The Role of Ovalbumin in Manganese Homeostasis during Chick Embryogenesis: An EPR Spectroscopic Study
Abstract
1. Introduction
2. Results and Discussion
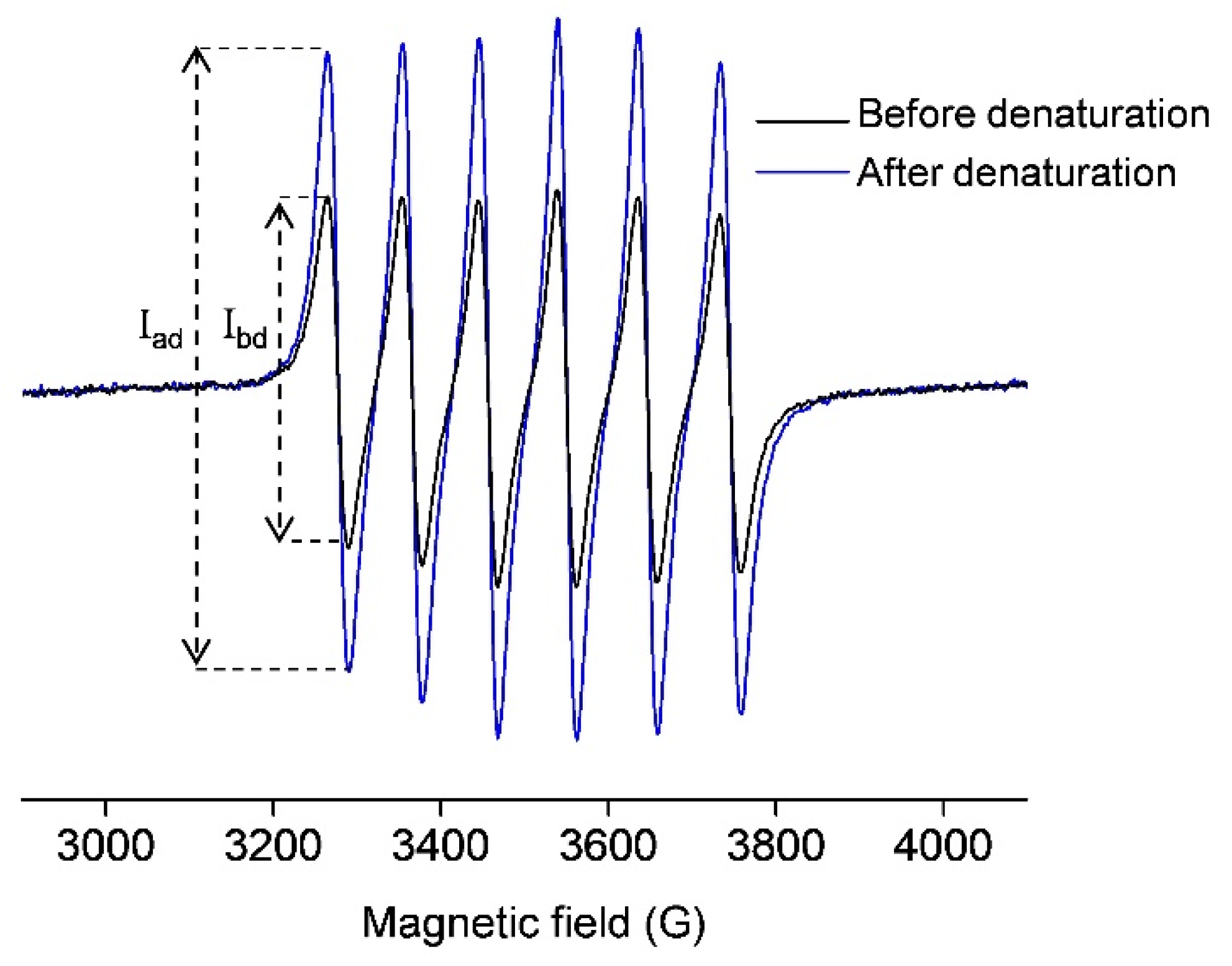
2.1. EPR Spectroscopy
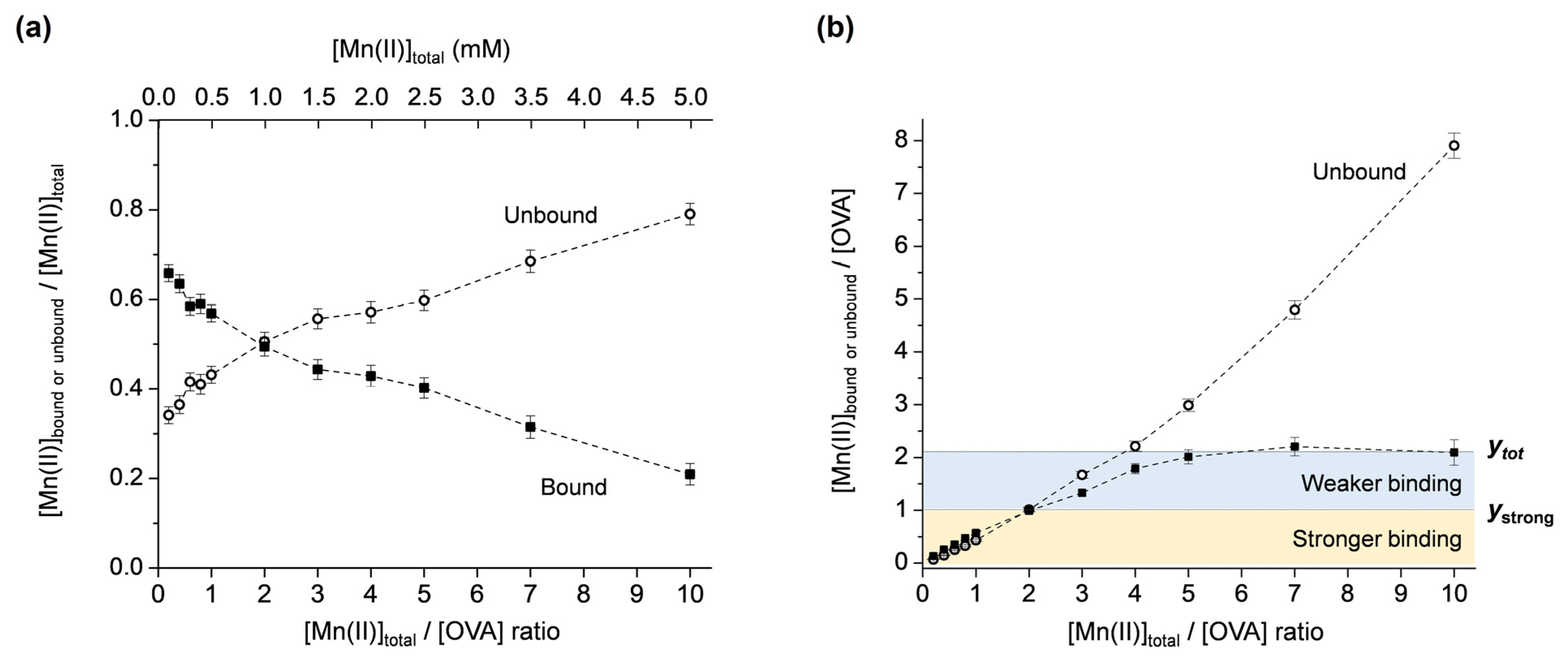
2.1.1. The Binding of Mn(II) to OVA
2.1.2. The Determination of the Total Number of Mn(II) Binding Sites on OVA
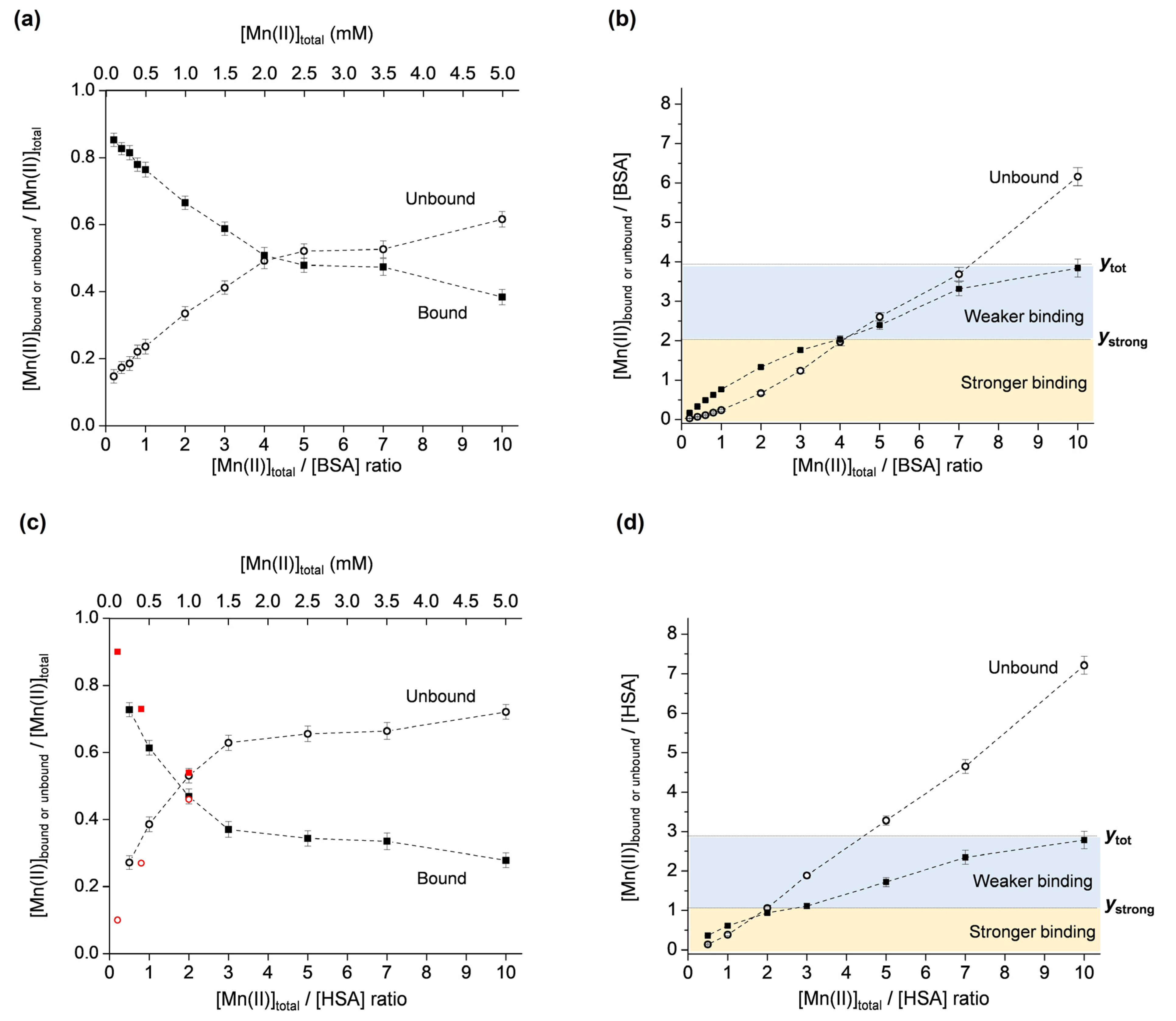
2.1.3. The Binding of Mn(II) to BSA and HSA
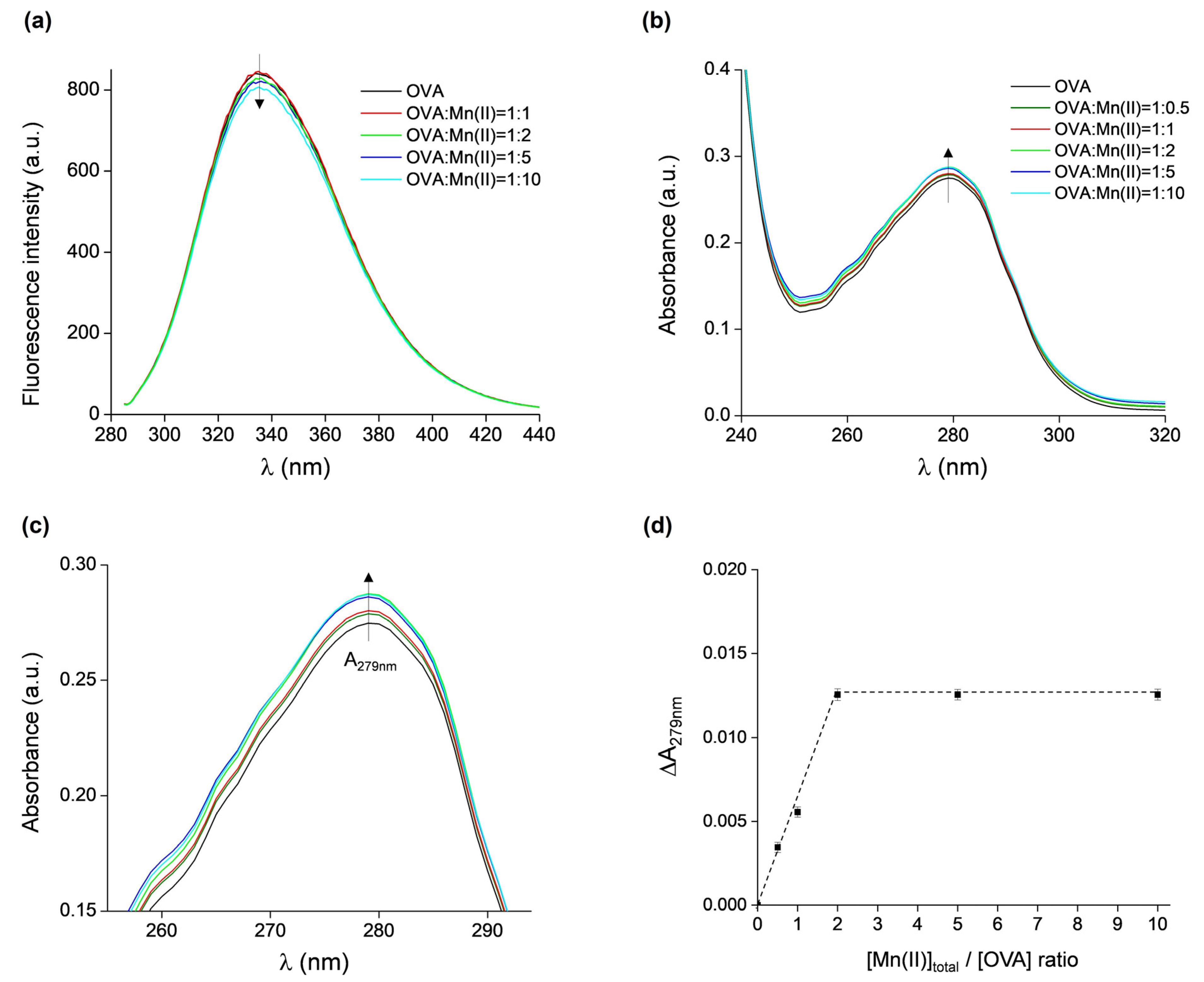
2.2. Fluorescence and UV/vis Absorption Spectra of OVA in Presence of Mn(II)
3. Materials and Methods
3.1. Chemicals
3.2. EPR Spectroscopy
3.3. Fluorescence Spectroscopy
3.4. UV/vis Spectrophotometry
3.5. Data Fitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guyot, N.; Da Silva, M.; Nys, Y.; Rehault-Godbert, S. Minireview The Family Secrets of Avian Egg-Specific Ovalbumin and Its Related Proteins Y and X. Biol. Reprod. 2015, 93, 130856. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Chaudhary, V.; Chhikara, N.; Sharma, N. Food Hydrocolloids Ovalbumin, an Outstanding Food Hydrocolloid: Applications, Technofunctional Attributes, and Nutritional Facts, A Systematic Review. Food Hydrocoll. 2023, 139, 108514. [Google Scholar] [CrossRef]
- Stein, P.E.; Leslie, A.G.W.; Finch, J.T.; Turnell, W.G.; McLaughlin, P.J.; Carrell, R.W. Crystal Structure of Ovalbumin as a Model for the Reactive Centre of Serpins. Nature 1990, 347, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.E.; Leslie, A.G.W.; Finch, J.T.; Carrell, R.W. Crystal Structure of Uncleaved Ovalbumin at 1·95 Å Resolution. J. Mol. Biol. 1991, 221, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, P.H.; Grey, A.; Carver, J.P.; Hakimi, J.; Ceccarini, C. Demonstration of Heterogeneity of Chick Ovalbumin Glycopeptides Using 360-MHz Proton Magnetic Resonance Spectroscopy. Biochemistry 1981, 20, 3979–3986. [Google Scholar] [CrossRef]
