMETRY | Selected Ion Monitoring. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 423–430. [Google Scholar]
- Kaufmann, A. High Mass Resolution Versus MS/MS. Compr. Anal. Chem. 2012, 58, 169–215. [Google Scholar] [CrossRef]
- Gumus, Z.P. Assessment of Toxic Pyrrolizidine and Tropane Alkaloids in Herbal Teas and Culinary Herbs Using LC-Q-ToF/MS. Foods 2023, 12, 3572. [Google Scholar] [CrossRef]
- Yi, Y.; Lu, Y.; Liu, H.; Wang, Z.; Li, S.; Huang, X.; Chai, Y.; Zhang, X.; Li, Z.; Chen, H. Insight into pyrrolizidine alkaloids degradation and the chemical structures of their degradation products using ultra high performance liquid chromatography and Q-Exactive Orbitrap mass spectrometry. J. Hazard. Mater. 2024, 471, 134260. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Wang, X.; Guo, W.; Jia, W.; Zhang, F. An analytical strategy for discovering structural analogues of alkaloids in plant food using characteristic structural fragments extraction by high resolution orbitrap mass spectrometry. LWT 2022, 154, 112329. [Google Scholar] [CrossRef]
- Rizzo, S.; Celano, R.; Piccinelli, A.L.; Russo, M.; Rastrelli, L. Target screening method for the quantitative determination of 118 pyrrolizidine alkaloids in food supplements, herbal infusions, honey and teas by liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Food Chem. 2023, 423, 136306. [Google Scholar] [CrossRef] [PubMed]
- Louisse, J.; Mulder, P.P.J.; Gerssen, A.; Stoopen, G.; Rijkers, D.; Van de Schans, M.G.M.; Peijnenburg, A.A.C.M. Bioassay-directed analysis-based identification of relevant pyrrolizidine alkaloids. Arch. Toxicol. 2022, 96, 2299–2317. [Google Scholar] [CrossRef] [PubMed]
- León, N.; Miralles, P.; Yusà, V.; Coscollà, C. A green analytical method for the simultaneous determination of 30 tropane and pyrrolizidine alkaloids and their N-oxides in teas and herbs for infusions by LC-Q-Orbitrap HRMS. J. Chromatogr. A 2022, 1666, 462835. [Google Scholar] [CrossRef]
- Cramer, L.; Beuerle, T. Detection and Quantification of Pyrrolizidine Alkaloids in Antibacterial Medical Honeys. Planta Med. 2012, 78, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Conradie, J.; Stewart, M.J.; Steenkamp, V. GC/MS identification of toxic pyrrolizidine alkaloids in traditional remedies given to two sets of twins. Ann. Clin.Biochem. Int. J. Lab. Med. 2005, 42, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Benamar, H.; Tomassini, L.; Frezza, C.; Marouf, A.; Bennaceur, M.; Nicoletti, M. First study on the pyrrolizidine alkaloids of Pardoglossum cheirifolium (L.) E.Barbier & Mathez.: GC-MS analysis of their volatile components in the whole plant. Nat. Prod. Res. 2021, 35, 4098–4103. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Manzinello, C.; Gallina, A.; Mutinelli, F. In-house validation and application of UHPLC-MS/MS method for the quantification of pyrrolizidine and tropane alkaloids in commercial honey bee-collected pollen, teas and herbal infusions purchased on Italian market in 2019–2020 referring to recent European Union regulations. Int. J. Food Sci. Technol. 2022, 57, 7505–7516. [Google Scholar] [CrossRef]
- Han, H.; Jiang, C.; Wang, C.; Wang, Z.; Chai, Y.; Zhang, X.; Liu, X.; Lu, C.; Chen, H. Development, optimization, validation and application of ultra high performance liquid chromatography tandem mass spectrometry for the analysis of pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides in teas and weeds. Food Control 2022, 132, 108518. [Google Scholar] [CrossRef]
- Kwon, Y.; Koo, Y.; Jeong, Y. Determination of Pyrrolizidine Alkaloids in Teas Using Liquid Chromatography–Tandem Mass Spectrometry Combined with Rapid-Easy Extraction. Foods 2021, 10, 2250. [Google Scholar] [CrossRef]
- Grüning, A.; Schad, G.J.; Stenzler, J. Determination of Pyrrolizidine Alkaloids in Plant Material Using SFC-MS/MS. 2019. Available online: https://www.shimadzu.com/an/literature/lcms/ego119034.html (accessed on 16 April 2024).
- Picron, J.-F.; Herman, M.; Van Hoeck, E.; Goscinny, S. Analytical strategies for the determination of pyrrolizidine alkaloids in plant based food and examination of the transfer rate during the infusion process. Food Chem. 2018, 266, 514–523. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Xiong, F.; Xiong, A.; Wang, Z.; Yang, L. Rapid identification and determination of pyrrolizidine alkaloids in herbal and food samples via direct analysis in real-time mass spectrometry. Food Chem. 2021, 334, 127472. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.M.; Gabler, A.M.; Rychlik, M.; Gottschalk, C.; Kaltner, F. A sensitive LC–MS/MS method for isomer separation and quantitative determination of 51 pyrrolizidine alkaloids and two tropane alkaloids in cow’s milk. Anal. Bioanal. Chem. 2022, 414, 8107–8124. [Google Scholar] [CrossRef] [PubMed]
- EURL EU Reference Laboratory WU&, R. Determination of Pyrrolizidine Alkaloids in Plant-Based Food and FEED Materials, Including (Herbal) Teas, Herbal Food Supplements, Fodder and Feedstuffs by LC-MS/MS. 2019. Available online: https://www.wur.nl/nl/show/eurl-mp-method_002-pyrrolizidine-alkaloids-by-lc-msms-v2.htm (accessed on 27 June 2024).
- Jiang, Z.; Liu, F.; Goh, J.J.L.; Yu, L.; Li, S.F.Y.; Ong, E.S.; Ong, C.N. Determination of senkirkine and senecionine in Tussilago farfara using microwave-assisted extraction and pressurized hot water extraction with liquid chromatography tandem mass spectrometry. Talanta 2009, 79, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Stashenko, E.E.; Martínez, J.R. LC/MS study of the diversity and distribution of pyrrolizidine alkaloids in Crotalaria species growing in Colombia. J. Sep. Sci. 2020, 43, 4322–4337. [Google Scholar] [CrossRef]
- European Commission. RASFF Window. 2024. Available online: https://food.ec.europa.eu/safety/rasff_en (accessed on 28 April 2024).

