Abstract
Heterocyclic aryl selenides have recently attracted considerable research interest owing to their applications in biological and pharmaceutical fields. Herein, we describe a simple and general synthesis of 3-selanylindoles via a novel regioselective C–H selenation of indoles using a bismuth reagent as a catalyst. The reactions of indoles with diselenides in the presence of 10 mol% BiI3 at 100 °C in DMF afforded the corresponding 3-selanylindoles in moderate-to-excellent yields. The reaction proceeded efficiently under aerobic conditions by adding only a catalytic amount of BiI3, which was non-hygroscopic and less toxic, and both selanyl groups of the diselenide were transferred to the desired products.
1. Introduction
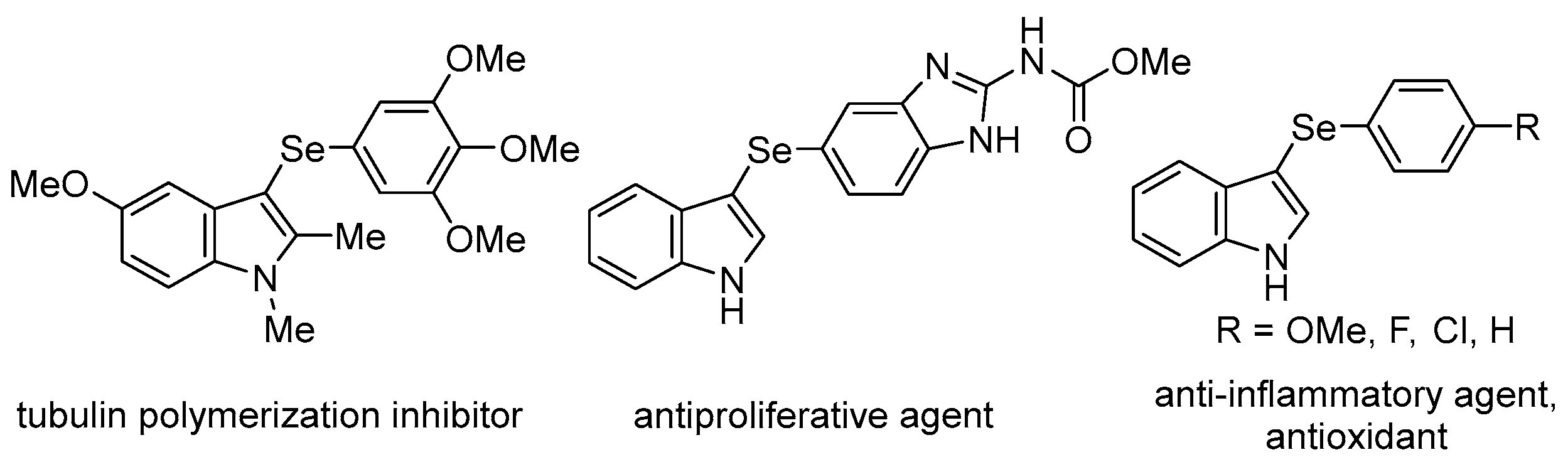
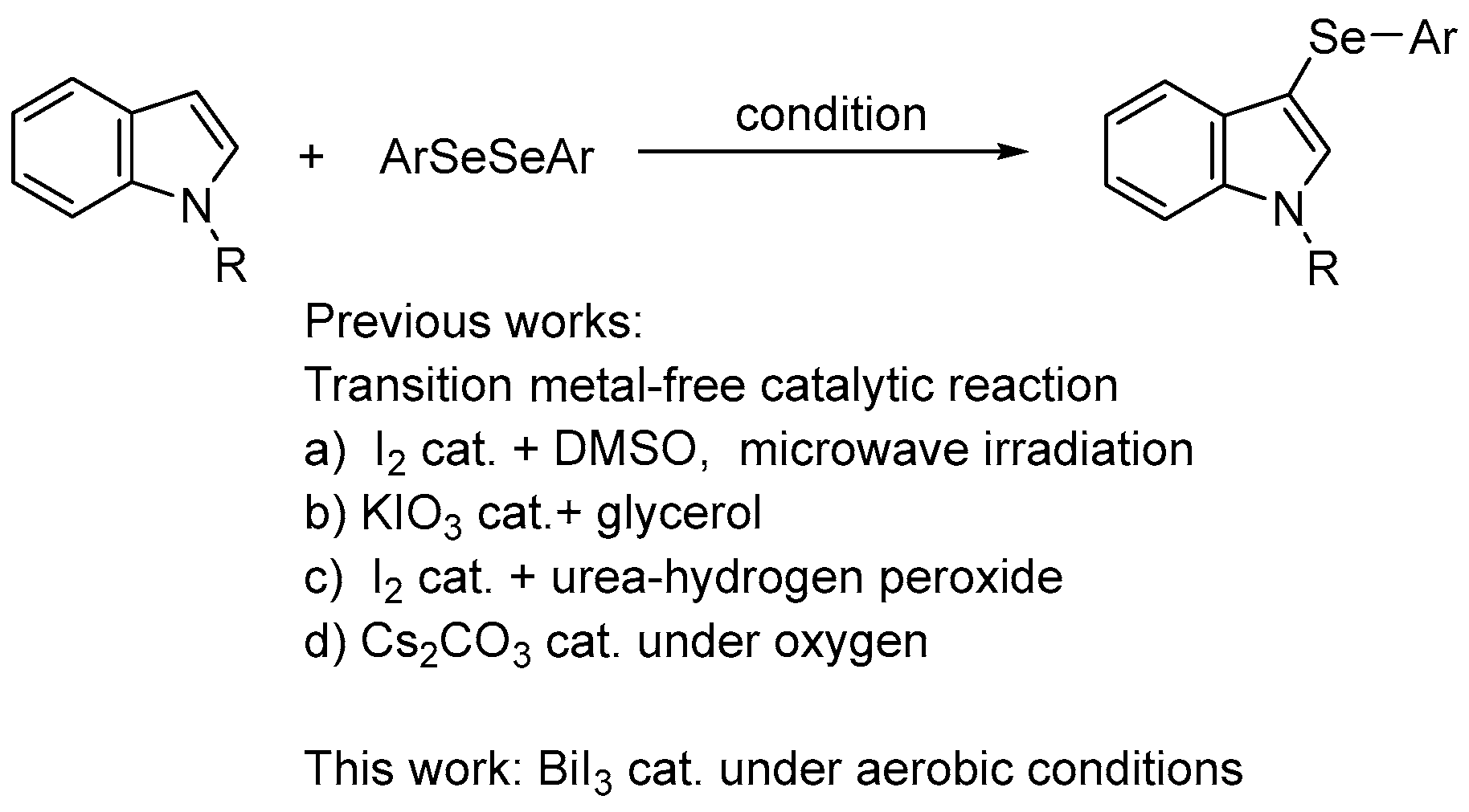
Organoselenium compounds have received considerable attention in organic chemistry, as well as in biological and pharmaceutical sciences [1,2,3,4,5,6,7,8,9,10,11,12,13,14], and there is growing interest in biologically active unsymmetrical diaryl selenides containing heterocyclic rings (i.e., aryl heteroaryl selenides). For example, 3-selanylindoles, compounds with a selenium side chain substituted at the 3-position of indoles, which are widely used as a basic skeleton in natural products and medicines, have been reported to have biological activities, such as the inhibition of tubulin polymerization, antiproliferative activity, anti-inflammatory properties, and antioxidant activity, and are expected to be used as drug discovery resources (Figure 1) [15,16,17,18,19,20]. Therefore, the development of synthetic methods for these compounds has attracted attention. Direct selenation into indoles has been reported since the 2010s and is a powerful and commonly used method involving the reaction of available indole derivatives with stable and easy-to-handle diselenides as selenium sources. These reactions can be broadly classified into those involving the addition of oxidants [21,22,23] or bases [24,25,26], radical reactions using photoreactors [27,28,29,30,31,32,33,34] or electrolytic devices [35,36], and those using transition metal catalysts containing Pd, Cu, Ag, and Fe [37,38,39,40,41,42]. However, these reactions use excessive reagents, additives, and transition metal catalysts of toxicological concern even in catalytic reactions, and require special equipment and expensive photocatalysts or supporting electrolytes for the photoreactions and electrolytic reactions, respectively. Recently, four transition metal-free catalytic reactions were reported (Scheme 1). Braga et al. developed a catalytic reaction using DMSO as the oxidant in the presence of a catalytic quantity of I2; however, the reaction required microwave irradiation [43]. The researchers also used KIO3 as a catalyst, but this reaction required an excess (4 equiv.) of glycerol [44]. Roehrs et al. reported an I2-catalyzed reaction that required the addition of stoichiometric amounts of urea hydrogen peroxide as an oxidant [45]. Jana et al. developed a reaction using Cs2CO3 as a catalyst, albeit in an oxygen atmosphere [46]. As mentioned above, catalytic reactions require additives; otherwise, the reaction conditions are restrictive.
