Iron-Catalyzed Sulfonylmethylation of Imidazo[1,2-α]pyridines with N,N-Dimethylacetamide and Sodium Sulfinates
Abstract
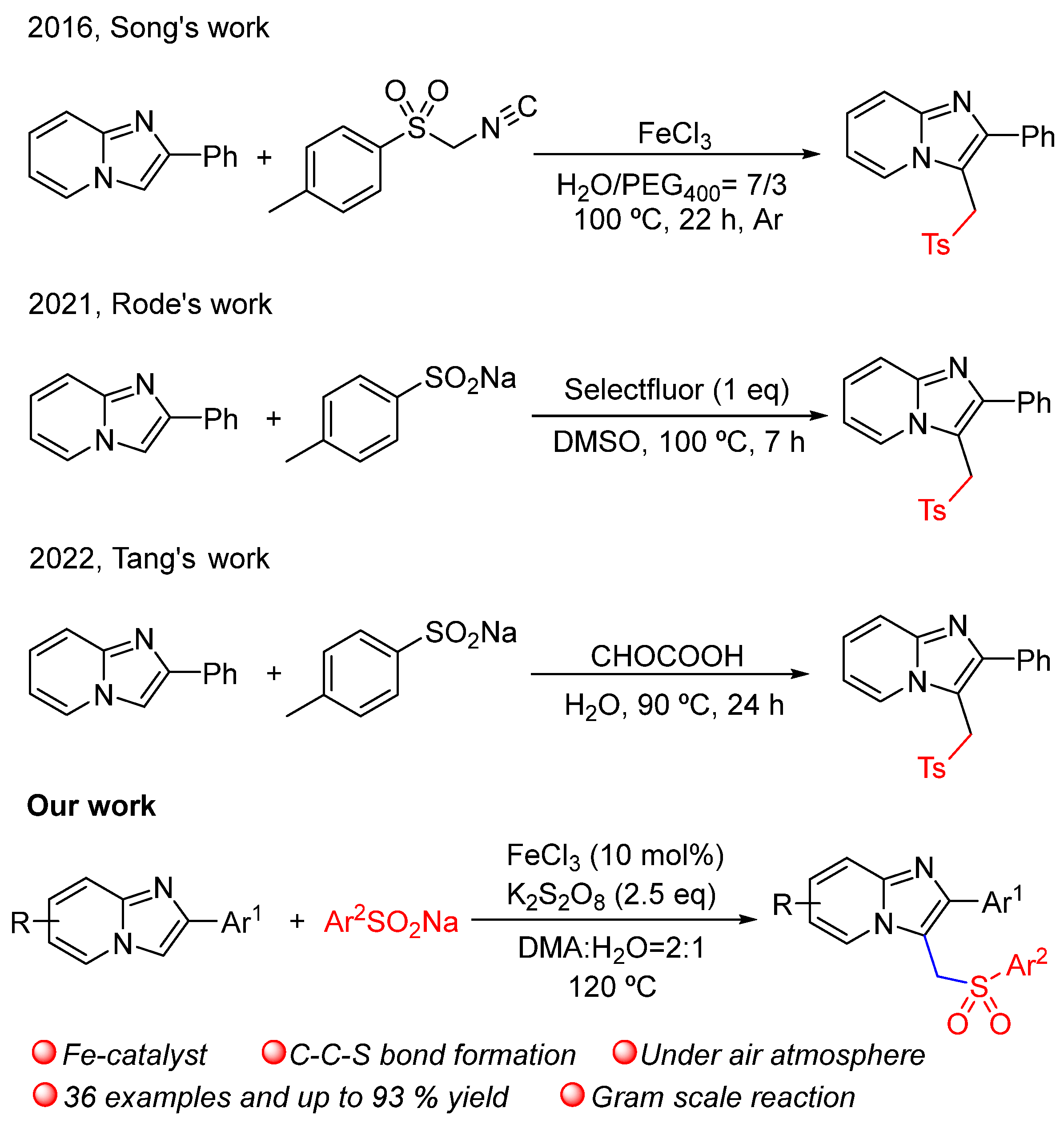
1. Introduction
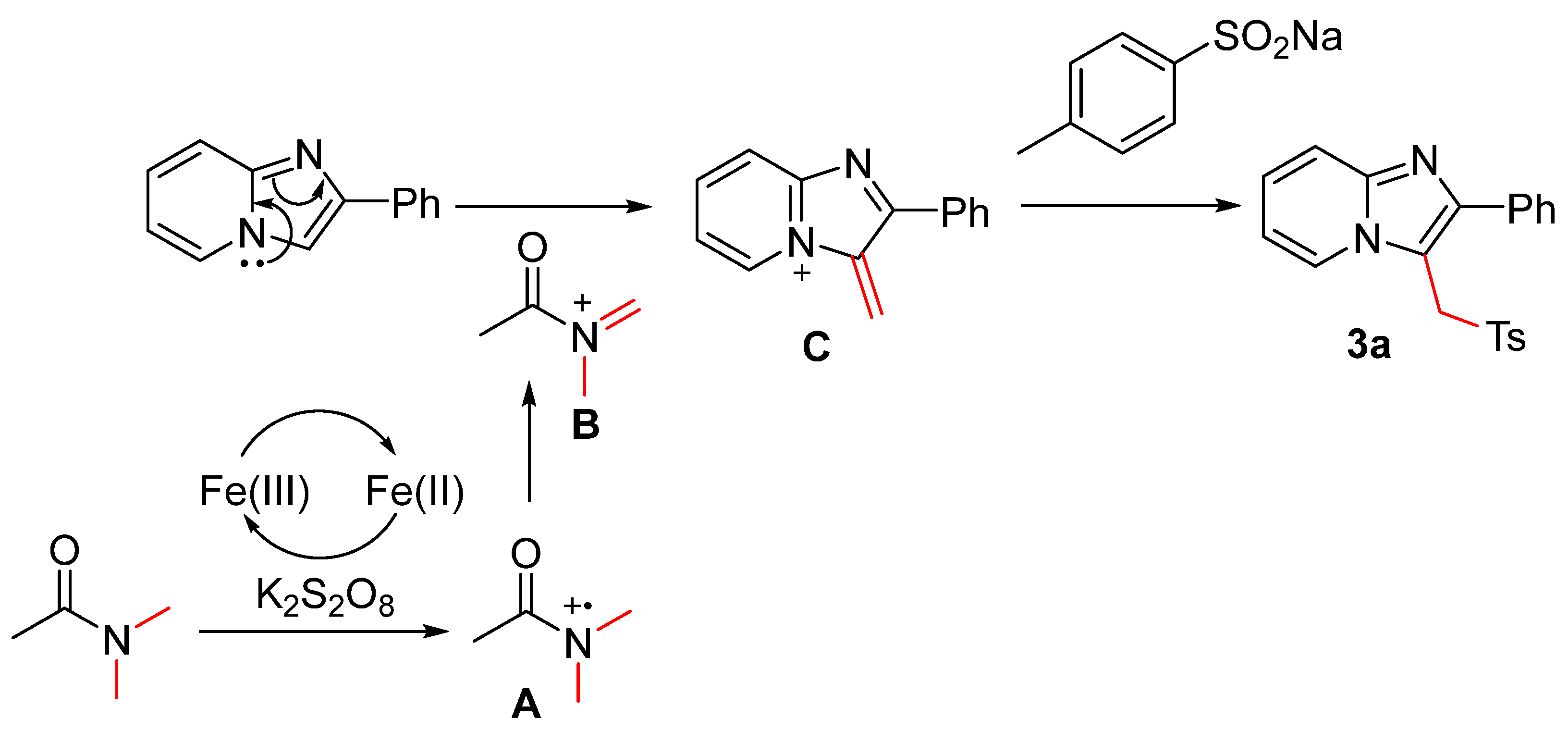
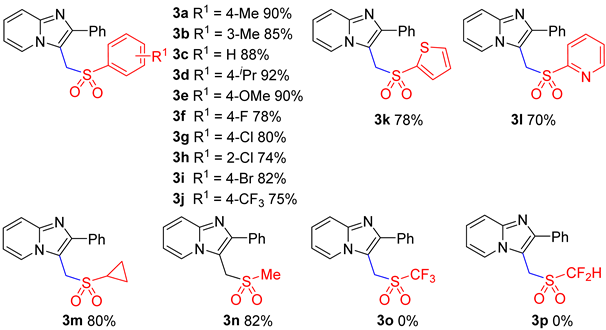
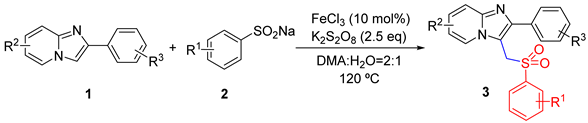
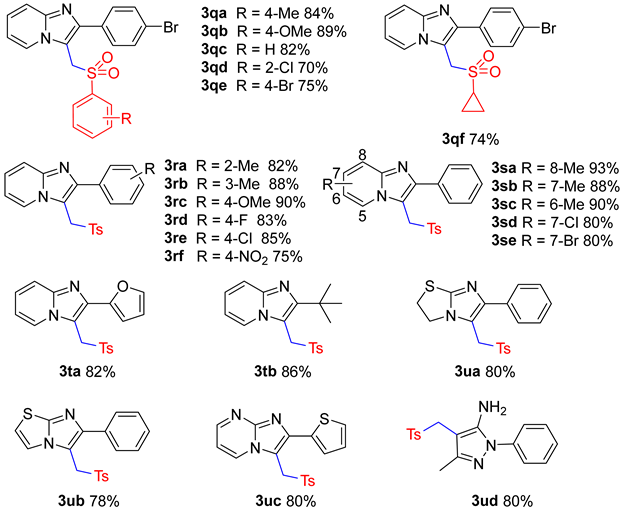
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gladysz, R.; Adriaenssens, Y.; De Winter, H.; Joossens, J.; Lambeir, A.-M.; Augustyns, K.; Van der Veken, P. Discovery and SAR of novel and selective inhibitors of urokinase plasminogen activator (uPA) with an imidazo[1,2-α]pyridine scaffold. J. Med. Chem. 2015, 58, 9238–9257. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.J.; Doweyko, A.M. Antiulcer agents. 6. Analysis of the in vitro biochemical and in vivo gastric antisecretory activity of substituted imidazo [1,2-α] pyridines and related analogues using comparative molecular field analysis and hypothetical active site lattice methodologies. J. Med. Chem. 1997, 40, 427–436. [Google Scholar] [PubMed]
- Chen, X.; Xu, W.; Wang, K.; Mo, M.; Zhang, W.; Du, L.; Yuan, X.; Xu, Y.; Wang, Y.; Shen, J. Discovery of a novel series of imidazo[1,2-α]pyrimidine derivatives as potent and orally bioavailable lipoprotein-associated phospholipase A2 inhibitors. J. Med. Chem. 2015, 58, 8529–8541. [Google Scholar] [CrossRef] [PubMed]
- Hamdouchi, C.; Blas, J.D.; Prado, M.D.; Gruber, J.; Heinz, B.A.; Vance, L. 2-Amino-3-substituted-6-[(E)-1-phenyl-2-(N-methylcarbamoyl) vinyl] imidazo [1,2-α] pyridines as a novel class of inhibitors of human rhinovirus: Stereospecific synthesis and antiviral activity. J. Med. Chem. 1999, 42, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Panda, M.; Mukherjee, K.; Choudhury, N.R.; Tantry, S.J.; Kedari, C.K.; Ramachandran, V.; Sharma, S.; Ramya, V.K.; Guptha, S.; et al. Synthesis and structure activity relationship of imidazo [1,2-α] pyridine-8-carboxamides as a novel antimycobacterial lead series. Bioorg. Med. Chem. Lett. 2013, 23, 4996–5001. [Google Scholar] [CrossRef] [PubMed]