| No. | Name | Alkaloid Chemical Structure | Chemical Structure of Corresponding N-Oxide |
|---|---|---|---|
| 1, 2 | Echimidine, echimidine-N-oxide | 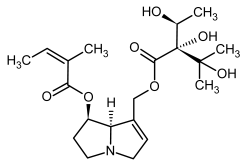 |  |
| Possible co-elution of 1 and 2 with, respectively: | |||
| 1a, 2a | Heliosupine, heliosupine-N-oxide | 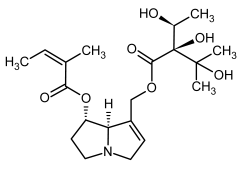 |  |
| 3, 4 | Heliotrine, heliotrine-N-oxide |  |  |
| 5, 6 | Intermedine, intermedine-N-oxide | 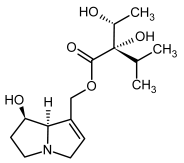 | 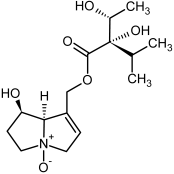 |
| 7, 8 | Lycopsamine, lycopsamine-N-oxide |  |  |
| Possible co-elution of 5, 6, 7, and 8 with, respectively: | |||
| 3a, 4a | Indicine, indicine-N-oxide | 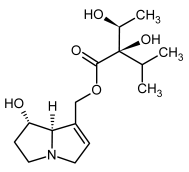 | 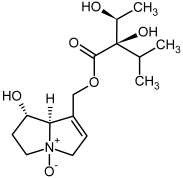 |
| 5a, 6a | Echinatine, echinatine-N-oxide | 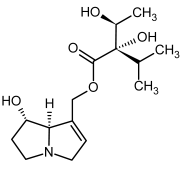 |  |
| 7a, 8a | Rinderine, rinderine-N-oxide | 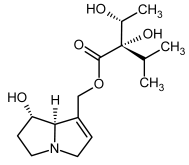 | 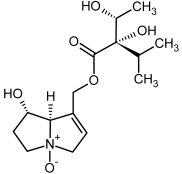 |
| 9, 10 | Retrorsine, retrorsine-N-oxide |  | 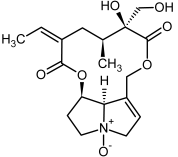 |
| Possible co-elution of 9 and 10 with, respectively: | |||
| 9a, 10a | Usaramine, usaramine-N-oxide | 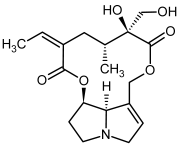 | 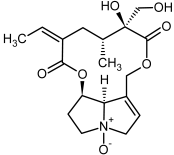 |
| 11, 12 | Senecionine, sencionine-N-oxide | 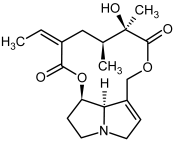 | 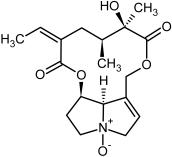 |
| 13, 14 | Senecivernine, senecivernine-N-oxide | 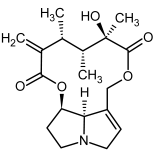 | 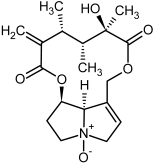 |
| Possible co-elution of 11, 12, 13 and 14 with: | |||
| 11a, 12a | Integerrimine, integerrimine-N-oxide | 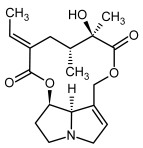 | 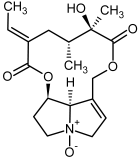 |
| 15, 16 | Seneciphylline, seneciphylline-N-oxide | 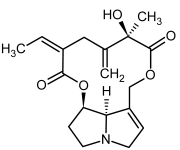 | 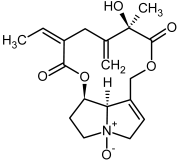 |
| Possible co-elution of 15 and 16 with, respectively: | |||
| 13a, 14a | Spartioidine, spartioidine N-oxide | 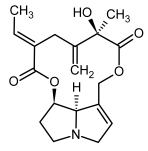 | 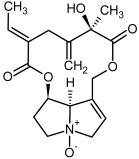 |
| 17, 18 | Europine, europine-N-oxide | 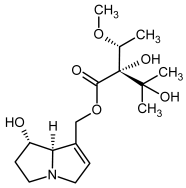 | 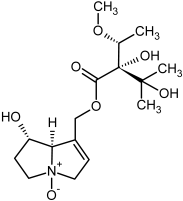 |
| 19, 20 | Lasiocarpine, lasiocarpine-N-oxide |  | 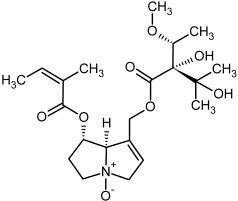 |
| 21 | Senkirkine |  | |
| No. | Name | Alkaloid Chemical Structure | Chemical Structure of Corresponding N-Oxide |
|---|---|---|---|
| 1b, 2b | Erucifoline, erucifoline-N-oxide | 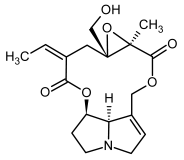 |  |
| 3b, 4b | Monocrotaline, monocrotaline-N-oxide | 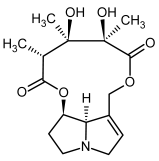 |  |
| 5b, 6b | Trichodesmine, trichodesmine-N-oxide | 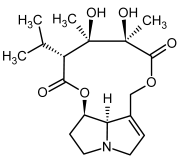 | 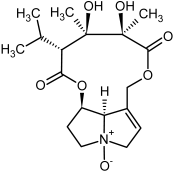 |
| 7b, 8b | Jacobine, jacobine-N-oxide | 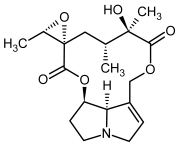 | 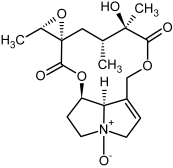 |
| 9b, 10b | Jaconine, jaconine-N-oxide |  | 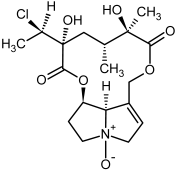 |
| In Silico Oral/Rat LD50 (mg/kg Body Weight) | In Vivo Intraperitoneal LD50 (mg/kg Body Weight) | Species | |
|---|---|---|---|
| Compound/Ref.: | [70] | [2] | |
| Echimidine | 616 | 200 | rat, male |
| Echinatine | 250 | 350 | rat, male |
| Europine | - | >1000 | rat, male |
| Heleurine | 616 | 140 | rat, male |
| Heliosupine | 708 | 60 | rat, male |
| Heliotridine | - | 1500 | rat, male |
| Heliotrine | 56 | 296 | rat, male |
| Heliotrine | - | 478 | rat, female |
| Indicine | 264 | >1000 | rat, male |
| Intermedine | 264 | 1500 | rat, male |
| Jacobine | 461 | 138 | rat, female |
| Jaconine | - | 168 | rat, female |
| Lasiocarpine | 555 | 77 | rat, male |
| Lasiocarpine | - | 79 | rat, female |
| Lycopsamine | 239 | 1500 | rat, male |
| Monocrotaline | - | 154 | mouse |
| Monocrotaline | 731 | 109 | rat, male |
| Monocrotaline | - | 230 | rat, female |
| Otonecine | 467 | - | - |
| Platyphylline | 443 | 252 | rat, male |
| Retronecine | 242 | - | - |
| Retrorsine | 320 | 34–38 | rat, male |
| Retrorsine | - | 153 | rat, female |
| Riddelliine | 616 | 80 | rat, male |
| Rinderine | 486 | 550 | rat, male |
| Senecionine | 127 | 50 | rat, male |
| Seneciphylline | 264 | 77 | rat, male |
| Seneciphylline | - | 83 | rat, female |
| Senecivernine | 592 | - | - |
| Senkirkine | 275 | 220 | rat, male |
| Spectabiline | 50 | rat, male | |
| Supinine | 215 | 450 | rat, male |
| Symphytine | - | 130 | rat, male |
| Usaramine | 264 | - | - |
| Trichodesmine | 324 | - | - |