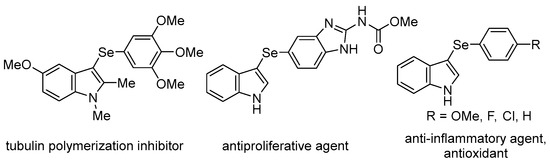
Figure 1.
Biologically active 3-selanylindoles.
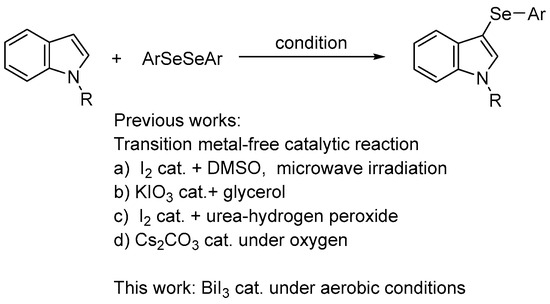
Scheme 1.
Selenation of indoles with diaryl diselenides.
Inorganic bismuth compounds have attracted attention in the field of organic synthesis since the 1980s because of their excellent reactivity as mild Lewis acids, nontoxicity, and environmental friendliness [47,48,49,50,51,52]. For example, BiCl3, a trivalent bismuth halide, has been reported to act as a catalyst for the following reactions: the Mukaiyama aldol reaction [53,54], the nucleophilic opening of epoxide [55], deoxygenative allylation [56], the Diels–Alder reaction [57,58], the three-component reaction of aldehydes, amines, and ketones or trimethylsilyl cyanide [59,60], the Friedel–Crafts reaction [61], the oxy-Michael addition [62], the aminooxygenation of propargyl amidine [63], and the tandem cyclization of tryptamine-ynamide [64]. More recently, BiCl3 has been utilized in the catalytic coupling reactions of aryl iodides or aminobenzimidazoles with arylboronic acids for C(Ar)–C(Ar) and C(Ar)–N bond formation [65,66]. By contrast, bismuth iodide (BiI3) is widely used in semiconductors and solar cell devices [67,68]. However, its chemical reactivity in organic reactions is largely unknown, and its use in catalytic reactions has been limited to the deprotection of acetals, guanylation with desulfurization using thioureas and amines, and S,S-acetalization of benzaldehyde [69,70,71]. Inspired by these reports, we present a facile Bi(III)-catalyzed regioselective C(Ar)–Se bond formation reaction of indoles with diaryl diselenides using BiI3 as the catalyst for the synthesis of 3-selanylindoles under mild conditions. The system was simple, containing only substrates and a Bi catalyst.
2. Results and Discussion
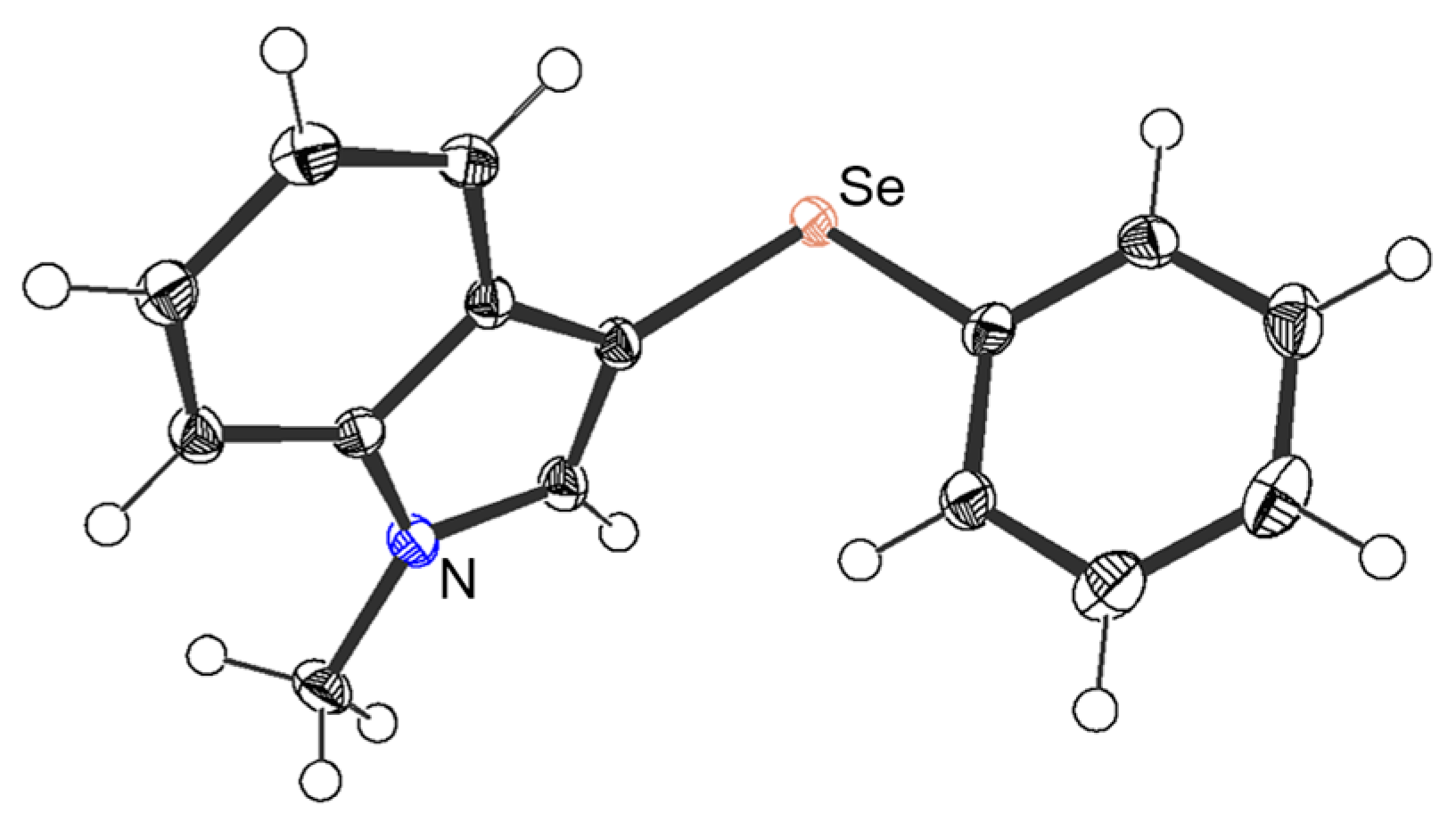
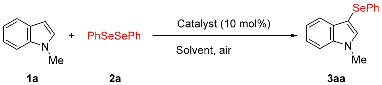
We initially focused on determining the optimal experimental conditions, including screening for suitable catalysts and solvents, for the synthesis of 3-selanylindole 3aa using N-methylindole 1a and diphenyl diselenide 2a as model substrates, the results of which, are summarized in Table 1. N-methylindole 1a (0.5 mmol) was reacted with 2a (0.25 mmol) in the presence of several Bi catalysts (0.05 mmol) in DMF at 100 °C under aerobic conditions (entries 1–7). BiCl3, BiBr3, BiI3, and Bi(OTf)3, which function as Lewis acids, afforded the corresponding 3-selanylindole 3aa in good-to-excellent yields (77–97%). BiI3 displayed the best yield and reaction time, and both selanyl groups were efficiently transferred from the diselenide to product 3aa (entry 3). Furthermore, although bismuth halides such as BiCl3 and BiBr3 are hygroscopic, BiI3 can be easily handled in air without such concerns. By contrast, antimony catalysts with the same group of atoms as bismuth and other Lewis acid catalysts were less effective than BiI3 (entries 8–12). A comparison to iodine (I2) was also attempted; however, the reaction barely progressed (entry 13). Solvent screening indicated that the reaction proceeded efficiently in DMF (97%), DMSO (89%), and THF (60%), whereas CH3CN, MeOH, dioxane, 1,2-DCE, and toluene were inefficient (entries 3 and 14–20). When the reaction was performed at 60 °C, the reaction time increased markedly to 8 h (entry 21). The reaction performed under oxygen produced 3aa in a high yield (94%), which was almost identical to that obtained under aerobic conditions (entries 3 and 22). However, the yield was notably suppressed (9%) under an argon atmosphere (entry 23). Decreasing the BiI3 loading from 10 to 5 and 1 mol% markedly prolonged the reaction time, although the reaction afforded the desired product (entries 24 and 25). The best result was obtained under aerobic conditions at 100 °C when 1a was treated with 0.5 equivalents of diselenide 2a in the presence of BiI3 (10 mol%) in DMF (entry 3). This selenation could also be scaled up to 10 mmol. The desired product 3aa was obtained in an excellent yield (99%), generating up to 2.84 g of the product. Furthermore, the reaction of 1a and 2a with 1 equivalent of TEMPO [(2,2,6,6-tetramethylpiperidin-1-yl)oxyl] or 1,1-diphenylethylene as radical scavengers afforded 3aa in yields of 94% and 96%, respectively (entries 26 and 27). These results indicate that the reaction system does not follow a radical mechanism. The regiochemistry of 3-selanylindole 3aa was elucidated using 1H-NMR and single-crystal X-ray analyses (Figure 2). The 1H-NMR spectrum of 3aa was consistent with that of the standard sample [41].