- Narasimhan, S.; Harpaz, N.; Longmore, G.; Carver, J.P.; Grey, A.A.; Schachter, H. Control of Glycoprotein Synthesis. The Purification by Preparative High Voltage Paper Electrophoresis in Borate of Glycopeptides Containing High Mannose and Complex Oligosaccharide Chains Linked to Asparagine. J. Biol. Chem. 1980, 255, 4876–4884. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, A.D.; Saundry, R.H.; Moir, A.J.G.; Fothergill, L.A.; Fothergill, J.E. The Complete Amino-Acid Sequence of Hen Ovalbumin. Eur. J. Biochem. 1981, 115, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Cann, J.R. Electrophoretic Analysis of Ovalbumin. J. Am. Chem. Soc. 1949, 71, 907–909. [Google Scholar] [CrossRef]
- Sharif, M.K.; Saleem, M.; Javed, K. Food Materials Science in Egg Powder Industry. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 505–537. ISBN 9780128115008. [Google Scholar]
- Ke, Y.; Li, Y.; Kapp, J.A. Ovalbumin Injected with Complete Freund’s Adjuvant Stimulates Cytolytic Responses. Eur. J. Immunol. 1995, 25, 549–553. [Google Scholar] [CrossRef]
- Gou, S.; Chen, Q.; Liu, Y.; Zeng, L.; Song, H.; Xu, Z.; Kang, Y.; Li, C.; Xiao, B. Green Fabrication of Ovalbumin Nanoparticles as Natural Polyphenol Carriers for Ulcerative Colitis Therapy. ACS Sustain. Chem. Eng. 2018, 6, 12658–12667. [Google Scholar] [CrossRef]
- Manivel, P.; Paulpandi, M.; Chen, X. Ovalbumin-Coated Fe3O4 Nanoparticles as a Nanocarrier for Chlorogenic Acid to Promote the Anticancer Efficacy on MDA-MB-231 Cells. New J. Chem. 2022, 46, 12609–12622. [Google Scholar] [CrossRef]
