Synthesis, Anticancer Activity, and Molecular Docking of New 1,2,3-Triazole Linked Tetrahydrocurcumin Derivatives
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Procedure
3.1. General Experimental Procedures
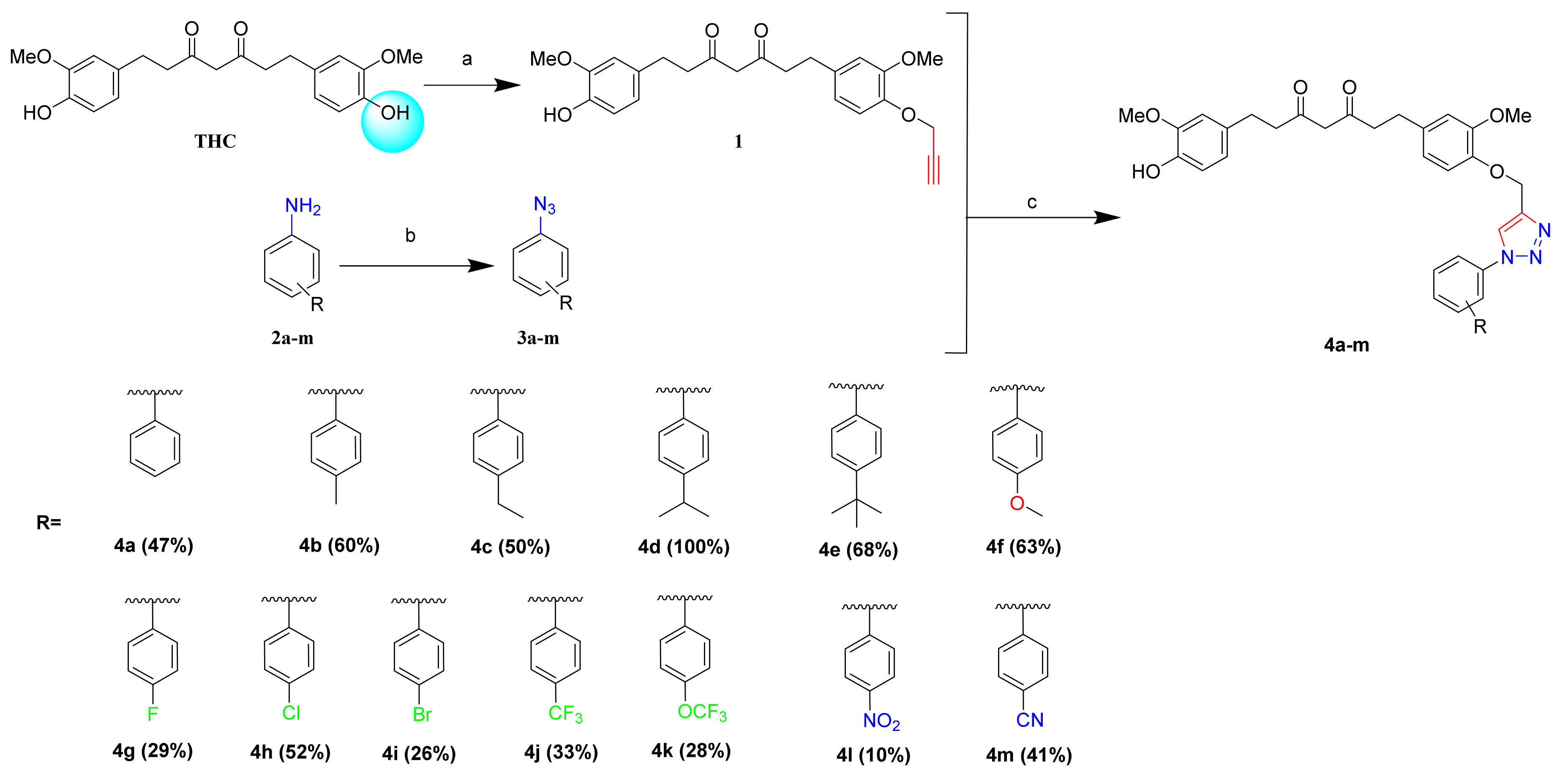
3.1.1. General Procedure for the Synthesis of 1-(4-Hydroxy-3-methoxyphenyl)-7-(3-methoxy-4-(prop-2-yn-1-yloxy)phenyl)heptane-3,5-dione (1)
3.1.2. General Procedure for the Synthesis of Azide Derivatives (3a~m)
3.1.3. General Procedure for the Synthesis of 1,2,3-Riazole Linked THC Derivatives (4a~m)
3.1.4. 1-(4-Hydroxy-3-methoxyphenyl)-7-(3-methoxy-4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy) phenyl)heptane-3,5-dione (4a)
3.1.5. 1-(4-Hydroxy-3-methoxyphenyl)-7-(3-methoxy-4-((1-(p-tolyl)-1H-1,2,3-triazol-5-yl)methoxy) phenyl)heptane-3,5-dione (4b)
3.1.6. 1-(4-((1-(4-Ethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione (4c)
3.1.7. 1-(4-Hydroxy-3-methoxyphenyl)-7-(4-((1-(4-isopropylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxyphenyl)heptane-3,5-dione (4d)
3.1.8. 1-(4-((1-(4-(Tert-butyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione (4e)
3.1.9. 1-(4-Hydroxy-3-methoxyphenyl)-7-(3-methoxy-4-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)heptane-3,5-dione (4f)
3.1.10. 1-(4-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione (4g)
3.1.11. 1-(4-((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione (4h)
3.1.12. 1-(4-((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione (4i)
3.1.13. 1-(4-Hydroxy-3-methoxyphenyl)-7-(3-methoxy-4-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)heptane-3,5-dione (4j)
3.1.14. 1-(4-Hydroxy-3-methoxyphenyl)-7-(3-methoxy-4-((1-(4-(trifluoromethoxy)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)heptane-3,5-dione (4k)
3.1.15. 1-(4-Hydroxy-3-methoxyphenyl)-7-(3-methoxy-4-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)heptane-3,5-dione (4l)
3.1.16. 4-(4-((4-(7-(4-Hydroxy-3-methoxyphenyl)-3,5-dioxoheptyl)-2-methoxyphenoxy)methyl)-1H-1,2,3-triazol-1-yl)benzonitrile (4m)
3.2. In Vitro Cytotoxicity Activity
3.3. Analysis of Cell Cycle by Flow Cytometry
3.4. Parameter Prediction of Drugs
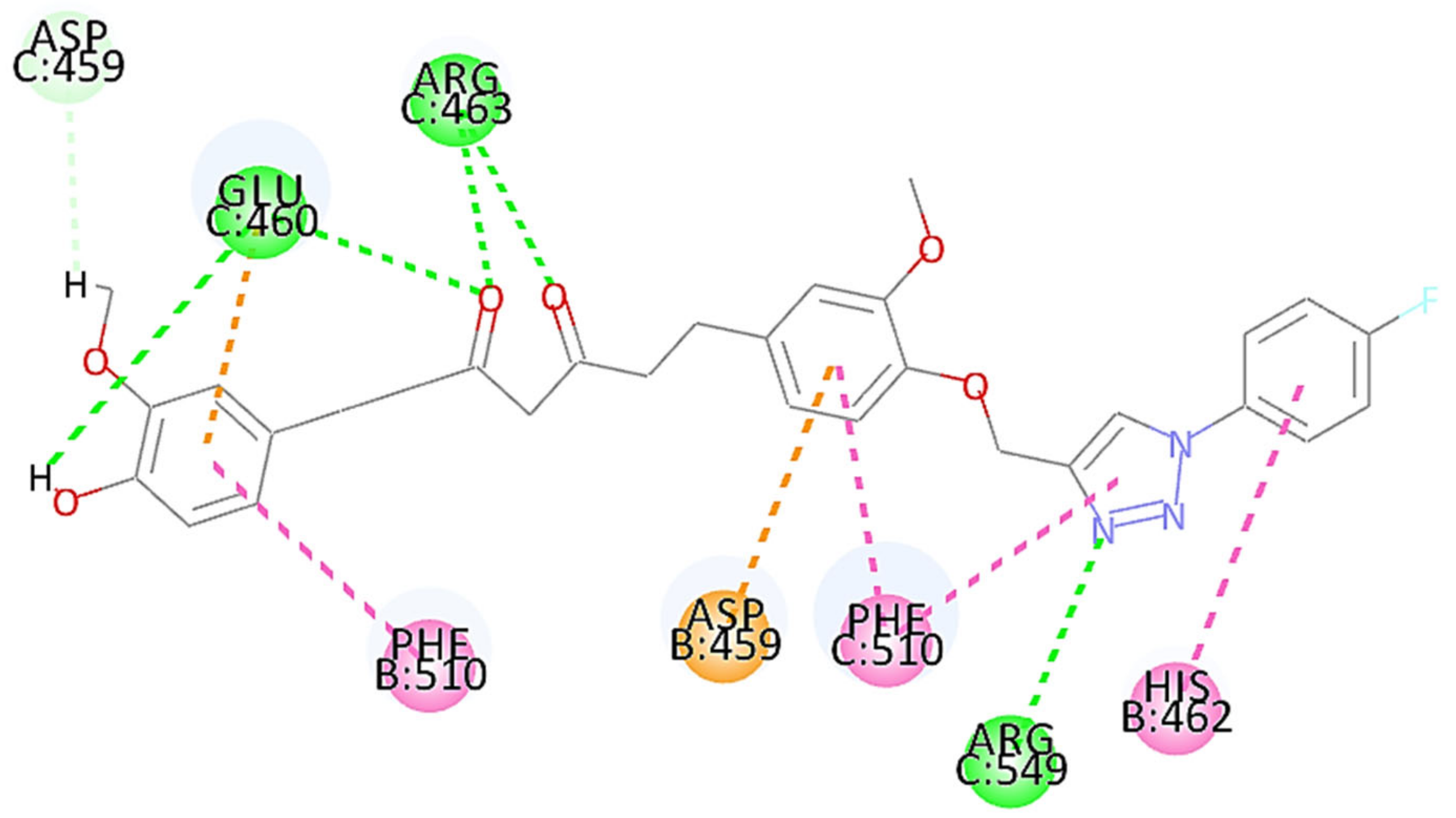
3.5. Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Sheikh, A.; Jaber, M.A.; Khalaf, H.; AlKhawaja, N.; Abuarqoub, D. Synthesis and biological evaluation of novel 2-morpholino-4-anilinoquinoline derivatives as antitumor agents against HepG2 cell line. RSC Adv. 2024, 14, 3304–3313. [Google Scholar] [CrossRef]