Table 1.
Optimization of the reaction conditions [a].
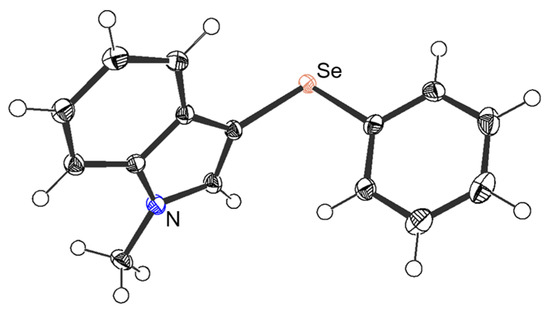
Figure 2.
ORTEP drawing of 3aa with 50% probability (CCDC 2291058).
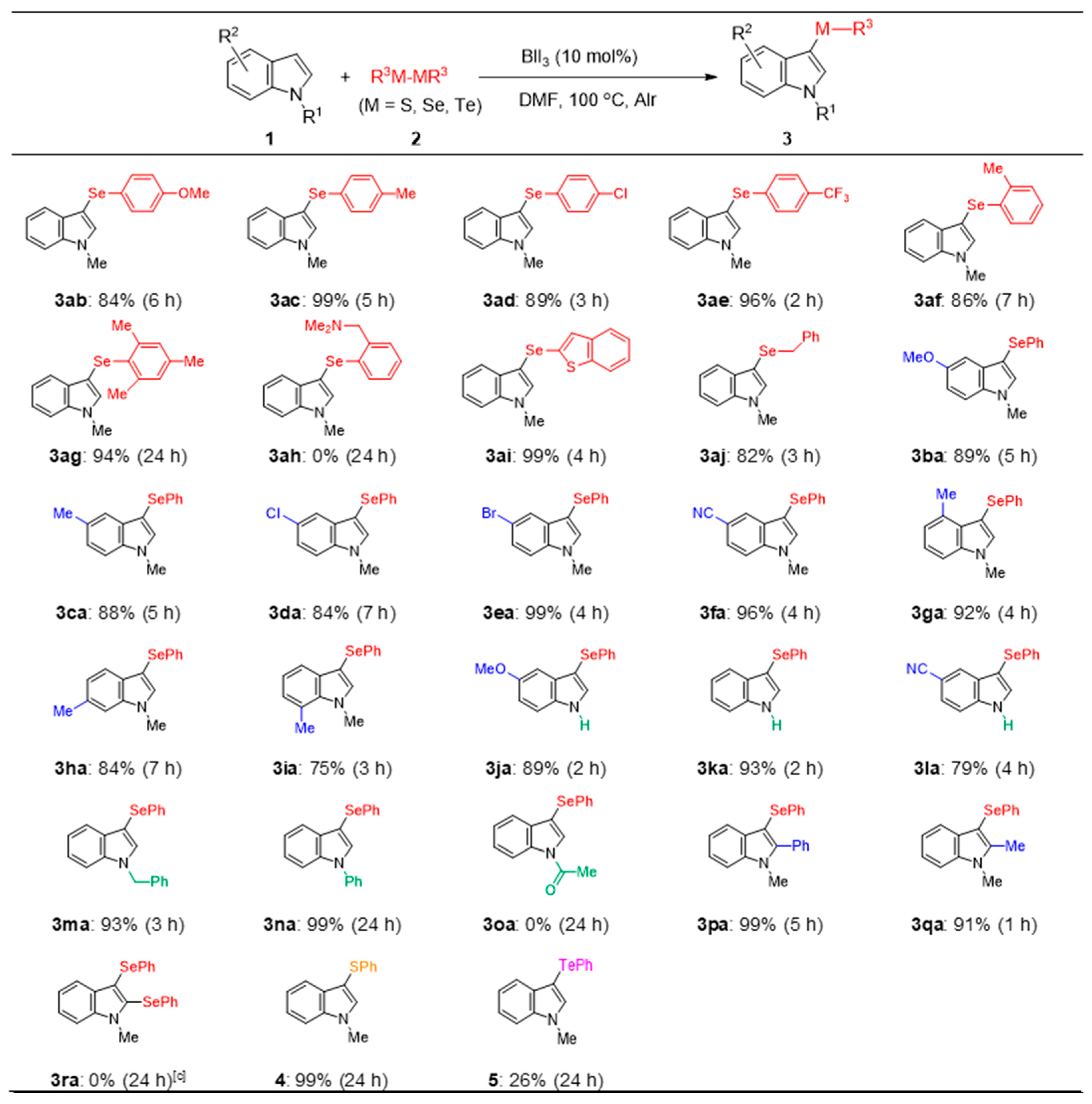
To understand the scope and limitations of the developed regioselective selenation reaction, various indoles 1 (0.5 mmol) were reacted with diselenides 2 (0.25 mmol) under the optimized conditions (Figure 3). The reaction of N-methylindole 1a with diaryl diselenides 2b–i afforded the corresponding products, i.e., 3ab–ai, in good-to-excellent yields, except for 3ah. For 3ab–ae, the presence of an electron-donating or electron-withdrawing group at the 4-position of the benzene ring of diselenides 3b–e did not affect the reaction progression, although the reaction time was slightly prolonged when electron-donating groups were substituted. Sterically hindered ortho-substituted diselenides 2f and 2g reacted to give selenides 3af and 3ag, respectively. By contrast, for 2h, which comprises a benzylamino group, the reaction did not proceed, and the starting materials were recovered. For the reaction using diaryl diselenide 2i, which bears a heterocyclic ring, 3ai was afforded in a good yield. Dibenzyl diselenide 2j, which contains a benzyl moiety as the alkyl group, also afforded 3aj in a good yield (82%). Next, the reaction of diphenyl diselenide 2a with various N-methylindoles, i.e., 1b–i, bearing electron-donating or electron-withdrawing groups on the benzene ring afforded the desired products 3ba–3ia in satisfactory yields (75–99%). The reaction proceeded smoothly from the unsubstituted indoles 1j–l to obtain the parent 3-selanylindoles 3ja–la (79–93%). Furthermore, the reaction of the N-substituted indoles 1m and 1n with benzyl or phenyl groups on the nitrogen also gave the corresponding products 3ma and 3na; however, N-acetylindole 1o with an electron-withdrawing group did not give 3oa, and the starting materials were recovered. These results suggest that the reaction is electrically influenced by the substituents on the indole nitrogen. 2-Phenyl- and 2-methylindoles 1p and 1q were treated with 2a to afford the 3-selanyl-2-substituted indoles 3pa and 3qa, respectively. The attempted double selenation of 1a using two equivalents of diphenyl diselenide 2a did not yield the corresponding 2,3-diselanylindole 3ra; instead, 3-selanylindole 3aa was isolated in a yield of 98%. These results suggest that this reaction proceeds only at the 3-position of the indole. Finally, the reaction of 1a with dichalcogenides containing sulfur and tellurium was attempted. The reaction with diphenyl disulfide afforded the desired 3-sulfanylindole 4 in an excellent yield (99%), although the reaction time (24 h) was longer than that with diselenide, which is a selenium reagent. By contrast, the reaction proceeded to a certain extent with diphenyl ditelluride, and indole 5 was obtained in a yield of 26%.
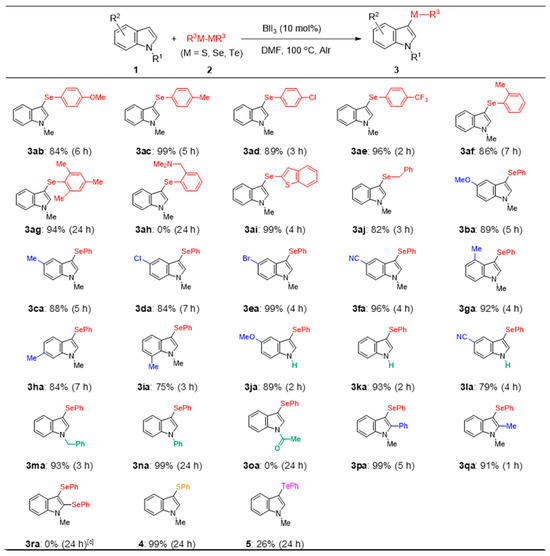
Figure 3.
Substrate scope: reaction of indoles with dichalcogenides [a,b]. [a] 1 (0.5 mmol), 2 (0.25 mmol), BiI3 (0.05 mmol), and DMF (2 mL). [b] Yield of isolated products. [c] 2a (0.5 mmol); 3aa was isolated in a yield of 98%.
