Abstract
The chromenopyridine scaffold represents an important class of heterocyclic compounds exhibiting a broad spectrum of biological properties. This review describes novel and efficient procedures for the synthesis of this scaffold. Herein, several methods were detailed and grouped according to their starting material (e.g., salicylaldehydes, chromones, chromanones and coumarins) and respective biological activity, when reported. This review highlights the potential of the reported synthetic strategies for preparing chromenopyridine derivatives with promising biological activity, paving the way for further developments in drug discovery.
1. Introduction
Drug discovery and development is a long and difficult process and medicinal chemists have been searching for inspiration in nature and synthetic compounds, with proven biological potential, to develop novel and significant molecules [1,2]. Indeed, a number of molecular scaffolds present in many natural and synthetic compounds have proven to be excellent promoters for hit/lead development [1,2,3,4,5]. Several “privileged structures”, an expression introduced by Evans et al. in 1988, are nowadays used as templates for the development of organic compounds with impacts in biology and medicine [6]. The use of these privileged scaffolds to construct disruptive compound libraries by enhancing structural diversity and physicochemical properties have ultimately led to highly potent and safe bioactive compounds.
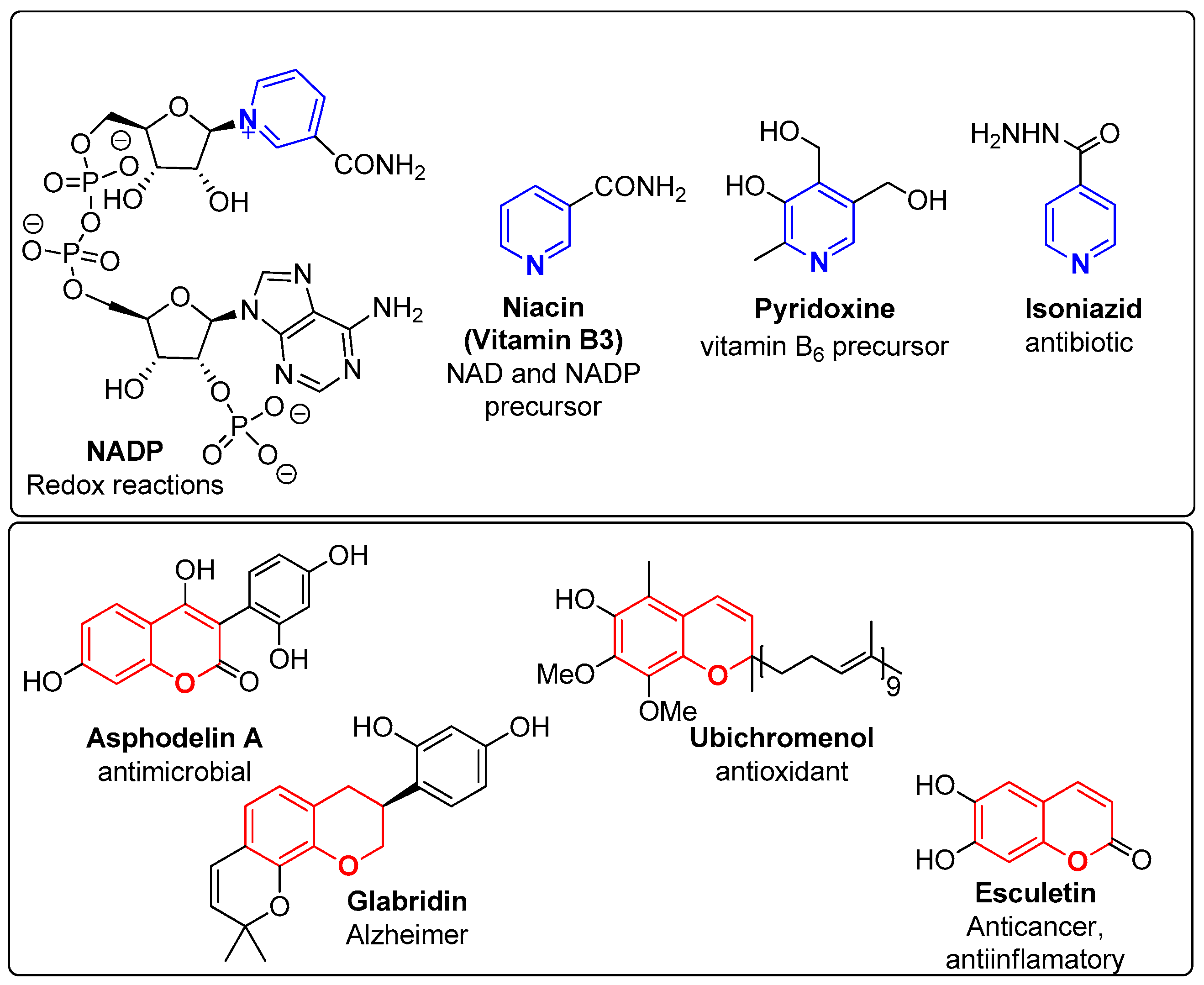
The pyridine unit can be found in natural and synthetic compounds, fused to other motifs, giving rise to compounds with interesting biological profiles [5,7,8,9,10] and is therefore considered an attractive scaffold [11,12]. This heterocyclic unit is present in a number of natural products such as nicotinamide adenine dinucleotide phosphate (NADP), vitamins B3 (niacin) and B6 (pyridoxine), in the alkaloid nicotine, as well as in several FDA-approved commercial drugs such as the antituberculotic isoniazid (Figure 1) [13]. Synthetic compounds incorporating the pyridine unit have also been developed and have presented diverse biological applications [2,3,4,5]. The synthesis of compounds bearing a 2,4-disubstituted pyridine ring as the central building block, presenting a potent inhibitory activity of the H4 histamine receptor (K1 < 10 nM), has been patented by Janssen Pharmaceutica [8,14]. Baraldi et al. [9] also reported the isolation of heterocyclic compounds bearing a pyridine ring fused with other aromatic moieties, such as pyrazolopyridines and imidazopyridines. These compounds proved to be potent antagonists of A1 and A2A adenosine receptors, important receptors for the treatment of several diseases such as chronic obstructive pulmonary disease (COPD), asthma and others. More recently, the work of Martinez-Gualda et al. [10], involving new isothiazolo [3,4-b]pyridines, has led to the discovery of new selective inhibitors of the cyclin G-associated kinase (GAK), which is associated with the treatment of viral infections caused by hepatitis C, dengue, the Zika virus from the Flaviviridae family, the Ebola filovirus and others. Radwan et al. [15] recently reported the synthesis, molecular docking studies and antimicrobial activity of new tetrasubstituted pyridines. In relation to pyrazolo [3,4-b]pyridines, Jian et al. [16] described the synthesis of these compounds based on the combretastatin structure, which were tested for their antiproliferative and tubulin polymerization inhibitory activities and demonstrated their potential for further development as novel anticancer agents.
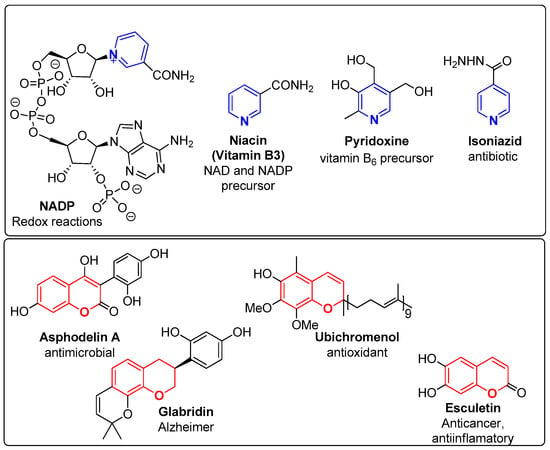
Figure 1.
Chemical structure of natural and synthetic pyridines (Blue) and chromenes (Red) with diverse biological applications.
On the other hand, the chromene unit comprises heterocyclic compounds that incorporate a benzene ring fused to a pyran nucleus. Chromenes are present in several natural and synthetic compounds [17], and have been widely studied as therapeutic agents due to their low toxicity, especially reported for natural derivatives, and the versatility of their pharmacological applications [17]. Similarly to the pyridine moiety, the chromene scaffold appears in the literature as an important building block for the development of new bioactive compounds. Various chromene-based compounds have revealed interesting anticancer [18,19,20,21,22,23,24], antimicrobial [17,25,26,27], antibacterial [25,28,29] and antioxidant [27,30] activity, as well as other relevant biological applications, such as monoamine oxidase inhibitors [31]. Haiba et al. [23] detailed a series of benzo[f]chromenes with cytotoxic activity against HepG-2 and MCF-7 cancer cell lines. Abu El-Azm et al. [19] reported a series of benzo[f]chromene, chromeno [2,3-d]pyrimidine and chromenotriazolo [1,5-c]pyrimidine derivatives with relevant antiproliferative activity against MCF-7, HePG2 and WI-38 cancer cell lines, stating that the most probable mechanism of action may involve tubulin inhibition. Thahn et al. [29] described a click chemistry approach to synthesize 1H-1,2,3-triazole-tethered 4H-chromene-D-glucose conjugates with remarkable activity against Gram-positive and Gram-negative bacteria, which has also been tested with fungi. Well-known examples of natural chromene-based compounds are esculetin (anticancer agent), asphodelin A (antimicrobial agent), ubichromenol (antioxidant agent) and glabridin (Alzheimer disease) (Figure 1).
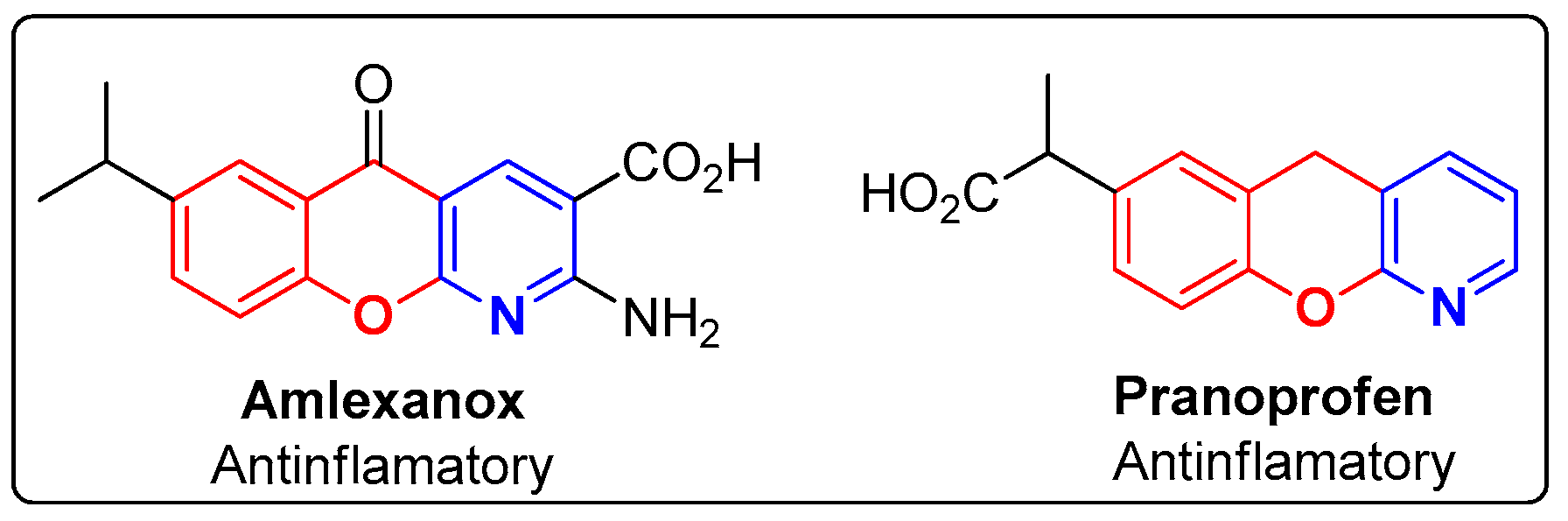
The design and synthesis of innovative compounds, combining the pyridine and chromene moieties, have been extensively reported in the literature, and these diversified structures have proven their potential as antinociceptive [32,33,34], alpha adrenergic antagonist [35], bronchodilator [36,37,38], antimicrobial [39] and antibiotic [40] agents, among others [41], including in FDA-approved drugs (Figure 2). Amlexanox, an antiallergic drug with clinical effectiveness against atopic diseases, allergic asthma and rhinitis, is a molecule with a chromenopyridine skeleton. Pranoprofen, a non-steroidal anti-inflammatory drug (NSAID) used in ophthalmology, constitutes another example of a chromenopyridine-based FDA approved drug [42].
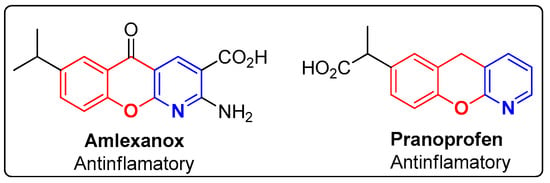
Figure 2.
FDA-approved compounds based on the chromenopyridine moieties.
This review is focused on these attractive scaffolds and presents a collection of synthetic methods for the preparation of chromenopyridines, including their potential as scaffolds for the development of novel bioactive compounds. A previously published review article, by Nunez-Vergara et al. [41], presents a good coverage of the synthesis of chromenopyridines up to 2011, which was mainly based on ANRORC-type mechanisms. This present work encompasses the latest updates on the involvement of chromenopyridines in the preparation of new compounds with biological activity, ranging from 2010 to 2023.
2. Synthesis and Biological Activity of Chromenopyridines
The different synthetic approaches to prepare this compound family were organized according to the starting material used. These include salicylaldehydes, chromones, chromanones, coumarins and miscellaneous/other sources. Whenever available, the biological activity of these chromenopyridines was reported, and also the reaction mechanism responsible for their formation.
2.1. Salicylaldehydes
The synthesis of chromenopyridines using salicylaldehydes as the starting material typically comprises a multi-component reaction (MCR), most commonly a three-component reaction, involving the appropriate salicylaldehyde, an active methylene compound and a nucleophile, under base catalysis. The presence of the nucleophile is paramount, as it promotes the intramolecular cyclization of the Knoevenagel adduct formed from the reaction between the active methylene compound and the salicylaldehyde into the final chromenopyridine scaffold.
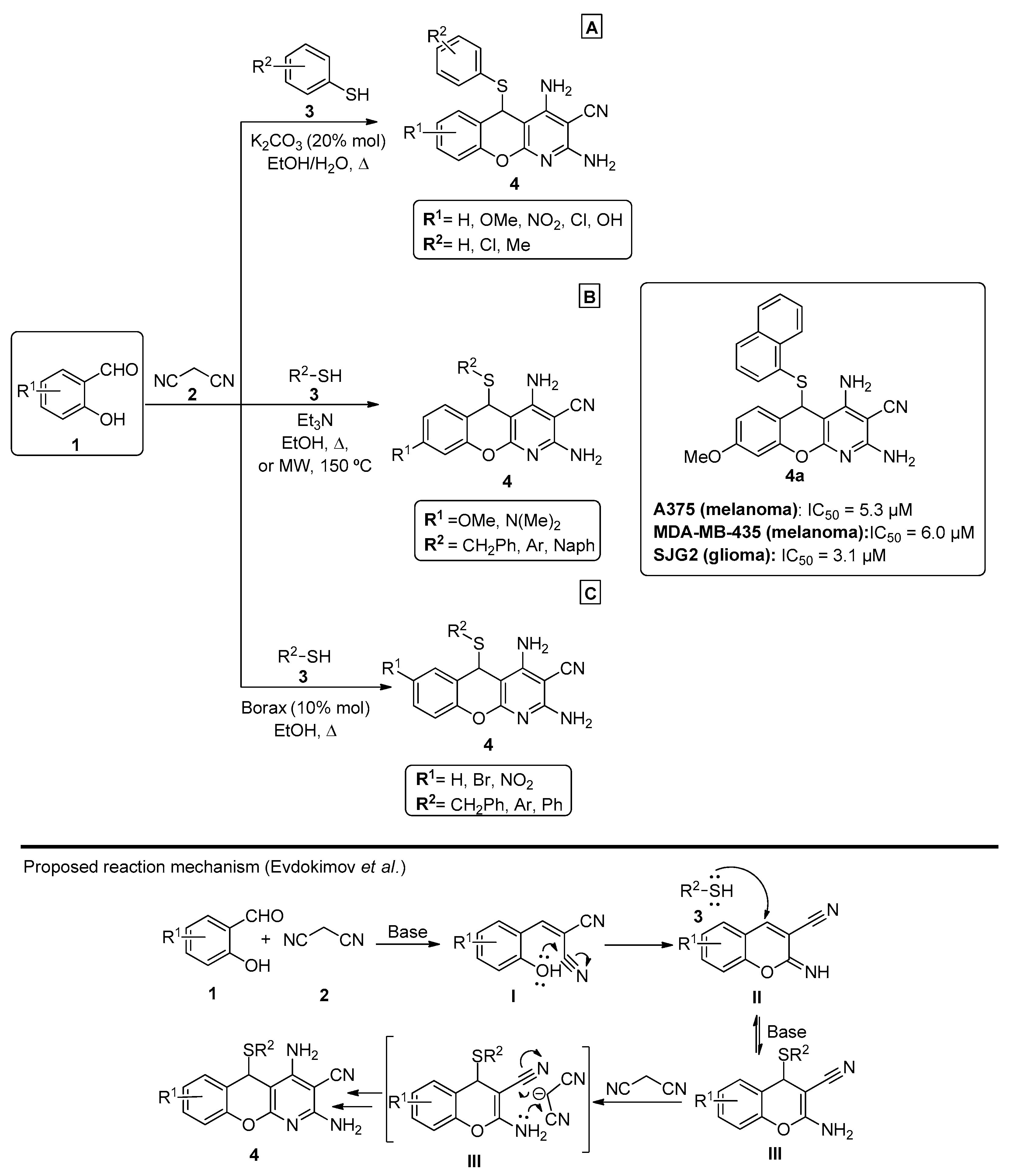
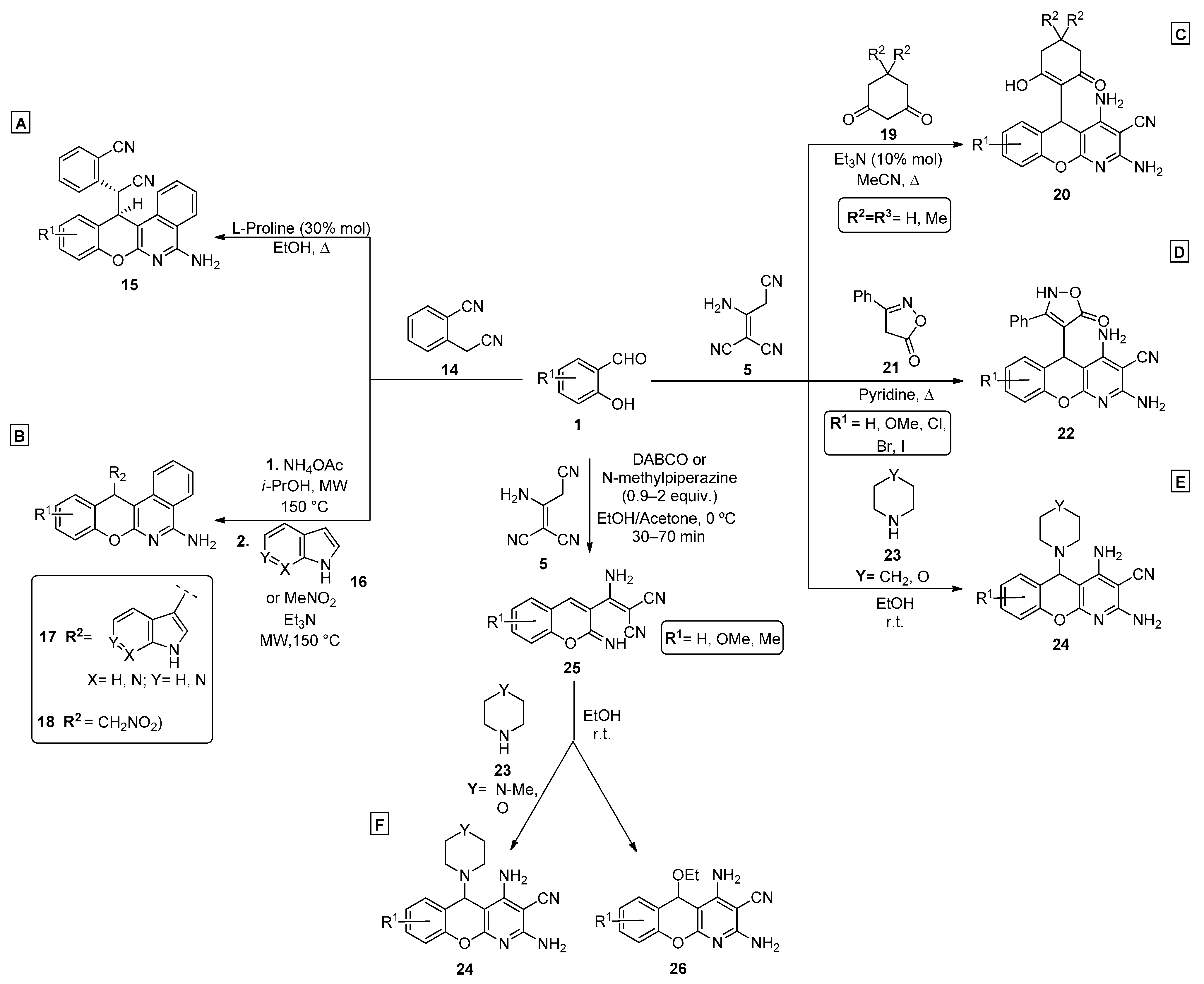
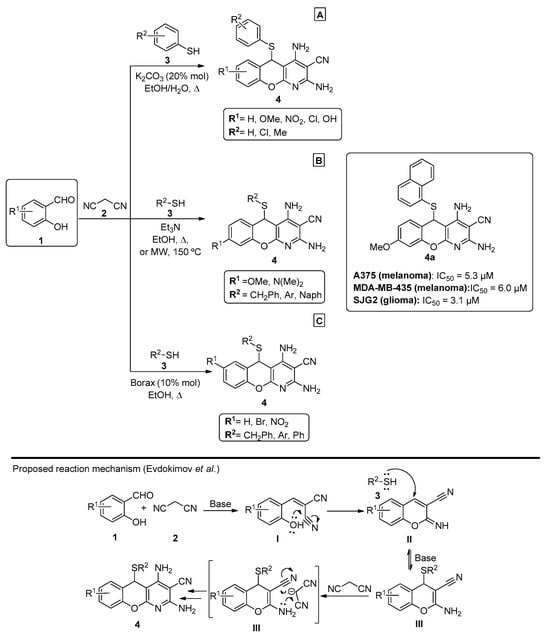
Regarding the use of malononitrile and its dimer, a 2011 publication by Mishra et al. [43] proposed an improvement to the method previously reported by Evdokimov et al. in 2006 and 2007 [12,44]. In this approach, a one-pot three-component reaction was explored using salicylaldehydes 1, malononitrile 2 and different thiols 3, in EtOH/H2O and under K2CO3 (20% mol) catalysis. In their work, the authors compared different bases that were previously reported for pyridine synthesis, including those evaluated in Evdokimov’s study, and concluded that K2CO3 afforded better reaction yields (up to 92%). Several chromenopyridines 4 were prepared in good-to-high yields (63–92%), under reflux in EtOH/H2O (Scheme 1A). A similar work was later reported by Banerjee et al. [45] (Scheme 1B), using triethylamine and either refluxing ethanol (88–90% yield) or microwave irradiation at 150 °C (30–45% yield). The anticancer potential of the compounds was tested for a series of melanoma and glioma cell lines, from which compound 4a (R1 = OMe, R2 = Nap) was highlighted (Scheme 1B). A more recent publication by Molla et al. [46] showed good-to-high yields (85–91%) of chromenopyridine 4 when the reaction was performed using borax (10% mol) as a catalyst (Scheme 1C).