- Raghavendra, N.M.; Pingili, D.; Kadasi, S.; Mettu, A.; Prasad, S.V.U.M. Dual or multi-targeting inhibitors: The next generation anticancer agents. Eur. J. Med. Chem. 2018, 143, 1277–1300. [Google Scholar] [CrossRef]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 15, 111653. [Google Scholar] [CrossRef]
- Mahal, A.; Wu, P.; Jiang, Z.-H.; Wei, X.Y. Synthesis and cytotoxic activity of new tetrahydrocurcumin derivatives bearing pyrazole moiety. Nat. Prod. Bioprospect. 2017, 7, 461–469. [Google Scholar] [CrossRef]
- Mahal, A.; Wu, P.; Jiang, Z.-H.; Wei, X.Y. Schiff bases of Tetrahydrocurcumin as potential anticacer agents. ChemistrySelect 2019, 4, 366–369. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Feng, L.; Li, Y.; Song, Z.-F.; Huai, Q.-Y.; Li, H.-J. Synthesis and biological evaluation of curcuminoid derivatives. Chem. Pharm. Bull. 2015, 63, 873–881. [Google Scholar] [CrossRef]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Curcumin-amino acid conjugates: Synthesis, antioxidant and antimutagenic attributes. Food Chem. 2010, 120, 523–530. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Shi, W.; Dolai, S.; Rizk, S.; Hussain, A.; Tariq, H.; Averick, S.; L’Amoreaux, W.; El Idrissi, A.; Banerjee, P.; Raja, K. Synthesis of monofunctional curcumin derivatives, clicked curcumin dimer, and a PAMAM dendrimer curcumin conjugate for therapeutic applications. Org. Lett. 2007, 9, 5461–5464. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Elangovan, N.; Manigandan, K.; Singh, S.; Shukla, S. CNB-001 a novel curcumin derivative, guards dopamine neurons in MPTP model of Parkinson’s disease. BioMed Res. Int. 2014, 2014, 236182. [Google Scholar] [CrossRef]
- Gomes, D.d.C.F.; Vilela Alegrio, L.; Edilson Freire de Lima, M.; Leon, L.L.; Araújo, C.A.C. Synthetic Derivatives of Curcumin and their Activity against Leishmania amazonensis. Arzneim. Forsch. Drug Res. 2002, 52, 120–124. [Google Scholar] [CrossRef]
- Banuppriya, G.; Sribalan, R.; Padmini, V.; Shanmugaiah, V. Biological evaluation and molecular docking studies of new curcuminoid derivatives: Synthesis and characterization. Bioorg. Med. Chem. Lett. 2016, 26, 1655–1659. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, Y.; Hu, Y.; Lu, M.; Wang, J.; Dong, J.; Chen, D.; Chen, L.; Fu, F.; Qiu, F. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: Translocation of nuclear factor-κB as potential target. Mol. Med. Rep. 2015, 11, 3087–3093. [Google Scholar] [CrossRef]