| Sample Type | PAs | Separation Technique | Chromatographic Conditions | Detection | LOD/LOQ | Ref. | |
|---|---|---|---|---|---|---|---|
| Column | Elution | ||||||
| Senecio brasiliensis beehive pollen honey | senecionine senecionine N-oxide retrorsine N-oxide | HPLC | C18 100 mm × 3.0 mm, 3.5 µm (manufacturer undefined) | Mobile phase A: water with 0.1% formic acid Mobile phase B: acetonitrile with 0.1% formic acid Gradient: 98% A from 0 to 2.0 min, 85% A from 2.0 to 5.0 min, 50% A from 5.0 to 8.0 min, 10% A from 8.0 to 9.0 min, 98% A from 9.0 to 11.0 min. | Q-TRAP; MS/MS; Mode: ESI + MRM | - | [34] |
| bee-collected pollen teas herbal infusions | acetyllycopsamine echimidine group europine heliotrine intermedine lasiocarpine lycopsamine group retrorsine group senecionine group seneciphylline group senkirkine trichodesmine | UHPLC | Accu-coreTM RP-MS (Thermo Scientific, Waltham, MA, USA) 100 mm × 2.1 mm, 2.6 μm | Mobile phase A: 0.1% formic acid in water Mobile phase B: methanol/acetonitrile 1:1 (v/v). Gradient: from 3% to 4% B (0–1 min), from 4% to 17% B (1–6 min), 17% B held for 2 min, from 17% to 44% B (8–10.5 min), from 44% to 95% B (in 0.1 min), 95% B held for 1 min, from 95% to 3% B in 0.1 min re-equilibration to 3% B for 4 min. | QqQ; MS/MS; Mode: ESI + MRM | LOD: 2.4–5.3 ng/g LOQ: 4.0–9.0 ng/g LOD: 0.04–0.08 ng/mL LOQ: 0.07–0.14 ng/mL | [111] |
| oregano | intermedine europine lycopsamine europine N-oxide intermedine N-oxide lycopsamine N-oxide retrorsine retrorsine N-oxide seneciphylline heliotrine heliotrine N-oxide senecivernine senecionine seneciphylline N-oxide senecivernine N-oxide senecionine N-oxide echimidine echimidine N-oxide lasiocarpine lasiocarpine N-oxide senkirkine | UHPLC | Luna Omega Polar C18 (Phenomenex, Torrance, CA, USA), 100 mm × 2.1 mm, 1.6 µm | Mobile phase A: 0.2% formic acid and 5 mM ammonium acetate in water Mobile phase B: 10 mM ammonium acetate in methanol Gradient: 5% B (0–0.5 min), 5–50% B (0.5–7 min), 50% B (7–7.5 min), 50–100% B (7.5–11 min), 100% B (11–12 min), 100–5% B (12–14 min). re-equilibrated with the initial composition for 1 min. | IT; MS/MS; Mode: ESI + TIC | LOD: 0.1–7.5 ng/g LOQ: 0.5–25.0 ng/g | [83] |
| black tea, green tea dark tea Chrysanthemum weed | heliotrine heliotrine-N-oxide retrorsine retrorsine-N-oxide senecionine senecionine-N-oxide jacobine jacobine-N-oxide intermedine intermedine-N-oxide seneciphylline seneciphylline-N-oxide europine senkirkine | UHPLC | Waters Acquity UPLC HSS T3 (Waters, Milford, MA, USA) 100 mm × 2.1 mm, 1.8 μm | Mobile phase A: methanol buffered with 0.1% formic acid and 1 mM ammonium formate. Mobile phase B: Water buffered with 0.1% formic acid and 1 mM ammonium formate. Gradient: MPB was applied: 0–1 min at 90%, 1–4 min from 90% to 40%, 4–7 min from 40% to 30%, 7–7.1 min from 30% to 2%, 7.1–11 min at 2%, 11–11.1 from 2% to 90%, held for 2.9 min before the next run. | QqQ; MS/MS; Mode: ESI + MRM | LOD: 0.001–0.4 ng/g LOQ: 1–5 ng/g | [112] |
| honey | echimidine intermedine lycopsamine retrorsine retrorsine N-oxide senecionine senecionine N-oxide echimidine N-oxide erucifoline erucifoline N-oxide europine europine N-oxide heliotrine heliotrine N-oxide intermedine intermedine N-oxide jacobine jacobine N-oxide lasiocarpine lasiocarpine N-oxide lycopsamine lycopsamine N-oxide monocrotaline monocrotaline N-oxide seneciphylline seneciphylline N-oxide senkirkine trichodesmine | UHPLC | Waters Acquity UPLC BEH C18 (Waters, Milford, MA, USA) 100 mm × 2.1 mm, 1.7 μm | Mobile phase A: 6.5 mM ammonium hydroxide in water Mobile phase B: 6.5 mM ammonium hydroxide in acetonitrile Gradient: 0 to 2 min: 0% B; 2 to 10 min: 0 to 50% B, maintained to 2 min; 12 to 14 min: 50 to 100% B, maintained to 16 min; 16 to 19 min: 100 to 0% B, maintained to 23 min. | QTOF-MS/MS; Mode: ESI + | LOD: 1–7 ng/g LOQ: 10–20 ng/g | [35] |
| Tussilago farfara Lithospermum erythrorhizon | echimidine echimidine N-oxide erucifoline erucifoline N-oxide europine europine N-oxide heliotrine heliotrine N-oxide intermedine intermedine N-oxide jacobine jacobine N-oxide lasiocarpine lasiocarpine N-oxide lycopsamine lycopsamine N-oxide monocrotaline monocrotaline N-oxide retrorsine retrorsine N-oxide senecionine senecionine N-oxide seneciphylline | HPLC | Shim-pack GIST-C18 (Shimadzu Corporation, Kyoto, Japan) 150 mm × 2.1 mm, 2 μm | Mobile phase A: 0.1% formic acid in 5 mM ammonium formate Mobile phase B: 0.1% formic acid plus 5 mM ammonium formate in 100% methanol Gradient: 1.5 min, 1% B; 1.5–3.0 min, 1–15% B; 3.0–18.0 min, 15–30% B; 18.0–19.0 min, from 30 to 95% B 19.0–21.0 min, 95% B; 21.1 min, 1% B. | QqQ; MS/MS Mode: ESI + MRM | LOD: 0.5–1.7 ng/g LOQ: 1.7–6.4 ng/g | [36] |
