Effective Transport Recovery of Palladium(II) from Hydrochloric Acid Solutions Using Polymer Inclusion Membrane with Tetrabutylammonium Bromide
Abstract
1. Introduction
2. Results and Discussion
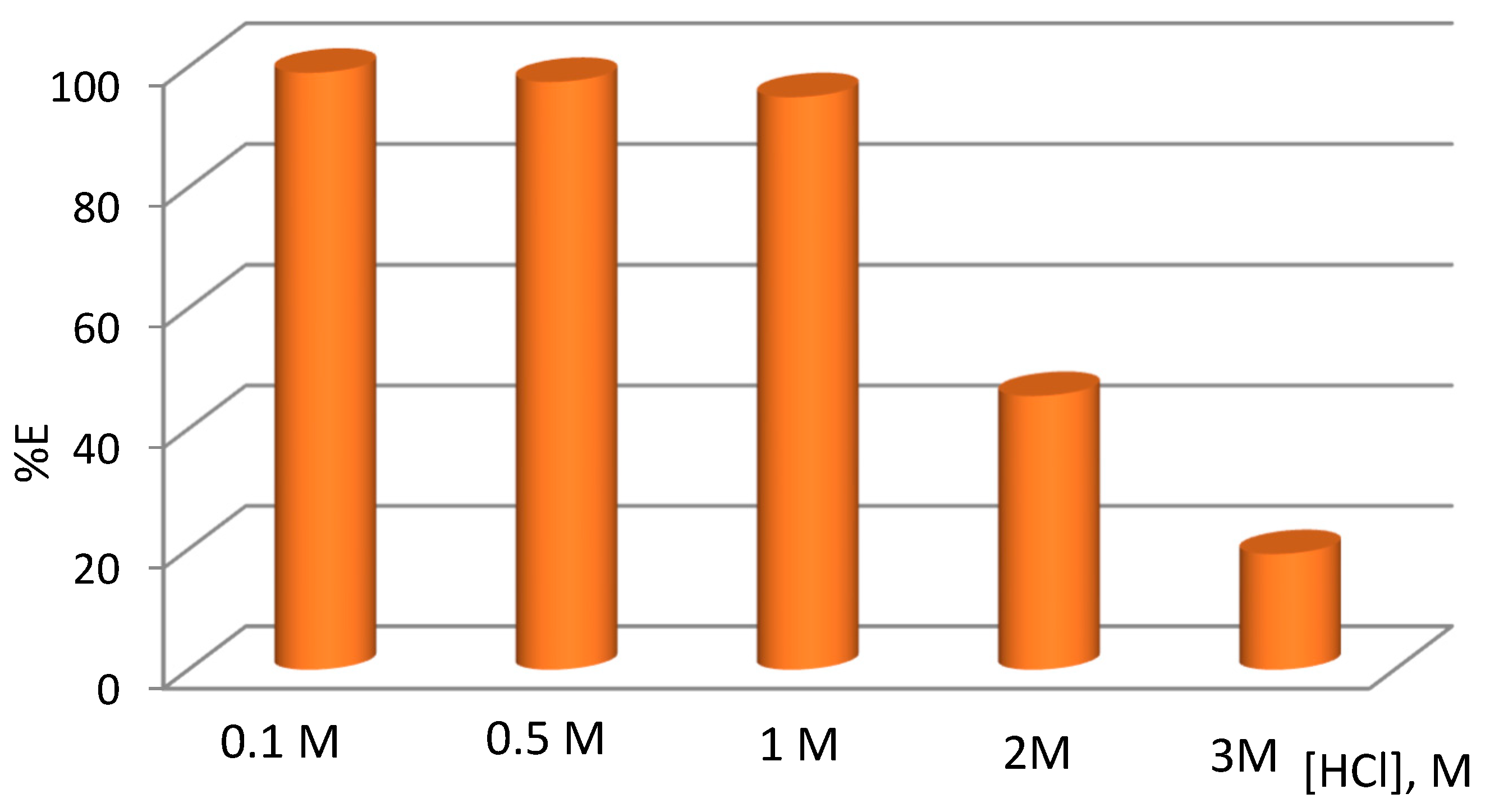
2.1. Solvent Extraction of Pd(II) by TBAB
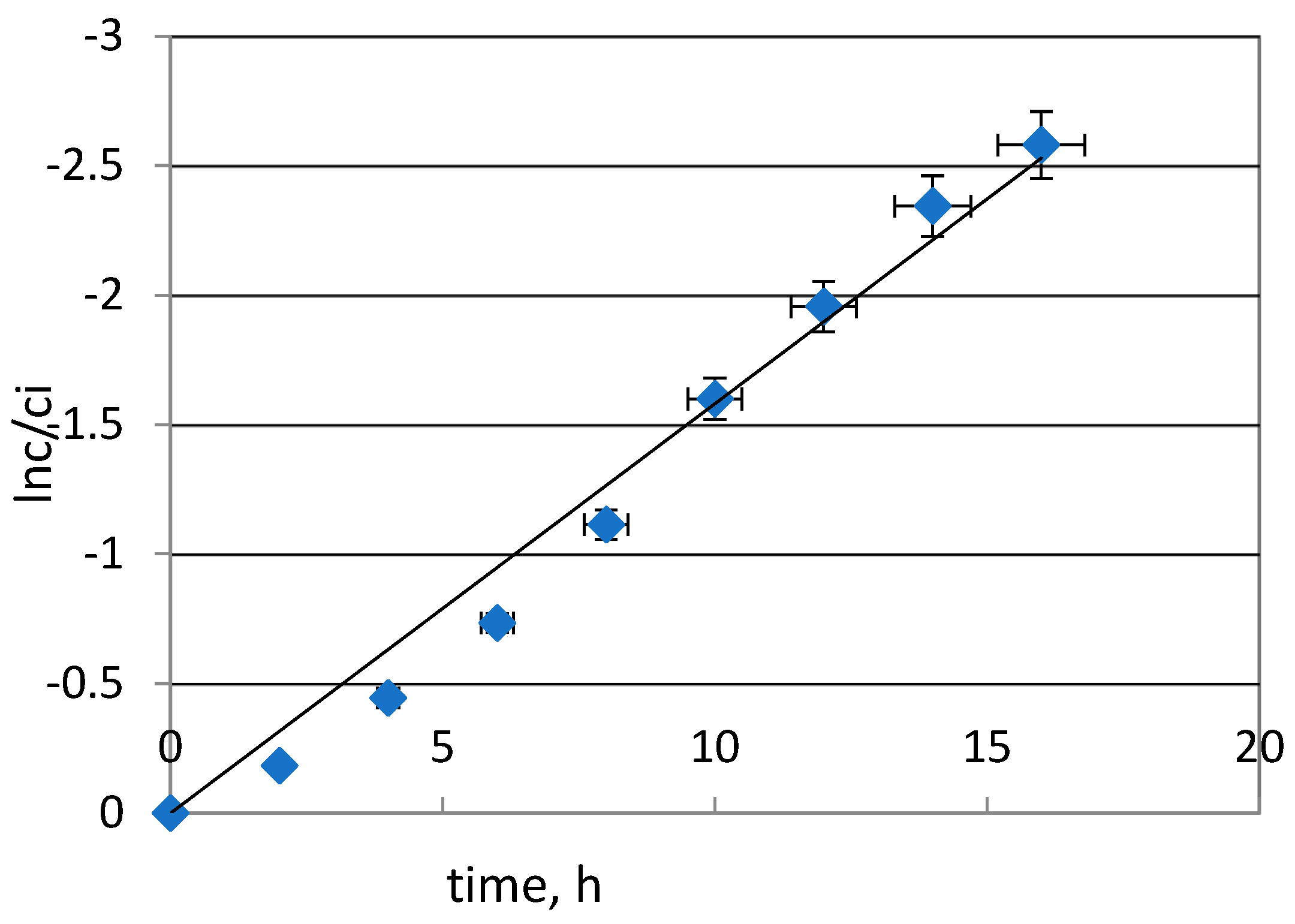
2.2. Transport Kinetics of Pd(II) from Hydrochloric Acid Solutions across PIM with TBAB
2.3. Effect of Thiourea Concentration in Stripping Phase
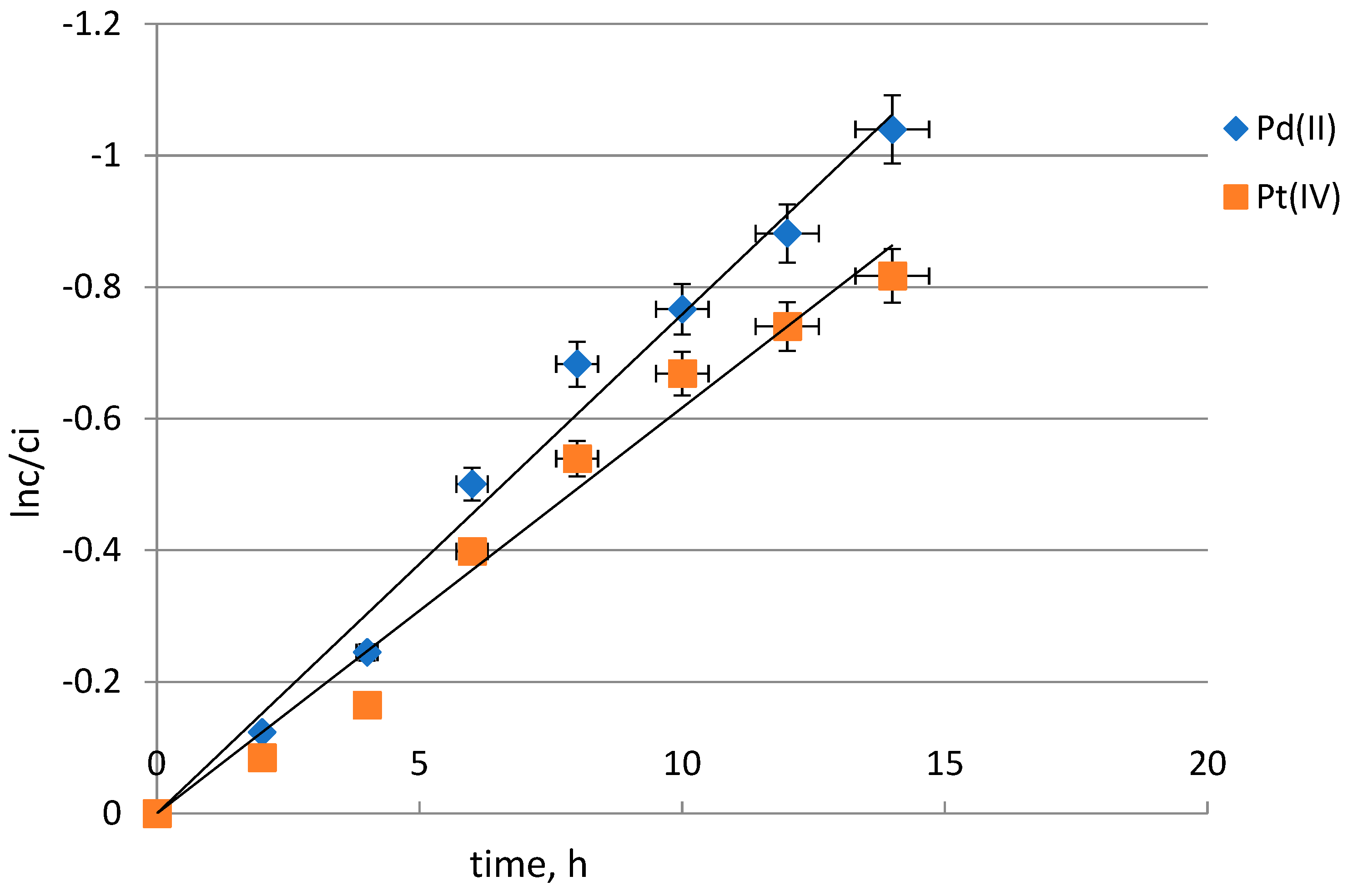
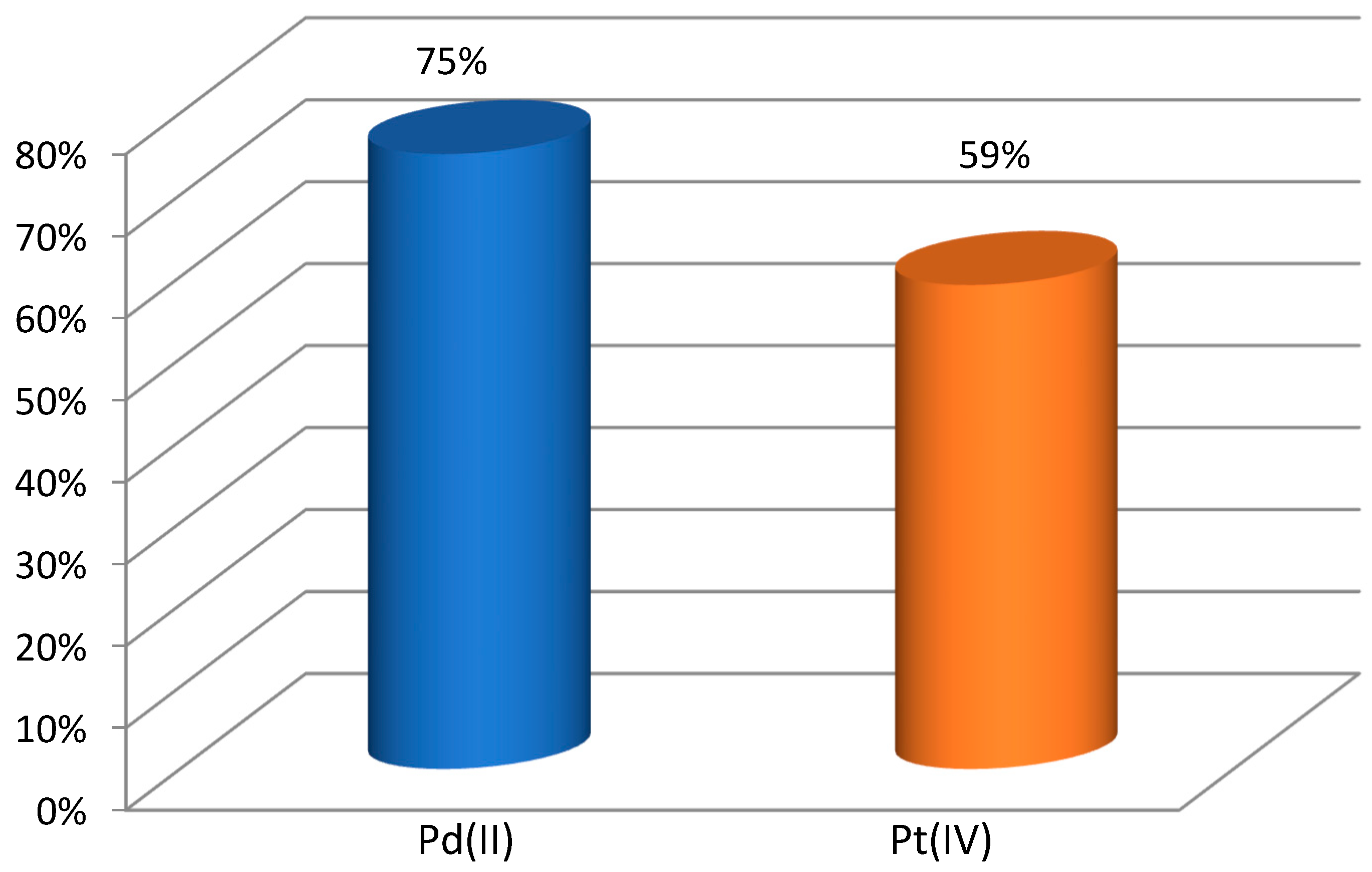
2.4. Selectivity Study
2.5. Stability of PIMs with TBAB
3. Materials and Methods
3.1. Reagents
3.1.1. Inorganic Reagents
3.1.2. Organic Reagents
3.2. Synthesis of Polymer Inclusion Membranes (PIMs)
3.3. Transport of Metal Ions Experiments
3.4. Solvent Extraction of Pd(II)
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolev, S.D.; Sakai, Y.; Cattrall, R.W.; Paimin, R.; Potter, I.D. Theoretical and experimental study of palladium(II) extraction from hydrochloric acid solutions into Aliquat 336/PVC membranes. Anal. Chim. Acta 2000, 413, 241–246. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Applicability of liquid membranes in chromium(VI) transport with amines as ion carriers. J. Membr. Sci. 2005, 266, 143–150. [Google Scholar] [CrossRef]
- Macías, M.; Rodríguez de San Miguel, E. On the Use of polymer inclusion membranes for the selective separation of Pb(II), Cd(II), and Zn(II) from seawater. Membranes 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, V.; Unal, A. The extraction and the removal of Cd(II) using polymer inclusion membrane containing symmetric room temperature ionic liquid as ion carrier. J. Environ. Chem. Eng. 2023; in press. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, A.; Fontas, C.; Matamoros, V.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Development of a polymer inclusion membrane-based passive sampler for monitoring of sulfamethoxazole in natural waters. Minimizing the effect of the flow pattern of the aquatic system. Microchem. J. 2016, 124, 175–180. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Alguacil, F.J. Recent trends in metal extraction. Rev. Metal. 2013, 49, 292–316. [Google Scholar] [CrossRef]
- Dudek, S.; Kołodyńska, D. Arsenate removal on the ion exchanger modified with cerium(III) ions. Physicochem. Probl. Miner. Process. 2022, 58, 147412. [Google Scholar] [CrossRef]
- Vazguez, M.I.; Romero, V.; Fontas, C.; Antico, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical-chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on extraction and permeation of cadmium(II) using Cyphos IL 104 as selective extractant and ion carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Wiecka, Z.; Rzelewska-Piekut, M.; Reis, M.T.A.; Ismael, M.R.C.; Wieszczycka, K.; Regel-Rosocka, M. Pd(II) and Pt(IV) dispersive or non-dispersive extraction from model and real leach solutions with alkoxyimine-1-propylpyridinium derivatives. Sep. Purif. Technol. 2023, 317, 123800. [Google Scholar] [CrossRef]
- Makowka, A.; Pospiech, B. Studies on extraction and competitive permeation of cerium(III) and lanthanum(III) using Cyphos IL104 as selective extractant and ion carrier. Sep. Sci. Technol. 2020, 55, 2193–2203. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, L.; Deng, T.; Zhang, C.; Xu, W.; Zhang, W. Polymer Inclusion Membranes with P507-TBP Carriers for Lithium Extraction from Brines. Membranes 2022, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, B.; Kujawski, W. Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation. Rev. Chem. Eng. 2015, 31, 179–191. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Pospiech, B. Separation of Co from Ni and Li from chloride media using polymer inclusion membrane system with thiosalicylate based ionic liquid. Physicochem. Probl. Miner. Process. 2022, 58, 152997. [Google Scholar] [CrossRef]
- Bashiri, A.; Nikzad, A.; Maleki, R.; Asadnia, M.; Razmjou, A. Rare earth elements recovery using selective membranes via extraction and rejection. Membranes 2022, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De San Miguel, E.R.; Hernández-Andaluz, A.M.; Bañuelos, J.G.; Saniger, J.M.; Aguilar, J.C.; de Gyves, J. LIX®-loaded polymer inclusion membrane for copper(II) transport: 1. Composition–performance relationships through membrane characterization and solubility diagrams. Mater. Sci. Eng. A 2006, 434, 30–38. [Google Scholar] [CrossRef]
- De Gyves, J.; Hernández-Andaluz, A.M.; de San Miguel, E.R. LIX®-loaded polymer inclusion membrane for copper (II) transport: 2. Optimization of the efficiency factors (permeability, selectivity, and stability) for LIX® 84-I. J. Membr. Sci. 2006, 268, 142–149. [Google Scholar] [CrossRef]
- Pospiech, B. Highly efficient facilitated membrane transport of palladium(II) ions from hydrochloric acid solutions through plasticizer membranes with Cyanex 471X. Physicochem. Probl. Miner. Process. 2015, 51, 281–291. [Google Scholar]