- Lozada-García, M.C.; Enríquez, R.G.; Ramírez-Apán, T.O.; Nieto-Camacho, A.; Palacios-Espinosa, J.F.; Custodio-Galván, Z.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis of curcuminoids and evaluation of their cytotoxic and antioxidant properties. Molecules 2017, 22, 633. [Google Scholar] [CrossRef]
- González, Y.; Mojica-Flores, R.; Moreno-Labrador, D.; Pecchio, M.; Rao, K.S.J.; Ahumedo-Monterrosa, M.; Fernández, P.L.; Larionov, O.V.; Lakey-Beitia, J. Tetrahydrocurcumin Derivatives Enhanced the Anti-Inflammatory Activity of Curcumin: Synthesis, Biological Evaluation, and Structure–Activity Relationship Analysis. Molecules 2023, 28, 7787. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef]
- Masuda, T.; Hidaka, K.; Shinohara, A.; Maekawa, T.; Takeda, Y.; Yamaguchi, H. Chemical studies on antioxidant mechanism of curcuminoid: Analysis of radical reaction products from curcumin. J. Agric. Food Chem. 1999, 47, 71–77. [Google Scholar] [CrossRef]
- Rege, S.A.; Varshneya, M.A.; Momin, S.A. A Mini-Review: Comparison between curcumin and tetrahydrocurcumin based on their activities. Croat. J. Food Sci. Technol. 2021, 13, 128–132. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Berrocal, R.; Rao, K.S.; Durant, A.A. Polyphenols as Therapeutic Molecules in Alzheimer’s Disease through Modulating Amyloid Pathways. Mol. Neurobiol. 2015, 51, 466–479. [Google Scholar] [CrossRef]
- Song, G.; Lu, H.; Chen, F.; Wang, Y.; Fan, W.; Shao, W.; Lu, H.; Lin, B. Tetrahydrocurcumin-induced autophagy via suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma cells. Mol. Med. Rep. 2018, 17, 5964–5969. [Google Scholar] [CrossRef]
- Pari, L.; Amali, D.R. Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J. Pharm. Pharm. Sci. 2005, 8, 115–123. [Google Scholar]
- Han, X.; Deng, S.; Wang, N.; Liu, Y.F.; Yang, X.B. Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells. Food Nutr. Res. 2016, 60, 30616. [Google Scholar] [CrossRef]
- Yoysungnoen, B.; Bhattarakosol, P.; Changtam, C.; Patumraj, S. Effects of Tetrahydrocurcumin on Tumor Growth and Cellular Signaling in Cervical Cancer Xenografts in Nude Mice. Biomed. Res. Int. 2016, 2016, 1781208. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chiou, S.S.; Weng, J.P.; Lin, P.C. Curcumin and tetrahydrocurcumin induce cell death in Ara-C-resistant acute myeloid leukemia. Phytother. Res. 2019, 33, 1199–1207. [Google Scholar] [CrossRef]