| Sorghum oregano mixed herbal tea | echimidine echinatine erucifoline europine heliotrine indicine intermedine jacobine lasiocarpine lycopsamine monocrotaline retronecine retrorsine senecionine seneciphylline senecivernine senkirkine trichodesmine echimidine N-oxide echinatine N-oxide erucifoline N-oxide europine N-oxide heliotrine N-oxide indicine N-oxide intermedine N-oxide jacobine N-oxide lasiocarpine N-oxide lycopsamine N-oxide monocrotaline N-oxide retrorsine N-oxide senecionine N-oxide seneciphylline N-oxide senecivernine N-oxide | UHPLC | Waters Acquity UPLC® BEH Amide (Waters, Milford, MA, USA) 100 mm × 2.1 mm; 1.7 μm | Mobile phase A: water with ammonium formate 5 mM Mobile phase B: acetonitrile: water 95:5, v/v, with formic acid (0.1%, v/v). Gradient: 1.5 min, 1% B; 1.5–3.0 min, 1–15% B; 3.0–18.0 min, 15–30% B; 18.0–19.0 min, from 30 to 95% B; 19.0–21.0 min 95% B; 21.1 min, return to 1% B. | Q-TRAP; MS/MS Mode: ESI + MRM | LOD: - LOQ: 0.5–10 ng/g | [33] |
| tea | echimedine heliotrine lasiocarpine lycopsamine monocrotaline monocrotaline N-oxide retrorsine-N-oxide retrorsine senecionine-N-oxide senecionine seneciphylline N-oxide seneciphylline senkirkine trichodesmine europine-N-oxide intermedine jacobine europine jacobine N-oxide lasiocarpine N-oxide heliotrine N-oxide | UPLC | Waters X-Bridge (Waters, Milford, MA, USA) C18, 100 mm × 2.1 mm, 3.5 µm | Mobile Phase A: 5 mM ammonium formate and 0.1% formic acid Mobile Phase B: 95% methanol with 5 mM ammonium formate and 0.1% formic acid Gradient: 5% B for 0.5 min, increasing B from 5% to 30% for 6.5 min, from 30% to 95% for 4 min and then holding for 2 min, decreasing to 5% for 0.1 min, and finally holding for 1.9 min | QqQ; MS/MS Mode: ESI + MRM | LOD: 0.1–3.0 ng/g LOQ: 0.3–9.0 ng/g | [113] |
| plant material tea | SFC | CHIRALPAK®, IG-3/SFC, (Daicel Chiral Technologies, Shanghai, China) 100 mm × 3 mm, 3 µm, | Mobile Phase A: CO2 Mobile Phase B: 50 mM Ammonium formate in methanol Mobile Phase C: Methanol Mobile Phase D: 0.1% Formic acid | QqQ; MS/MS Mode: ESI + | LOQ: 2–200 ng/g | [114] | |
| plant based food herbal tea | erucifoline N-oxide europine europine N-oxide, jacobine, retrorsine, retrorsine N-oxide, seneciphylline N-oxide, senecivernine N-oxide trichodesmine | UHPLC | Waters Acquity UPLC® BEH C18 (Waters, Milford, MA, USA) 100 mm × 2.1 mm, 1.7 μm | Mobile phase A: water with 0.1% ammonia Mobile phase B: acetonitrile. Gradient: Starting at 5% of phase B, kept for 1 min, rising to 15% till 2 min before a new isocratic separation for 1 min, increasing to 20% (from 3 to 5 min), 25% (from 5 to 6 min), 50% (from 6 to 9 min) and 95% (from 9 to 10 min). | QqQ; MS/MS Mode: ESI + MRM | LOD: - LOQ: 0.5–1 ng/g | [115] |
| maize | total | HPLC | Synergy Max-RP 80 Å (Phenomenex, Aschaffenburg, Germany) 150 mm × 2.1 mm, 4 μm, | Mobile phase A: 0.3% formic acid in water Mobile phase B: 0.3% formic acid in acetonitrile) Gradient: 2 min (95% A), 14 min (95–40% A), 15 min (40–0% A), 18 min (0% A), 19 min (95% A), 30 min (reequilibration 95% A). | Q-TRAP; MS/MS Mode: ESI + MRM | - | [81] |
| Gynura japonica milk | senecionine, seneciphylline, senkirkine, retrorsine | DART-MS HPLC-MS | Waters Acquity UHPLC BEH C18 (Waters, Milford, MA, USA) 2.1 mm × 100 mm, 1.7 μm | Mobile phase A: water with 0.1% formic acid Mobile phase B: acetonitrile Gradient: 0–3 min, B 3%; 3–6 min, B 3–10%; 6–8 min, B 10–100%; 8–10 min, B 100–3%; 10–15 min, Re-equilibration, B 3%. | IT; MS/MS Mode: ESI + | LOD: 0.55–0.85 ng/mL LOQ: 1.83–2.82 ng/mL | [116] |
| herbal food supplements | monocrotaline, intermedine, monocrotaline N-oxide, indicine, lycopsamine, europine, europine N-oxide, indicine N-oxide, riddelliine, junction, riddelline N-oxide, trichodesmine, retrorsine, retrorsine N-oxide, heliotrine, seneciphylline, heliotrine N-oxide, seneciphylline N-oxide, integerrimine, senecionine, senecionine N-oxide, senkirkine, echimidine, lasiocarpine, lasiocarpine N-oxide | UHPLC | Agilent Poroshell 120 EC-C18 (Agilent Technologies, Palo Alto, CA, USA) 2.1 mm × 150 mm, 2.7 μm | Mobile phase A: water with 0.1% formic acid Mobile phase B: acetonitrile with 0.1% formic acid Gradient: 0–23 min, 3–4% B; 23–45 min, 4–15% B; 45–55 min, 15–25% B 55–57 min 25–100% B. 3 min wash-100% B 5 min Re-equilibration 3% B | QToF-MS/MS Mode: ESI + TIC | LOD: 0.05–5 ng/mL LOQ: - | [78] |