- Baviskar, A.T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S.K.; Kundu, C.N.; Banerjee, U.C.; Bharatam, P.V. N-fused imidazoles as novel anticancer agents that inhibit catalytic activity of topoisomerase IIα and induce apoptosis in G1/S phase. J. Med. Chem. 2011, 54, 5013–5030. [Google Scholar] [CrossRef] [PubMed]
- Bode, M.L.; Gravestock, D.; Moleele, S.S.; van der Westhuyzen, C.W.; Pelly, S.C.; Steenkamp, P.A.; Hoppe, H.C.; Khan, T.; Nkabinde, L.A. Imidazo [1,2-α] pyridin-3-amines as potential HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2011, 19, 4227–4237. [Google Scholar] [CrossRef] [PubMed]
- Moraski, G.C.; Markley, L.D.; Cramer, J.; Hipskind, P.A.; Boshoff, H.; Bailey, M.A.; Alling, T.; Ollinger, J.; Parish, T.; Miller, M.J. Advancement of imidazo[1,2-α]pyridines with improved pharmacokinetics and nM activity vs. mycobacterium tuberculosis. ACS Med. Chem. Lett. 2013, 4, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Koubachi, J.; El Kazzouli, S.; Bousmina, M.; Guillaumet, G. Functionalization of imidazo [1,2-α] pyridines by means of metal-catalyzed cross-coupling reactions. Eur. J. Org. Chem. 2014, 2014, 5119–5123. [Google Scholar] [CrossRef]
- Cao, H.; Zhan, H.; Lin, Y.; Lin, X.; Du, Z.; Jiang, H. Direct arylation of imidazo [1,2-α] pyridine at C-3 with aryl iodides, bro-mides, and triflates via copper (I)-catalyzed C–H bond functionali-zation. Org. Lett. 2012, 14, 1688–1692. [Google Scholar] [CrossRef]
- Fu, H.Y.; Chen, L.; Doucet, H. Phosphine-free palladium-catalyzed direct arylation of imidazo [1,2-α] pyridines with aryl bromides at low catalyst loading. J. Org. Chem. 2012, 77, 4473–4478. [Google Scholar] [CrossRef] [PubMed]
- Choy, P.Y.; Luk, K.C.; Wu, Y.; So, C.M.; Wang, L.-L.; Kwong, F.Y. Regioselective direct C-3 arylation of imidazo[1,2-α]pyridines with aryl tosylates and mesylates promoted by palladium-phosphine complexes. J. Org. Chem. 2015, 80, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, D.; Deperasińska, I.; Vakuliuk, O.; Banasiewicz, M.; Tasior, M.; Makarewicz, A.; Cyrański, M.K.; Kozankiewicz, B.; Gryko, D.T. Double head-to-tail direct arylation as a viable strategy towards the synthesis of the aza-analog of dihydrocyclopenta [hi] aceanthrylene-an intriguing antiaromatic heterocycle. Chem. Commun. 