- Tang, C.; Liu, J.; Yang, C.; Ma, J.; Chen, X.; Liu, D.; Zhou, Y.; Zhou, W.; Lin, Y.; Yuan, X. Curcumin and Its Analogs in Non-Small Cell Lung Cancer Treatment: Challenges and Expectations. Biomolecules 2022, 12, 1636. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Garbisa, S.; Limtrakul, P. Tetrahydrocurcumin inhibits HT1080 cell migration and invasion via downregulation of MMPs and uPA. Acta Pharmacol. Sin. 2008, 29, 853–860. [Google Scholar] [CrossRef]
- Kim, J.M.; Araki, S.; Kim, D.J.; Park, C.B.; Takasuka, N.; Baba-Toriyama, H.; Ota, T.; Nir, Z.; Khachik, F.; Shimidzu, N.; et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 1998, 19, 81–85. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Rao, A.B.; Prasad, E.; Deepthi, S.S.; Ansari, I.A. Synthesis and biological evaluation of glucosyl curcuminoids. Arch. Pharm. Chem. Life Sci. 2014, 347, 834–839. [Google Scholar] [CrossRef]
- Plyduang, T.; Lomlim, L.; Yuenyongsawad, S.; Wiwattanapatapee, R. Carboxymethylcellulose–tetrahydrocurcumin conjugates for colon-specific delivery of a novel anti-cancer agent, 4-amino tetrahydrocurcumin. Eur. J. Pharm. Biopharm. 2014, 88, 351–360. [Google Scholar] [CrossRef]
- Manjunatha, J.R.; Bettadaiah, B.K.; Negi, P.S.; Srinivas, P. Synthesis of amino acid conjugates of tetrahydrocurcumin and evaluation of their antibacterial and anti-mutagenic properties. Food Chem. 2013, 139, 332–338. [Google Scholar] [CrossRef]
- Manjunatha, J.R.; Bettadaiah, B.K.; Negi, P.S.; Srinivas, P. Synthesis of quinoline derivatives of tetrahydrocurcumin and zingerone and evaluation of their antioxidant and antibacterial attributes. Food Chem. 2013, 136, 650–658. [Google Scholar] [CrossRef]
- Mohri, K.; Watanabe, Y.; Yoshida, Y.; Satoh, M.; Isobe, K.; Sugimoto, N.; Tsuda, Y. Synthesis of Glycosylcurcuminoids. Chem. Pharm. Bull. 2003, 51, 1268–1272. [Google Scholar] [CrossRef]
- Yutthaseri, T.; Ajavakom, A.; Ajavakom, V. Natural Tetrahydrocurcumin in Multi-Component Synthesis of 1,4-Dihydropyridine Derivatives. Heterocycles 2016, 92, 1512–1520. [Google Scholar] [CrossRef]
- Baker, M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar] [CrossRef]
- Zinad, D.S.; Mahal, A.; Siswodihardjo, S.; Pratama, M.R.F.; Mohapatra, R.K. 3D-Molecular Modeling, Antibacterial Activity and Molecular Docking Studies of Some Imidazole Derivatives. Egypt. J. Chem. 2021, 64, 93–105. [Google Scholar] [CrossRef]