| black tea, green tea mixed tea flavoured tea herbal tea (chamomile, sage linden, fennel, rosehips) culinary herb samples (thyme, peppermint) | 29 pyrrolizidine alkaloids | UHPLC | Agilent Poroshell 120 EC-C18 (Agilent Technologies, Palo Alto, CA, USA) 2.1 mm × 150 mm, 2.7 μm | Mobile phase A: 0.1% formic acid in water Mobile phase B: 0.1% formic acid in acetonitrile Gradient: 0–23 min, 3–4% B; 23–45 min, 4–15% B; 45–55 min, 15–25% B 55–57 min to 100% B. 3 min wash with 100% B 5 min reequilibration with 3% B. | Q-TOF/MS Mode: ESI + Product Ion | LOD: 0.105–0.867 ng/g LOQ: 0.357–2.890 ng/g | [102] |
| milk | 51 pyrrolizidine alkaloids | HPLC | Kinetex EVO C18 (Phenomenex, Torrance, CA, USA), 100 mm × 2.1 mm, 2.6 μm. Kinetex EVO C18 (Phenomenex, Torrance, CA, USA), 150 mm × 2.1 mm, 5 μm. | acidic conditions: Mobile phase A: water with ammonium formate and formic acid 5 mmol/L Mobile phase B: acetonitrile/water (95/5, v/v), 26.5 mmol/L alkaline conditions: Mobile phase A: ammonium carbonate in water 10 mmoL/L Mobile phase B: acetonitrile | QqQ; MS/MS Mode: ESI + MRM | LOD: 0.005–0.054 ng/g LOQ: 0.009–0.123 ng/g | [117] |
| black tea, peppermint tea, mixed herbal tea, valerian herbal supplement, alfalfa, hay, sunflower expeller, bovine compound feed | 43 pyrrolizidine alkaloids | UPLC | alkaline conditions: Waters Acquity UPLC BEH C18 (Waters, Milford, MA, USA) 2.1 mm × 150 mm, 1.7 μm acidic conditions: Waters Acquity UPLC CSH C18 (Waters, Milford, MA, USA) 2.1 mm × 150 mm, 1.7 μm | alkaline conditions: Mobile phase A: 10 mM ammonium carbonate in water, pH 9 Mobile phase B: acetonitrile acidic conditions: Mobile phase A: 0.1% formic acid in water Mobile phase B: acetonitrile | QqQ; MS/MS Mode: ESI + MRM | LOD: - LOQ: 10 ng/g | [118] |
| honey | retronecine | GC | Zebron ZB-5MS (Phenomenex, Torrance, CA, USA), 30 m × 0.25 mm; film 0.25 μm | - | Q; MS Mode: positive SIM | LOD: 2 ng/g LOQ: 6 ng/g | [108] |
| honey | echimidine heliotrine intermedine lasiocarpine lycopsamine retrorsine seneciphylline senecionine senkirkine | UHPLC | Supelco Analytical C8 (Supelco, Bellefonte, PA, USA), 150 mm × 3 mm, 2.7 μm | Mobile phase A: 0.5% formic acid in water Mobile phase B: acetonitrile | Q; MS Mode: ESI + SIM | LOD: - LOQ: 0.08–4.3 ng/g | [98] |
| honey | lycopsamine senecionine senecionine N-oxide heliosupine echimidine | HPLC | Phenomenex Synergi hydro-RP C18, (Phenomenex, Torrance, CA, USA), 100 mm × 30 mm, 2.5 μm | Mobile phase A: 0.1% formic acid in water Mobile phase B: 0.1% formic acid in acetonitrile | QqQ; MS/MS Mode: ESI + SIM | LOD: 0.45–0.67 ng/mL LOQ: 1.21–1.79 ng/mL | [99] |
| Tussilago farfara | senecionine senkirkine | HPLC-DAD | Waters Xterra C18 (Waters, Milford, MA, USA) 3.9 mm × 150 mm, 5 μm | Mobile phase A: 0.1% formic acid in 20 mM NH4CH3CO2; Mobile phase B: 0.1% formic acid in acetonitrile | Q; MS Mode: ESI + SIM | LOD: 0.26/1.32 ng/g LOQ: 1.04/5.29 ng/g | [119] |
| Pardoglossum cheirifolium | 9 pyrrolizidine alkaloids | GC | Restek Rxi-1 ms (Restek, Bellefonte, PA, USA), 30 m × 0.25 mm; film 0.25 μm | - | Q; MS TIC | LOD: - LOQ: - | [110] |
| tea, potato, beans. | 15 pyrrolizidine alkaloids | UHPLC | Waters Acquity HSS T3 (Waters, Milford, MA, USA) 2.1 mm × 50 mm, 1.7 μm | Mobile phase A: water with 0.1 % formic acid and ammonium formate 4 mmol/L. Mobile phase B: methanol | Q-Orbitrap-MS/MS Mode: ESI + HRMS | LOD: 1.18–13.28 ng/g LOQ: - | [104] |
| herbal infusions, rooibos, anise, lemon balm, chamomile, thyme, peppermint, lemon verbena, mixtures of teas of Camellia sinensis, flavoured teas, 73 plant-based food supplements (formulated as solid forms, infusions, and sirups). | 118 pyrrolizidine alkaloids | UHPLC | Phenomenex Luna Omega Polar C18 (Phenomenex, Torrance, CA, USA), 2.1 mm × 100 mm, 1.6 μm | Mobile phase A: 0.1% formic acid in water Mobile phase B: 0.1% formic acid in acetonitrile | Q-Orbitrap-HRMS/MS Mode: HESI-II + Full MS/dd-MS2 | LOD: 0–1.5 ng/mL LOQ: 0.1–2.1 ng/g in solids; 1–12 ng/g in infusions | [105] |