- Pospiech, B. Facilitated transport of palladium(II) across polymer inclusion membranes with ammonium ionic liquid as effective carrier. Chem. Pap. 2018, 72, 301–308. [Google Scholar] [CrossRef]
- Hanada, T.; Firmansyah, M.L.; Yoshida, W.; Kubota, F.; Kolev, S.D.; Goto, M. Transport of rhodium(III) from chloride media across a polymer inclusion membrane containing an ionic liquid metal ion carrier. ACS Omega 2020, 5, 12989–12995. [Google Scholar] [CrossRef] [PubMed]
- Fajar, A.T.N.; Hanada, T.; Goto, M. Recovery of platinum group metals from a spent automotive catalyst using polymer inclusion membranes containing an ionic liquid carrier. J. Membr. Sci. 2021, 629, 119296. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. Extraction of gold(III) from hydrochloric acid solutions with a PVC-based polymer inclusion membrane (PIM) containing Cyphos® IL 104. Membranes 2015, 5, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Saternus, M.; Fornalczyk, A. Possible ways of refining precious group metals (PGM) obtained from recycling of the used auto catalytic converters. Metalurgija 2013, 52, 267–270. [Google Scholar]
- Firmansyah, M.L.; Kubota, F.; Yoshida, W.; Goto, M. Application of a novel phosphonium-based ionic liquid to the separation of platinum group metals from automobile catalyst leach liquor. Ind. Eng. Chem. Res. 2019, 58, 3845–3852. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.C.; Chagnes, A.; Kim, M.S.; Jeong, J.; Cote, G. Highly selective separation of individual Platinum Group Metals (Pd, Pt, Rh) from acidic chloride media using phosphonium-based ionic liquid in aromatic diluent. RSC Adv. 2016, 6, 62717–62728. [Google Scholar] [CrossRef]