- Zinad, D.S.; Mahal, A.; Shareef, O.A. Antifungal activity and theoretical study of synthesized pyrazole-imidazole hybrids. IOP Conf. Ser. Mater. Sci. Eng. 2020, 770, 012053. [Google Scholar] [CrossRef]
- Salman, G.A.; Zinad, D.S.; Mahal, A. Design, synthesis, and biological evaluation of new quinoline-based heterocyclic derivatives as novel antibacterial agents. Monatsh. Chem. 2020, 151, 1621–1628. [Google Scholar] [CrossRef]
- Buchanan, D.; Pham, A.M.; Singh, S.K.; Panda, S.S. Molecular Hybridization of Alkaloids Using 1,2,3-Triazole-Based Click Chemistry. Molecules 2023, 28, 7593. [Google Scholar] [CrossRef]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzyme Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Tahlan, S.; Narasimhan, B.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Kakkar, S. Synthesis and biological evaluation of heterocyclic 1,2,4-triazole scaffolds as promising pharmacological agents. BMC Chem. 2021, 15, 5. [Google Scholar] [CrossRef]
- Liang, T.; Sun, T.; Li, W.; Hou, G.; Gao, F. 1,2,3-Triazole-Containing Compounds as Anti–Lung Cancer Agents: Current Developments, Mechanisms of Action, and Structure–Activity Relationship. Front. Pharmacol. 2021, 12, 661173. [Google Scholar] [CrossRef]
- Wen, X.; Zho, Y.; Zeng, J.; Liu, X. Recent Development of 1,2,4-triazole-containing Compounds as Anticancer Agents. Curr. Top. Med. Chem. 2020, 20, 1441–1460. [Google Scholar] [CrossRef]
- Mahal, A.; Al-Janabi, M.; Eyüpoğlu, V.; Alkhouri, A.; Chtita, S.; Kadhim, M.M.; Obaidullah, A.J.; Alotaibi, J.M.; Wei, X.; Pratama, M.R.F. Molecular docking, drug-likeness and DFT study of some modified tetrahydrocurcumins as potential anticancer agents. Saudi Pharm. J. 2024, 32, 101889. [Google Scholar] [CrossRef] [PubMed]
- Zinad, D.S.; Mahal, A.; Salman, G.A. Synthesis and Antibacterial Activity of Novel 1,3-Oxazine Derivatives. Org. Prep. Proced. Int. 2021, 53, 578–584. [Google Scholar] [CrossRef]
- Zinad, D.S.; Mahal, A.; Salman, G.A.; Alduhan, I.A.; Yahya, M.; Meitao, D.; Alkhouri, A.; Zinad, Y.S. Synthesis of Novel Quinolines with Antibacterial Activity. Org. Prep. Proced. Int. 2024, 56, 180–186. [Google Scholar] [CrossRef]