Scheme 1.
Preparation of chromenopyridines 4 with the anticancer potential (IC50) of compound 4a highlighted and the reaction mechanism proposed by Evdokimov et al. (adapted from Refs. [12,43,44,45,46]). (A)—Mishra et al.; (B)—Banerjee et al.; (C)—Molla et al.
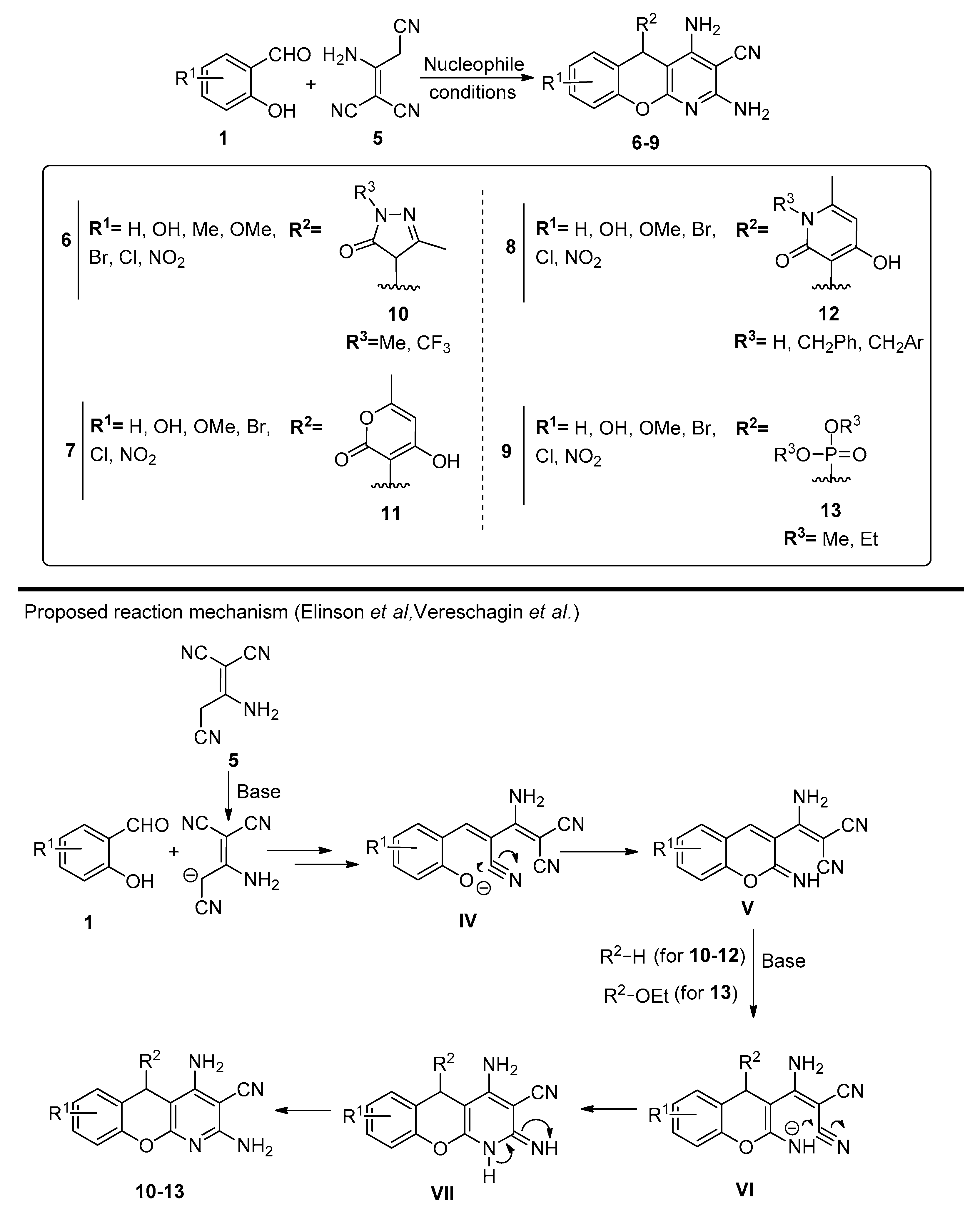
Several studies by Vereschagin et al. and Elinson et al. [47,48,49,50,51,52,53,54,55,56] report the preparation of new chromenopyridines from a three-component reaction involving the dimer of malononitrile, 2-aminoprop-1-ene-1,1,3-tricarbonitrile. This dimer can be previously synthesized or generated in situ [48], and was reacted with salicylaldehydes 1 by Knoevenagel condensation. Formation of the 3-substituted 2-iminochromene, followed by intramolecular cyclization and Michael addition of a nucleophile to the chromene ring generated the chromenopyridine scaffold (Scheme 2). These reactions are characterized by their simplicity, facile work-up methods and high atom economy. Over the last 12 years, several studies have used this methodology to prepare different substituted chromenopyridines, mainly varying the reaction conditions and the nucleophile used, usually under basic conditions. Elinson et al. published a selection of articles on this subject [50,51,52,53,54,56], using different nucleophiles and reaction conditions.
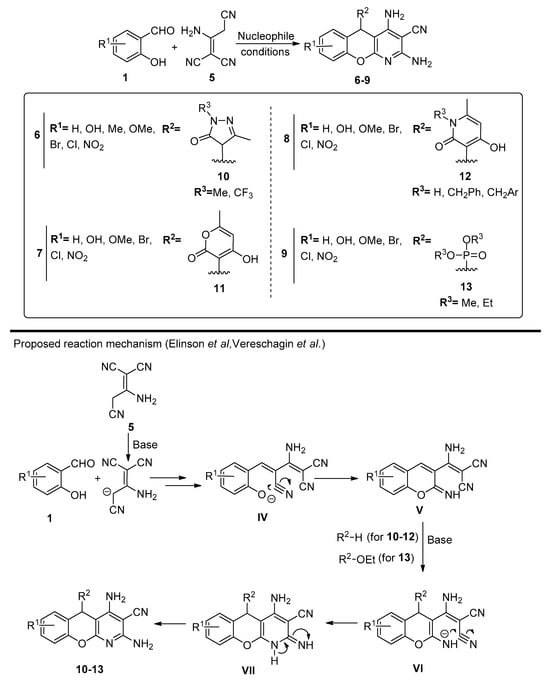
Scheme 2.
Preparation of chromenopyridines 6-9 and reaction mechanism proposed by the authors (adapted from Refs. [47,48,49,50,51,52,53,54,55,56]). Reaction conditions: synthesis of 6: 10, Et3N (10% mol), PrOH or MeCN; synthesis of 7: 11, EtOH/pyridine (3:1), 80 °C; synthesis of 8: 12, EtOH/pyridine (3:1), 80 °C; synthesis of 9: 13, morpholine (10% mol), MeCN, reflux (adapted from Refs. [46,47,48,49,50,51,52,53,54,55,56]).
The synthesis of chromenopyridines 6 was performed by reaction of salycilaldehydes 1, 2-aminoprop-1-ene-1,1,3-tricarbonitrile 5 and pyrazoline-5-ones 10, under triethylamine catalysis (10% mol), in either propanol or acetonitrile, in high-to-almost quantitative yields [50,51]. Chromenopyridines 7 and 8 were also prepared in good yields (49–85% for 7, up to 97% for 8) by reacting salicylaldehydes 1, 2-aminoprop-1-ene-1,1,3-tricarbonitrile 5 and 4-hydroxy-6-methyl-2H-pyran-2-one 11 or substituted 4-hydroxy-6-methylpyridin-2(1H)-ones 12, in a mixture of EtOH/pyridine 3:1 at 80 °C [53]. Chromenopyridines 9 [54,56] were prepared in good-to-high yields (56–86%) by a similar approach, using trialkyl phosphites 13 as nucleophiles, with morpholine (10% mol) catalysis and under reflux conditions in acetonitrile.
The use of homophtalonitrile 14 instead of malononitrile or derivates, as a precursor in the synthesis of chromenopyridines, has also been studied in the past years. Two recent publications [57,58] describe the experimental conditions to prepare chromenopyridines 15, 17 and 18 by a multicomponent reaction involving 14, salicylaldehydes 1 and different nucleophiles (Scheme 3A,B). The proposed methodologies are conceptually similar to those involving other active methylene compounds and are also based on the formation of an adduct from Knoevenagel condensation, followed by a tandem intramolecular cyclization promoted by Michael addition of a nucleophile, leading to the formation of the chromenopyridine scaffold. For the synthesis of compound 15, salicylaldehydes 1 and two equivalents of homophtalonitrile 14 were refluxed in ethanol, under L-Proline (30% mol) catalysis. The authors suggest that L-proline may aid in the Michael addition step of the second homophtalonitrile unit, acting as a base (Scheme 3A). For the synthesis of compounds 17 and 18, the best results were obtained by a two-step process where homophtalonitrile 14 and the appropriate salicylaldehyde 1 were reacted at 150 °C for 10 min, under microwave irradiation and ammonium acetate catalysis. Addition of an excess of the appropriate nucleophile (16) and one equivalent of triethylamine and heating at 150 °C for a further 10 min period, under microwave irradiation, led to product 17 (Scheme 3B).
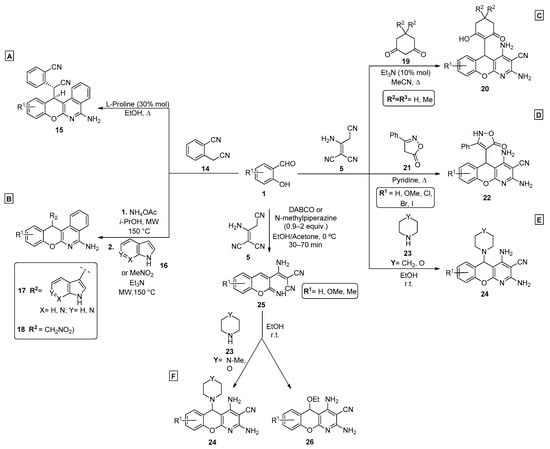
Scheme 3.
Preparation of chromenopyridines 15, 17, 18, 20, 22, 24, and 26. (A,B)—Festa et al.; (C,D)—Vereshchagin et al.; (E)—Shaabani et al.; (F)—Lopes et al. (adapted from Refs. [47,48,49,57,58,59,60]).
Vereshchagin et al. [48,49], in collaboration with Elinson’s group, developed similar procedures to prepare chromenopyridines from malononitrile dimer in a one-pot three-component reaction. One of the methods [49] combined salicylaldehydes 1, aminoprop-1-ene-1,1,3-tricarbonitrile 5 and 1,3-cyclohexanediones 19 in acetonitrile and under reflux conditions, with a catalytic amount of triethylamine, to prepare chromenopyridines 20 in good-to-high yields (59–88%) (Scheme 3C). Molecular docking studies of compounds 20 with mitogen-activated protein kinases 1 and 2 binding pockets showed favorable binding free energy values, which highlight their biological potential for the development of anticancer agents. In another work [48], the preparation of chromenopyridines 20 involved the use of malononitrile and formation of the dimer was considered to occur in situ under the experimental conditions that were used. This study showed that the best results were obtained when using triethylamine (10% mol) and acetonitrile, under reflux, leading to compound 20 in 53–87% yields. The same author also presented a method [47] to prepare chromenopyridines 22, by performing the reaction with 5 and 3-phenylisoxazol-5(4H)-one 21 in pyridine and under reflux, where pyridine behaved both as solvent and catalyst (Scheme 3D). The final products were obtained in 65–74% yields. A different work, by Shaabani et al. [59], described a multicomponent reaction involving salicylaldehydes 1, aminoprop-1-ene-1,1,3-tricarbonitrile 5 and secondary cyclic amines 23 (Scheme 3E). With this method, piperidine and morpholine were incorporated into the chromenopyridine scaffold, by reacting as nucleophiles in the intramolecular cyclization steps, in a reaction performed in ethanol, at room temperature, affording chromenopyridines 24 in high yields (80–94%).
A similar work was published by Lopes et al. [60], where different nucleophiles were incorporated in the chromenopyridine moiety. The procedure involved a two-step reaction that starts from 2-aminoprop-1-ene-1,1,3-tricarbonitrile 5 and salicylaldehydes 1, from which compound 25 was isolated as a pure product in 71–84% yields only when the reaction was performed at 0 °C. Intramolecular cyclization involving either N-methylpiperazine, morpholine, or ethanol, acting as nucleophiles, resulted in the final chromenopyridine scaffold (compounds 24 and 26, Scheme 3F). A selection of these compounds were tested for their anticancer activity against breast cancer cell lines where an interesting anticancer profile for the luminal breast subtype was identified for derivative 24 (R1 = Me or OMe, Y = N-Me). These compounds presented the capacity to inhibit cell proliferation (low micromolar range) and induced cell cycle arrest, promoting apoptosis and microtubule destabilization, having also demonstrated a good safety profile in the in vivo C. elegans toxicity assay.
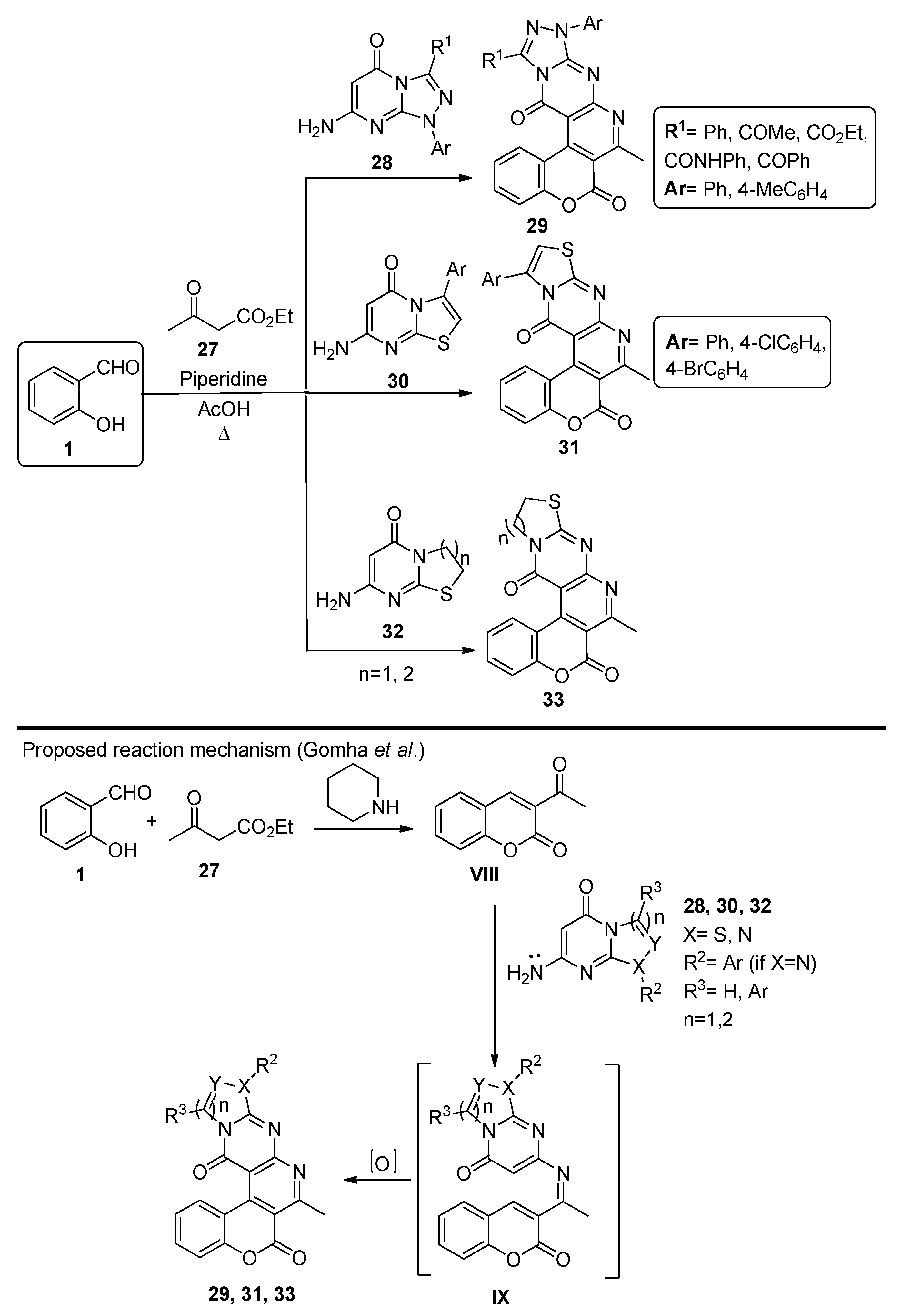
In 2014, Gomha et al. [61] developed a three-component reaction involving salicylaldehyde, ethyl acetoacetate 27 and compounds 28, 30 and 32, to originate chromenopyridines 29, 31 and 33, respectively (Scheme 4). The reaction occurred under reflux conditions, using acetic acid as solvent and with piperidine catalysis, and afforded compounds 29, 31 and 33 in 66–77, 73–84, and 73–78% isolated yields, respectively. The authors proposed that the reaction starts with the base-catalyzed condensation of salicylaldehyde 1 with ethyl acetoacetate 27, followed by intramolecular cyclization involving the ester group and elimination of ethanol. Addition of the heterocyclic enamine afforded the chromenopyridines 29, 31, or 33.