2016, 52, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Naskar, A.; Mitra, S.; Hajra, A. Palladium-catalyzed α-selective alkenylation of imidazo[1,2-α]pyridines through aerobic cross-dehydrogenative coupling reaction. Eur. J. Org.Chem. 2015, 2015, 715–718. [Google Scholar] [CrossRef]
- Zhan, H.; Zhao, L.; Li, N.; Chen, L.; Liu, J.; Liao, J.; Cao, H. Ruthenium-catalyzed direct C-3 oxidative olefination of imidazo [1,2-α] pyridines. RSC Adv. 2014, 4, 32013–32016. [Google Scholar] [CrossRef]
- Cao, H.; Lei, S.; Liao, J.; Huang, J.; Qiu, H.; Chen, Q.; Qiu, S.; Chen, Y. Palladium (II)-catalyzed intermolecular oxidative C-3 alkenylations of imidazo [1,2-α] pyridines by substrate-contolled regioselective C-H functionalization. RSC Adv. 2014, 4, 50137–50140. [Google Scholar] [CrossRef]
- Monir, K.; Bagdi, A.K.; Ghosh, M.; Hajra, A. Regioselective oxidative trifluoromethylation of imidazoheterocycles via C(sp2)-H bond functionalization. J. Org. Chem. 2015, 80, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, H.-R.; Jin, R.-X.; Lan, Q.; Wang, X.-S. Nickel-catalyzed C-H trifluoromethylation of electron-rich heteroarenes. Adv. Synth. Catal. 2016, 358, 3528–3533. [Google Scholar] [CrossRef]
- Cao, H.; Lei, S.; Li, N.; Chen, L.; Liu, J.; Cai, H.; Qiu, S.; Tan, J. Cu-catalyzed selective C3-formylation of imidazo [1,2-α] pyridine C-H bonds with DMSO using molecular oxygen. Chem. Commun. 2015, 51, 1823–1825. [Google Scholar] [CrossRef]
- Mondal, S.; Samanta, S.; Jana, S.; Hajra, A. (Diacetoxy) iodobenzene-mediated oxidative C-H amination of imidazopyridines at ambient temperature. J. Org. Chem. 2017, 82, 4504–4510. [Google Scholar] [CrossRef]
- Lu, S.; Tian, L.-L.; Cui, T.-W.; Zhu, Y.-S.; Zhu, X.; Hao, X.-Q.; Song, M.-P. Copper-mediated C-H amination of imidazopyridines with N-fluorobenzenesulfonimide. J. Org. Chem. 2018, 83, 13991–14000. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.S.S.; Balaguez, R.A.; Franco, M.S.; Machado, V.C.S.; Saba, S.; Rafique, J.; Galetto, F.Z.; Braga, A.L. Trihaloisocyanuric acids in ethanol: An eco-friendly system for the regioselective halogenation of imidazo-heteroarenes. Green Chem. 2020, 22, 3410–3415. [Google Scholar] [CrossRef]