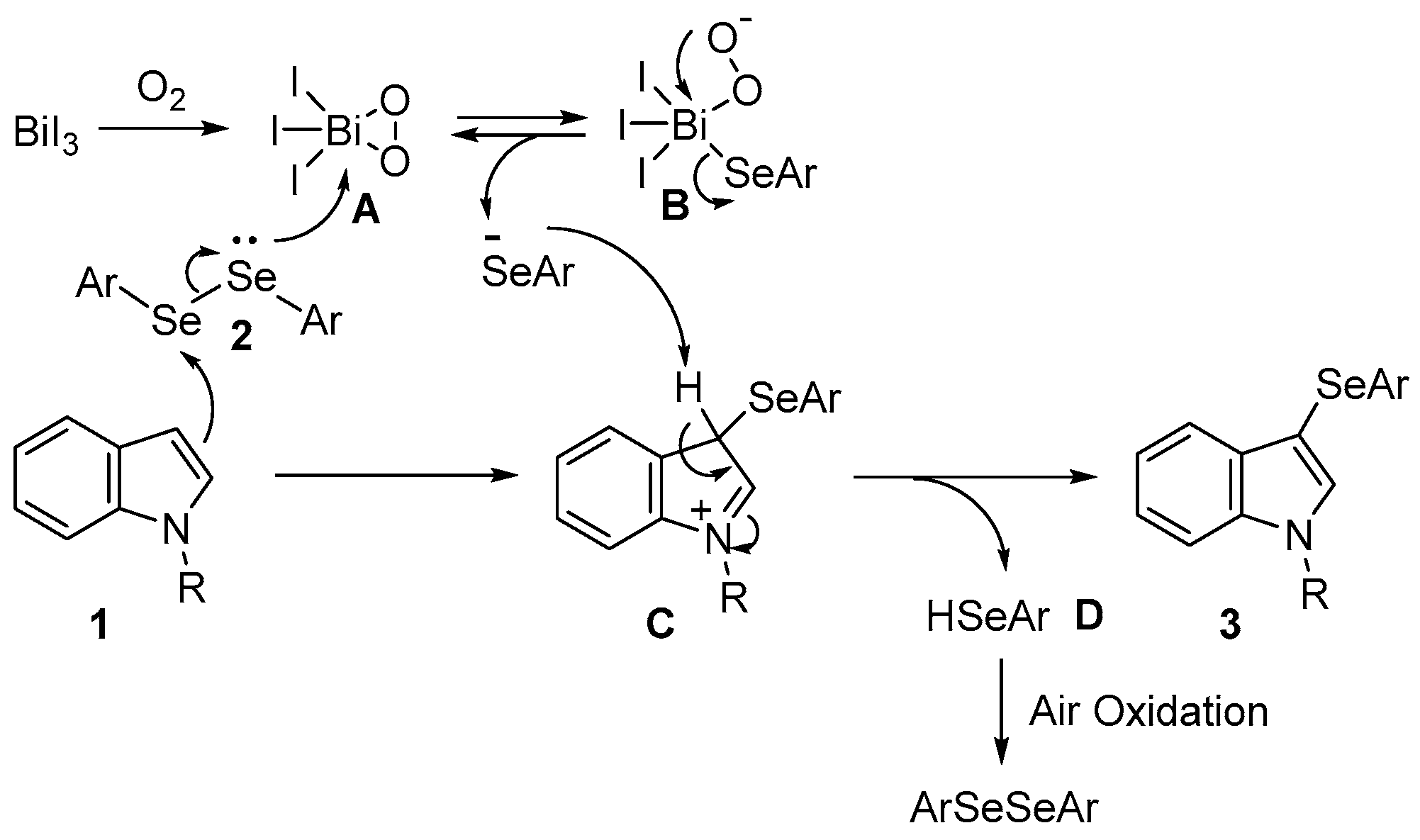
However, the reaction mechanism for this selenation remains unclear. Circumstantial evidence indicates that the reaction was affected by the gaseous atmosphere and proceeded smoothly in the presence of a molecular oxygen atmosphere while being notably suppressed in an inert gas atmosphere (Table 1: entries 3, 22, and 23). BiI3 forms a pentacoordinated complex with bismuth, the central atom, and the oxygen atoms of reagents and solvents such as Mo8O26 and THF [72,73]. Therefore, a possible mechanism for this reaction is illustrated in Scheme 2. The initial step was the generation of the pentacoordinated Bi–peroxo complex A from BiI3 and oxygen. While the selenium atom of the diselenide coordinates with complex A, the 3-position of the indole nucleophilically attacks another selenium atom, forming complex B and intermediate C. The aryl selenide anion formed during the interconversion between complexes B and A attacks intermediate C to form 3-selanylindole 3 and selenol D. Selenol D is converted to diselenide 2 via oxidation in air. Therefore, the reaction proceeds with 0.5 equivalents of diselenide, and both selanyl groups are used for the reaction. Bismuth complexes A and B, which are expected to form during this process, have not yet been confirmed or isolated.
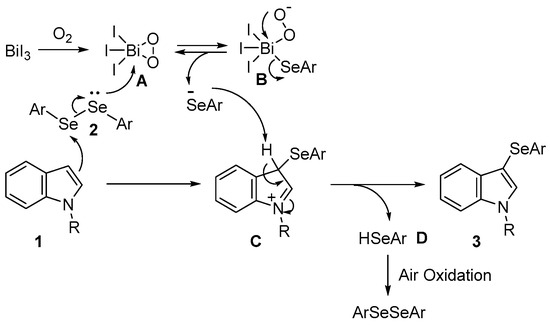
Scheme 2.
Possible mechanism.
3. Conclusions
Herein, we report a simple Bi-catalyzed regioselective selenation protocol for the synthesis of 3-selanylindoles under mild reaction conditions. The reaction is atom-economical, with the participation of both selanyl groups of the diaryl diselenide. Indoles and diselenides bearing different functional groups afforded the corresponding products in satisfactory yields. This reaction is the first example of the Bi-catalyzed C–H selenation of aromatic heterocycles. Detailed studies on the exact mechanism of this reaction and the synthesis of asymmetric selenides containing other heterocyclic rings using this protocol are currently underway.
4. Materials and Methods
4.1. General Information
All the chemicals, including organic solvents, were obtained from commercial vendors and used as received without further purification. All chromatographic separations were accomplished with Silica Gel 60N (Kanto Chemical Co., Inc., Tokyo, Japan). Thin-layer chromatography (TLC) was performed using Macherey–Nagel Pre-coated TLC plates Sil G25 UV254. Melting point measurements were conducted on a Yanagimoto micro-melting point hot-stage apparatus (MP-S3) and reported as uncorrected values. In addition, 1H NMR (TMS: δ = 0.00 ppm as an internal standard), 13C NMR (CDCl3: δ = 77.00 ppm as an internal standard), 19F NMR (376 MHz, benzotrifluoride; δ = −64.0 ppm as an external standard), and 77Se NMR (76 MHz, diphenyldiselenide; δ = 463.15 ppm as an external standard) spectra were recorded on JEOL ECZ-400S (400, 100, 376, and 76 MHz for 1H-, 13C-, 19F-, and 77Se NMR, respectively) spectrometers (JEOL Ltd., Tokyo, Japan). GC-MS (EI) spectra were recorded on Agilent 5977 E Diff-SST MSD-230 V spectrometer. HRMS (ESI) spectra were recorded on Agilent 6230 (Agilent Technologies Japan, Ltd., Tokyo, Japan). X-ray measurements were recorded on Rigaku XtaLAB Synergy with a HyPix3000 diffractometer (Rigaku, Corp., Tokyo, Japan). IR spectra were recorded on an FTIR-8400S or IRAffinity-1S system from a Shimadzu spectrometer (SHIMADZU Corp, Kyoto, Japan) and are reported as the frequencies of absorption (cm−1). Only selected IR absorbencies are reported. The spectroscopic data of the calcogenated indoles 3aa–ad, 3ag, 3ka, 3ma, 3qa, 4 [41], 3aj [74], 3ba [42], 3ea, 3ja, 3na [35], 3la [27], 3pa [75], and 5 [76] are in accordance with those in the literature, and their characterization data are in Supplementary Materials.
4.2. General Procedure for the Synthesis of Calcogenated Indoles
The indole derivative (1) (0.5 mmol) was added to a solution of dichalcogenide (2) (0.25 mmol, 0.5 eq.) and bismuth(III) iodide (30 mg, 0.05 mmol, and 10 mol%) in anhydrous dimethylformamide (2 mL). After stirring at 100 °C in an oil bath, the mixture was cooled to room temperature and evaporated to dryness under reduced pressure. The crude product was purified on a silica gel column chromatography to give the desired product 3.