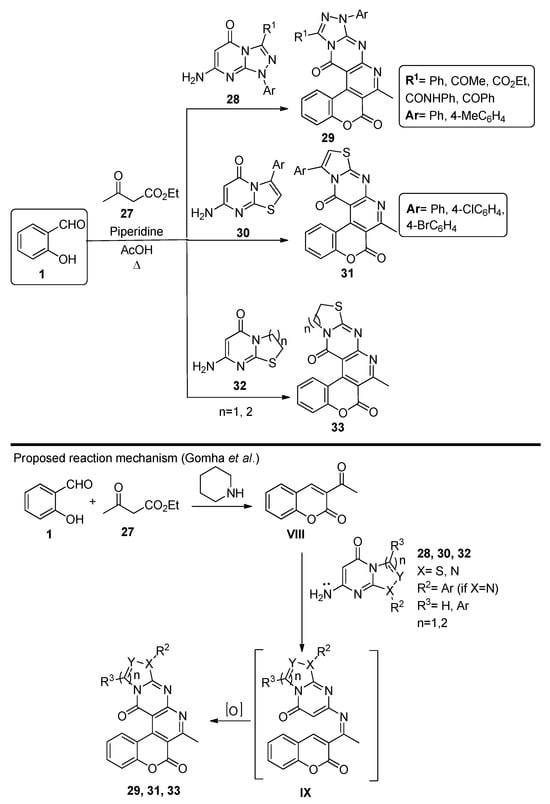
Scheme 4.
Preparation of chromenopyridines 29, 31 and 33 and reaction mechanism proposed by Gomha et al. (adapted from Ref. [61]).
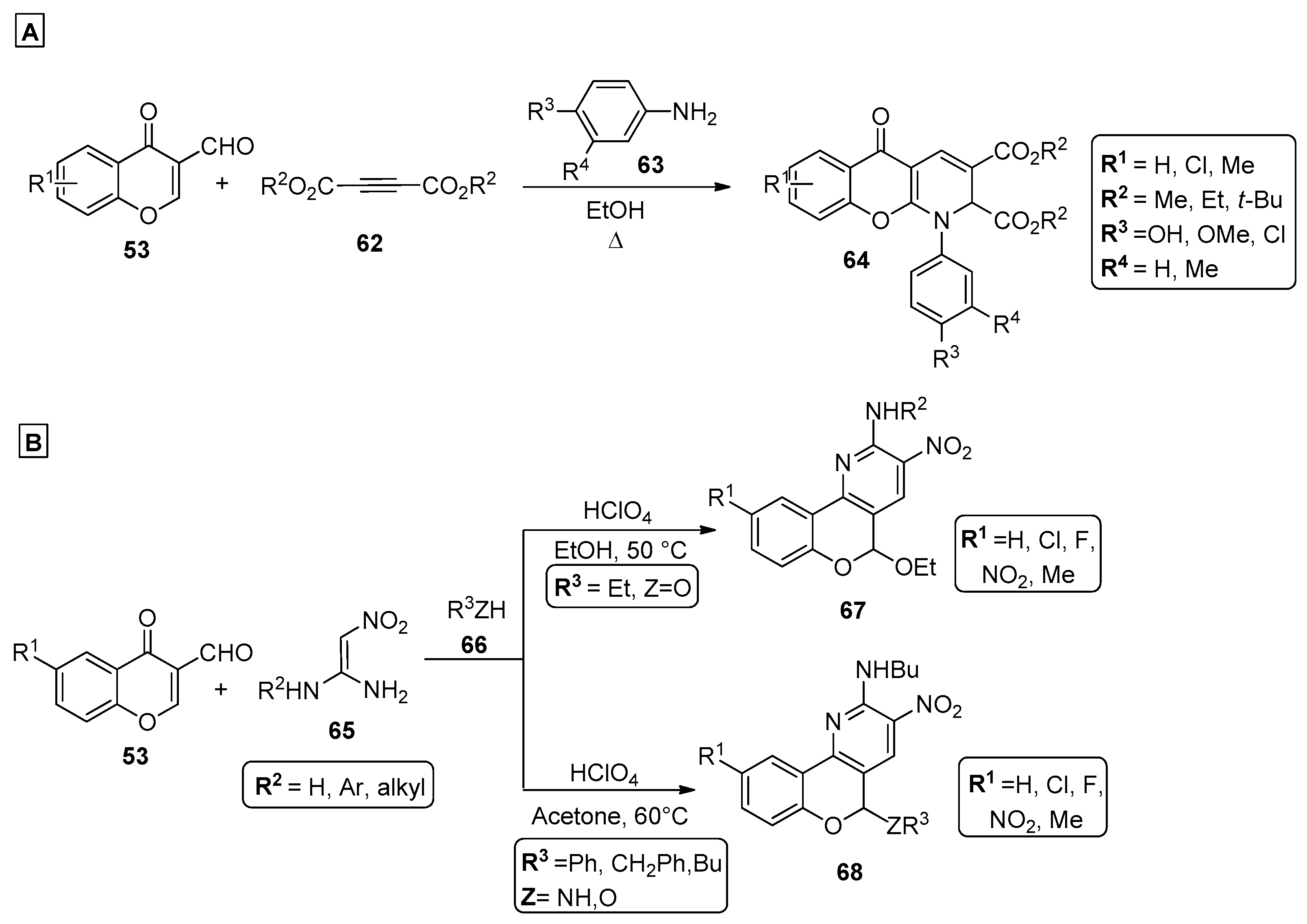
In the typical Hantzsch pyridine synthesis, a one-pot three-component reaction between an aldehyde, two molar equivalents of a β-ketoester and a nitrogen donor occurs to generate the pyridine ring. This procedure was directly applied to the synthesis of chromenopyridines by using salicylaldehydes. In their work, Navarrete-Encina et al. demonstrated that chromeno [3,4-c]pyridine-3-carboxylates 35 and chromeno [4,3-b]pyridine-3-carboxylates 37 could be synthetized from the reaction of salycilaldehydes 1 with either two equivalents of ethylaminocrotonate 34 or three equivalents of ethyl acetoacetate 36, respectively (Scheme 5A,B) [62]. The authors suggested that the reaction was initiated by the formation of the benzopyran ring by condensation of 36 with the aldehyde. The formation of the pyridine ring started by a 1,4-addition of either the Cα or the amino group of ethylaminocrotonate 34, to the benzopyran C4 atom. This determined the regioselectivity of the general process that is mainly favored by the reaction conditions used. The use of ethylaminocrotonate 34 in a reaction performed at 60 °C, and using acetic acid as solvent, led to the formation of regioisomer 35 (Scheme 5A). Reacting salicylaldehyde 1 with ammonium acetate and ethyl acetoacetate 36 under reflux, in a 1:1 mixture of acetic acid/ethanol, led to the formation of regioisomer 37, which was further oxidized to the final chromenopyridine moiety (38, Scheme 5B).

Scheme 5.
Preparation of chromenopyridines 35, 37, 38 and 42 (adapted from Refs. [62,63]). (A,B)—Navarrete-Encina et al.; (C)—Adolfsson et al.
The Povarov reaction [63] is a useful tool to prepare nitrogen-containing six-membered rings through a cycloaddition reaction. This method has been recently used by Adolfsson et al. [64] to generate the chromenopyridine-fused 2-pyridones 42 by the reaction of amino-2-pyridones 40 and O-alkylated salicylaldehydes 41 under BF3·OEt2 catalysis, followed by DDQ oxidation (Scheme 5C). Using this methodology, 11 derivatives of 42 (R1 = Ar, R2 = H, cPr) were prepared in good-to-high yields (53–93%) when the O-alkylated salicylaldehyde 41 contained a terminal allyl moiety, while 5 other derivatives of 42 (R1 = H, R2 = H, cPr) were isolated in low-to-moderate yields (13–54%) from the O-alkylated salicylaldehyde 41.
2.2. Chromones
Chromones, and in particular substituted chromones, have been widely used as precursors for the chromenopyridine scaffold and most of the reactions proceed through an ANRORC-type mechanism [43]. The first reaction step typically involves Michael addition of an active methylene compound to either position 2 or position 4 of the chromone derivative. This is followed by ring opening, bond rotation and ring closure to generate the chromene ring. For the formation of the chromenopyridine ring, the chromone precursor is typically substituted with a nitrile group at position 3 that will afford the nitrogen in the pyridine moiety.
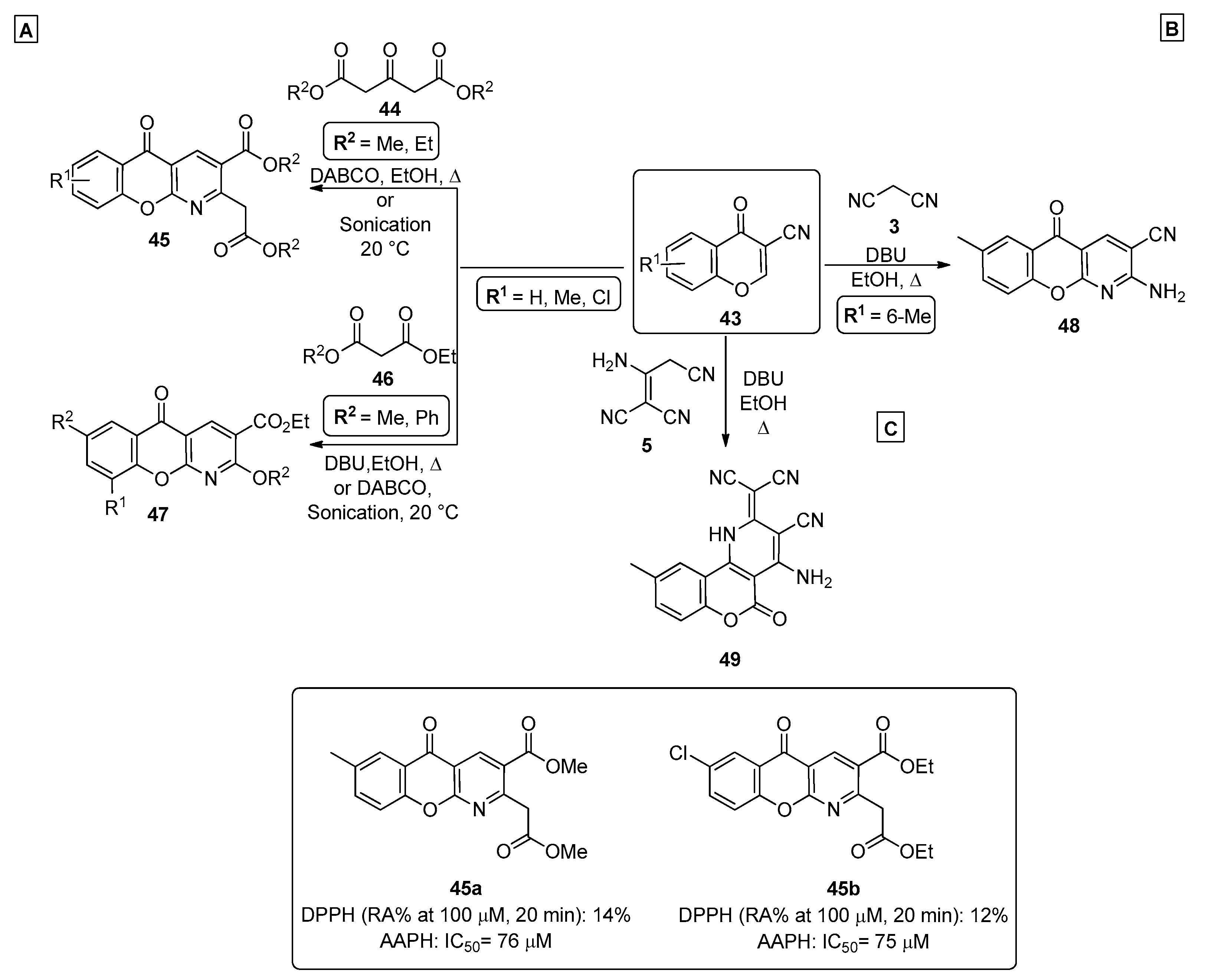
In 2014, Dimitriadou et al. [65] prepared a series of chromenopyridine derivatives 45 and 47, by reacting 3-cyanochromone 43 with dimethyl- or diethyl 3-oxopentanedioates 44 or 1,3-dicarbonyl compounds 46 in the presence of base catalysis (DABCO or DBU) (Scheme 6A). Products 45 and 47 were prepared from chromone 43 under reflux in ethanol in overall moderate-to-good yields (47–54%). For these products, an additional method using ultrasound sonication and DABCO, at 20 °C, was also reported, leading to higher reaction yields (70–78%). A batch of compounds 45 and 47 was prepared and tested for their antioxidant activity, where their capacity to interact with 1,1-diphenylpicrylhydrazyl (DPPH) and with the azo compound 2,2″-azobis(2-amidinopropane) dihydrochloride (APPH) was measured. The results were compared with a standard, nordihydroguairetic acid (NDGA). The best results were obtained for derivatives 45a (R1 = 7-CH3, R2 = CH3) and 45b (R1 = 7-Cl, R2 = CH2CH3).
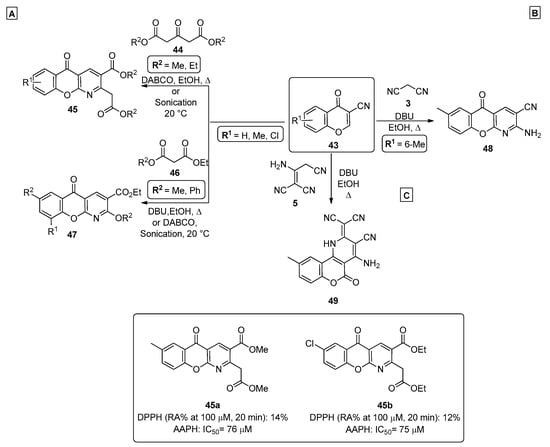
Scheme 6.
Preparation of chromenopyridines 45, 47, 48 and 49 and antioxidant activity (IC50, RA = reducing agent) of compounds 45a and 45b highlighted (adapted from Refs. [65,66,67]). (A)—Dimitriadou et al.; (B,C)—Ibrahim et al.
Ibrahim et al. reported the synthesis of diverse chromenopyridines by a cascade process starting from a 3-cyanochromone 43 and involving ring opening of the pyran ring initiated by nucleophilic attack of the malononitrile anion (Scheme 6B) [66]. This is followed by intramolecular cyclization, with nucleophilic attack of the phenolate oxygen to the nitrile group in position 3, generating an imine that cyclized through nucleophilic attack to another nitrile group. The reaction was performed in ethanol, under reflux, using DBU as a base, leading to chromenopyridine 48. Ibrahim et al. also reported the preparation of new chromenopyridine derivatives 49 by reacting 6-methylchromone-3-carbonitrile 43 with the malononitrile dimer 5 [67]. The reaction followed an ANRORC-type mechanism, where the nitrile group was involved in the formation of the pyridine ring, fused to C3 and C4 carbons of the newly formed coumarin ring (Scheme 6C).
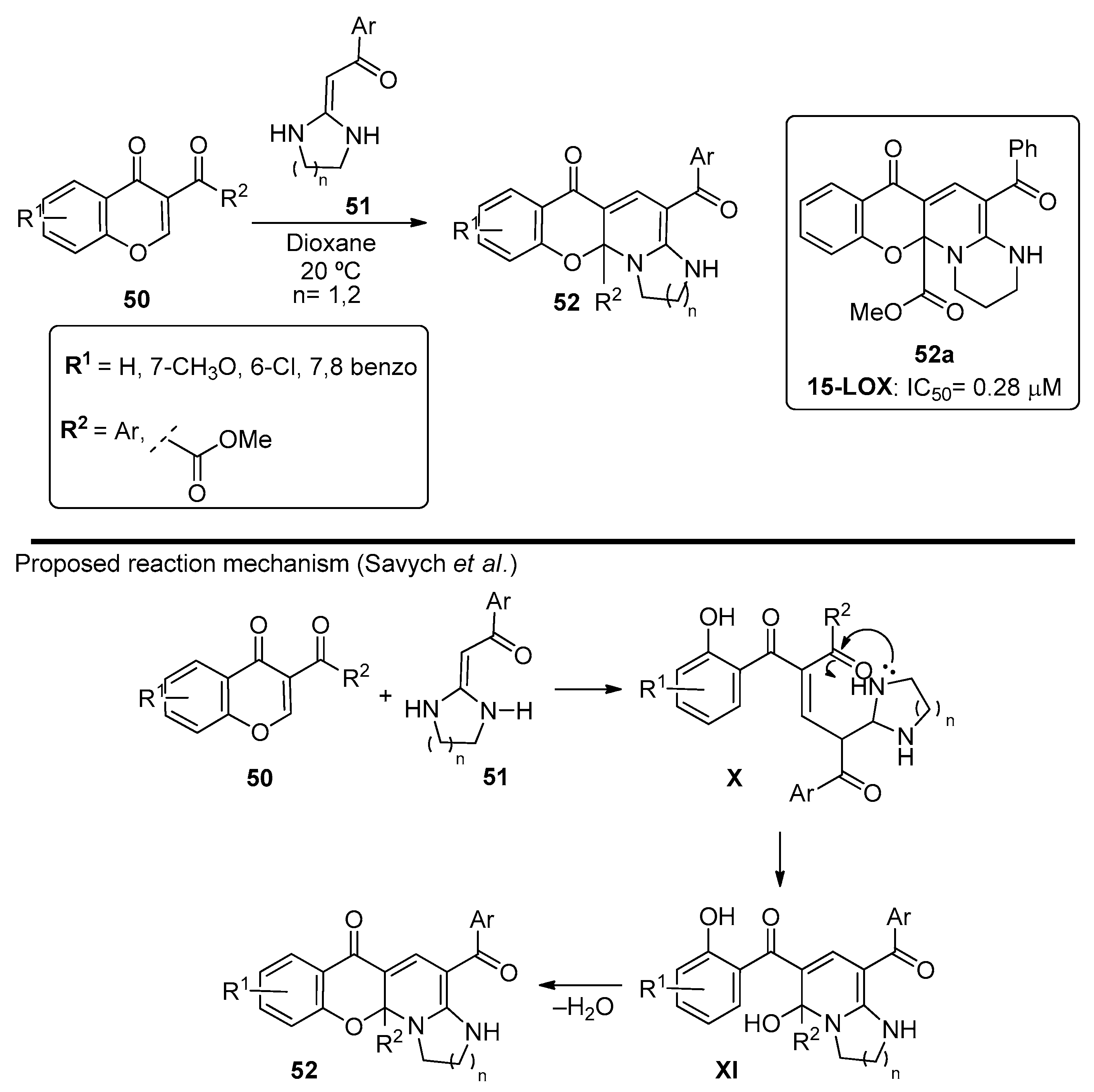
The synthesis of tetracyclic chromonopyridines via Michael addition using heterocyclic ketene aminals as nucleophiles was also reported by Savych et al. [68]. The chromonopyridine structure was generated from nucleophilic attack of the aminal 51 to chromones 50. Ring opening of the pyran ring followed by ring closure to the ketone in the 3-position ultimately led to chromenopyridines 52 (Scheme 7) in good-to-high yields (75–92%). These compounds were studied by DFT and molecular docking calculations, and the structure was confirmed by X-ray crystallography. The anti-inflammatory properties were also assessed through 15-Lipoxigenase (15-LOX) inhibition studies, and the best results (56-fold higher than the standard, quercetin) were obtained for R1 = H, R2 = COOCH3, Ar = Ph, and n = 2 (Scheme 7, compound 52a).
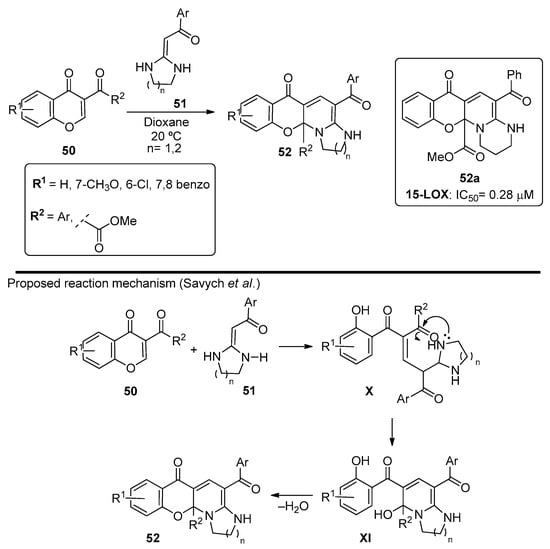
Scheme 7.