- Semwal, R.; Ravi, C.; Kumar, R.; Meena, R.; Adimurthy, S. Sodium salts (NaI/NaBr/NaCl) for the halogenation of imidazo-fused heterocycles. J. Org. Chem. 2019, 84, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wei, X.-N.; Zhang, M.; Liu, Y.; Zhu, L.-M.; Zhao, Y.-H. Catalyst and additive-free oxidative dual C-H sulfenylation of imidazoheterocycles with elemental sulfur using DMSO as a solvent and an oxidant. Chem. Commun. 2020, 56, 5751–5754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ding, Y.; Bian, Z.; Xie, P.; Xu, B.; Tang, Q.; Wu, W.; Zhou, A. One-pot three-component synthesis of alkylthio-/arylthio-substituted imidazo[1,2-α]pyridine derivatives via C(sp2)-H functionalization. Adv. Synth. Catal. 2017, 359, 2215–2221. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Berteina-Raboin, S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG 400. Green Chem. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, X.-J.; Li, K.; Guo, Y.-J.; Wang, M.-D.; Zhao, X.-M.; Hao, X.-Q.; Song, M.-P. Reactivity of p-toluenesulfonylmethyl isocyanide: Iron-involved C-H tosylmethylation of imidazopyridines in nontoxic media. J. Org. Chem. 2016, 81, 8370–8377. [Google Scholar] [CrossRef]
- Kalari, S.; Shinde, A.U.; Rode, H.B. Methylene-tethered arylsulfonation and benzotriazolation of aryl/heteroaryl C-H bonds with DMSO as a one-carbon surrogate. J. Org. Chem. 2021, 86, 17684–17695. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Wen, K.-M.; Wu, Y.-R.; Shi, J.; Yao, X.-G.; Tang, X.-D. Transition metal catalyst-free C-3 sulfonylmethylation of imidazo[1,2-α]pyridines with glyoxylic acid and sodium sulfinates in water. J. Org. Chem. 2022, 87, 3780–3787. [Google Scholar] [CrossRef]
- Lou, S.-J.; Xu, D.-Q.; Shen, D.-F.; Wang, Y.-F.; Liu, Y.-K.; Xu, Z.-Y. Highly efficient vinylaromatics generation via Iron-catalyzed sp3 C-H bond functionalization CDC reaction: A novel approach to preparing substituted benzo[α]phenazines. Chem. Commun. 2012, 48, 11993–11995. [Google Scholar] [CrossRef]