- Buszewski, B.; Žuvela, P.; Krol-Gorniak, A.; Railean-Plugaru, V.; Rogowska, A.; Wah Wong, M.; Yi, M.; Rodzik, A.; Sprynskyy, M.; Pomastowski, P. Applied Surface Science Interactions of Zinc Aqua Complexes with Ovalbumin at the Forefront of the Zn2+/ZnO-OVO Hybrid Complex Formation Mechanism. Appl. Surf. Sci. 2021, 542, 148641. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Huang, L.; Ma, L.; Chen, G. Au Nanoclusters for Sensitive Detection of Ascorbic. Anal. Methods 2015, 7, 4123–4129. [Google Scholar] [CrossRef]
- Nakum, R.; Ghosh, A.K.; Ranjan, B.; Sahoo, S.K. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Fluorescent Ovalbumin-Functionalized Gold Nanocluster as a Highly Sensitive and Selective Sensor for Relay Detection of Salicylaldehyde, Hg (II) and Folic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 313, 124143. [Google Scholar] [CrossRef]
- Nyemb, K.; Guérin-Dubiard, C.; Dupont, D.; Jardin, J.; Rutherfurd, S.M.; Nau, F. The Extent of Ovalbumin In Vitro Digestion and the Nature of Generated Peptides Are Modulated by the Morphology of Protein Aggregates. Food Chem. 2014, 157, 429–438. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, M.H.A.; El-Shibiny, S. Natural Biopolymers as Nanocarriers for Bioactive Ingredients Used in Food Industries. In Nanotechnology in the Agri-Food Industry, Encapsulations; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 793–829. ISBN 9780128043073. [Google Scholar]
- Vesković, A.; Nakarada, Đ.; Popović Bijelić, A. Fatty Acid Binding to Ovalbumin Studied by EPR. In Proceedings of the Regional Biophysics Conference 2022, Pécs, Hungary, 22–26 August 2022. [Google Scholar]
- Vesković, A.; Bajuk-Bogdanović, D.; Arion, V.B.; Popović Bijelić, A. Spectroscopic Characterization of the Binding and Release of Hydrophilic, Hydrophobic and Amphiphilic Molecules from Ovalbumin Supramolecular Hydrogels. Gels 2023, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Malik, W.U.; Jindal, M.R. Polarographic Studies on the Binding of Copper(II) and Cadmium(II) with Ovalbumin. J. Electroanal. Chem. 1968, 19, 436–438. [Google Scholar] [CrossRef]