4.3. Characterization Data of Novel Compounds
4.3.1. 3-(4-Trifluoromethylphenyl)selanyl-1-methyl-1H-indole (3ae)
Yield: 171 mg (96%); Colorless prism (from CH2Cl2-Hexane); m.p. 133.0–135.0 °C; Rf = 0.54 (CH2Cl2-Hexane, 1:2). 1H NMR (400 MHz, CDCl3): δ = 7.57 (d, J = 7.8 Hz, 1H; Ar-H), 7.41 (d, J = 8.2 Hz, 1H; Ar-H), 7.36 (s, 1H; Ar-H), 7.35–7.30 (m, 3H; Ar-H), 7.26 (d, J = 8.2 Hz, 2H; Ar-H), 7.19 (td, J = 8.2, 0.9 Hz, 1H; Ar-H), 3.88 ppm (s, 3H; N-CH3). 13C NMR (100 MHz, CDCl3): δ = 140.0 (C), 137.5 (C), 135.9 (CH), 130.4 (C), 128.0 (CH), 127.5 (q, J = 32 Hz, C), 125.5 (q, J = 3.9 Hz, CH), 124.2 (q, J = 272 Hz, C), 122.7 (CH), 120.7 (CH), 120.2 (CH), 109.7 (CH), 94.6 (C), 33.2 ppm (CH3). 19F NMR (376 MHz, CDCl3): δ = −63.7 ppm. 77Se NMR (76 MHz, CDCl3): δ = 223.4 ppm. IR (ATR): ν~ = 739, 822, 1072, 1105, 1321 cm−1. MS (EI, 70 eV): m/z (%) = 355 (21) [M]+, 275 (100), 130 (14). HRMS (ESI): m/z calcd for C16H12F3NSe: 355.0087 [M]+; found: 355.0088.
4.3.2. 1-Methyl-3-(2-methylphenyl)selanyl-1H-indole (3af)
Yield: 129 mg (86%); Colorless plate (from CH2Cl2-Hexane); m.p. 129.0–132.0 °C; Rf = 0.20 (CH2Cl2-Hexane, 1:5). 1H NMR (400 MHz, CDCl3): δ = 7.59 (d, J = 7.8 Hz, 1H; Ar-H), 7.39 (d, J = 8.2 Hz, 1H; Ar-H), 7.31 (s, 1H; Ar-H), 7.30 (td, J = 8.2, 0.9 Hz, 1H; Ar-H), 7.17 (td, J = 7.3, 0.9 Hz, 1H; Ar-H), 7.10 (d, J = 7.3 Hz, 1H; Ar-H), 7.00 (td, J = 7.8, 2.3 Hz, 1H; Ar-H), 6.86–6.80 (m, 2H; Ar-H), 3.85 (s, 3H; N-CH3), 2.46 ppm (s, 3H; CH3). 13C NMR (100 MHz, CDCl3): δ = 137.6 (C), 135.92 (C), 135.87 (CH), 134.8 (C), 130.8 (C), 129.7 (CH), 127.8 (CH), 126.4 (CH), 125.2 (CH), 122.4 (CH), 120.5 (CH), 120.4 (CH), 109.5 (CH), 94.9 (C), 33.1 (CH3), 21.2 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 178.3 ppm. IR (ATR): ν~ = 411, 426, 729, 746, 1456 cm−1. MS (EI, 70 eV): m/z (%) = 301 (40) [M]+, 221 (60), 131 (100), 91 (30). HRMS (ESI): m/z calcd for C16H15NSe: 301.0370 [M]+; found: 301.0370.
4.3.3. 3-(2-Benzothienyl)selanyl-1-methyl-1H-indole (3ai)
Yield: 171 mg (99%); Yellow needle (from CH2Cl2-Hexane); m.p. 144.0–147.0 °C; Rf = 0.21 (CH2Cl2-Hexane, 1:5). 1H NMR (400 MHz, CDCl3): δ = 7.78 (d, J = 7.8 Hz, 1H; Ar-H), 7.62 (d, J = 6.9 Hz, 1H; Ar-H), 7.60 (d, J = 7.3 Hz, 1H; Ar-H), 7.39 (s, 1H; Ar-H), 7.36 (d, J = 7.8 Hz, 1H; Ar-H), 7.32–7.16 (m, 5H; Ar-H), 3.83 ppm (s, 3H; N-CH3). 13C NMR (100 MHz, CDCl3): δ = 142.2 (C), 140.5 (C), 137.2 (C), 135.1 (CH), 132.1 (C), 130.3 (C), 126.3 (CH), 124.1 (CH), 123.5 (CH), 122.51 (CH), 122.47 (CH), 121.5 (CH), 120.5 (CH), 120.3 (CH), 109.6 (CH), 97.1 (C), 33.1 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 179.9 ppm. IR (ATR): ν~ = 426, 486, 556, 723, 735, 1236 cm−1. MS (EI, 70 eV): m/z (%) = 343 (14) [M]+, 263 (100), 207 (21), 131 (43), 89 (21), 44 (29). HRMS (ESI): m/z calcd for C17H13NSSe: 342.9934 [M]+; found: 342.9932.
4.3.4. 1,5-Dimethyl-3-phenylselanyl-1H-indole (3ca)
Yield: 133 mg (88%); Colorless plate (from CH2Cl2-Hexane); m.p. 104.0–105.0 °C; Rf = 0.58 (CH2Cl2-Hexane, 1:5). 1H NMR (400 MHz, CDCl3): δ = 7.41 (s, 1H; Ar-H), 7.27–7.20 (m, 4H; Ar-H), 7.14–7.05 (m, 4H; Ar-H), 3.81 (s, 3H; N-CH3), 2.43 ppm (s, 3H; CH3). 13C NMR (100 MHz, CDCl3): δ = 135.83 (C), 135.76 (CH), 134.5 (C), 130.9 (C), 129.8 (C), 128.9 (CH), 128.3 (CH), 125.4 (CH), 124.1 (CH), 120.0 (CH), 109.2 (CH), 95.0 (C), 33.1 (CH3), 21.4 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 206.4 ppm. IR (ATR): ν~ = 424, 457, 731, 793, 1474, 1506 cm−1. MS (EI, 70 eV): m/z (%) = 301 (20) [M]+, 221 (100), 144 (13). HRMS (ESI): m/z calcd for C16H15NSe: 301.0370 [M]+; found: 301.0369.
4.3.5. 5-Chloro-1-methyl-3-phenylselanyl-1H-indole (3da)
Yield: 135 mg (84%); Colorless plate (from CH2Cl2-Hexane); m.p. 132.0–133.5 °C; Rf = 0.54 (CH2Cl2-Hexane, 1:5). 1H NMR (400 MHz, CDCl3): δ = 7.60 (s, 1H; Ar-H), 7.33 (s, 1H; Ar-H), 7.28–7.19 (m, 4H; Ar-H), 7.15–7.08 (m, 3H; Ar-H), 3.82 ppm (s, 3H; N-CH3). 13C NMR (100 MHz, CDCl3): δ = 136.9 (CH), 135.9 (C), 133.7 (C), 131.9 (C), 129.0 (CH), 128.6 (CH), 126.5 (C), 125.7 (CH), 122.8 (CH), 119.9 (CH), 110.7 (CH), 95.7 (C), 33.3 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 209.6 ppm. IR (ATR): ν~ = 422, 457, 689, 733, 795, 1422, 1474 cm−1. MS (EI, 70 eV): m/z (%) = 321 (25) [M]+, 241 (100), 164 (15). HRMS (ESI): m/z calcd for C15H12ClNSe: 320.9823 [M]+; found: 320.9825.
4.3.6. 1-Methyl-3-phenylselanyl-1H-indole-5-carbonitrile (3fa)
Yield: 149 mg (96%); Colorless prism (from CH2Cl2-Hexane); m.p. 198.0–199.5 °C; Rf = 0.34 (CH2Cl2-Hexane, 1:1). 1H NMR (400 MHz, CDCl3): δ = 7.95 (d, J = 1.4 Hz, 1H; Ar-H), 7.49 (dd, J = 8.7, 1.4 Hz, 1H; Ar-H), 7.45 (s, 1H; Ar-H), 7.41 (d, J = 8.7 Hz, 1H; Ar-H), 7.23–7.20 (m, 2H; Ar-H), 7.17–7.12 (m, 3H; Ar-H), 3.88 ppm (s, 3H; N-CH3). 13C NMR (100 MHz, CDCl3): δ = 139.0 (C), 137.6 (CH), 132.8 (C), 130.5 (C), 129.11 (CH), 129.07 (CH), 126.14 (CH), 126.08 (CH), 125.4 (CH), 120.4 (C), 110.5 (CH), 103.6 (C), 97.9 (C), 33.3 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 212.5 ppm. IR (ATR): ν~ = 461, 474, 631, 692, 741 cm−1. MS (EI, 70 eV): m/z (%) = 312 (17) [M]+, 232 (100), 155 (16). HRMS (ESI): m/z calcd for C16H12N2Se: 312.0166 [M]+; found: 312.0166.