- Modi, A.; Ali, W.; Patela, B.K. N,N-dimethylacetamide (DMA) as a methylene synthon for regioselective linkage of imidazo[1,2-α]pyridine. Adv. Synth. Catal. 2016, 358, 2100–2107. [Google Scholar] [CrossRef]
- Kaswan, P.; Nandwana, N.K.; DeBoef, B.; Kumar, A. Vanadyl acetylacetonate catalyzed methylenation of imidazo[1,2-α]pyridines by using dimethylacetamide as a methylene source: Direct access to bis(imidazo[1,2-α]pyridin-3-yl)methanes. Adv. Synth. Catal. 2016, 358, 2108–2115. [Google Scholar] [CrossRef]
- Sun, S.-N.; Li, J.-C.; Gou, Y.-B.; Gao, Z.-H.; Bi, X.-J. Controllable synthesis of disulfides and thiosulfonates from sodium sulfinates mediated by hydroiodic acid using ethanol and H2O as solvents. Org. Biomol. Chem. 2022, 20, 8885–8892. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Tian, J.-H.; Wen, K.-M.; Gao, Q.-W.; Shi, J.; Yao, X.-G.; Wu, T.; Tang, X.-D. A new method for C (sp2)-H sulfonylmethylation with glyoxylic acid and sodium sulfinates. Org. Biomol. Chem. 2022, 20, 1652–1655. [Google Scholar] [CrossRef]

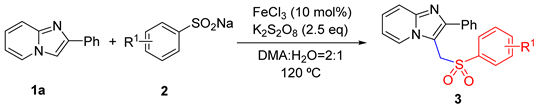
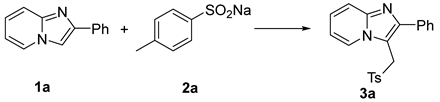 | ||||
| Entry | Catalyst/mol% | Oxidant/Equiv | Solvent | Yield/% b |
| 1 | - | - | DMA:H2O/2:1 | 0 |
| 2 | - | K2S2O8/2.5 | DMA:H2O/2:1 | 5 |
| 3 | FeCl3/20 | - | DMA:H2O/2:1 | 0 |
| 4 | CuI/20 | K2S2O8/2.5 | DMA:H2O/2:1 | 18 |
| 5 | Cu(acac)2/20 | K2S2O8/2.5 | DMA:H2O/2:1 | 18 |
| 6 | FeCl3/20 | K2S2O8/2.5 | DMA:H2O/2:1 | 73 |
| 7 | FeCl2/20 | K2S2O8/2.5 | DMA:H2O/2:1 | 67 |
| 8 | Fe3O4/20 | K2S2O8/2.5 | DMA:H2O/2:1 | 50 |
| 9 | V(acac)2/20 | K2S2O8/2.5 | DMA:H2O/2:1 | 63 |
| 10 | AgNO3/20 | K2S2O8/2.5 | DMA:H2O/2:1 | 6 |
| 11 | FeCl3/5 | K2S2O8/2.5 | DMA:H2O/2:1 | 41 |
| 12 | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 73 |
| 13 | FeCl3/30 | K2S2O8/2.5 | DMA:H2O/2:1 | 70 |
| 14 | FeCl3/10 | H2O2/2.5 | DMA:H2O/2:1 | 0 |
| 15 | FeCl3/10 | TBHP/2.5 | DMA:H2O/2:1 | 0 |
| 16 | FeCl3/10 | O2/air | DMA:H2O/2:1 | 0 |