- Goux, W.J.; Venkatasubramanian, P.N. Metal Ion Binding Properties of Hen Ovalbumin and S-Ovalbumin: Characterization of the Metal Ion Binding Site by 31P NMR and Water Proton Relaxation Rate Enhancements. Biochemistry 1986, 25, 84–94. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Sanuki, S.; Ohsako, S.; Higashimoto, Y.; Kondo, M.; Kurawaki, J.; Ibrahim, H.R.; Aoki, T.; Kusakabe, T.; Koga, K. Ovalbumin in Developing Chicken Eggs Migrates from Egg White to Embryonic Organs While Changing Its Conformation and Thermal Stability *. J. Biol. Chem. 1999, 274, 11030–11037. [Google Scholar] [CrossRef]
- Uni, Z.; Yadgary, L.; Yair, R. Embryonic Development 1. J. Appl. Poult. Res. 2012, 21, 175–184. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Babadi, V.Y.; Sadeghi, L.; Shirani, K.; Malekirad, A.A.; Rezaei, M. The Toxic Effect of Manganese on the Acetylcholinesterase Activity in Rat Brains. J. Toxicol. 2014, 2014, 946372. [Google Scholar]
- Li, L. Review Article The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed]
- Olgun, O. Manganese in Poultry Nutrition and Its Effect on Performance and Eggshell Quality. World’s Poult. Sci. J. 2017, 73, 45–56. [Google Scholar] [CrossRef]
- Voica, C.; Cristea, G.; Iordache, A.M.; Roba, C.; Curean, V. Elemental Profile in Chicken Egg Components and Associated Human Health Risk Assessment. Toxics 2023, 11, 900. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Krawczyk, J.; Puchała, M.; Józefiak, D. Dietary Factors Improving Eggshell Quality: An Updated Review with Special Emphasis on Microelements and Feed Additives. World’s Poult. Sci. J. 2015, 71, 83–94. [Google Scholar] [CrossRef]