4.3.7. 1,4-Dimethyl-3-phenylselanyl-1H-indole (3ga)
Yield: 138 mg (92%); Colorless plate (from CH2Cl2-Hexane); m.p. 84.0–85.0 °C; Rf = 0.25 (CH2Cl2-Hexane, 1:5). 1H NMR (400 MHz, CDCl3): δ = 7.29 (s, 1H; Ar-H), 7.24–7.18 (m, 4H; Ar-H), 7.15–7.11 (m, 2H; Ar-H), 7.07 (tt, J = 6.9, 1.4 Hz, 1H; Ar-H), 6.89 (d, J = 7.3 Hz, 1H; Ar-H), 3.80 (s, 3H; N-CH3), 2.69 ppm (s, 3H; CH3). 13C NMR (100 MHz, CDCl3): δ = 137.9 (C), 136.8 (CH), 136.4 (C), 132.3 (C), 129.0 (CH), 127.9 (CH), 125.2 (CH), 122.4 (CH), 122.0 (CH), 107.5 (CH), 94.6 (C), 33.1 (CH3), 18.7 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 251.5 ppm. IR (ATR): ν~ = 457, 667, 689, 727, 739, 1474 cm−1. MS (EI, 70 eV): m/z (%) = 301 (33) [M]+, 221 (100), 144 (44). HRMS (ESI): m/z calcd for C16H15NSe: 301.0370 [M]+; found: 301.0369.
4.3.8. 1,6-Dimethyl-3-phenylselanyl-1H-indole (3ha)
Yield: 126 mg (84%); Colorless plate (from CH2Cl2-Hexane); m.p. 86.0–87.5 °C; Rf = 0.45 (CH2Cl2-Hexane, 1:5). 1H NMR (400 MHz, CDCl3): δ = 7.50 (d, J = 8.2 Hz, 1H; Ar-H), 7.26–7.22 (m, 3H; Ar-H), 7.18 (s, 1H; Ar-H), 7.14–7.06 (m, 3H; Ar-H), 7.01 (d, J = 7.8 Hz, 1H; Ar-H), 3.80 (s, 3H; N-CH3), 2.52 ppm (s, 3H; CH3). 13C NMR (100 MHz, CDCl3): δ = 137.8 (C), 135.1 (CH), 134.3 (C), 132.4 (C), 128.9 (CH), 128.5 (C), 128.4 (CH), 125.4 (CH), 122.1 (CH), 120.1 (CH), 109.5 (CH), 95.6 (C), 32.9 (CH3), 21.8 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 209.3 ppm. IR (ATR): ν~ = 430, 598, 689, 729, 797 cm−1. MS (EI, 70 eV): m/z (%) = 301 (19) [M]+, 221 (100), 144 (17). HRMS (ESI): m/z calcd for C16H15NSe: 301.0370 [M]+; found: 301.0371.
4.3.9. 1,7-Dimethyl-3-phenylselanyl-1H-indole (3ia)
Yield: 113 mg (75%); Colorless plate (from CH2Cl2-Hexane); m.p. 110.0–111.0 °C; Rf = 0.34 (CH2Cl2-Hexane, 1:5). 1H NMR (400 MHz, CDCl3): δ = 7.46 (d, J = 7.8 Hz, 1H; Ar-H), 7.24–7.20 (m, 3H; Ar-H), 7.13–7.05 (m, 3H; Ar-H), 7.01 (t, J = 7.3 Hz, 1H; Ar-H), 6.96 (d, J = 6.9 Hz, 1H; Ar-H), 4.08 (s, 3H; N-CH3), 2.79 ppm (s, 3H; CH3). 13C NMR (100 MHz, CDCl3): δ = 137.2 (CH), 136.1 (C), 134.2 (C), 131.8 (C), 128.9 (CH), 128.5 (CH), 125.4 (CH), 125.1 (CH), 121.5 (C), 120.6 (CH), 118.7 (CH), 95.7 (C), 37.0 (CH3), 19.6 ppm (CH3). 77Se NMR (76 MHz, CDCl3): δ = 208.8 ppm. IR (ATR): ν~ = 689, 733, 748, 781, 1450 cm−1. MS (EI, 70 eV): m/z (%) = 301 (18) [M]+, 221 (100), 144 (17). HRMS (ESI): m/z calcd for C16H15NSe: 301.0370 [M]+; found: 301.0370.
4.4. Single-Crystal X-ray Diffraction Experiment of 3aa
A suitable crystal was selected and measured on an XtaLAB Synergy, Single source at home/near, HyPix3000 diffractometer. The crystal was kept at 103 K in an N2 cold stream during data collection. Using Olex2 [77], the structure was solved with the SHELXT [78] structure solution program using Intrinsic Phasing and refined with the SHELXL [79] refinement package using Least Squares minimization. Crystal Data for 3aa: C15H13NSe (M = 286.22 g/mol), monoclinic, space group P21/n (no. 14), a = 7.73810(10) Å, b = 9.03610(10) Å, c = 18.1620(2) Å, β = 101.9160(10)°, V = 1242.56(3) Å3, Z = 4, T = 103 K, μ(Cu Kα) = 3.873 mm−1, Dcalc = 1.530 g/cm3, 6253 reflections measured (9.954° ≤ 2Θ ≤ 136.378°), and 2263 unique reflections (Rint = 0.0255 and Rsigma = 0.0218), which were used in all calculations. The final R1 was 0.0243 (I > 2σ(I)), and wR2 was 0.0652 (all data).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29133227/s1. The characterization data of known compounds and 1H- and 13C-NMR spectra are available online. The crystal structures have been deposited to the CCDC with the number 2291058, and the CIF files are also provided.
Author Contributions
All authors contributed to the writing and gave approval for the final version of the manuscript. M.M. and S.Y. designed chemical synthesis, analyzed results, and wrote the manuscript. M.M., A.U. and Y.S. performed chemical synthesis experiments and analyzed the results. Y.M. and N.A. analyzed the results and wrote the manuscript. M.M. performed single-crystal X-ray diffraction analysis and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a research grant from the Institute of Pharmaceutical Life Sciences, Aichi Gakuin University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in the manuscript and Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef] [PubMed]
- Rampon, D.S.; Luz, E.Q.; Lima, D.B.; Balaguez, R.A.; Schneider, P.H.; Alves, D. Transition Metal Catalysed Direct Selanylation of Arenes and Heteroarenes. Dalton Trans. 2019, 48, 9851–9905. [Google Scholar] [CrossRef]
- Hellwig, P.S.; Peglow, T.J.; Penteado, F.; Bagnoli, L.; Perin, G.; Lenardão, E.J. Recent Advances in the Synthesis of Selenophenes and Their Derivatives. Molecules 2020, 25, 5907. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.E.; Kanchana, U.S.; Mathew, T.V.; Anilkumar, G. Recent Developments and Perspectives in the C-Se Cross Coupling Reactions. Curr. Org. Chem. 2020, 24, 1230–1262. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Ninomiya, M.; Koketsu, M. Diorganyl Diselenides: A Powerful Tool for the Construction of Selenium Containing Scaffolds. Dalton Trans. 2021, 50, 12764–12790. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Li, Z.; Bi, L.; Fan, L.; Zhang, P. Recent Advances in Organic Synthesis Applying Elemental Selenium. Tetrahedron 2022, 112, 132752. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formations via Cross-Coupling and Atom-Economic Addition Reactions. Achievements and Challenges. Chem. Rev. 2022, 122, 16110–16293. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Adak, L.; Mukherjee, N.; Ghosh, T. Benign-Metal-Catalyzed Carbon–Carbon and Carbon–Heteroatom Bond Formation. Synlett 2023, 34, 601–621. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.-W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Thiol Cofactors for Selenoenzymes and Their Synthetic Mimics. Org. Biomol. Chem. 2008, 6, 965–974. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Rocha, J.B.T. Toxicology and Pharmacology of Selenium: Emphasis on Synthetic Organoselenium Compounds. Arch. Toxicol. 2011, 85, 1313–1359. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small Molecule Selenium-Containing Compounds: Recent Development and Therapeutic Applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Guan, Q.; Han, C.; Zuo, D.; Zhai, M.; Li, Z.; Zhang, Q.; Zhai, Y.; Jiang, X.; Bao, K.; Wu, Y.; et al. Synthesis and Evaluation of Benzimidazole Carbamates Bearing Indole Moieties for Antiproliferative and Antitubulin Activities. Eur. J. Med. Chem. 2014, 87, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xu, J.; Wang, Z.; Qi, H.; Xu, Q.; Bai, Z.; Zhang, Q.; Bao, K.; Wu, Y.; Zhang, W. 3-(3,4,5-Trimethoxyphenylselenyl)-1H-indoles and Their Selenoxides as Combretastatin A-4 Analogs: Microwave-Assisted Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2015, 90, 184–194. [Google Scholar] [CrossRef]