Preparation of chromenopyridines 52 with the antioxidant activity (IC50) of compound 52a highlighted and reaction mechanism proposed by Savych et al. (adapted from Ref. [68]).
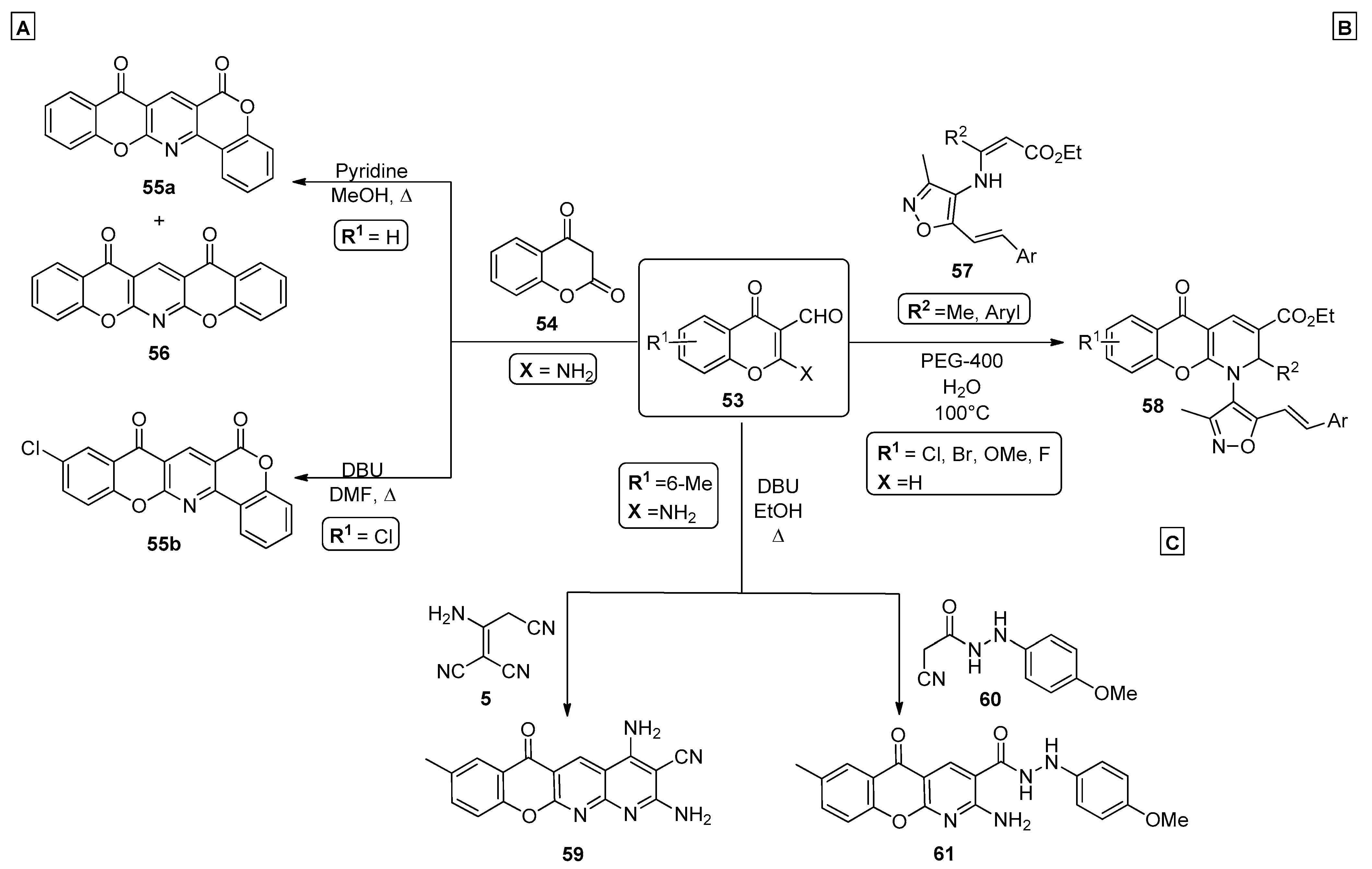
A simple and convenient procedure for the preparation of chromenopyridine derivatives involves the formation of the pyridine ring either by reaction of a chromone with a nucleophile incorporating an amino group or by reaction of a chromone incorporating an amino group with a nucleophile. For example, Siddiqui et al. [69] synthesized two new chromenopyridines (mixture of 55a and 56) in a 1:2 ratio by reaction of 2-amino-4-oxo-4H-chromene-3-carbaldehyde 53 with chroman-2,4-dione 54, in refluxing methanol and in the presence of a catalytic amount of pyridine (Scheme 8A). In 2016, Ibrahim et al. [70] developed a simple condensation procedure to afford pentacyclic chromenopyridines 55b from the DBU-catalyzed reaction of 4H-chromene-3-carbaldehyde 53 and chroman-2,4-dione 54. By performing the reaction in DMF and under reflux conditions, compound 55b was obtained in a 54% yield (Scheme 8A). Further optical studies proceeded, and the results indicated that this scaffold could be tuned to enhance its photochemical properties, to be used as a photodetector.
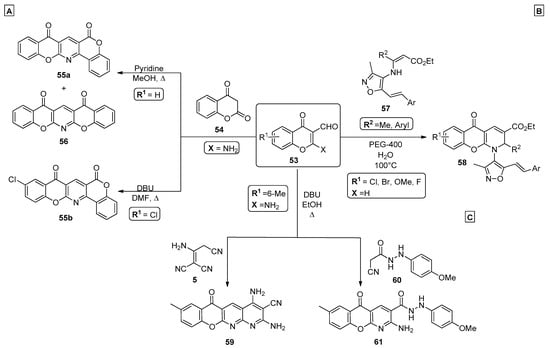
Scheme 8.
Preparation of chromenopyridines 55, 56, 58, 59 and 61 (adapted from Refs. [67,69,70,71]). (A)—Siddiqui et al., Ibrahim et al.; (B)—Pondurii et al.; (C)—Ibrahim et al.
Ponduri et al. explored the reactivity of isoxazolyl enamino esters 57 with 3-formylchromones 53 to generate new chromenopyridine-based compounds 58 (Scheme 8B) [71]. Optimization of the reaction conditions showed that water was the best solvent, while the use of PEG-400 (10% mol) catalysis at 100 °C was also optimal. This procedure allowed the preparation of isoxazolyl-substituted chromenopyridines 58 in good-to-high yields (75–92%). Mechanistically, it was suggested that PEG catalyzes the condensation of isoxazolyl enamino esters and 3-formylchromones by facilitating the nucleophilic attack between the enamine nucleophile 57 and the aldehyde group of 53. After condensation, a 6π-electrocyclization occurs to afford the pyridine ring of 58. Alternatively, the chromenopyridine can be formed by condensation of a substituted 2-aminochromone with a nucleophile, followed by an intramolecular cyclization reaction. In these reactions, the nitrogen of the pyridine ring can come from the chromone or chromane moiety, typically in the form of an amine group, or from the nucleophile that is condensed with the chromene ring. Ibrahim et al. also reported the preparation of new chromenopyridine derivatives 59 by reacting 4H-chromone 53 with the malononitrile dimer 5 (Scheme 8C). Compound 53 was also reacted with 2-cyano-acetohydrazide 60 in the presence of DBU and reflux in ethanol, to afford chromenopyridines 61 (Scheme 8C) [67].
In a methodology reported by Dolatkhah et al. [72], benzochromonodiazocines were reported as the only product of the reaction of 3-formylchromones 53, dialkyl acetylenedicarboxylates 62 and aromatic amines 63 under reflux in ethanol (Scheme 9A). Through a convenient and simple reaction, also with simple work-up protocols, chromenopyridines 64 were obtained in high yields (70–86%). Although no mechanistic studies were discussed in this work, it is suggested that the most probable mechanism involves a Michael addition of amine 63 to chromone 53, followed by condensation of the resulting intermediate with 62 and subsequent cyclization into the final product 64. Chromenopyridines incorporating a nitro group could be synthesized in moderate-to-high yields via a perchloric acid-catalyzed three-component reaction (Scheme 9B) [73]. This starts from the reaction of 3-formylchromenes 53, 1,1-enediamines 65 and nucleophiles 66, under mild conditions (ethanol or acetone, 50–60 °C). After optimizing the reaction conditions, the authors highlight acetone as the best solvent when ethanol is not used as a nucleophile, and perchloric acid as the best catalyst among several other acids used (the product was formed with a 81% yield). It was also reported that using temperatures higher than 50 °C did not favor the chromenopyridine formation. It was suggested that the reaction starts by Michael addition of 65 to the aldehyde of 53, generating an intermediate with high degree of regioselectivity. The reaction proceeds through intramolecular cyclization, generating the 6-membered ring, which after imine-enamine tautomerization, acid-catalyzed water elimination and oxidation, leads to the chromenopyridine moiety of compounds 67 or 68, depending on the experimental conditions used.
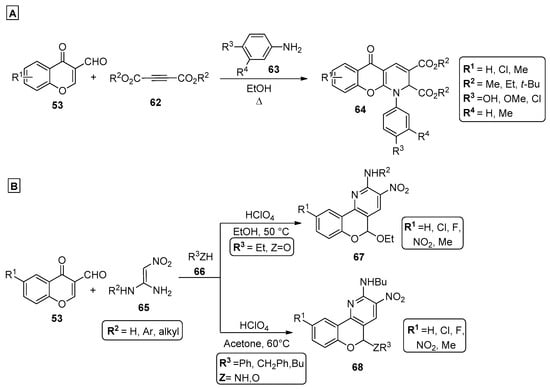
Scheme 9.
Preparation of chromenopyridines 64, 67 and 68 (adapted from Refs. [72,73]). (A) Dolatkhah et al.; (B) Zhang et al.
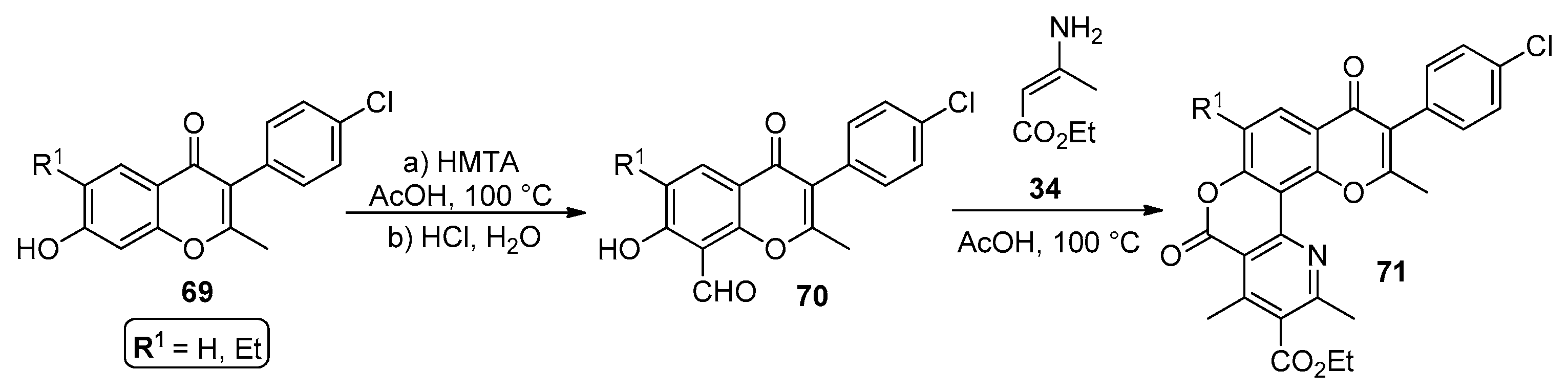
In the work performed by Lozinski et al. [74], a modified version of the Hantzsch synthesis afforded chromenopyridines 71 in good-to-moderate yields (43–69%). The procedure starts with the reaction of compound 69 with hexamethylenetetramine (HMTA) in acetic acid, followed by water addition and acidification with HCl, leading to 70 in a high yield (80–90%). Then, the modified Hantzsch reaction of 70 with an excess of 3-aminocrotonate 34 afforded chromenopyridines 71. According to the authors, a predominance of C–N addition over the C–C addition pathway led to 71, which was explained by the electronic effects within the 8-formyl-7-hydroxychromone scaffold 70 (Scheme 10).
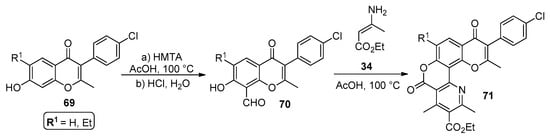
Scheme 10.
Preparation of chromenopyridines 71 as proposed by Lozinski et al. (adapted from Ref. [74]).
2.3. Chromanones
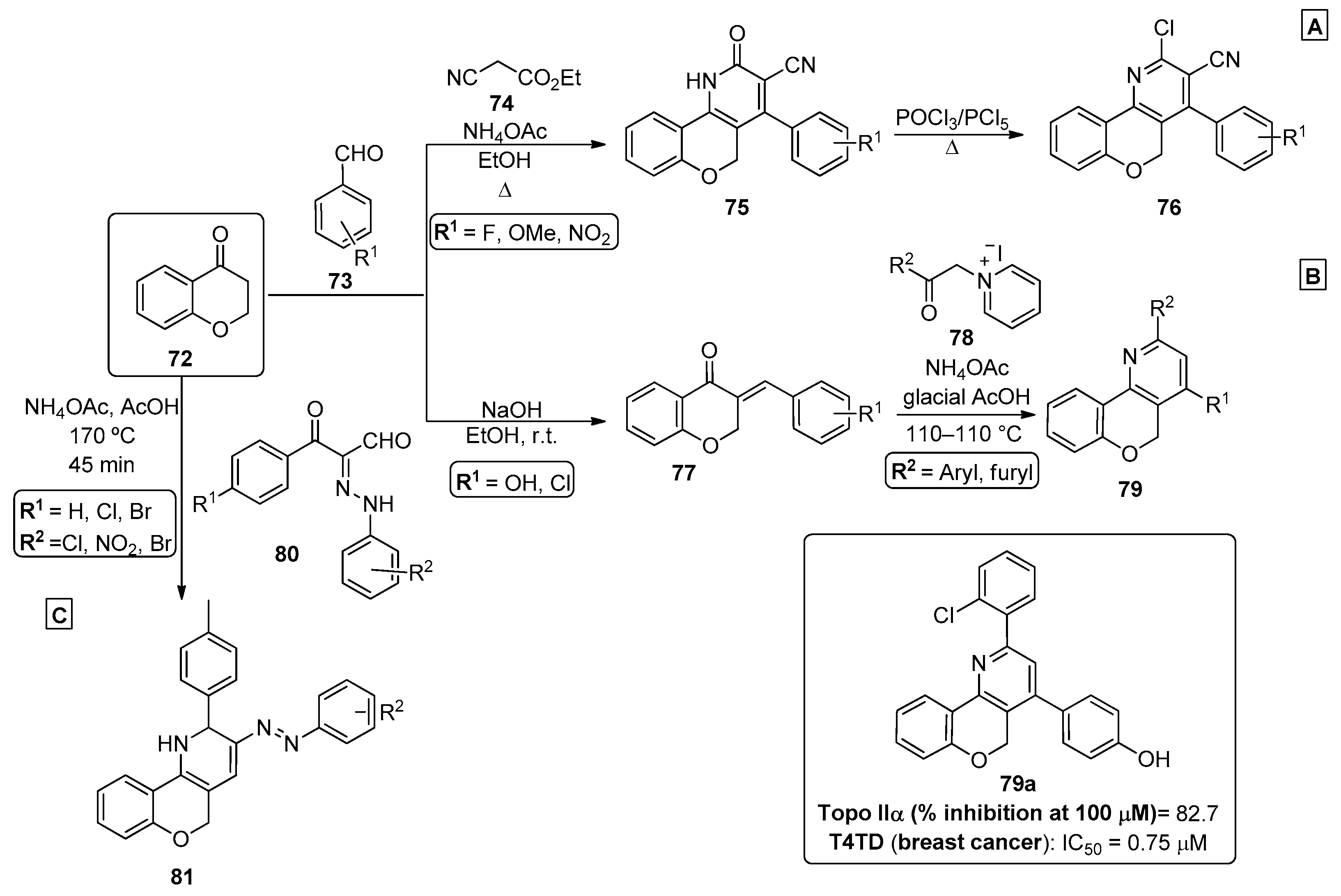
The use of chromanones as starting material for the preparation of chromenopyridines was also reported in the literature, and they present a similar chemical reactivity to that observed for chromones and substituted chromones. For example, to prepare new chromenopyridine derivatives 76, a four-component reaction was developed [75]. Chroman-4-one 72 reacted with aromatic aldehydes 73, ethyl cyanoacetate 74 and ammonium acetate under reflux in ethanol, affording compounds 75 in 65–82% isolated yield (Scheme 11A). These products were converted into the chlorinated chromenopyridines 76 (69–79% yields) by reaction with phosphonyl chloride, in a POCl3/PCl5 mixture under reflux conditions.
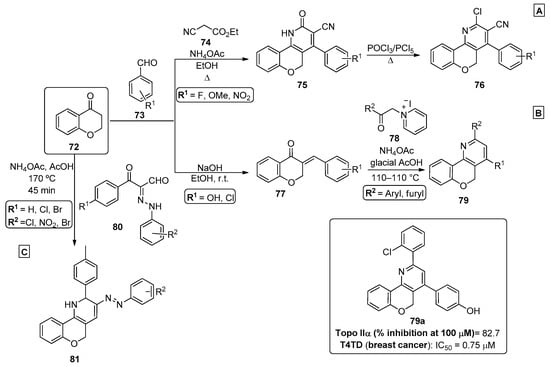
Scheme 11.
Preparation of chromenopyridines 76, 79 and 81 with the biological activity of compound 79a highlighted (adapted from Refs. [75,76,77,78,79,80,81]). (A) Ali et al.; (B) Thapa et al.; (C) Behbehani et al.
In their work, Thapa et al. [76,77] developed a new multicomponent reaction to prepare α-terthiophene bioisosters based on the chromenopyridine moiety, in order to enhance the rigidity of the scaffold predicted to improve its biological activity (Scheme 11B). Aldol condensation of chroman-4-one 77 with diverse aldehydes 73 under base conditions led to chromanones 77 that evolved to chromenopyridines 79 by heating at 100–110 °C with diverse 2-oxoalkylpyridinium iodides 78 in the presence of ammonium acetate and glacial acetic acid. The resulting products 79 were tested for their activity as topoisomerase (Topo) I and II inhibitors, as well as for their cytotoxicity against several human cancer cell lines. The authors considered the isolated compounds as novel chromenopyridine-based Topo I and II inhibitors [78,79,80]. Compounds 79, with phenyl or thienyl substituents, were among the most relevant in terms of selectivity against Topo II. Moreover, this new batch of chromenopyridine derivatives showed interesting and improved results in various cancer cell lines, including breast cancer cell lines T47D. The selectivity towards breast cancer cell lines versus non-tumorigenic breast cell line MCF-10A was noteworthy, with some compounds having an 18-fold enhanced inhibitory capacity when compared with the reference compound. Further SAR studies proceeded with compound 79a, highlighted for its Topo I and IIα inhibitory capacity, as well as for its potent and selective antiproliferative activity via Topo Iiα inhibition (Scheme 11B) [80]. In 2021, Behbehani et al. [81] proposed a green protocol for the development of thiochromeno [4,3-b]pyridine and chromeno [4,3-b]pyridine derivatives, in which a sealed Q-tube reactor was used to perform the reaction under high pressure. In this procedure, a three-component reaction between chroman-4-one 72, arylhydrazonals 80 and ammonium acetate, in acetic acid and at 170 °C, led to chromenopyridines 81 isolated in 84–92% yields (Scheme 11C).