- Gu, J.; Yang, G.; Huang, X.; He, Q. Revealing the Complexity of Distinct Manganese Species-Protein Interactions through Multi-Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119981. [Google Scholar] [CrossRef] [PubMed]
- Weil, J.A.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 9780471754961. [Google Scholar]
- Wieghardt, K. The Active Sites in Manganese-Containing Metalloproteins and Inorganic Model Complexes. Angew. Chem. Int. Ed. Engl. 1989, 28, 1153–1172. [Google Scholar] [CrossRef]
- Hagen, W.R. Biomolecular EPR Spectroscopy; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-5957-1. [Google Scholar]
- Reed, G.H.; Markham, G.D. EPR of Mn(II) Complexes with Enzymes and Other Proteins. In Biological Magnetic Resonance; Berliner, L.J., Reuben, J., Eds.; Springer: Boston, MA, USA, 1984; pp. 73–142. [Google Scholar]
- Cohn, M.; Townsend, J. A Study of Manganous Complexes by Paramagnetic Resonance Absorption. Nature 1954, 173, 1090–1091. [Google Scholar] [CrossRef]
- Malmström, B.G.; Vänngård, T.; Larsson, M. An Electron-Spin-Resonance Study of the Interaction of Manganous Ions with Enolase and Its Substrate. Biochim. Biophys. Acta 1958, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.H.; Cohn, M. Electron Paramagnetic Resonance Spectra of Manganese(II)-Protein Complexes. J. Biol. Chem. 1970, 245, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.H.; Cohn, M. Electron Paramagnetic Resonance Studies of Manganese(II)-Pyruvate Kinase-Substrate Complexes. J. Biol. Chem. 1973, 248, 6436–6442. [Google Scholar] [CrossRef] [PubMed]