| 17 | FeCl3/10 | I2O5/2.5 | DMA:H2O/2:1 | 0 |
| 18 | FeCl3/10 | K2S2O8/1.0 | DMA:H2O/2:1 | 57 |
| 19 | FeCl3/10 | K2S2O8/1.5 | DMA:H2O/2:1 | 64 |
| 20 | FeCl3/10 | K2S2O8/3.5 | DMA:H2O/2:1 | 73 |
| 21 | FeCl3/10 | K2S2O8/2.5 | DMA/10 eq | 0 |
| 22 | FeCl3/10 | K2S2O8/2.5 | DMA/20 eq | 0 |
| 23 | FeCl3/10 | K2S2O8/2.5 | DMA/50 eq | 43 |
| 24 | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/1:1 | 43 |
| 25 | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/5:1 | 70 |
| 26 c | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 80 |
| 27 d | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 90 |
| 28 e | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 90 |
| 29 d,f | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 60 |
| 30 d,g | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 70 |
| 31 d,h | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 93 |
| 32 d,i | FeCl3/10 | K2S2O8/2.5 | DMA:H2O/2:1 | 93 |
| 33 d,h | FeCl3/10 | K2S2O8/2.5 | DMF:H2O/2:1 | 33 |
| 34 d,h | FeCl3/10 | K2S2O8/2.5 | DMSO:H2O/2:1 | 0 |
| 35 d,h | FeCl3/10 | K2S2O8/2.5 | TMEDA:H2O/2:1 | 0 |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Ye, H.; Liu, H.; Li, J.; Bi, X. Iron-Catalyzed Sulfonylmethylation of Imidazo[1,2-α]pyridines with N,N-Dimethylacetamide and Sodium Sulfinates. Molecules 2024, 29, 3196. https://doi.org/10.3390/molecules29133196
Sun S, Ye H, Liu H, Li J, Bi X. Iron-Catalyzed Sulfonylmethylation of Imidazo[1,2-α]pyridines with N,N-Dimethylacetamide and Sodium Sulfinates. Molecules. 2024; 29(13):3196. https://doi.org/10.3390/molecules29133196
Chicago/Turabian StyleSun, Shengnan, Hexia Ye, Haibo Liu, Junchen Li, and Xiaojing Bi. 2024. "Iron-Catalyzed Sulfonylmethylation of Imidazo[1,2-α]pyridines with N,N-Dimethylacetamide and Sodium Sulfinates" Molecules 29, no. 13: 3196. https://doi.org/10.3390/molecules29133196
APA StyleSun, S., Ye, H., Liu, H., Li, J., & Bi, X. (2024). Iron-Catalyzed Sulfonylmethylation of Imidazo[1,2-α]pyridines with N,N-Dimethylacetamide and Sodium Sulfinates. Molecules, 29(13), 3196. https://doi.org/10.3390/molecules29133196