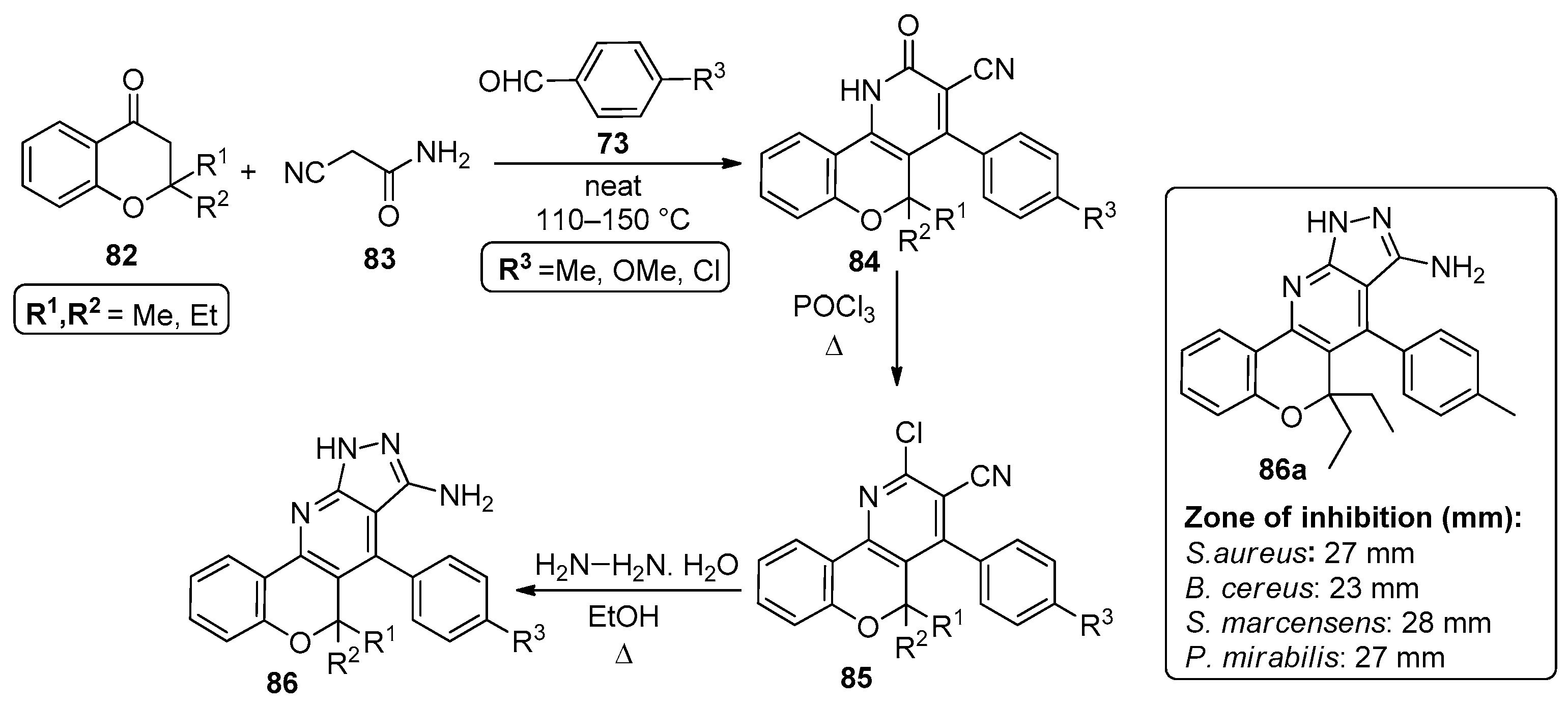
In 2014, El-Essawy’s group published a one-pot three-component reaction involving 2,2-dialkylchroman-4-ones 82, 2-cyanoacetamide 83 and 4-substituted aldehydes 73, in a solventless reaction performed at 110–150 °C, leading to chromenopyridines 85 in 62–73% yields (Scheme 12) [82]. The synthesis of compounds 84 had been previously reported [83] and in the more recent work, compounds 85 were prepared through POCl3 chlorination of 84 and used in the synthesis of 86 by reaction with hydrazine monohydrate under reflux in ethanol. Chromenopyridines 84-86 were tested for their antibacterial activity against S. aureus (SA), B. cereus (BC), S. marcensens (SM) and P. mirabilis (PM), revealing an interesting antibacterial profile that was transversal to all the tested chromenopyridine subfamilies. Several compounds revealed relevant activity against more than one bacterial strain. Among the tested compounds, 86a (R1 = R2 = Et) was highlighted for the diameter of its inhibition zone and announced as a promising drug candidate when compared to the standard ampicillin (Scheme 12).
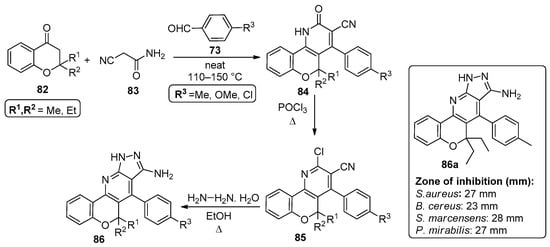
Scheme 12.
Preparation of chromenopyridines 85 and 86 with the antibacterial activity (zone of inhibition, mm) of compound 86a highlighted (adapted from Ref. [82]).
2.4. Coumarins
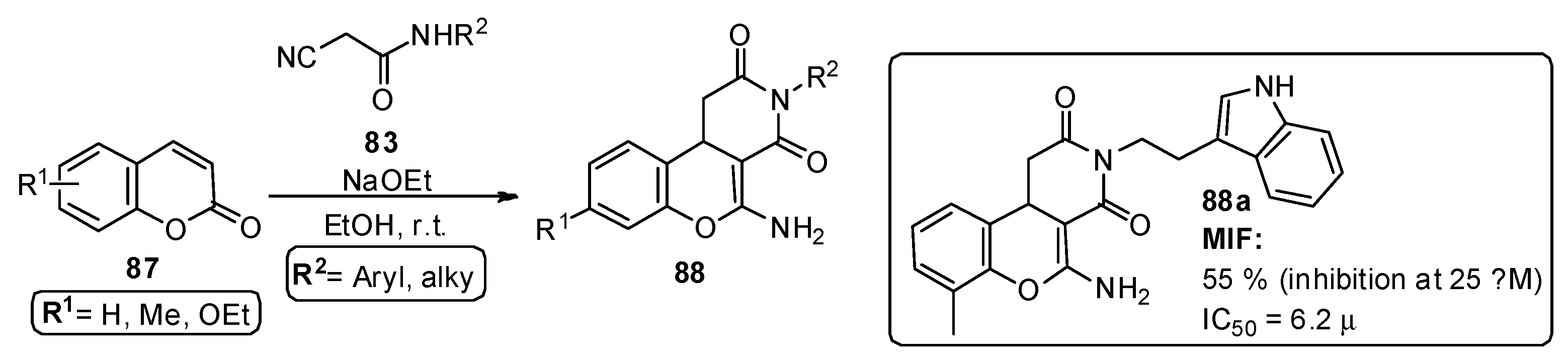
Kok et al. [84] reported the development of new macrophage migration inhibitory factor (MIF) inhibitors, referenced as an important target in the inflammatory process, signaling tumor cell growth in inflammatory disease models. In this study, 57 amino-2H-chromenopyridine-dione derivatives 88 were prepared from the reaction of cyanoacetamides 83 and substituted coumarins 87, under mild conditions and in the presence of sodium ethoxide, with good and very good yields (Scheme 13). Most of the tested compounds showed MIF tautomerase inhibitory capacity, with IC50 in the low micromolar range, particularly compound 88a (Scheme 13).
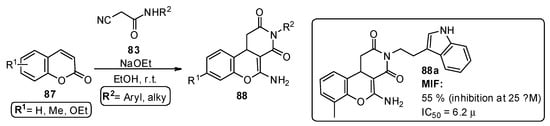
Scheme 13.
Preparation of chromenopyridines 88, with the respective biological activity (MIF inhibition at 25 µM and respective IC50) highlighted for compound 88a (adapted from Ref. [84]).
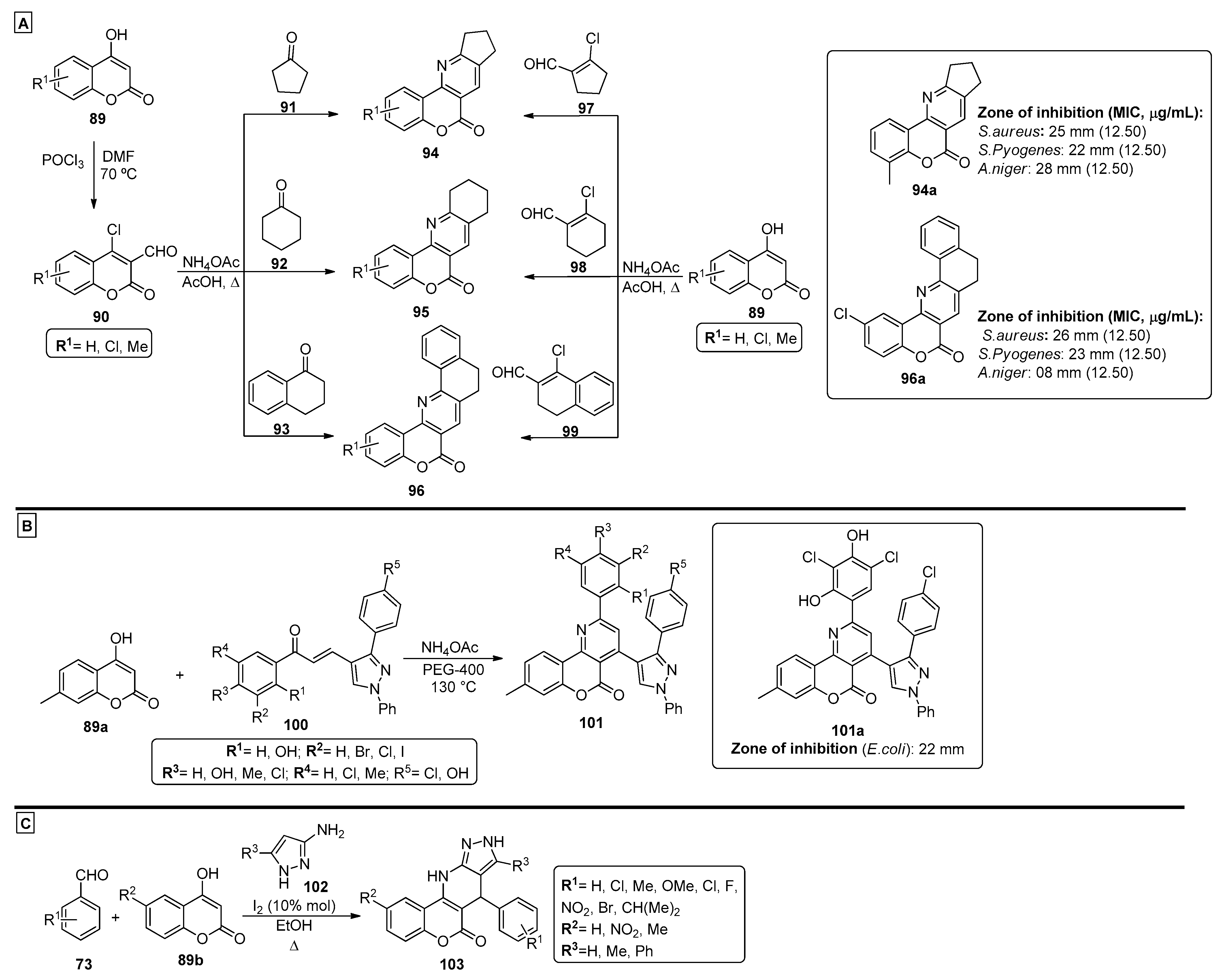
Patel et al. [85] proposed a methodology for the preparation of novel chromenopyridines 94–96 from substituted 4-hydroxy-2H-chromen-2-ones 89 (Scheme 14A). The synthetic route starts by converting compounds 89 into the formylated and chlorinated coumarins 90 using POCl3 in DMF, followed by reaction with cyclic ketones 91-93 and ammonium acetate. Alternatively, the direct reaction of compounds 89 with cyclic chloroformyl compounds 97–99 and ammonium acetate was also used. The products were evaluated for their antimicrobial activity, and all tested compounds presented better inhibitory activity for Gram-positive bacteria than for Gram-negative bacteria. For the tested compounds, products 94a and 96a were among the most active structures, with interesting minimum inhibitory concentrations (MIC) and zone of inhibition results against S. aureus (SA), S. pyogenes (SP) and A. niger (AN) (Scheme 14A). Dawane et al. also proposed the formation of chromenopyridines 101 by a PEG-400-catalyzed MCR [40]. In this case, 4-hydroxy-7-methyl-coumarin 89a was reacted with different chalcones 100 and ammonium acetate, at 130 °C, using PEG-400 as solvent (Scheme 14B). This method produced diarylsubstituted chromenopyridines 101 in 81–89% yields, in a process that allowed a facile separation of the final product and reusage of the PEG-400 solvent. The proposed mechanism for the formation of compounds 101 involved the in situ generation of 4-amino-7-methylcoumarin and subsequent Michael addition of the enamine unit to 100, finishing with a ring closing step to form the pyridine ring. These compounds were tested against an array of microorganisms (e.g., E. coli and A. flavus), and several compounds demonstrated good results in one or more tested bacteria strains. A promising germination inhibition in several tested fungi was also reported. Compound 101a (R1 = R3 = OH, R2 = R4 = R5 = Cl) showed significant inhibition of E. coli when compared with penicillin and was able to reduce or inhibit growth in all fungi tested. The use of iodine as a catalyst was also explored by Pal et al. [86]. In this work, the iodine-catalyzed reaction between aromatic aldehydes 73, 4-hydroxycoumarins 89b and different amino pyrazole derivatives 102 was studied. Optimization studies showed that the use of 10% mol of I2 as catalyst, under reflux in ethanol, were the best conditions for this reaction (Scheme 14C). Chromenopyridines 103 were prepared by a green and convenient method, with environmentally friendly catalysts and solvents and no chromatographic purification methods required. The authors also suggested that compounds 103 could be prepared from a four-component one-pot reaction, where the amino pyrazoles 102 were generated in situ from the reaction of benzoylacetonitrile and hydrazine monohydrate. The most likely mechanism to originate compounds 103 involves a Knoevenagel condensation between aldehydes 73 and 4-hydroxycoumarins 89b, followed by Michael addition of aminopyrazole 102 to the newly formed adduct, which then undergoes a tandem intramolecular cyclization reaction. The authors also suggested that iodine acts as catalyst by coordinating to the carbonyl moieties, enhancing their electrophilicity, which facilitates nucleophilic attack in the Knoevenagel condensation, Michael addition and intramolecular condensation steps.
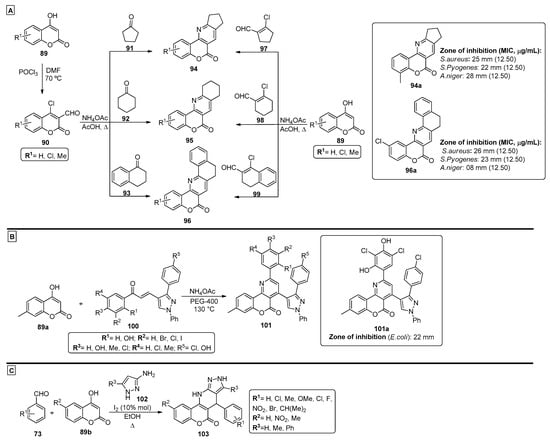
Scheme 14.
Preparation of chromenopyridines 94–96, 101 and 103, with the respective biological activity (zone of inhibition, mm and MIC, µg/mL) highlighted for compounds 94a, 96a and 101a (adapted from Refs. [40,85,86]). (A)—Patel et al.; (B)—Dawane et al.; (C)—Pal et al.
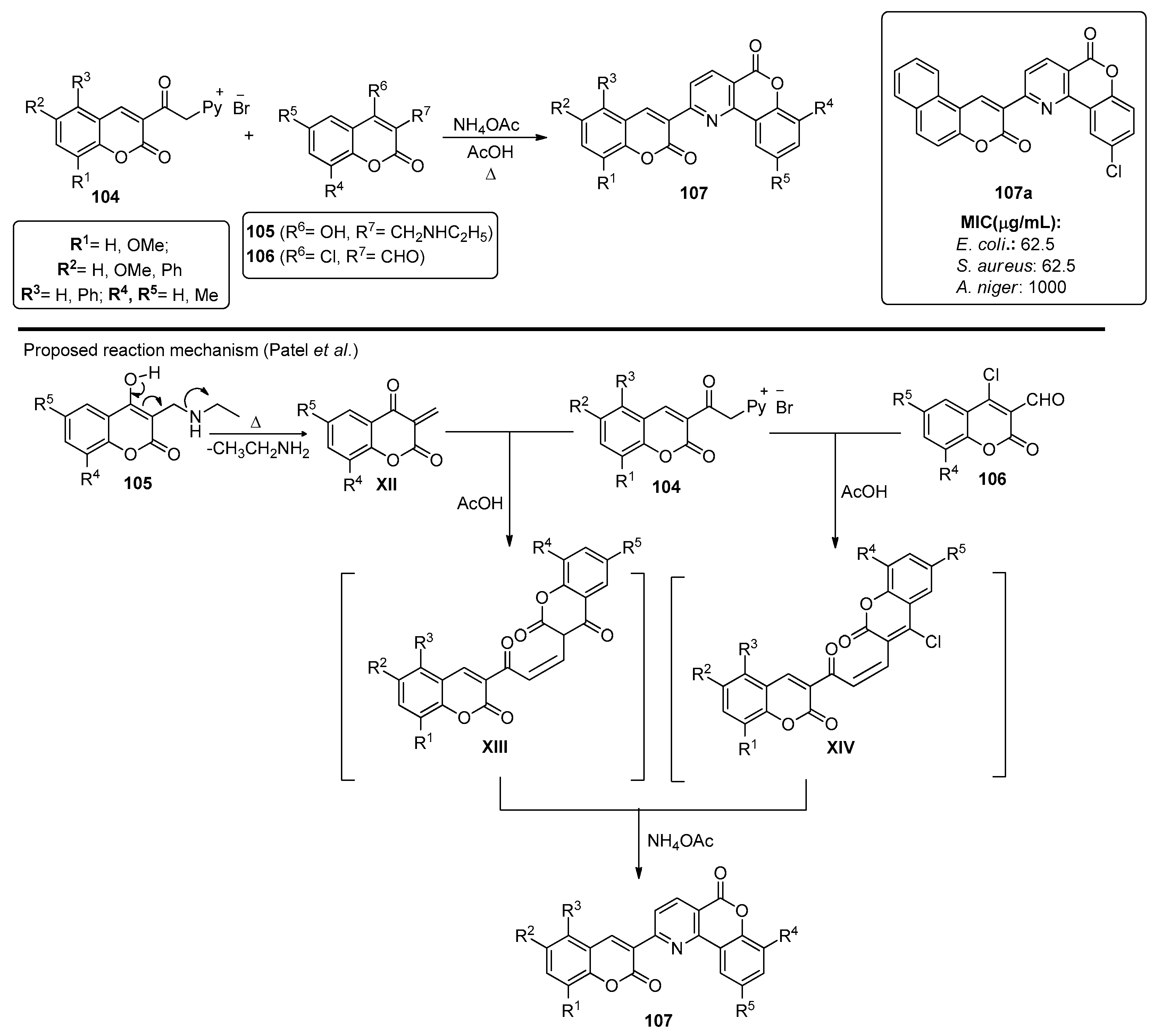
Patel et al. also presented a method for preparing bischromeno structures 107, starting from chromenylpyridinium salts 104 [87]. Compounds 104 were either reacted with chromen-2-ones 105 or chromene-3-carbaldehydes 106, in the presence of ammonium acetate and reflux in acetic acid, to afford chromenopyridines 107. The reaction proceeded through bischromene adduct XIII or XIV, respectively, which then generated the chromenopyridine scaffold by reacting with ammonium acetate (Scheme 15). The use of compound 106 afforded 107 in higher yields (54–79% vs. 38–57%). A series of chromenopyridine derivatives 107 were synthesized and tested for their antimicrobial activity for S. aureus and E. coli, as well as for their antifungal activity against A. niger and C. albicans, with some compounds, such as 107a, presenting MIC for S. aureus up to 4-fold lower than the standard, ampicillin (Scheme 15).
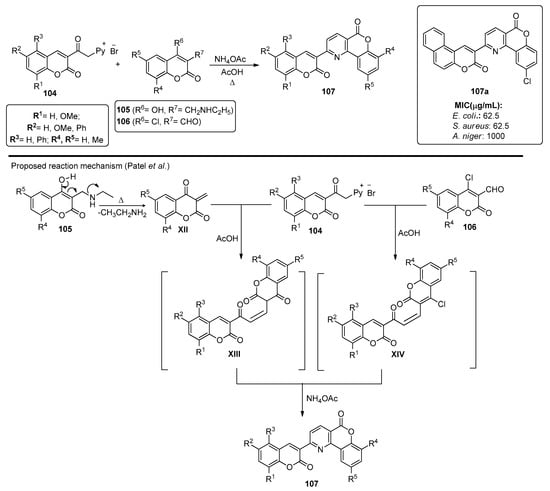
Scheme 15.