- Pianowska, K.; Kluczka, J.; Benke, G.; Goc, K.; Malarz, J.; Ochmański, M.; Leszczyńska-Sejda, K. Solvent extraction as a method of recovery and separation of Platinum Group Metals. Materials 2023, 16, 4681. [Google Scholar] [CrossRef] [PubMed]
- Cieszyńska, A.; Wieczorek, D. Efficiency and mechanism of palladium(II) extraction from chloride media with n-hexadecylpiperidinium chloride. J. Sol. Chem. 2020, 49, 486–503. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Rzelewska, M.; Baczynska, M.; Janus, M.; Wisniewski, M. Removal of palladium(II) from aqueous chloride solutions with Cyphos phosphonium ionic liquids as metal ion carriers for liquid-liquid extraction and transport across polymer inclusion membranes. Physicochem. Probl. Min. Process. 2015, 51, 621–631. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wisniewski, M. Extractive recovery of palladium(II) from hydrochloric acid solutions with Cyphos IL 104. Hydrometallurgy 2012, 113–114, 79–85. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wisniewski, M. Selective extraction of palladium(II) from hydrochloric acid solutions with phosphonium extractants. Sep. Purif. Technol. 2011, 80, 385–389. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of palladium(II) with a magnetic sorbent based on polyvinyl alcohol gel, metallic iron, and an environmentally friendly polydentate phosphazene-containing extractant. Gels 2022, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.P.; Lee, M.S. Separation of Pt(IV) and Pd(II) from the loaded Alamine 336 by stripping. Hydrometallurgy 2011, 109, 181–184. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Ionic liquids as components of systems for metal extraction. ChemEngineering 2022, 6, 6. [Google Scholar] [CrossRef]