- Hussain, I.; Yawer, M.Y.; Appel, B.; Sher, M.; Mahal, A.; Villinger, A.; Fischer, C.; Langer, P. Synthesis of 4-hydroxy- and 2,4-dihydroxy-homophthalates by [4+2] cycloaddition of 1,3-bis(silyloxy)-1,3-butadienes with dimethyl allene-1,3-dicarboxylate. Tetrahedron 2008, 64, 8003. [Google Scholar] [CrossRef]
- Duan, M.; Mahal, A.; Mohammed, B.; Zhu, Y.; Tao, H.; Mai, S.; Al-Haideri, M.; Zhu, Q. Synthesis and antitumor activity of new tetrahydrocurcumin derivatives via click reaction. Nat. Prod. Res. 2021, 36, 5268–5276. [Google Scholar] [CrossRef]
- Rupireddy, V.; Chittireddy, V.R.R.; Dongamanti, A. An Efficient Approach for the Synthesis of Triazole Conjugated Pyrazole Chalcone Derivatives. Chem. Afr. 2020, 3, 45–52. [Google Scholar] [CrossRef]
- Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Apoptosis and APC in colorectal tumorigenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 7950–7954. [Google Scholar] [CrossRef] [PubMed]
- Noe, O.; Filipiak, L.; Royfman, R.; Campbell, A.; Lin, L.; Hamouda, D.; Stanbery, L.; Nemunaitis, J. Adenomatous polyposis coli in cancer and therapeutic implications. Oncol. Rev. 2021, 15, 534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Deng, R.; Yang, X.; Shang, J.; Lu, S.; Zhao, Y.; Song, K.; Liu, X.; Zhang, Q.; Chen, Y.; et al. Peptidomimetic inhibitors of APC-Asef interaction block colorectal cancer migration. Nat. Chem. Biol. 2017, 13, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhou, Q.; He, J.; Jiang, Z.; Peng, C.; Tong, R.; Shi, J. Recent advances in the development of protein-protein interactions modulators: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2020, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.E.; Bayly, A.R.; Abell, C.; Skidmore, J. Small molecules, big targets: Drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 2016, 15, 533–550. [Google Scholar] [CrossRef]
- Jadav, S.S.; Macalino, S.J.Y.; Alluri, R. Structure-based discovery of small molecule APC-Asef interaction inhibitors: In silico approaches and molecular dynamics simulations. J. Mol. Model 2020, 26, 207. [Google Scholar] [CrossRef]

| Compound | IC50 (μM) | |||
|---|---|---|---|---|
| HCT116 | HeLa | A549 | HepG2 | |
| Tetrahydrocurcumin | 50.96 ± 0.23 | 33.88 ± 0.35 | 69.25 ± 0.48 | 60.39 ± 0.19 |
| Cisplatin | 8.22 ± 0.86 | 10.12 ± 0.06 | 28.10 ± 1.58 | 98.22 ± 1.33 |
| 4a | 62.63 ± 3.06 | >200 | >200 | >200 |
| 4b | >200 | >200 | >200 | >200 |
| 4c | >200 | >200 | >200 | >200 |
| 4d | >200 | >200 | >200 | >200 |
| 4e | >200 | >200 | 162.40 ± 2.20 | >200 |