Preparation of chromenopyridines 107, with the biological activity (MIC, µg/mL) highlighted for compound 107a adapted from Ref. [87]).
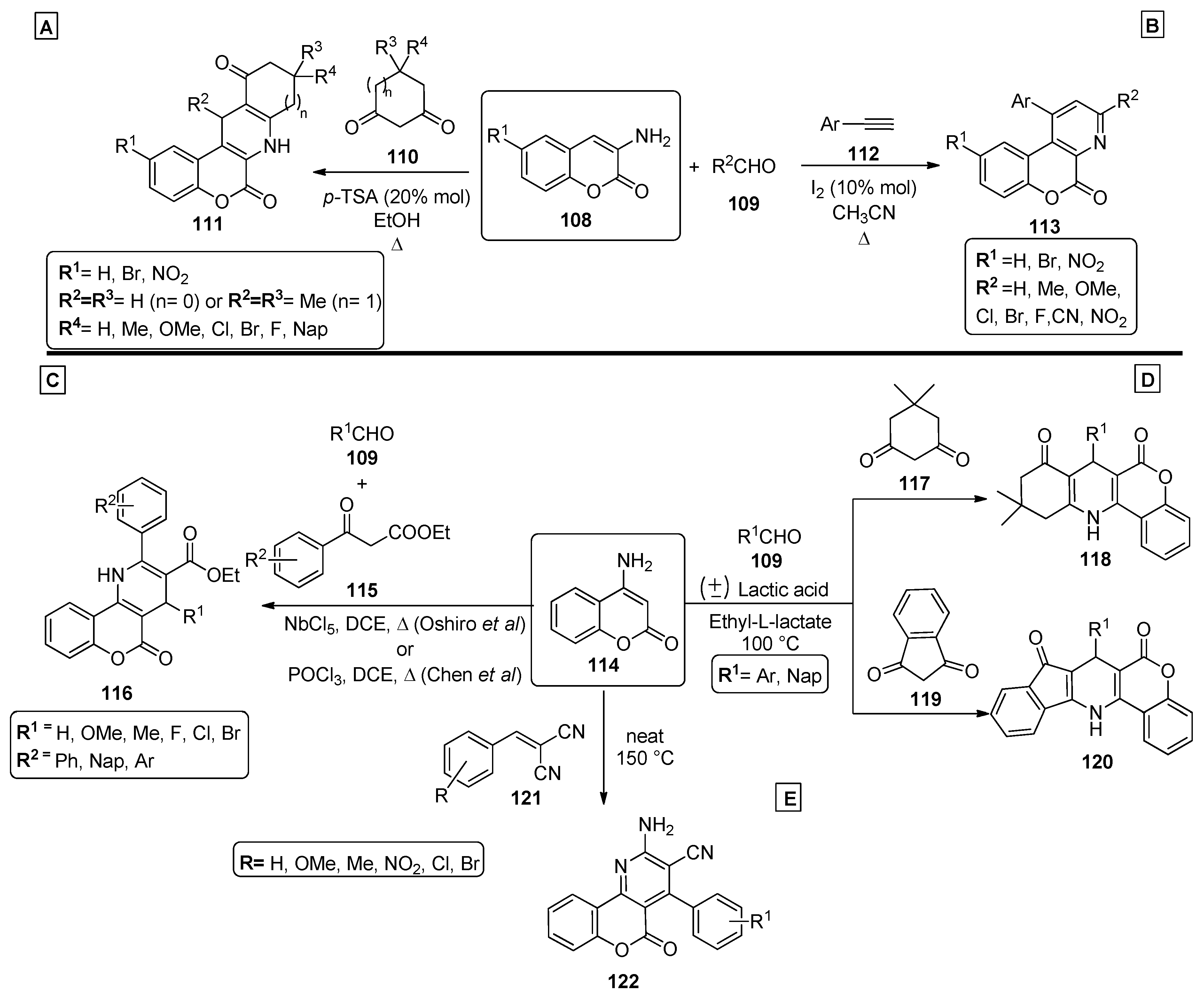
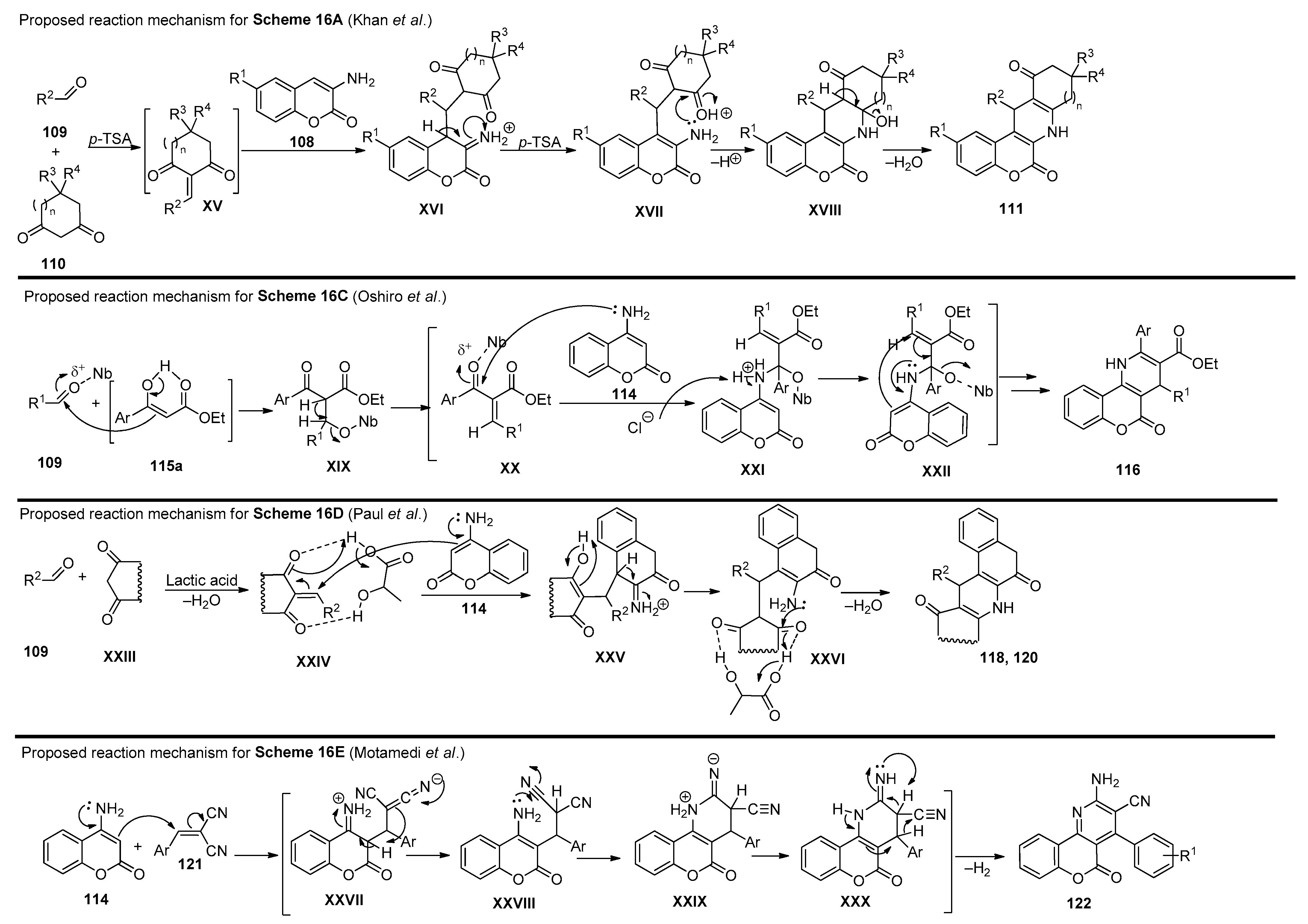
In 2012, Khan and coworkers [88] presented a methodology to obtain chromenopyridine derivatives 111 (72–82% yield) from a three-component reaction involving 3-aminocoumarins 108, aldehydes 109 and cyclic diketones 110 under reflux in ethanol, catalyzed by p-toluenesulfonic acid (p-TSA) (20% mol) (Scheme 16A). These chromenopyridines were obtained by condensation of 109 with 110, followed by Michael addition of coumarin 108 via an enamine intermediate. The adduct formed during this process was not isolated and undergoes intramolecular cyclization, followed by dehydration, to afford the desired product 111. Later, the same author. presented a procedure for the preparation of chromenopyridines 113 [89]. In this work, aminocoumarins 108 were reacted with phenylacetylenes 112, and the reaction was performed under iodine catalysis and acetonitrile reflux (Scheme 16B). Similarly to their previous work [88], mainly aromatic aldehydes were used. Several exploratory reactions to optimize the reaction conditions were also performed, confirming that a catalyst was necessary for the reaction to occur, and I2 (10% mol) was the best catalyst. The proposed mechanism for the formation of chromenopyridines 113 involved an imine intermediate, which in turn undergoes intramolecular cyclization followed by oxidation, to afford the final product. In 2013, Chen et al. [90] prepared chromenopyridines 116 from the reaction of 4-amino-2H-chromen-2-one 114, aldehydes 106 and ethyl benzoylacetates 115 (Scheme 16C). A series of protic and Lewis acids were tested as catalysts and POCl3 was selected as the best catalyst, in a reaction performed under reflux in dichloroethane. Chromenopyridine 116 was oxidized using DDQ under reflux in ethanol. Using this method, 20 derivatives of 116 were prepared in 52–63% yields. Later, Oshiro et al. [91] further explored this procedure, evaluating the performance of other metal-containing Lewis acids, from which niobium chloride (100 mol%) emerged as the most effective catalyst for the three-component reaction of 114, aldehydes 109 and ethyl benzoylacetate 115, affording chromenopyridines 116 in 38–58% yields (Scheme 16C). The authors proposed that the Knoevenagel condensation between 109 and the enolic form of ethyl benzoylacetate 115, as well as the nucleophilic attack of the amine group of 114 to the carbonyl moiety of the Knoevenagel adduct, are facilitated by the niobium catalyst used, which increases electrophilicity in both steps. The work published by Paul et al. [92] also involved aminocoumarins as precursors of chromenopyridines (Scheme 16D). In this work, 4-aminocoumarin 114 was combined with several aldehydes 109, and with either indane-1,3-dione 117 (to prepare 118) or cyclohexane-1,3-dione 119 (to prepare 120), in a reaction performed in ethyl-L-lactate at 100 °C and with (±) lactic acid catalysis (Scheme 16D). These chromenopyridines were isolated in high yields (73–85% for 119, 83–91% for 120). The reaction mechanism is similar to that proposed for the reaction in Scheme 16A, starting from the condensation between the diketone compound 117 or 119 and aldehydes 109, followed by Michael addition of the aminocoumarin 114 and intramolecular cyclization. It was suggested that lactic acid aids the process by interacting with the adduct via hydrogen bonding, facilitating the Michael addition step. Motamendi presented a solvent-free methodology for the synthesis of chromenopyridine derivatives 122, through the reaction of 4-aminocoumarin 114 with arylidenemalononitriles 121, at 150 °C [93]. The final product was obtained after flash chromatography purification, with isolated yields of 60–80%. The authors propose that the reaction occurs via Michael addition, where the arylidenemalononitrile acts as the Michael acceptor and the enamine group as the Michael donor, with an intramolecular cyclization step followed by isomerization leading to the chromenopyridine scaffold (Scheme 16E). The respective reaction mechanisms, as proposed by the authors, are presented in Scheme 17.
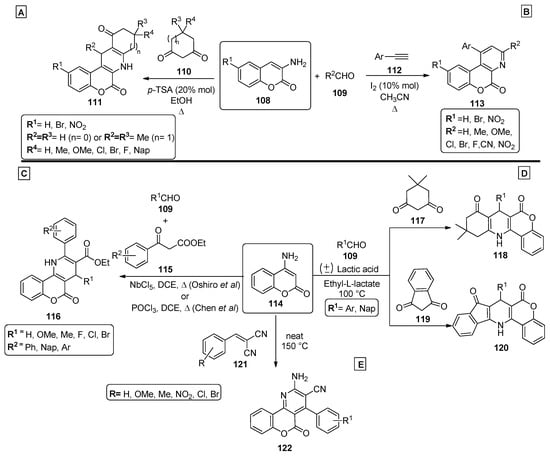
Scheme 16.
Preparation of chromenopyridines 111, 113, 116, 118, 120 and 122 (adapted from Refs. [88,89,90,91,92,93]). (A,B)—Khan et al.; (C)—Chen et al., Oshiro et al.; (D)—Paul et al.; (E)—Motamendi et al.
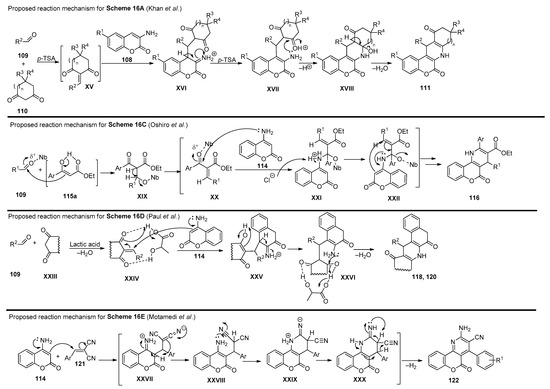
Scheme 17.
Proposed reaction mechanisms for the formation of compounds 111, 116, 118, 120 and 122 (adapted from Refs. [88,89,90,91,92,93]).
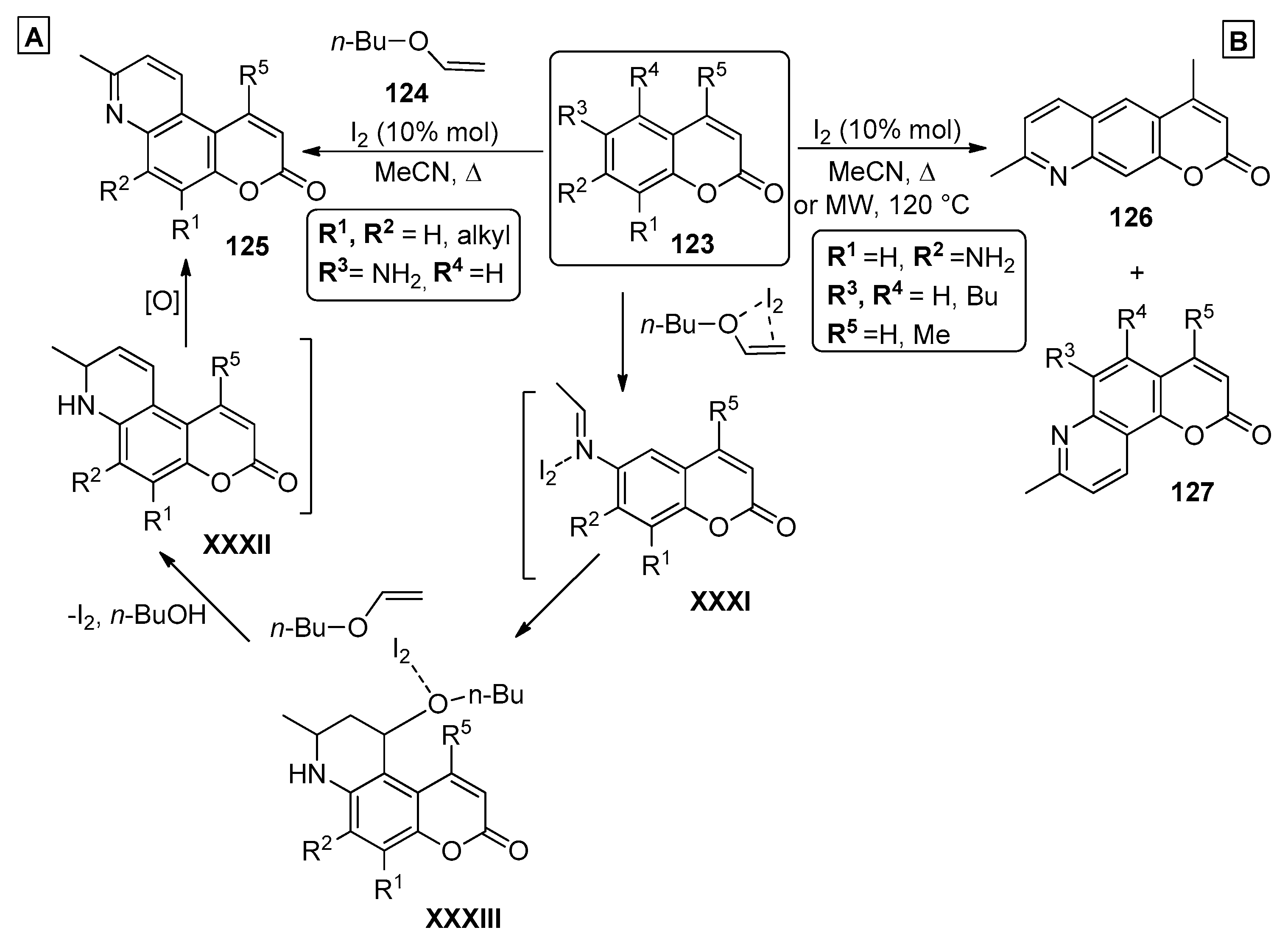
In the past decade, several examples of the use of olefins and aminocoumarins to prepare chromenopyridines have been reported in the literature. One of these procedures was presented by Symeonidis et al. [94], describing an MCR to prepare chromenopyridines. The reaction of 6-amino-7-methylcoumarins 123 (R3 = NH2) with two equivalents of n-butyl vinyl ether 124, in the presence of a catalytic amount of iodine, in acetonitrile, and under reflux, led to the isolation of compounds 125 in 45–87% yields (Scheme 18A). The reaction starts by a Lewis acid-catalyzed imine formation from one equivalent of both 123 and n-butyl vinyl ether 124, followed by the incorporation of a second equivalent of 124 through a Lewis acid-catalyzed Aza-Diels–Alder reaction. The final product 125 is generated by Lewis acid-catalyzed elimination of n-butanol and subsequent oxidation. The authors note that the reaction did not occur in the absence of iodine catalysis, as it is essential in aiding the nucleophilic attack of the amine group to the vinyl ether moiety. An optimization study indicated 10% mol equivalent as the optimal conditions. This methodology was also applied to the preparation of chromenopyridines 126 and 127 from 7-amino-4-methylcoumarins 123 (R2 = NH2), which could additionally be prepared by performing the reaction under microwave irradiation at 120 °C instead of reflux. Both methods presented similar results, although none of the proposed methods were regioselective, affording the chromenopyridines 127 as secondary products (Scheme 18B).
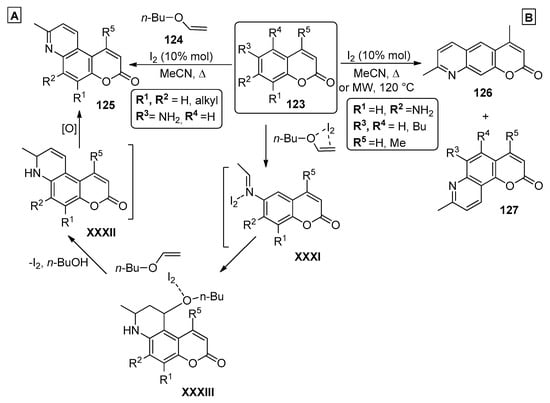
Scheme 18.
Preparation of chromenopyridines 125–127, with the mechanism proposed by Symeonidis et al. (adapted from Ref. [94]).