| Kinetic Parameters | Values |
|---|---|
| Rate constant (k), h−1 | 0.158 |
| Permeability coefficient (P), μmol∙s−1 | 3.50 |
| Kinetic Parameters | Pd(II) | Pt(IV) | Selectivity Order and Selectivity Coefficient |
|---|---|---|---|
| Rate constant (k), h− | 0.079 | 0.06 | Pd(II) > Pt(IV) |
| Permeability coefficient (P), μmol∙s−1 | 1.75 | 1.32 | SPd/Pt = 1.3 |
| Cycle Number | Initial Flux, μmol∙s−1∙m−2 |
|---|---|
| 1 | 3.5 |
| 2 | 3.1 |
| 3 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pospiech, B. Effective Transport Recovery of Palladium(II) from Hydrochloric Acid Solutions Using Polymer Inclusion Membrane with Tetrabutylammonium Bromide. Molecules 2024, 29, 3009. https://doi.org/10.3390/molecules29133009
Pospiech B. Effective Transport Recovery of Palladium(II) from Hydrochloric Acid Solutions Using Polymer Inclusion Membrane with Tetrabutylammonium Bromide. Molecules. 2024; 29(13):3009. https://doi.org/10.3390/molecules29133009
Chicago/Turabian StylePospiech, Beata. 2024. "Effective Transport Recovery of Palladium(II) from Hydrochloric Acid Solutions Using Polymer Inclusion Membrane with Tetrabutylammonium Bromide" Molecules 29, no. 13: 3009. https://doi.org/10.3390/molecules29133009
APA StylePospiech, B. (2024). Effective Transport Recovery of Palladium(II) from Hydrochloric Acid Solutions Using Polymer Inclusion Membrane with Tetrabutylammonium Bromide. Molecules, 29(13), 3009. https://doi.org/10.3390/molecules29133009