- Wen, Z.; Li, X.; Zuo, D.; Lang, B.; Wu, Y.; Jiang, M.; Ma, H.; Bao, K.; Wu, Y.; Zhang, W. Ultrasound-Promoted Two-Step Synthesis of 3-Arylselenylindoles and 3-Arylthioindoles as Novel Combretastatin A-4 Analogues. Sci. Rep. 2016, 6, 23986. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Ignasiak, M.T.; Chuang, C.Y.; Vieira, B.; Padilha, N.B.; Carroll, L.; Lenardão, E.J.; Savegnago, L.; Davies, M.J. Selenium-Containing Indolyl Compounds: Kinetics of Reaction with Inflammation-Associated Oxidants and Protective Effect against Oxidation of Extracellular Matrix Proteins. Free Radic. Biol. Med. 2017, 113, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.M.; Thurow, S.; da Costa, M.; Casaril, A.M.; Domingues, M.; Schumacher, R.F.; Perin, G.; Alves, D.; Savegnago, L.; Lenardão, E.J. Ultrasound-Assisted Synthesis and Antioxidant Activity of 3-Selanyl-1H-indole and 3-Selanylimidazo [1,2-a]pyridine Derivatives. Asian J. Org. Chem. 2017, 6, 1635–1646. [Google Scholar] [CrossRef]
- Pedroso, G.J.; Costa, D.M.S.; Felipe Kokuszi, L.T.; da Silva, E.B.V.; Cavalcante, M.F.O.; Junca, E.; Moraes, C.A.O.; Pich, C.T.; de Lima, V.R.; Saba, S.; et al. Selenylated Indoles: Synthesis, Effects on Lipid Membrane Properties and DNA Cleavage. New J. Chem. 2023, 47, 2719–2726. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Wolf, L.; Martins, G.M.; von Mühlen, L. Efficient Synthesis of 3-Selanyl- and 3-Sulfanylindoles Employing Trichloroisocyanuric Acid and Dichalcogenides. Tetrahedron 2012, 68, 10464–10469. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Yan, J. Selective Synthesis of 3-Selanylindoles from Indoles and Diselenides Using IK/mCPBA System. Appl. Organomet. Chem. 2017, 31, e3864. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, Y.-Q.; Zhou, C.-F.; Jiang, Y.-Q.; Xu, Y.; Zeng, X.; Liu, G.-Q. Iodine Pentoxide-Mediated Oxidative Selenation and Seleno/Thiocyanation of Electron-Rich Arenes. Org. Biomol. Chem. 2022, 20, 5463–5469. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.L.; Azeredo, J.B.; Fiorentin, B.L.; Braga, A.L. Synthesis of 3-Selenylindoles under Ecofriendly Conditions. Eur. J. Org. Chem. 2015, 2015, 5070–5074. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Song, Z.; Liang, G. An Efficient t-BuOK Promoted C3-Chalcogenylation of Indoles with Dichalcogenides. Org. Biomol. Chem. 2018, 16, 4958–4962. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yi, R.; Zeng, C.; Cui, Y.; Xu, X.; Wang, X.-Q.; Li, N. CsOH-Promoted Regiospecific Sulfenylation, Selenylation, and Telluration of Indoles in H2O. Synlett 2023, 34, 124–132. [Google Scholar]
- Zhang, Q.-B.; Ban, Y.-L.; Yuan, P.-F.; Peng, S.-J.; Fang, J.-G.; Wu, L.-Z.; Liu, Q. Visible-Light-Mediated Aerobic Selenation of (Hetero)Arenes with Diselenides. Green Chem. 2017, 19, 5559–5563. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Ramesh, V.; Gangadhar, M.; Vijaykumar, S. Catalyst and Sensitizer-Free Visible-Light-Induced C(sp2)−H Chalcogenation of Arenes/Heteroarenes with Dichalcogenides. Asian J. Org. Chem. 2018, 7, 1689–1697. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Franco, M.S.; Schneider, A.R.; Espíndola, L.; Silva, D.O.; Braga, A.L. Rose Bengal Catalysed Photo-Induced Selenylation of Indoles, Imidazoles and Arenes: A Metal Free Approach. Org. Biomol. Chem. 2018, 16, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Kumar, S. Visible-Light-Induced Metal and Reagent-Free Oxidative Coupling of sp2 C–H Bonds with Organo-Dichalcogenides: Synthesis of 3-Organochalcogenyl Indoles. Green Chem. 2019, 21, 2670–2676. [Google Scholar] [CrossRef]
- Lemir, I.D.; Castro-Godoy, W.D.; Heredia, A.A.; Schmidt, L.C.; Argüello, J.E. Metal- and Photocatalyst-Free Synthesis of 3-Selenylindoles and Asymmetric Diarylselenides Promoted by Visible Light. RSC Adv. 2019, 9, 22685–22694. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.A.; Soria-Castro, S.M.; Castro-Godoy, W.D.; Lemir, I.D.; López-Vidal, M.; Bisogno, F.R.; Argüello, J.E.; Oksdath-Mansilla, G. Multistep Synthesis of Organic Selenides under Visible Light Irradiation: A Continuous-Flow Approach. Org. Process Res. Dev. 2020, 24, 540–545. [Google Scholar] [CrossRef]
- Huang, Q.; Peng, X.; Li, H.; He, H.; Liu, L. Visible-Light-Induced, Graphene Oxide-Promoted C3-Chalcogenylation of Indoles Strategy under Transition-Metal-Free Conditions. Molecules 2022, 27, 772. [Google Scholar] [CrossRef] [PubMed]
- Quadros, G.T.; de Medeiros, S.P.; de Oliveira, C.A.; Rambo, M.W.; Abenante, L.; Lenardão, E.J.; Penteado, F. Benzeneseleninic Acids (BSA) and Photocatalysis: An Alternative Duo for the Synthesis of 3-Selanylindoles. Asian J. Org. Chem. 2023, 12, e202300517. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Jiang, H.; Sun, L. Convenient Synthesis of Selenyl-Indoles via Iodide Ion-Catalyzed Electrochemical C–H Selenation. Chem. Commun. 2018, 54, 8781–8784. [Google Scholar] [CrossRef] [PubMed]
- Meirinho, A.G.; Pereira, V.F.; Martins, G.M.; Saba, S.; Rafique, J.; Braga, A.L.; Mendes, S.R. Electrochemical Oxidative C(sp2)–H Bond Selenylation of Activated Arenes. Eur. J. Org. Chem. 2019, 2019, 6465–6469. [Google Scholar] [CrossRef]
- Fang, X.-L.; Tang, R.-Y.; Zhong, P.; Li, J.-H. Iron-Catalyzed Sulfenylation of Indoles with Disulfides Promoted by a Catalytic Amount of Iodine. Synthesis 2009, 24, 4183–4189. [Google Scholar]
- Vieira, B.M.; Thurow, S.; Brito, J.S.; Perin, G.; Alves, D.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Sonochemistry: An Efficient Alternative to the Synthesis of 3-Selanylindoles Using CuI as Catalyst. Ultrason. Sonochem. 2015, 27, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Céspedes, S.; Ferry, A.; Candish, L.; Glorius, F. Heterogeneously Catalyzed Direct C-H Thiolation of Heteroarenes. Angew. Chem. Int. Ed. 2015, 54, 5772–5776. [Google Scholar] [CrossRef] [PubMed]
- Luz, E.Q.; Seckler, D.; Araújo, J.S.; Angst, L.; Lima, D.B.; Rios, E.A.M.; Ribeiro, R.R.; Rampon, D.S. Fe(III)-Catalyzed Direct C3 Chalcogenylation of Indole: The Effect of Iodide Ions. Tetrahedron 2019, 75, 1258–1266. [Google Scholar] [CrossRef]
- Rios, E.A.M.; Gomes, C.M.B.; Silvério, G.L.; Luz, E.Q.; Ali, S.; D’Oca, C.d.R.M.; Albach, B.; Campos, R.B.; Rampon, D.S. Silver-Catalyzed Direct Selanylation of Indoles: Synthesis and Mechanistic Insights. RSC Adv. 2023, 13, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Benchawan, T.; Maneewong, J.; Saeeng, R. Selective Synthesis of 3-Chalcogenylindoles via Silver-Catalyzed Direct Chalcogenation of Indoles with Dichalcogenides. ChemistrySelect 2023, 8, e202301988. [Google Scholar] [CrossRef]
- Azeredo, J.B.; Godoi, M.; Martins, G.M.; Silveira, C.C.; Braga, A.L. A Solvent- and Metal-Free Synthesis of 3-Chacogenyl-Indoles Employing DMSO/I2 as an Eco-Friendly Catalytic Oxidation System. J. Org. Chem. 2014, 79, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Rafique, J.; Saba, S.; Franco, M.S.; Bettanin, L.; Schneider, A.R.; Silva, L.T.; Braga, A.L. Direct, Metal-free C(sp2)−H Chalcogenation of Indoles and Imidazopyridines with Dichalcogenides Catalysed by KIO3. Chem. Eur. J. 2018, 24, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.R.; Gularte, M.M.; dos Santos, F.C.; Roehrs, J.A.; Azeredo, J.B. Synthesis of 3-Chalcogenyl-Indoles Mediated by the Safer Reagent Urea-Hydrogen Peroxide (UHP). Tetrahedron Lett. 2023, 120, 154446. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Das, P.; Jana, R. Atom-Economical Selenation of Electron-Rich Arenes and Phosphonates with Molecular Oxygen at Room Temperature. Org. Biomol. Chem. 2018, 16, 9243–9250. [Google Scholar] [CrossRef] [PubMed]