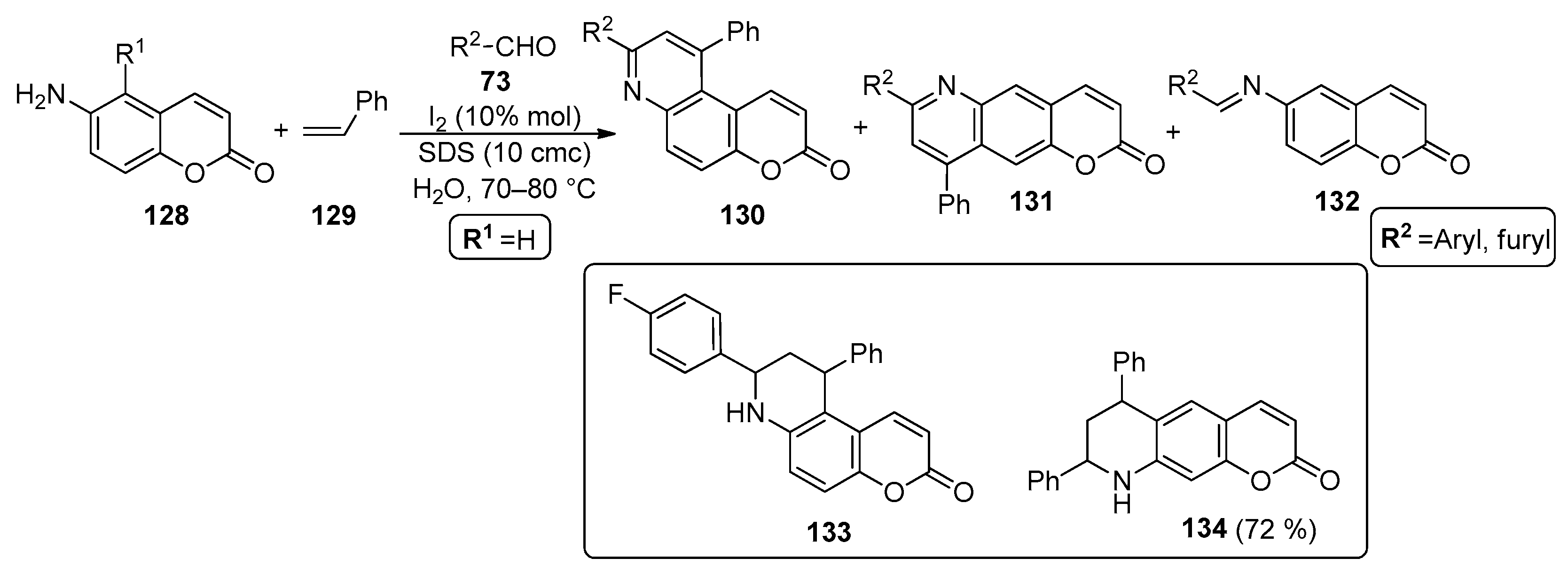
Iodine catalysis was also used to prepare chromenopyridines 130–132 in an MCR between 6-aminocoumarins 128, aromatic aldehydes 73 and styrene 129 (Scheme 19). Ganguly and Chandra [95] reported that the reaction occurs in an aqueous medium, using SDS as a micellar auxiliar. The optimized reaction conditions involved the use of coumarin/aldehyde/styrene in a 1:1.1:4 ratio, in aqueous SDS (10 cmc) and I2 (10% mol), at 70–80 °C. It was mentioned that SDS behaves as an activator of iodine, important in the formation of the intermediate Schiff base from the reaction of 128 with the aromatic aldehyde 73. These conditions were applied to a wide variety of aromatic aldehydes, which afforded mixtures of 130, 131 and 132 in variable yields (25–72% for 130, 10–40% for 131 and 7–40% for 132). Nevertheless, compound 130 was always isolated as the major product. Compounds 131 and 132 were also isolated when aldehydes 73 were used. In the reaction of 6-aminocoumarin and p-iodobenzaldehyde, the non-oxidized form was isolated (133) and in the reaction of 6-amino-5-bromocoumarin with benzaldehyde, a product incorporating two equivalents of styrene was isolated (134) (Scheme 19).
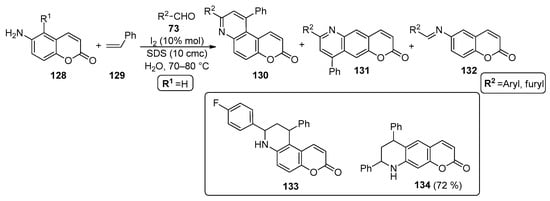
Scheme 19.
Preparation of chromenopyridines 130–132, with the chemical structures of the side products 133 (identified in a mixture with 130 and 132) and 134 highlighted (adapted from Ref. [95]).
2.5. Miscellaneous
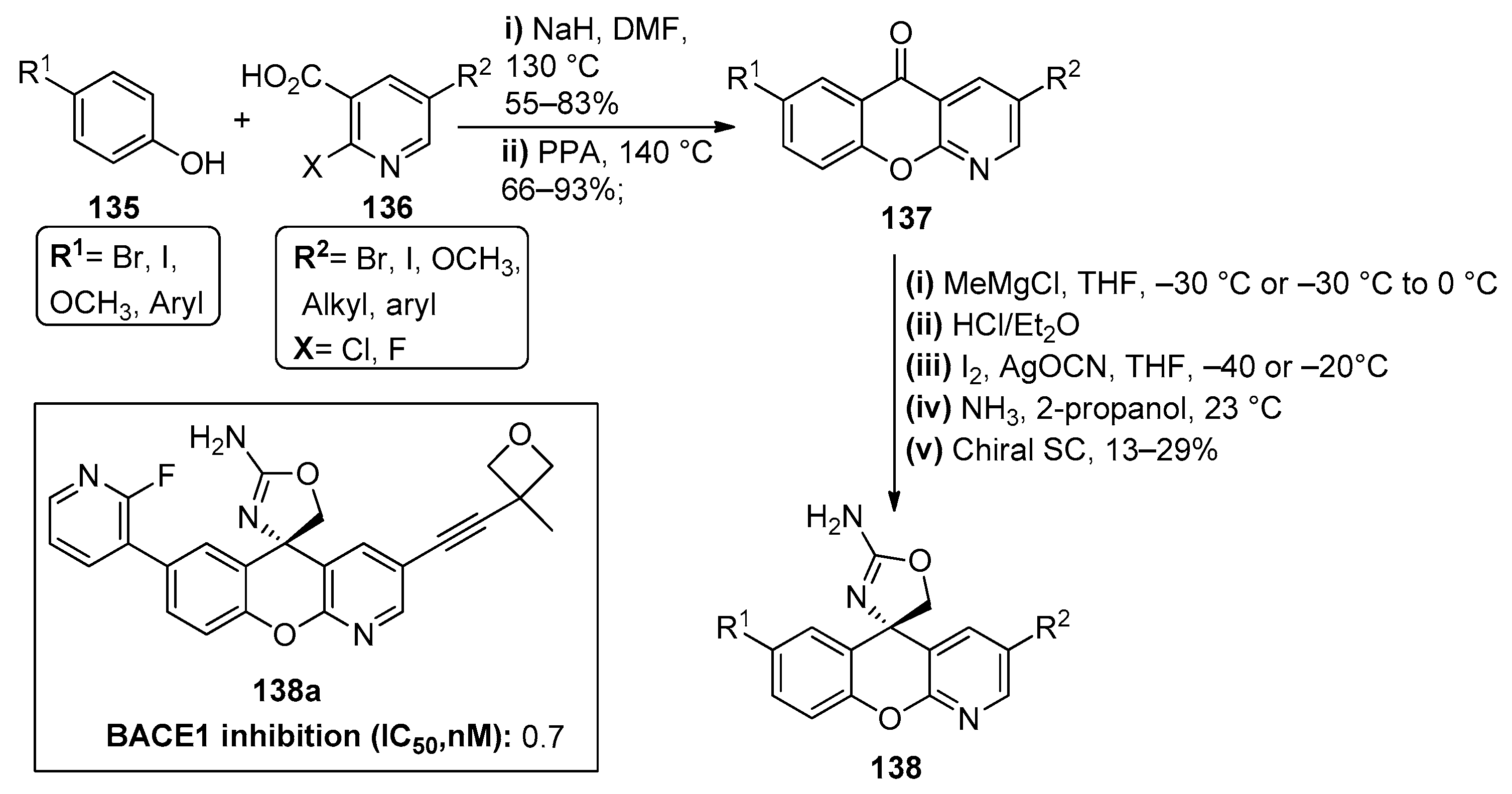
The literature also reports the formation of the chromenopyridine moiety through coupling reactions. Several publications [96,97,98] on the development of new β-site amyloid precursor protein cleaving enzyme (BACE1) inhibitors reported the synthesis of a set of xanthene derivatives. However, after a series of molecular optimization studies, it was observed that the best results for balancing BACE1 potency, P-glycoprotein-mediated efflux and human Ether-à-go-go-Related Gene (hERG) binding affinity could be achieved using a chromenopyridine scaffold [99]. The chromenopyridines 137 prepared in this study were generated by coupling a selection of p-substituted phenols 135 (R1 = Br, I, OCH3) with 2,5-substituted nicotinic acids 136, using NaH as catalyst, followed by ring closure of the adduct by neat polyphosphoric acid at 140 °C (Scheme 20). The enantioselective preparation of chromenopyridines 138 with an aminooxazole moiety was obtained by a three-step methodology: (i) addition of a Grignard reagent (CH3MgCl) to 137 and subsequent treatment with HCl in diethyl ether to afford an unstable exo-olefin; (ii) treatment of the olefin with the in situ generated iodine isocyanate at −20 °C; (iii) treatment with a solution of ammonia in 2-propanol at 23 °C, to generate a racemic mixture of the 2-aminooxazole chromenopyridine derivative. The pure (S)-enantiomer of compound 138 was obtained after chiral supercritical fluid (SCF) separation, in 13–29% yields, depending on the substituents present in the product. These chromenopyridines were then used in a variety of subsequent reactions to prepare a library of compounds with different substituents R1 and R2. From this library, compound 138a is highlighted, with a promising inhibitory capacity of BACE1, among other important physicochemical properties (Scheme 20).
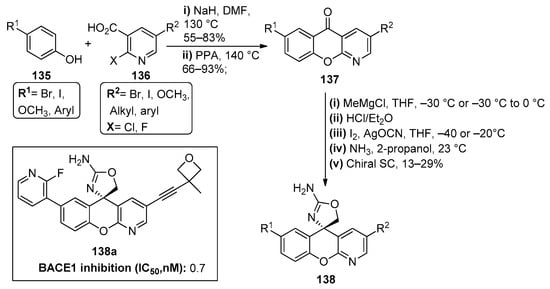
Scheme 20.
Preparation of chromenopyridine scaffold 138, used for the preparation of 16 derivatives, as proposed by Dineen et al., with the respective biological activity (BACE1 inhibition, µg/mL) highlighted for compound 138a (adapted from Refs. [96,97,98,99]).
3. Conclusions
Chromono- and chromenopyridines combine two of the most widely recognized biologically active structural motifs, the chromene and pyridine nucleus. Bibliographical information from the years of 2010–2023 on different strategies for chromenopyridine synthesis, as well as their promising associated biological properties, have reinforced what was observed until 2010: there is a strong interest in the scientific community concerning this type of compound and its biological use. This interest has led not only to improvements on already known synthetic methods, but also to developments of new insightful methods. Furthermore, this review contributes to a thorough compilation of those discoveries, to fuel further advancements on the development of novel bioactive chromenopyridines with the potential for clinical translation.
Author Contributions
Conceptualization, F.P.d.L., M.F.P. and M.C.; literature search and study selection, F.P.d.L., M.F.P. and A.S.; writing—original draft preparation, F.P.d.L., M.F.P., A.S. and M.C.; writing—review and editing, F.P.d.L., M.F.P., A.S. and M.C; final approval, F.P.d.L., A.S., M.C. and M.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by National funds, through the Foundation for Science and Technology (FCT)—project UIDB/50026/2020 and UIDP/50026/2020 and by the project NORTE-01-0145-FEDER-000055, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). We also acknowledge the financial support from University of Minho, FCT and FEDERCOMPETE for financial support through Centro de Química (UID/QUI/00686/2020 and UID/QUI/0686/2016) and project UIDB/50026/2020 and UIDP/50026/2020 and also by the project NORTE-01-0145-FEDER-000055, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Re-gional Development Fund (ERDF). We also acknowledge the financial support received from FCT for the PhD grant awarded to Beatriz Sousa (2020.07919.BD) and research contract to Marta Costa (DOI: 10.54499/DL57/2016/CP1377/CT0074).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and Drug Discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Park, S.B. Privileged Structures: Efficient Chemical “Navigators” toward Unexplored Biologically Relevant Chemical Spaces. J. Am. Chem. Soc. 2014, 136, 14629–14638. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, S.; Pingaew, R.; Worachartcheewan, A.; Sinthupoom, N.; Prachayasittikul, V.; Ruchirawat, S.; Prachayasittikul, V. Roles of Pyridine and Pyrimidine Derivatives as Privileged Scaffolds in Anticancer Agents. Mini-Rev. Med. Chem. 2017, 17, 869–901. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagoniststs. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, C.; Sitkoff, D.; Cheadle, N.L.; Xu, S.; Muckelbauer, J.K.; Adam, L.P.; Wexler, R.R.; Quan, M.L. Identification of 5H-chromeno [3,4-c]pyridine and 6H-isochromeno[3,4-c]pyridine derivatives as potent and selective dual ROCK inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127474. [Google Scholar] [CrossRef] [PubMed]
- Marson, C.M. Targeting the histamine H4 receptor. Chem. Rev. 2011, 111, 7121–7156. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Tabrizi, M.A.; Gessi, S.; Borea, P.A. Adenosine receptor antagonists: Translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev. 2008, 108, 238–263. [Google Scholar] [CrossRef]
- Martinez-Gualda, B.; Pu, S.Y.; Froeyen, M.; Herdewijn, P.; Einav, S.; De Jonghe, S. Structure-activity relationship study of the pyridine moiety of isothiazolo[4,3-b]pyridines as antiviral agents Targeting cyclin G-associated kinase. Bioorg. Med. Chem. 2020, 28, 115188. [Google Scholar] [CrossRef]
- Barreiro, E.J. Chapter 1: Privileged Scaffolds in Medicinal Chemistry: An Introduction. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; The Royal Society of Chemistry: London, UK, 2016; pp. 1–15. ISBN 978-1-78262-030-3. [Google Scholar]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. One-Step Synthesis of Heterocyclic Privileged Medicinal Scaffolds by a Multicomponent Reaction of Malononitrile with Aldehydes and Thiols. J. Org. Chem. 2007, 72, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.D. De Novo Synthesis of Substituted Pyridines. Tetrahedron 2004, 60, 6043–6061. [Google Scholar] [CrossRef]
- Edwards, J.P.; Kindrachuk, D.E.; Venable, J.D. Benzo-Imidazolyl Pyridines as Modulators of the Histamine H4 Receptor. Hong. Kong Patent HK1124767A1, 24 July 2009. [Google Scholar]
- Radwan, M.A.A.; Alshubramy, M.A.; Abdel-Motaal, M.; Hemdan, B.A.; El-Kady, D.S. Synthesis, molecular docking and antimicrobial activity of new fused pyrimidine and pyridine derivatives. Bioorg. Chem. 2020, 96, 103516. [Google Scholar] [CrossRef]
- Jian, X.E.; Yang, F.; Jiang, C.S.; You, W.W.; Zhao, P.L. Synthesis and biological evaluation of novel pyrazolo[3,4-b]pyridines as cis-restricted combretastatin A-4 analogues. Bioorg. Med. Chem. Lett. 2020, 30, 127025. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Pontes, O.; Costa, M.; Santos, F.; Sampaio-Marques, B.; Dias, T.; Ludovico, P.; Proença, F.; Baltazar, F. Exploitation of new chalcones and 4H-chromenes as agents for cancer treatment. Eur. J. Med. Chem. 2018, 157, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Azm, F.S.M.; El-Shahawi, M.M.; Elgubbi, A.S.; Madkour, H.M.F. Design, synthesis, anti-proliferative activity, and molecular docking studies of novel benzo[f]chromene, chromeno[2,3-d]pyrimidines and chromenotriazolo[1,5-c]pyrimidines. Synth. Commun. 2020, 50, 669–683. [Google Scholar] [CrossRef]
- Alblewi, F.F.; Okasha, R.M.; Hritani, Z.M.; Mohamed, H.M.; El-Nassag, M.A.A.; Halawa, A.H.; Mora, A.; Fouda, A.M.; Assiri, M.A.; Al-Dies, A.A.M.; et al. Antiproliferative effect, cell cycle arrest and Aaoptosis generation of novel synthesized anticancer heterocyclic derivatives based 4H-benzo[h]chromene. Bioorg. Chem. 2019, 87, 560–571. [Google Scholar] [CrossRef]
- Halawa, A.H.; Elaasser, M.M.; El Kerdawy, A.M.; Abd El-Hady, A.M.A.I.; Emam, H.A.; El-Agrody, A.M. Anticancer activities, molecular docking and structure–activity relationship of novel synthesized 4H-chromene, and 5H-chromeno[2,3-d]pyrimidine candidates. Med. Chem. Res. 2017, 26, 2624–2638. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Haiba, M.E.; Al-Abdullah, E.S.; Ahmed, N.S.; Ghabbour, H.A.; Awad, H.M. Efficient and easy synthesis of new Benzo[h]chromene and Benzo[h]quinoline derivatives as a new class of cytotoxic agents. J. Mol. Struct. 2019, 1195, 702–711. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Albarrán-Velo, J.; Light, M.E.; Padrón, J.M.; Román, E.; Serrano, J.A.; Gil, M.V. Synthesis and antiproliferative activity of new 2-glyco-3-nitro-2H-chromenes. Bioorg. Chem. 2019, 87, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Okasha, R.M.; Alsehli, M.; Ihmaid, S.; Althagfan, S.S.; El-Gaby, M.S.A.; Ahmed, H.E.A.; Afifi, T.H. First example of Azo-Sulfa conjugated chromene moieties: Synthesis, characterization, antimicrobial assessment, docking simulation as potent class I histone deacetylase inhibitors and antitumor agents. Bioorg. Chem. 2019, 92, 103262. [Google Scholar] [CrossRef] [PubMed]
- Sabry, N.M.; Mohamed, H.M.; Khattab, E.S.A.E.H.; Motlaq, S.S.; El-Agrody, A.M. Synthesis of 4H-Chromene, Coumarin, 12H-Chromeno[2,3-d]Pyrimidine Derivatives and Some of Their Antimicrobial and Cytotoxicity Activities. Eur. J. Med. Chem. 2011, 46, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Zachariah, S.M. Pharmacological Activities of Chromene Derivatives: An Overview. Asian J. Pharm. Clin. Res. 2013, 6, 11–15. [Google Scholar]
- Li, M.; Zhao, X.; Yang, W.; Zhong, F.; Yuan, L.; Ren, Q. Asymmetric synthesis and biological evaluation of 3-nitro-2H-chromenes as potential antibacterial agents. Tetrahedron Lett. 2018, 59, 3511–3515. [Google Scholar] [CrossRef]
- Thanh, N.D.; Hai, D.S.; Ngoc Bich, V.T.; Thu Hien, P.T.; Ky Duyen, N.T.; Mai, N.T.; Dung, T.T.; Toan, V.N.; Kim Van, H.T.; Dang, L.H.; et al. Efficient click chemistry towards novel 1H-1,2,3-triazole-tethered 4H-chromene−D-glucose conjugates: Design, synthesis and evaluation of in vitro antibacterial, MRSA and antifungal activities. Eur. J. Med. Chem. 2019, 167, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Subbareddy, C.V.; Subashini, R.; Sumathi, S. Synthesis of substituted 2H-chromenes by a three-component reaction as potential antioxidants. Mol. Divers. 2017, 21, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Yahagi, H.; Uesawa, Y.; Sugita, Y. 3-(E)-Styryl-2H-chromene derivatives as potent and selective monoamine oxidase B inhibitors. Bioorg. Chem. 2018, 77, 436–442. [Google Scholar] [CrossRef]
- Razdan, R.K.; Pars, H.G.; Granchelli, F.E.; Harris, L.S. Steroidal Analog of a Tetrahydrocannabinol. J. Med. Chem. 1968, 11, 377–378. [Google Scholar] [CrossRef]
- Pars, H.G.; Granchelli, F.E.; Keller, J.K.; Razdan, R.K. Physiologically Active Nitrogen Analogs of Tetrahydrocannabinols. Tetrahydrobenzopyrano[3,4-d]Pyridines. J. Am. Chem. Soc. 1966, 88, 3664–3665. [Google Scholar] [CrossRef]
- Pars, H.G.; Granchelli, F.E.; Razdan, R.K.; Keller, J.K.; Teiger, D.G.; Rosenberg, F.J.; Harris, L.S. Drugs derived from cannabinoids. 1. Nitrogen analogs, benzopyranopyridines and benzopyranopyrroles. J. Med. Chem. 1976, 19, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Altenbach, R.J.; Basha, F.Z.; Carroll, W.A.; Drizin, I.; Kerwin, J.F., Jr.; Wendt, M.D.; Haight, A.R.; Zhang, W. Benzopyranopyrrole and Benzopyranopyridine Alpha-1 Adrenergic Compounds. WO Patent WO9824791A1, 11 June 1998. [Google Scholar]
- Brown, R.E.; Puchalski, C.; Shavel, J., Jr. Novel Substituted Benzopyranopyridine. U.S. Patent 3,962,266, 8 June 1976. [Google Scholar]
- Connor, D.T.; Unangst, P.C.; Schwender, C.F.; Sorenson, R.J.; Carethers, M.E.; Puchalski, C.; Brown, R.E. Synthesis of 1,2,3,4-tetrahydro-5H-[1]benzopyrano[3,4-c]pyridin-5-ones. II. Substitution at the 3-position with 2-aminoethyl and 2-aminopropyl side chains. J. Heterocycl. Chem. 1984, 21, 1561–1564. [Google Scholar] [CrossRef]
- Connor, D.T.; Unangst, P.C.; Schwender, C.F.; Sorenson, R.J.; Carethers, M.E.; Brown, R.E.; Puchalski, C. Synthesis of 1,2,3,4-tetrahydro-5H-[1]benzopyrano[3,4-c]pyridin-5-ones. I. 3-unsubstituted compounds. J. Heterocycl. Chem. 1984, 21, 1557–1559. [Google Scholar] [CrossRef]
- Radulovic, N.; Stojanovic, G.; Vukicevic, R.; Dekic, V.; Dekic, B.; Palic, R. New 3,4-Annelated Coumarin Derivatives: Synthesis, Antimicrobial Activity, Antioxidant Capacity, and Molecular Modeling. Monatshefte Chem./Chem. Mon. 2006, 137, 1477–1486. [Google Scholar] [CrossRef]
- Dawane, B.S.; Konda, S.G.; Bodade, R.G.; Bhosale, R.B. An efficient one-pot synthesis of some new 2,4-diaryl pyrido[3,2-c]coumarins as potent antimicrobial agents. J. Heterocycl. Chem. 2010, 47, 237–241. [Google Scholar] [CrossRef]
- Nunez-Vergara, L.J.; Squella, J.A.; Navarrete-Encina, P.A.; Vicente-Garcia, E.; Preciado, S.; Lavilla, R. Chromenopyridines: Promising Scaffolds for Medicinal and Biological Chemistry. Curr. Med. Chem. 2011, 18, 4761–4785. [Google Scholar] [CrossRef] [PubMed]
- Delost, M.D.; Smith, D.T.; Anderson, B.J.; Njardarson, J.T. From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem. 2018, 61, 10996–11020. [Google Scholar] [CrossRef]