| Common heliotrope (Heliotropium europaeum) Heliotropium popovii Chamomile (Matricaria recutita) | 35 pyrrolizidine alkaloids | UHPLC | Waters Acquity UPLC BEH C18 (Waters, Milford, MA, USA) 150 mm × 2.1 mm, 1.7 μm | Mobile phase A: 10 mM ammonium carbonate in water, pH 9 Mobile phase B: acetonitrile | Q-Orbitrap-MS/MS Mode: HESI-II + Full MS Scan | LOD: - LOQ: - | [106] |
| rooibos, chamomile, red tea, black tea, green tea, white tea, linden, horsetail, mixture of herbs. | 28 pyrrolizidine alkaloids | HPLC | C18 | Mobile phase A: 0.1% formic acid in water Mobile phase B: 0.1% formic acid in acetonitrile | Q-Orbitrap-MS/MS Mode: ESI + HRMS | LOD: - LOQ: 5 ng/g | [107] |
| Crotalaria (Fabaceae) species | 45 pyrrolizidine alkaloids | UHPLC | Hypersil GOLD aQ C18 (Thermo Scientific, Waltham, MA, USA) 100 mm × 2.1 mm, 1.9 μm | Mobile phase A: formic acid in water Mobile phase B: formic acid in acetonitrile (various formic acid concentrations: 0.05, 0.2, and 0.35% v/v) | Orbitrap-MS Mode: HESI-II + Full MS Scan | LOD: 0.05 ng/mL LOQ: - | [120] |
| No. | Product | Country of Origin | Notifying Country | Determined Level of PAs (µg/kg—ppb) | Maximum Level (µg/kg—ppb) | Notification Date |
|---|---|---|---|---|---|---|
| 1 | Psyllium Fibre Food Supplement | UK | Ireland | 1177.0 ± 111.7 1113.5 ± 109.3 | 400 | 31 May 2024 |
| 2 | Cumin | Turkey | Germany | 8374 ± 3685 | 400 | 22 May 2024 |
| 3 | Dill tops rubbed | Poland | Germany | 1300 2000 | 400 | 16 May 2024 |
| 4 | Ground oregano | Romania, Turkey | France | 2563 ± 560 | 1000 | 7 May 2024 |
| 5 | Dill | Poland, Spain | The Netherlands | 840 | 400 | 3 May 2024 |
| 6 | Cumin powder | Turkey | Bulgaria | 3248.5 ± 1299.4 3232.5 ± 1293 | 400 | 29 April 2024 |
| 7 | Herbes de Provence | France | France | 2800 ± 700 | 600 | 19 April 2024 |
| 8 | Ground cumin | Belgium | Belgium | 773 | 400 | 18 April 2024 |
| 9 | Pollen | France | Switzerland | 3300 | 500 | 18 April 2024 |
| 10 | Black tea—naturally flavoured maple | India | Belgium | 347 | 150 | 15 April 2024 |
| 11 | Dried oregano | Turkey | France | 7861 ± 3931 | 1000 | 11 April 2024 |
| 12 | Cumin seeds | Turkey | France | 34,149.4 ± 17,074.7 | 400 | 11 April 2024 |
| 13 | Cumin powder | Germany | Belgium | 8860 | 400 | 29 March 2024 |
| 14 | Oregano | Turkey | Switzerland | 8062 | 1000 | 28 March 2024 |
| 15 | Oregano | Turkey | Switzerland | 24,231 | 1000 | 26 March 2024 |
| 16 | Cumin | India | Czech Republic | 985 | 400 | 11 March 2024 |
| 17 | Black Tea | Kenya | Poland | 540 ± 291 | 150 | 5 March 2024 |
| 18 | Dried oregano | Belgium | France | 1781.5 | 1000 | 5 March 2024 |
| 19 | Gokshura/Lifepower/Karela | The Netherlands | The Netherlands | 3920 | 400 | 28 February 2024 |
| 20 | Kmin rzymski mielony (Ground cumin) | Poland | Poland | 3340 ± 1169 | 400 | 22 February 2024 |
| 21 | Herbata Czarna Earl Grey (Earl Gray Tea black tea) | Kenya | Poland | 525 ± 180, 540 ± 291 | 150 | 20 February 2024 |
| 22 | Cumin | India | Poland | 1914 ± 670 | 400 | 20 February 2024 |
| 23 | Parsley leaves | Poland | Romania | 1400 | 400 | 15 February 2024 |
| 24 | Kmin rzymski (Cumin) | Austria | Poland | 776 ± 273 | 400 | 14 February 2024 |
| 25 | Dried parsley leaves | Poland | Poland | 3249 ± 459 | 400 | 26 January 2024 |
| 26 | Oregano | Turkey | The Netherlands | 2400 | 1000 | 19 January 2024 |
| 27 | Green tea | Germany | The Netherlands | 165 | 150 | 17 January 2024 |
| 28 | Food Supplement | Norway | Sweden | 1100 | 400 | 11 January 2024 |
| 29 | Chamomile herbal tea | Czech Republic | Czech Republic | 1936 | 400 | 10 January 2024 |
| 30 | Pollen | Spain | Belgium | 1430 | 500 | 9 January 2024 |
| 31 | Oregano | Greece | The Netherlands | 2600 ± 1300 | 1000 | 3 January 2024 |
| 32 | Oregano | Turkey | The Netherlands | 21,000 | 1000 | 28 December 2023 |
| 33 | Ground cumin | Belgium | Belgium | 752 | 400 | 22 December 2023 |
| 34 | Oregano | Turkey | The Netherlands | 1245 | 1000 | 21 December 2023 |
| 35 | Oregano | Turkey | Poland | 7941 ± 1571 | 1000 | 21 December 2023 |
| 36 | Oregano | Jordan | Ireland | 49,432.8 ± 5776.1 | 1000 | 21 December 2023 |
| 37 | Cumin | Turkey | Germany | 711 | 400 | 20 December 2023 |
| 38 | Cumin, ground | Turkey | Germany | 6080 | 400 | 13 December 2023 |
| 39 | Black cumin seeds | Turkey | France | 1054.6 ± 527.3 | 400 | 12 December 2023 |
| 40 | Blackberry leaves | Albania | Germany | 5170 ± 1293 | 200 | 8 December 2023 |
| 41 | Mint tea (Mentha bruh, Mentha piperita) | Serbia | Croatia | >8550.5 | 400 | 5 December 2023 |
| 42 | Dried oregano | Turkey | France | 3626.4 ± 1813.2 | 1000 | 27 November 2023 |