| 4f | 15.59 ± 0.45 | >200 | >200 | 53.64 ± 0.27 |
| 4g | 1.09 ± 0.17 | 190 ± 0.67 | 45.16 ± 0.92 | 66.82 ± 0.42 |
| 4h | 69.74 ± 7.12 | >200 | >200 | >200 |
| 4i | >200 | >200 | >200 | >200 |
| 4j | 89.38 ± 1.39 | 175.9 ± 1.21 | 131.20 ± 1.00 | >200 |
| 4k | 72.07 ± 3.88 | 129.5 ± 3.46 | 57.96 ± 3.78 | 104.23 ± 0.23 |
| 4l | >200 | >200 | >200 | >200 |
| 4m | >200 | >200 | 135.60 ± 4.80 | >200 |
| Compound | MW | Natoms | miLogP | nON | nOHNH | Nrotb | TPSA | MV |
|---|---|---|---|---|---|---|---|---|
| 4a | 529.59 | 39 | 3.58 | 9 | 1 | 14 | 112.79 | 482.19 |
| 4b | 543.62 | 40 | 4.02 | 9 | 1 | 14 | 112.79 | 498.76 |
| 4c | 557.65 | 41 | 4.49 | 9 | 1 | 15 | 112.79 | 515.56 |
| 4d | 571.67 | 42 | 5.09 | 9 | 1 | 15 | 112.79 | 532.14 |
| 4e | 585.70 | 43 | 5.28 | 9 | 1 | 15 | 112.79 | 548.38 |
| 4f | 559.62 | 41 | 3.63 | 10 | 1 | 15 | 122.02 | 507.74 |
| 4g | 547.58 | 40 | 3.74 | 9 | 1 | 14 | 112.79 | 547.58 |
| 4h | 564.04 | 40 | 4.25 | 9 | 1 | 14 | 112.79 | 495.73 |
| 4i | 608.49 | 40 | 4.38 | 9 | 1 | 14 | 112.79 | 500.08 |
| 4j | 613.59 | 44 | 4.54 | 10 | 1 | 16 | 122.02 | 613.59 |
| 4k | 579.59 | 43 | 4.47 | 9 | 1 | 15 | 112.79 | 513.49 |
| 4l | 576.56 | 42 | 2.89 | 13 | 1 | 15 | 167.85 | 497.71 |
| 4m | 554.60 | 41 | 3.33 | 10 | 1 | 14 | 136.58 | 499.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Mahal, A.; Alkouri, A.; Wang, C.; Zhang, Z.; Ren, J.; Obaidullah, A.J. Synthesis, Anticancer Activity, and Molecular Docking of New 1,2,3-Triazole Linked Tetrahydrocurcumin Derivatives. Molecules 2024, 29, 3010. https://doi.org/10.3390/molecules29133010
Duan M, Mahal A, Alkouri A, Wang C, Zhang Z, Ren J, Obaidullah AJ. Synthesis, Anticancer Activity, and Molecular Docking of New 1,2,3-Triazole Linked Tetrahydrocurcumin Derivatives. Molecules. 2024; 29(13):3010. https://doi.org/10.3390/molecules29133010
Chicago/Turabian StyleDuan, Meitao, Ahmed Mahal, Anas Alkouri, Chen Wang, Zhiqiang Zhang, Jungang Ren, and Ahmad J. Obaidullah. 2024. "Synthesis, Anticancer Activity, and Molecular Docking of New 1,2,3-Triazole Linked Tetrahydrocurcumin Derivatives" Molecules 29, no. 13: 3010. https://doi.org/10.3390/molecules29133010
APA StyleDuan, M., Mahal, A., Alkouri, A., Wang, C., Zhang, Z., Ren, J., & Obaidullah, A. J. (2024). Synthesis, Anticancer Activity, and Molecular Docking of New 1,2,3-Triazole Linked Tetrahydrocurcumin Derivatives. Molecules, 29(13), 3010. https://doi.org/10.3390/molecules29133010