- Mishra, S.; Ghosh, R. K2CO3-Mediated, One-Pot, Multicomponent Synthesis of Medicinally Potent Pyridine and Chromeno[2,3-b]Pyridine Scaffolds. Synth. Commun. 2012, 42, 2229–2244. [Google Scholar] [CrossRef]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. Convenient one-step synthesis of a medicinally relevant benzopyranopyridine system. Tetrahedron Lett. 2006, 47, 9309–9312. [Google Scholar] [CrossRef]
- Banerjee, S.; Wang, J.; Pfeffer, S.; Ma, D.; Pfeffer, L.M.; Patil, S.A.; Li, W.; Miller, D.D. Design, Synthesis and Biological Evaluation of Novel 5H-Chromenopyridines as Potential Anti-Cancer Agents. Molecules 2015, 20, 17152–17165. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Ranjan, S.; Rao, M.S.; Dar, A.H.; Shyam, M.; Jayaprakash, V.; Hussain, S. Borax Catalysed Domino Synthesis of Highly Functionalised Spirooxindole and Chromenopyridine Derivatives: X-Ray Structure, Hirshfeld Surface Analysis and Molecular Docking Studies. ChemistrySelect 2018, 3, 8669–8677. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Pot, atom and step economic (PASE) synthesis of 5-isoxazolyl-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2015, 25, 424–426. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Novikov, R.A.; Egorov, M.P. PASE Pseudo-Four-Component Synthesis and Docking Studies of New 5-C-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]Pyridine-3-Carbonitriles. ChemistrySelect 2017, 2, 4593–4597. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Goloveshkin, A.S.; Ushakov, I.E.; Egorov, M.P. PASE facile and efficient multicomponent approach to the new type of 5-C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2018, 28, 372–374. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Goloveshkin, A.S.; Ushakov, I.E.; Egorov, M.P. Multicomponent transformation of salicylaldehydes, 2-aminoprop-1-ene-1,1,3-tricarbonitrile, and pyrazolin-5-ones into substituted 2,4-diamino-5-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitriles. Russ. Chem. Bull. 2018, 67, 1695–1703. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Egorov, M.P. Selective multicomponent ‘one-pot’ approach to the new 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)chromeno[2,3-b]pyridine scaffold in pyridine–ethanol catalyst/solvent system. Monatshefte Chem./Chem. Mon. 2019, 150, 1073–1078. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent Approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]Pyridine Scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot, atom and step economic (PASE) assembly of salicylaldehydes, malononitrile dimer and 4-hydroxypyridine-2(1H)-ones into medicinally relevant 5H-chromeno[2,3-b]pyridine scaffold. Mol. Divers. 2019, 24, 617–626. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Dorofeeva, E.O.; Goloveshkin, A.S.; Egorov, M.P. Pseudo six-component stereoselective synthesis of 2,4,6-triaryl-3,3,5,5-tetracyanopiperidines. Mendeleev Commun. 2018, 28, 384–386. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. A facile and efficient multicomponent approach to 5-[5-hydroxy-3-(trifluoromethyl)-1H-pyrazol-4-yl]-5H-chromeno[2,3-b]pyridines. J. Fluor. Chem. 2018, 213, 31–36. [Google Scholar] [CrossRef]
- Festa, A.A.; Storozhenko, O.A.; Golantsov, N.E.; Subramani, K.; Novikov, R.A.; Zaitseva, S.O.; Baranov, M.S.; Varlamov, A.V.; Voskressensky, L.G. Homophtalonitrile for Multicomponent Reactions: Syntheses and Optical Properties of o-Cyanophenyl- or Indol-3-Yl-SubstitutedChromeno[2,3-c]Isoquinolin-5-Amines. ChemistryOpen 2019, 8, 23–30. [Google Scholar] [CrossRef]
- Festa, A.A.; Storozhenko, O.A.; Bella Ndoutoume, D.R.; Varlamov, A.V.; Voskressensky, L.G. Sequential three-component reaction of homophthalonitrile, salicylaldehydes and nitromethane. Mendeleev Commun. 2017, 27, 451–453. [Google Scholar] [CrossRef]
- Shaabani, A.; Hajishaabanha, F.; Mofakham, H.; Maleki, A. A new one-pot three-component synthesis of 2,4-diamino-5H-chromeno[2,3-b] pyridine-3-carbonitrile derivatives. Mol. Divers. 2010, 14, 179–182. [Google Scholar] [CrossRef]
- Lopes, D.; Oliveira-Pinto, S.; Pontes, O.; Sampaio-Marques, B.; Costa, M.D.; Carvalho, L.; Gonçalves, C.S.; Costa, B.M.; Maciel, P.; Ludovico, P.; et al. Unravelling the anticancer potential of functionalized chromeno[2,3-b]pyridines for breast cancer treatment. Bioorg. Chem. 2020, 100, 103942. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Multicomponent Synthesis of Novel Penta-Heterocyclic Ring Systems Incorporating a Benzopyranopyridine Scaffold. Synthesis 2014, 46, 258–262. [Google Scholar] [CrossRef]
- Navarrete-Encina, P.A.; Salazar, R.; Vega-Retter, C.; Pérez, K.; Squella, J.A.; Nuñez-Vergara, L.J. On the one pot syntheses of chromeno[4,3-b]pyridine-3-carboxylate and chromeno[3,4-c]pyridine-3-carboxylate and dihydropyridines. J. Braz. Chem. Soc. 2010, 21, 413–418. [Google Scholar] [CrossRef]
- Povarov, L.S.; Grigos, V.I.; Mikhailov, B.M. Reaction of benzylideneaniline with some unsaturated compounds. Russ. Chem. Bull. 1963, 12, 1878–1880. [Google Scholar] [CrossRef]
- Adolfsson, D.E.; Tyagi, M.; Singh, P.; Deuschmann, A.; Ådén, J.; Gharibyan, A.L.; Jayaweera, S.W.; Lindgren, A.E.G.; Olofsson, A.; Almqvist, F. Intramolecular Povarov Reactions for the Synthesis of Chromenopyridine Fused 2-Pyridone Polyheterocycles Binding to α-Synuclein and Amyloid-β Fibrils. J. Org. Chem. 2020, 85, 14174–14189. [Google Scholar] [CrossRef]
- Dimitriadou, E.; Raftopoulou, M.; Kasapidou, P.M.; Tsoleridis, C.A.; Stephanidou-Stephanatou, J.; Hadjipavlou-Litina, D.J.; Kontogiorgis, C.; Pritsa, A.; Papadopoulos, A. Ultrasound promoted synthesis of chromeno[2,3-b]pyridines and their evaluation as lipid peroxidation inhibitors. Arkivoc 2014, 2014, 372–384. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M.; Ibrahim, S.S.; Said, S. Synthesis of Some Novel Heteroannelated Chromones by Basic Rearrangement of 6-Methylchromone-3-Carbonitrile. Chem. Heterocycl. Compd. 2015, 50, 1624–1633. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M. Studies on the Chemical Transformations of 6-Methylchromone-3-Carbonitrile under Nucleophilic Conditions. J. Heterocycl. Chem. 2016, 53, 859–864. [Google Scholar] [CrossRef]
- Savych, I.; Ejaz, S.A.; Shah, S.J.A.; Iaroshenko, V.O.; Villinger, A.; Sosnovskikh, V.Y.; Iqbal, J.; Abbasi, A.; Langer, P. Reactions of 3-Acylchromones with Heterocyclic Ketene Aminals: One-Pot Synthesis and Phosphatase Inhibitory Activity of Fused Pyridine Derivatives. Eur. J. Org. Chem. 2017, 2017, 186–202. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Praveen, S.; Farooq, F. Novel benzopyranopyridine derivatives of 2-amino-3-formylchromone. Chem. Pap. 2010, 64, 818–824. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Farag, A.A.M.; Roushdy, N.; El-Gohary, N.M. Synthesis, optical and photoelectrical characterizations of the novel 10-chloro-6H,8H-dichromeno[2,3-b:3′,4′-e]pyridine-6,8-dione (CDPD) and its photodiode application. Opt. Mater. 2016, 51, 70–77. [Google Scholar] [CrossRef]
- Ponduri, R.; Kumar, P.; Vadali, L.R.A.O.; Modugu, N.R. Water-PEG-400 Mediated an Efficient One-Pot Eco-Friendly Synthesis of Functionalized Isoxazole Substituted Chromeno[2,3-b]pyridine-3-carboxylate Derivatives. ChemistrySelect 2018, 3, 7766–7770. [Google Scholar] [CrossRef]
- Dolatkhah, Z.; Nasiri-Aghdam, M.; Bazgir, A. A Three-Component Synthesis of Benzochromenodiazocines and Chromenopyridines. Tetrahedron Lett. 2013, 54, 1960–1962. [Google Scholar] [CrossRef]
- Zhang, C.H.; Huang, R.; Hu, X.M.; Lin, J.; Yan, S.J. Three-Component Site-Selective Synthesis of Highly Substituted 5H-Chromeno-[4,3-b]Pyridines. J. Org. Chem. 2018, 83, 4981–4989. [Google Scholar] [CrossRef]
- Lozinski, O.A.; Shokol, T.V.; Zubatyuk, R.I.; Shishkin, O.V.; Khilya, V.P. An alternative approach to the synthesis of 5H-chromeno[4,3-b]pyridin-5-one system using the cleavage of 5H,9H-pyrano[2′,3′:5,6]chromeno[4,3-b]pyridine-5,9-diones with binucleophiles. Chem. Heterocycl. Comp. 2018, 54, 96–99. [Google Scholar] [CrossRef]
- Ali, K.A.; Abdel Hafez, N.A.; Elsayed, M.A.; Ibrahim, A.A. Microwave-assisted synthesis and heterocyclic functionalization of chromenopyridines on calixarene scaffold. J. Heterocycl. Chem. 2020, 57, 1838–1844. [Google Scholar] [CrossRef]
- Thapa, U.; Thapa, P.; Karki, R.; Yun, M.; Choi, J.H.; Jahng, Y.; Lee, E.; Jeon, K.H.; Na, Y.; Ha, E.M.; et al. Synthesis of 2,4-diaryl chromenopyridines and evaluation of their topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship. Eur. J. Med. Chem. 2011, 46, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Lee, E.S. 2,4-Diaryl-5,6-Dihydro-1,10-Phenanthrolines with Furyl or Thienyl Moiety at 4-Position: Synthesis, Topoisomerase I and II Inhibitory Activity, and Cytotoxicity. Bull. Korean Chem. Soc. 2012, 33, 1769–1772. [Google Scholar] [CrossRef][Green Version]
- Thapa, P.; Jun, K.Y.; Kadayat, T.M.; Park, C.; Zheng, Z.; Thapa Magar, T.B.; Bist, G.; Shrestha, A.; Na, Y.; Kwon, Y.; et al. Design and synthesis of conformationally constrained hydroxylated 4-phenyl-2-aryl chromenopyridines as novel and selective topoisomerase II-targeted antiproliferative agents. Bioorg. Med. Chem. 2015, 23, 6454–6466. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.B.; Park, C.; Jeon, K.H.; Lee, E.; Park, S.E.; Jun, K.Y.; Kadayat, T.M.; Thapa, P.; Karki, R.; Na, Y.; et al. A Series of Novel Terpyridine-Skeleton Molecule Derivants Inhibit Tumor Growth and Metastasis by Targeting Topoisomerases. J. Med. Chem. 2015, 58, 1100–1122. [Google Scholar] [CrossRef] [PubMed]
- Magar, T.B.T.; Seo, S.H.; Kadayat, T.M.; Jo, H.; Shrestha, A.; Bist, G.; Katila, P.; Kwon, Y.; Lee, E.S. Synthesis and SAR study of new hydroxy and chloro-substituted 2,4-diphenyl 5H-chromeno[4,3-b]pyridines as selective topoisomerase IIα-targeting anticancer agents. Bioorg. Med. Chem. 2018, 26, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, H.; Dawood, K.M.; Aryan, F.A.; Ibrahim, H.M. Green Protocol for the Novel Synthesis of Thiochromeno[4,3-b]Pyridine and Chromeno[4,3-b]Pyridine Derivatives Utilizing a High-Pressure System. ACS Omega 2021, 6, 34065–34074. [Google Scholar] [CrossRef] [PubMed]
- El-Essawy, F.; El-Etrawy, A.S. Synthesis of New Chromeno[4,3-b]pyrazolo[4,3-e]pyridines Derivatives with Antimicrobial Evaluation. J. Heterocycl. Chem. 2013, 51, 191–195. [Google Scholar] [CrossRef]
- Rong, L.; Han, H.; Jiang, H.; Zhang, Q.; Tu, S. Efficient one-pot synthesis of 4-aryl-3-cyano-2,5-dihydro-1H-indeno[1,2-b]pyridin-2-one and 4-aryl-3-cyano-1,2,5,6-tetrahydrobenzo[h] quinolin-2-one derivatives under solvent-free conditions. Synth. Commun. 2009, 39, 1027–1034. [Google Scholar] [CrossRef][Green Version]
- Kok, T.; Wapenaar, H.; Wang, K.; Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Proietti, G.; Eleftheriadis, N.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Cool, R.H.; et al. Discovery of chromenes as inhibitors of macrophage migration inhibitory factor. Bioorg. Med. Chem. 2018, 26, 999–1005. [Google Scholar] [CrossRef]
- Patel, M.A.; Bhila, V.G.; Patel, N.H.; Patel, A.K.; Brahmbhatt, D.I. Synthesis, characterization and biological evaluation of some pyridine and quinoline fused chromenone derivatives. Med. Chem. Res. 2012, 21, 4381–4388. [Google Scholar] [CrossRef]
- Pal, S.; Khan, M.N.; Karamthulla, S.; Choudhury, L.H. Molecular iodine catalyzed one-pot multicomponent reactions for the synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-ones. RSC Adv. 2013, 3, 15705–15711. [Google Scholar] [CrossRef]
- Patel, A.A.; Lad, H.B.; Pandya, K.R.; Patel, C.V.; Brahmbhatt, D.I. Synthesis of a new series of 2-(2-oxo-2H-chromen-3-yl)-5H-chromeno[4,3-b]pyridin-5-ones by two facile methods and evaluation of their antimicrobial activity. Med. Chem. Res. 2013, 22, 4745–4754. [Google Scholar] [CrossRef]
- Khan, A.T.; Das, D.K. Michael Initiated Ring Closure (MIRC) reaction on in situ generated benzylidenecyclohexane-1,3-diones for the construction of chromeno[3,4-b]quinoline derivatives. Tetrahedron Lett. 2012, 53, 2345–2351. [Google Scholar] [CrossRef]
- Khan, A.T.; Das, D.K.; Islam, K.; Das, P. A simple and expedient synthesis of functionalized pyrido[2,3-c] coumarin derivatives using molecular iodine catalyzed three-component reaction. Tetrahedron Lett. 2012, 53, 6418–6422. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, J.; Su, W. An Efficient Protocol for Multicomponent Synthesis of 1H-Chromeno[4,3-b]Pyridin-5(4H)-Ones Derivatives. J. Chem. Res. 2013, 37, 327–330. [Google Scholar] [CrossRef]
- Oshiro, P.B.; Bregadiolli, B.A.; da Silva-Filho, L.C. A facile one-step synthesis of chromeno[4,3-b]pyridine derivatives promoted by niobium pentachloride. J. Heterocycl. Chem. 2020, 57, 2795–2800. [Google Scholar] [CrossRef]
- Paul, S.; Das, A.R. An efficient green protocol for the synthesis of coumarin fused highly decorated indenodihydropyridyl and dihydropyridyl derivatives. Tetrahedron Lett. 2012, 53, 2206–2210. [Google Scholar] [CrossRef]
- Motamedi, R. Solvent-free synthesis of novel 5-oxo-5H-chromeno [4,3-b]pyridine derivatives. Chem. Heterocyc. Compd. 2013, 48, 1839–1843. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Litinas, K.E. Synthesis of methyl substituted [5,6]- and [7,8]-fused pyridocoumarins via the iodine-catalyzed reaction of aminocoumarins with n-butyl vinyl ether. Tetrahedron Lett. 2013, 54, 6517–6519. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Chandra, S. One-pot access to pyridocoumarins via Povarov-hydrogen transfer cascade under auto-tandem catalysis of iodine in aqueous micelles. Tetrahedron Lett. 2014, 55, 1564–1568. [Google Scholar] [CrossRef]
- Epstein, O.; Bryan, M.C.; Cheng, A.C.; Derakhchan, K.; Dineen, T.A.; Hickman, D.; Hua, Z.; Human, J.B.; Kreiman, C.; Marx, I.E.; et al. Lead Optimization and Modulation of HERG Activity in a Series of Aminooxazoline Xanthene β-Site Amyloid Precursor Protein Cleaving Enzyme (BACE1) Inhibitors. J. Med. Chem. 2014, 57, 9796–9810. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Brown, J.; Judd, T.C.; Lopez, P.; Qian, W.; Powers, T.S.; Chen, J.J.; Bartberger, M.D.; Chen, K.; Dunn, R.T.; et al. An Orally Available BACE1 Inhibitor That Affords Robust CNS Aβ Reduction without Cardiovascular Liabilities. ACS Med. Chem. Lett. 2015, 6, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; La, D.S.; Cheng, A.C.; Whittington, D.A.; Patel, V.F.; Chen, K.; Dineen, T.A.; Epstein, O.; Graceffa, R.; Hickman, D.; et al. Structure- and Property-Based Design of Aminooxazoline Xanthenes as Selective, Orally Efficacious, and Cns Penetrable BACE Inhibitors for the Treatment of Alzheimers Disease. J. Med. Chem. 2012, 55, 9156–9169. [Google Scholar] [CrossRef]
- Dineen, T.A.; Chen, K.; Cheng, A.C.; Derakhchan, K.; Epstein, O.; Esmay, J.; Hickman, D.; Kreiman, C.E.; Marx, I.E.; Wahl, R.C.; et al. Inhibitors of β-Site Amyloid Precursor Protein Cleaving Enzyme (BACE1): Identification of (S)-7-(2-Fluoropyridin-3-yl)-3-((3-methyloxetan-3-yl)ethynyl)-5′H-Spiro[Chromeno[2,3-b]pyridine-5,4′-oxazol]-2′-amine (AMG-8718). J. Med. Chem. 2014, 57, 9811–9831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).