| 43 | Ground cumin | Spain | Belgium | 2790 | 400 | 24 November 2023 |
| 44 | Ground oregano | Greece | Germany | 23,350 | 1000 | 24 November 2023 |
| 45 | Chili powder | India, The Netherlands, Spain, Turkey | Belgium | 2790 | 0 | 20 November 2023 |
| 46 | Pollen | Spain | Belgium | 1070 | 500 | 13 November 2023 |
| 47 | Spiskummin (Cumin) | Lebanon | Sweden | 1060, 1850, 2160 | 400 | 10 November 2023 |
| 48 | Dried oregano | Turkey | Italy | 3910 ± 773 | 1000 | 9 November 2023 |
| 49 | Whole lovage leaf | Germany | The Netherlands | 1310 | 1000 | 6 November 2023 |
| 50 | Chives, grinded | Germany | The Netherlands | 553 | 0 | 2 November 2023 |
| 51 | Rosemary | France | The Netherlands | 967 | 400 | 30 October 2023 |
| 52 | Cumin and Organic Cumin | Egypt, India | Denmark | 16,000, 1600 | 400 | 27 October 2023 |
| 53 | Dried oregano | Turkey | Poland | 3640 ± 1274 | 1000 | 26 October 2023 |
| 54 | Cumin | Lebanon | Denmark | 12,000 | 400 | 24 October 2023 |
| 55 | Herbal tea | Morocco | Germany | 594 ± 148 | 200 | 19 October 2023 |
| 56 | Cumin seed | Turkey | Belgium | 1306 | 400 | 9 October 2023 |
| 57 | Cumin powder | Turkey | Bulgaria | >16,221 | 400 | 6 October 2023 |
| 58 | Dried oregano | Turkey | Bulgaria | 8640.7 | 1000 | 21 September 2023 |
| 59 | Peppermint herbal tea | Poland | Czech Republic | 657 | 400 | 20 September 2023 |
| 60 | Oregano | Turkey | Luxembourg | 3292 ± 745 | 1000 | 14 September 2023 |
| 61 | Herbal infusion | China | Belgium | 786 | 200 | 21 August 2023 |
| 62 | Tarragon | France | Belgium | 1120 | 400 | 11 August 2023 |
| 63 | Cumin seeds crushed or ground | Turkey | Greece | 2074 ± 415 | 400 | 7 August 2023 |
| 64 | Dried oregano | Turkey | Greece | 4285 ± 857 | 1000 | 7 August 2023 |
| 65 | Kmin rzymski mielony (Cumin) | India, Poland | Poland | 1217 | 400 | 11 July 2023 |
| 66 | Ground cumin | Turkey | Belgium | 23,813 | 400 | 26 June 2023 |
| 67 | Ground cumin | Turkey | Greece | 8281 | 400 | 26 June 2023 |
| 68 | Cumin | Turkey | Germany | 13,600 | 400 | 21 June 2023 |
| 69 | Cumin | Turkey | Belgium | 2259 ± 890 | 400 | 14 June 2023 |
| 70 | Cumin seeds | Spain | Luxembourg | 717 ± 108 | 400 | 14 June 2023 |
| 71 | Herbata Loyd Earl grey | Poland | Poland | 240 ± 40 | 150 | 12 May 2023 |
| 72 | Ground cumin | Turkey | Bulgaria | 1553.4 | 400 | 2 May 2023 |
| 73 | Dried oregano | Turkey | Sweden | 2263 | 1000 | 12 April 2023 |
| 74 | Organic oregano, rubbed | Germany, Greece | Germany | 24,000 | 1000 | 28 March 2023 |
| 75 | Dried oregano | Poland | Czech Republic | 1448 | 1000 | 28 March 2023 |
| 76 | Oregano rubbed | Greece | Germany | 17,000 | 1000 | 22 March 2023 |
| 77 | Cumin grain | Belgium, France | France | 10,000 | 400 | 7 March 2023 |
| 78 | Cumin Whole | India | Ireland | 527.1 ± 87.9 | 400 | 17 February 2023 |
| 79 | Borage | Italy | Germany | >59,999 | 1000 | 7 February 2023 |
| 80 | Ground cumin | Belgium, Syria | Belgium | 16,596, 13,551.4 | 400 | 3 February 2023 |
| 81 | Ginkgo biloba extract | France | Belgium | 702 | 400 | 30 January 2023 |
| 82 | Camomille tea | France | Belgium | 2470 | 400 | 27 January 2023 |
| 83 | Cumin seeds | Turkey | France | 1148.9 ± 574.4, 660.9 ± 330.5, 563.7 ± 281.9 | 400 | 27 January 2023 |
| 84 | Licorice root ground Zoethoutwortel gemalen | France | The Netherlands | 1558 | 400 | 23 January 2023 |
| 85 | Herbal tea mix | Morocco | Norway | 11,608.3 | 200 | 12 January 2023 |
| 86 | Black Tea (ceai negru) | Poland | Romania | 700 | 150 | 10 January 2023 |
| 87 | Pollen | Poland | Poland | 1187 ± 301 | 500 | 3 January 2023 |
| 88 | Cumin fines | Turkey | Spain | 7290 ± 3650 | 400 | 29 December 2022 |
| 89 | Ground cumin | - | Belgium | 5298, 2926 | 400 | 16 December 2022 |
| 90 | Dried oregano | Turkey | Poland | 13,921 ± 2735 | 1000 | 13 December 2022 |
| 91 | Cumin | - | Greece | 17,512 | 400 | 13 December 2022 |
| 92 | Ground cumin | India | Germany | 4040 ± 1620 | 400 | 1 December 2022 |
| 93 | Ground cumin | Afghanistan, France | Belgium | 23,899, 14,249 | 400 | 22 November 2022 |
| 94 | Oregano (dried) | Turkey | Belgium | 1983.5 | 1000 | 21 November 2022 |
| 95 | Dried oregano | Turkey | France | 5174 ± 2587 | 1000 | 17 November 2022 |
| 96 | Dried oregano | Turkey | Poland | 8236 ± 1564 | 1000 | 15 November 2022 |
| 97 | Ground cumin | - | Belgium | 3697 ± 1395, 10,118 ± 3915 | 400 | 3 November 2022 |
| 98 | Oregano | Greece | The Netherlands | 30,313 | 1000 | 2 November 2022 |