- Leonard, N.M.; Wieland, L.C.; Mohan, R.S. Applications of Bismuth(III) Compounds in Organic Synthesis. Tetrahedron 2002, 58, 8373–8397. [Google Scholar] [CrossRef]
- Gaspard-Iloughmane, H.; Le Roux, C. Bismuth(III) Triflate in Organic Synthesis. Eur. J. Org. Chem. 2004, 2004, 2517–2532. [Google Scholar] [CrossRef]
- Bothwell, J.M.; Krabbe, S.W.; Mohan, R.S. Applications of Bismuth(III) Compounds in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 4649–4707. [Google Scholar] [CrossRef] [PubMed]
- Ondet, P.; Lemière, G.; Duñach, E. Cyclisations Catalysed by Bismuth(III) Triflate. Eur. J. Org. Chem. 2017, 2017, 761–780. [Google Scholar] [CrossRef]
- Raţ, C.I.; Soran, A.; Varga, R.A.; Silvestru, C. C–H Bond Activation Mediated by Inorganic and Organometallic Compounds of Main Group Metals. Adv. Organomet. Chem. 2018, 70, 233–311. [Google Scholar]
- Takasawa, R.; Jona, A.; Inoue, M.; Azuma, M.; Akahane, H.; Ueno, Y.; Nakagawa, Y.; Chimori, R.; Mano, Y.; Murata, Y.; et al. Triphenylbismuth Dichloride Inhibits Human Glyoxalase I and Induces Cytotoxicity in Cultured Cancer Cell Lines. J. Toxicol. Sci. 2022, 47, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Ohki, H.; Wada, M.; Akiba, K.Y. Bismuth Trichloride as a New Efficient Catalyst in the Aldol Reaction. Tetrahedron Lett. 1988, 29, 4719–4722. [Google Scholar] [CrossRef]
- Wada, M.; Takeichi, E.; Matsumoto, T. Bismuth Trichloride as a New Efficient Catalyst in the Aldol Reaction and the Michael Reaction. Bull. Chem. Soc. Jpn. 1991, 64, 990–994. [Google Scholar] [CrossRef]
- Ollevier, T.; Lavie-Compin, G. An Efficient Method for the Ring Opening of Epoxides with Aromatic Amines Catalyzed by Bismuth Trichloride. Tetrahedron Lett. 2002, 43, 7891–7893. [Google Scholar] [CrossRef]
- De, S.K.; Gibbs, R.A. Bismuth(III) Chloride-Catalyzed Direct Deoxygenative Allylation of Substituted Benzylic Alcohols with Allyltrimethylsilane. Tetrahedron Lett. 2005, 46, 8345–8350. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, E.V.; Maruthi, C.; Yadav, J.S. Bismuth(III) Chloride-Catalyzed Intramolecular Hetero-Diels–Alder Reactions: A Novel Synthesis of Hexahydrodibenzo[b,h][1,6]Naphthyridines. Tetrahedron Lett. 2002, 43, 1573–1575. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, E.V.; Yadav, J.S.; Rama Krishna, K.V.S.; Ravi Sankar, A. Stereoselective Synthesis of Octahydro-3bH-[1,3]dioxolo [4″,5″:4′,5′]furo [2′,3′:5,6]pyrano [4,3-b]quinolines via Intramolecular Hetero-Diels–Alder Reactions Catalyzed by Bismuth(III) Chloride. Tetrahedron Lett. 2002, 43, 4029–4032. [Google Scholar] [CrossRef]
- Li, Z.; Wei, C.; Chen, L.; Varma, R.S.; Li, C.-J. Three-Component Coupling of Aldehyde, Alkyne, and Amine Catalyzed by Silver in Ionic Liquid. Tetrahedron Lett. 2004, 45, 2443–2446. [Google Scholar] [CrossRef]
- Li, H.; Zeng, H.-Y.; Shao, H.-W. Bismuth(III) Chloride-Catalyzed One-Pot Mannich Reaction: Three-Component Synthesis of β-Amino Carbonyl Compounds. Tetrahedron Lett. 2009, 50, 6858–6860. [Google Scholar] [CrossRef]
- Wu, F.; Huang, W.; Yiliqi; Yang, J.; Gu, Y. Relay Catalysis of Bismuth Trichloride and Byproduct Hydrogen Bromide Enables the Synthesis of Carbazole and Benzo[α]carbazoles from Indoles and α-Bromoacetaldehyde Acetals. Adv. Synth. Catal. 2018, 360, 3318–3330. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, X.-X.; Wang, Q.-D.; Yun, J.-J.; Rao, W.; Yang, J.-M.; Shen, Z.-L. Bismuth Trichloride-Catalyzed Oxy-Michael Addition of Water and Alcohol to α,β-Unsaturated Ketones. Chin. Chem. Lett. 2020, 31, 1297–1300. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Feng, B.; Liang, J.; You, G.; Liu, X.; Xian, L. Bi(III)-Catalyzed Aminooxygenation of Propargyl Amidines to Synthesize 2-Fluoroalkyl Imidazole-5-carbaldehydes and Their Decarbonylations. Chem. Commun. 2020, 56, 6400–6403. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-T.; Zhao, C.; Wang, D.-R.; Wu, G.-C.; Chen, G.-S.; Chen, S.-J.; Ren, H.; Deng, D.-S.; Xu, Y.-B.; Hu, X.-W.; et al. BiCl3-Mediated Tandem Cyclization of Tryptamine-Derived Ynamide: Concise Synthesis of Pentacyclic Spiroindolines and Tricyclic Indole Derivatives. Adv. Synth. Catal. 2022, 364, 890–896. [Google Scholar] [CrossRef]
- Malik, P.; Joseph, D.; Chakraborty, D. BiCl3-catalyzed Carbon–Carbon Cross-Coupling of Organoboronic Acids with Aryl Iodides. Appl. Organometal. Chem. 2013, 27, 519–522. [Google Scholar] [CrossRef]
- Riyaz, M.A.B.; Swu, T. Bismuth-catalyzed N-Arylation of 2-Aminobenzimidazole and Phosphorylation of Substituted Coumarins via C-H Functionalization. ChemistrySelect 2022, 7, e202203281. [Google Scholar] [CrossRef]
- Zhang, J.Z. Interfacial Charge Carrier Dynamics of Colloidal Semiconductor Nanoparticles. J. Phys. Chem. B 2000, 104, 7239–7253. [Google Scholar] [CrossRef]
- Ünlü, F.; Deo, M.; Mathur, S.; Kirchartz, T.; Kulkarni, A. Bismuth-Based Halide Perovskite and Perovskite-Inspired Light Absorbing Materials for Photovoltaics. J. Phys. D Appl. Phys. 2022, 55, 113002. [Google Scholar] [CrossRef]
- Komatsu, N.; Uda, M.; Suzuki, H. Bismuth(III) Halides and Sulfate as Highly Efficient Catalyst for the Sulfenylation of Carbonyl and Related Compounds1. Synlett 1995, 9, 984–986. [Google Scholar] [CrossRef]
- Cunha, S.; Rodrigues, M.T., Jr. The First Bismuth(III)-Catalyzed Guanylation of Thioureas. Tetrahedron Lett. 2006, 47, 6955–6956. [Google Scholar] [CrossRef]
- Bailey, A.D.; Baru, A.R.; Tasche, K.K.; Mohan, R.S. Environmentally Friendly Organic Synthesis Using Bismuth Compounds: Bismuth(III) Iodide Catalyzed Deprotection of Acetals in Water. Tetrahedron Lett. 2008, 49, 691–694. [Google Scholar] [CrossRef]
- Adonin, S.A.; Peresypkina, E.V.; Sokolov, M.N.; Korolkov, I.V.; Fedin, V.P. Polyoxomolybdate-Supported Bismuth Trihalides [Mo8O26(BiX3)2]4– (X = Cl, Br, I): Syntheses and Study of Polymorphism. Inorg. Chem. 2014, 53, 6886–6892. [Google Scholar] [CrossRef] [PubMed]
- Wedal, J.C.; Ziller, J.W.; Evans, W.J. Expanding Bismuth Trihalide Coordination Chemistry with Trimethyltriazacyclohexane and Trimethyltriazacyclononane. Inorg. Chem. 2022, 61, 11766–11774. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Dong, Z.; Zhang, P.; Xing, W.; Li, L. Direct Selenation of Imidazoheterocycles and Indoles with Selenium Powder in a Copper-Catalyzed Three-Component One-Pot System. Tetrahedron Lett. 2018, 59, 2554–2558. [Google Scholar] [CrossRef]
- Lin, M.; Kang, L.; Gu, J.; Dai, L.; Tang, S.; Zhang, T.; Wang, Y.; Li, L.; Zheng, X.; Zhu, W.; et al. Heterogeneous Synergistic Catalysis by Ru-RuOx Nanoparticles for Se–Se Bond Activation. Nano Res. 2017, 10, 922–932. [Google Scholar] [CrossRef]
- Chen, J.; Hu, L.; Wang, H.; Tan, H. Iodine-Catalyzed Telluration of Indole Derivatives with Diarylditellurides for Synthesis of 3-Aryltellurylindoles. Chin. J. Org. Chem. 2019, 39, 2048–2052. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).