- Von Goldammer, E.; Zorn, H. Electron-Paramagnetic-Resonance Study of Manganese Ions Bound to Concanavalin A. Eur. J. Biochem. 1974, 44, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Whiting, A.K.; Boldt, Y.R.; Hendrich, M.P.; Wackett, L.P.; Que, L. Manganese(II)-Dependent Extradiol-Cleaving Catechol Dioxygenase from Arthrobacter Globiformis CM-2. Biochemistry 1996, 35, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: London, UK, 1970. [Google Scholar]
- Eaton, G.R.; Eaton, S.S.; Barr, D.P.; Weber, R.T. Quantitative EPR; Springer: Vienna, Austria, 2010; ISBN 978-3-211-92947-6. [Google Scholar]
- Voevodskaya, N.; Lendzian, F.; Ehrenberg, A.; Gräslund, A. High Catalytic Activity Achieved with a Mixed Manganese–Iron Site in Protein R2 of Chlamydia Ribonucleotide Reductase. FEBS Lett. 2007, 581, 3351–3355. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; Enríquez-Navas, P.M.; Nieto, P.M. Ligand–Receptor Binding Affinities from Saturation Transfer Difference (STD) NMR Spectroscopy: The Binding Isotherm of STD Initial Growth Rates. Chem.-Eur. J. 2010, 16, 7803–7812. [Google Scholar] [CrossRef] [PubMed]
- Voet, D.; Voet, J.G. Biochemistry, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-57095-1. [Google Scholar]
- Sekula, B.; Zielinski, K.; Bujacz, A. Crystallographic Studies of the Complexes of Bovine and Equine Serum Albumin with 3,5-Diiodosalicylic Acid. Int. J. Biol. Macromol. 2013, 60, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-Y.; Yang, C.-T.; Chu, L.-K. Differentiating the Protein Dynamics Using Fluorescence Evolution of Tryptophan Residue(s): A Comparative Study of Bovine and Human Serum Albumins upon Temperature Jump. Chem. Phys. Lett. 2021, 781, 138998. [Google Scholar] [CrossRef]
- Fanali, G.; Cao, Y.; Ascenzi, P.; Fasano, M. Mn(II) Binding to Human Serum Albumin: A 1H-NMR Relaxometric Study. J. Inorg. Biochem. 2012, 117, 198–203. [Google Scholar] [CrossRef]