| 99 | Origano secco | Turkey | Italy | 5591 ± 1177 | 1000 | 19 October 2022 |
| 100 | Comino | Turkey | Spain | 8170 ± 4090 | 400 | 11 October 2022 |
| 101 | Ground cumin | Turkey | Switzerland | 4436 | 400 | 10 October 2022 |
| 102 | Dried oregano | Turkey | Bulgaria | >2500 | 400 | 10 October 2022 |
| 103 | Ground cumin | Turkey | Ireland | 1191.4 ± 197.8 | 400 | 25 August 2022 |
| 104 | Cumin seeds | India | Switzerland | 154,000, 2780, 14,100 | 400 | 15 June 2022 |
| 105 | Cumin | Turkey | Sweden | 12,350, 10,560 | 0 | 10 June 2022 |
| 106 | Ground cumin | Turkey | Bulgaria | >2500 | 0 | 12 May 2022 |
| 107 | Dried oregano | Turkey | Bulgaria | 2154 | 400 | 10 May 2022 |
| 108 | Dried oregano | Turkey | Bulgaria | 2644.1 | 400 | 7 May 2022 |
| 109 | Ground cumin | Turkey | Bulgaria | 1505.4 | 400 | 24 April 2022 |
| 110 | Ground Cumin | Turkey | Ireland | 1723.8, 4810.6 ± 801.4 | 400 | 22 April 2022 |
| 111 | Dried oregano | Turkey | Finland | 6970 | 0 | 30 March 2022 |
| 112 | Semillas de comino (Cumin seeds) | Turkey | Spain | 50,000 | 400 | 7 March 2022 |
| 113 | Ground cumin | Turkey | Czech Republic | 11,907.7 | 0 | 1 March 2022 |
| 114 | Organic bee feed | Spain | The Netherlands | 97, 42, 880 | 500 | 5 January 2022 |
| 115 | Oregano | Spain | Denmark | 14,000 ± 5000 | 0 | 23 December 2021 |
| 116 | Chamomile Tea | Uzbekistan | Denmark | 5400 | 0 | 22 December 2021 |
| 117 | Oregano | Turkey | Germany | 2785, 2568 | 0 | 28 October 2021 |
| 118 | Cumin seeds | Turkey | Germany | 9474 | 0 | 19 October 2021 |
| 119 | Oregano | Turkey | Switzerland | 4879 | 0 | 2 June 2021 |
| 120 | Oregano | Turkey | Germany | 2079 | 0 | 20 May 2021 |
| 121 | Organic cumin | Turkey | Germany | 10,483.39 | 5000 | 14 May 2021 |
| 122 | Cumin | Turkey | Germany | 10,906.77 | 0 | 7 May 2021 |
| 123 | Cumin | Turkey | Germany | 10,406.94 | 5000 | 5 May 2021 |
| 124 | Kräutertee (Herbal tea) | Czech Republic | Germany | 2928.10 | 0 | 1 April 2021 |
| 125 | Oregano | Turkey | Switzerland | 8895 | 0 | 26 March 2021 |
| 126 | Kreuzkümmel, gemahlen (Ground cummin) | Turkey | Germany | 27,500 ± 970 | 0 | 12 February 2021 |
| 127 | Kreuzkümmel, gemahlen (Ground cummin) | The Netherlands | Germany | 21,200 ± 5300 | 0 | 21 January 2021 |
| 128 | Ground cumin | Turkey | Switzerland | 9948 | 0 | 24 December 2020 |
| 129 | Ground cumin | Turkey | Switzerland | 20,377, 5786 | 0 | 23 December 2020 |
| 130 | Ground cumin | Turkey | Switzerland | 5522 | 0 | 23 December 2020 |
| 131 | Kreuzkümmel, gemahlen (Ground cummin) | Turkey | Germany | 11,700 ± 2900 | 0 | 4 December 2020 |
| 132 | Ground cumin | The Netherlands | Germany | 55,176 | 0 | 18 November 2020 |
| 133 | Cumin (Kreuzkümmel) | Lebanon | Germany | 22,000, 18,900 | 0 | 18 November 2020 |
| 134 | Anissamen (Anise seeds) | Egypt | Germany | 12,184, 15,114, 1206 ± 188 | 0 | 20 August 2020 |
| 135 | Cumin ganz | Syria | Germany | 57,827 | 0 | 19 August 2020 |
| 136 | Cumin, Organic | Turkey | Switzerland | 29,120 | 0 | 30 June 2020 |
| 137 | Bio Cumin | Turkey | Germany | 56.100 | 0 | 30 April 2020 |
| 138 | Ground cumin and dry oregano | Turkey | Denmark | 15,000, 7200 | 0 | 24 April 2020 |
| 139 | Oregano | Turkey | Germany | 6620 | 0 | 30 March 2020 |
| 140 | Dried camomile tea | Poland | Belgium | 530 | 0 | 11 February 2020 |
| 141 | Oregano getrocknet | Turkey | Germany | 16,962 ± 8481 | 0 | 5 February 2020 |
| 142 | Rubbed oregano | Turkey | Germany | 8836 | 0 | 4 February 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis-Cieplak, A.; Trześniowska, K.; Stolarczyk, K.; Stolarczyk, E.U. Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations. Molecules 2024, 29, 3269. https://doi.org/10.3390/molecules29143269
Lis-Cieplak A, Trześniowska K, Stolarczyk K, Stolarczyk EU. Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations. Molecules. 2024; 29(14):3269. https://doi.org/10.3390/molecules29143269
Chicago/Turabian StyleLis-Cieplak, Agnieszka, Katarzyna Trześniowska, Krzysztof Stolarczyk, and Elżbieta U. Stolarczyk. 2024. "Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations" Molecules 29, no. 14: 3269. https://doi.org/10.3390/molecules29143269
APA StyleLis-Cieplak, A., Trześniowska, K., Stolarczyk, K., & Stolarczyk, E. U. (2024). Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations. Molecules, 29(14), 3269. https://doi.org/10.3390/molecules29143269