- Mildvan, A.S.; Cohn, M. Magnetic Resonance Studies of the Interaction of the Manganous Ion with Bovine Serum Albumin. Biochemistry 1963, 2, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.E.; MacDermott, T.E.; O’Sullivan, W.J. Studies on Manganese Complexes of Human Serum Albumin. Bioinorg. Chem. 1973, 3, 27–38. [Google Scholar] [CrossRef]
- Hong, L.; Chu-Qiao, T.; Hong-Zhi, Z.; Xing-Can, S.; Yong-Qia, Z.; Pan-Wen, S. Binding Equilibrium Study between Mn(II) and HSA or BSA. Chin. J. Chem. 2000, 18, 35–41. [Google Scholar] [CrossRef]
- Aime, S.; Canton, S.; Geninatti Crich, S.; Terreno, E. 1H and 17O Relaxometric Investigations of the Binding of Mn(II) Ion to Human Serum Albumin. Magn. Reson. Chem. 2002, 40, 41–48. [Google Scholar] [CrossRef]
- Gale, E.M.; Zhu, J.; Caravan, P. Direct Measurement of the Mn(II) Hydration State in Metal Complexes and Metalloproteins through 17O NMR Line Widths. J. Am. Chem. Soc. 2013, 135, 18600–18608. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y. Comparative Study of the Interactions between Ovalbumin and Five Antioxidants by Spectroscopic Methods. J. Fluoresc. 2017, 27, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ma, J.; Wang, S.; Lou, R.; Wu, S.; He, J.; Kang, H.; Liu, L.; Xiao, F. Interaction Mechanism between Resveratrol and Ovalbumin Based on Fluorescence Spectroscopy and Molecular Dynamic Simulation. LWT 2021, 146, 111455. [Google Scholar] [CrossRef]
- Cen, C.; Chen, J.; Wang, W.; Zhang, J.; Yang, X.; Fu, L.; Wang, Y. Exploring the Interaction Mechanism of Dietary Protein Ovalbumin and Folic Acid: A Combination Research of Molecular Simulation Technology and Multispectroscopy. Food Chem. 2022, 385, 132536. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, X.; Fu, C.; Gong, Y.; Huang, X.; Zhang, J.; Li, X.; Song, H.; Huang, Q. Binding Mechanism of Monascus Pigment and Ovalbumin: Spectral Analysis, Molecular Docking and Molecular Dynamics Simulation. Food Sci. Anim. Prod. 2023, 1, 9240038. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, M.; Li, C.; Xiao, F.; He, J.; Liu, L.; Niu, H.; Ma, J. Study of the Weak Interaction Mechanism of Ovalbumin and Caffeic Acid Using Fluorescence Spectroscopy and Molecular Dynamics Simulation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122966. [Google Scholar] [CrossRef]
- Joshi, D.; Soni, R.K. Nanosecond Laser-Assisted Fabrication of Colloidal Gold and Silver Nanoparticles and Their Conjugation with S-Ovalbumin. Plasmonics 2018, 13, 1297–1308. [Google Scholar] [CrossRef]
- Tsykhanovska, I.; Stabnikova, O.; Gubsky, S. Spectroscopic Studies of Interactions of Iron Oxide Nanoparticles with Ovalbumin Molecules. Mater. Proc. 2022, 9, 2. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesković, A.; Bondžić, A.M.; Popović Bijelić, A. The Role of Ovalbumin in Manganese Homeostasis during Chick Embryogenesis: An EPR Spectroscopic Study. Molecules 2024, 29, 3221. https://doi.org/10.3390/molecules29133221
Vesković A, Bondžić AM, Popović Bijelić A. The Role of Ovalbumin in Manganese Homeostasis during Chick Embryogenesis: An EPR Spectroscopic Study. Molecules. 2024; 29(13):3221. https://doi.org/10.3390/molecules29133221
Chicago/Turabian StyleVesković, Ana, Aleksandra M. Bondžić, and Ana Popović Bijelić. 2024. "The Role of Ovalbumin in Manganese Homeostasis during Chick Embryogenesis: An EPR Spectroscopic Study" Molecules 29, no. 13: 3221. https://doi.org/10.3390/molecules29133221
APA StyleVesković, A., Bondžić, A. M., & Popović Bijelić, A. (2024). The Role of Ovalbumin in Manganese Homeostasis during Chick Embryogenesis: An EPR Spectroscopic Study. Molecules, 29(13), 3221. https://doi.org/10.3390/molecules29133221








