Fragmentation Patterns of Phenolic C-Glycosides in Mass Spectrometry Analysis
Abstract
1. Introduction
2. Results
2.1. Phenolic C-Glycoside Selection
2.2. Fragmentation Patterns of Dietary Phenolic C-Glycosides
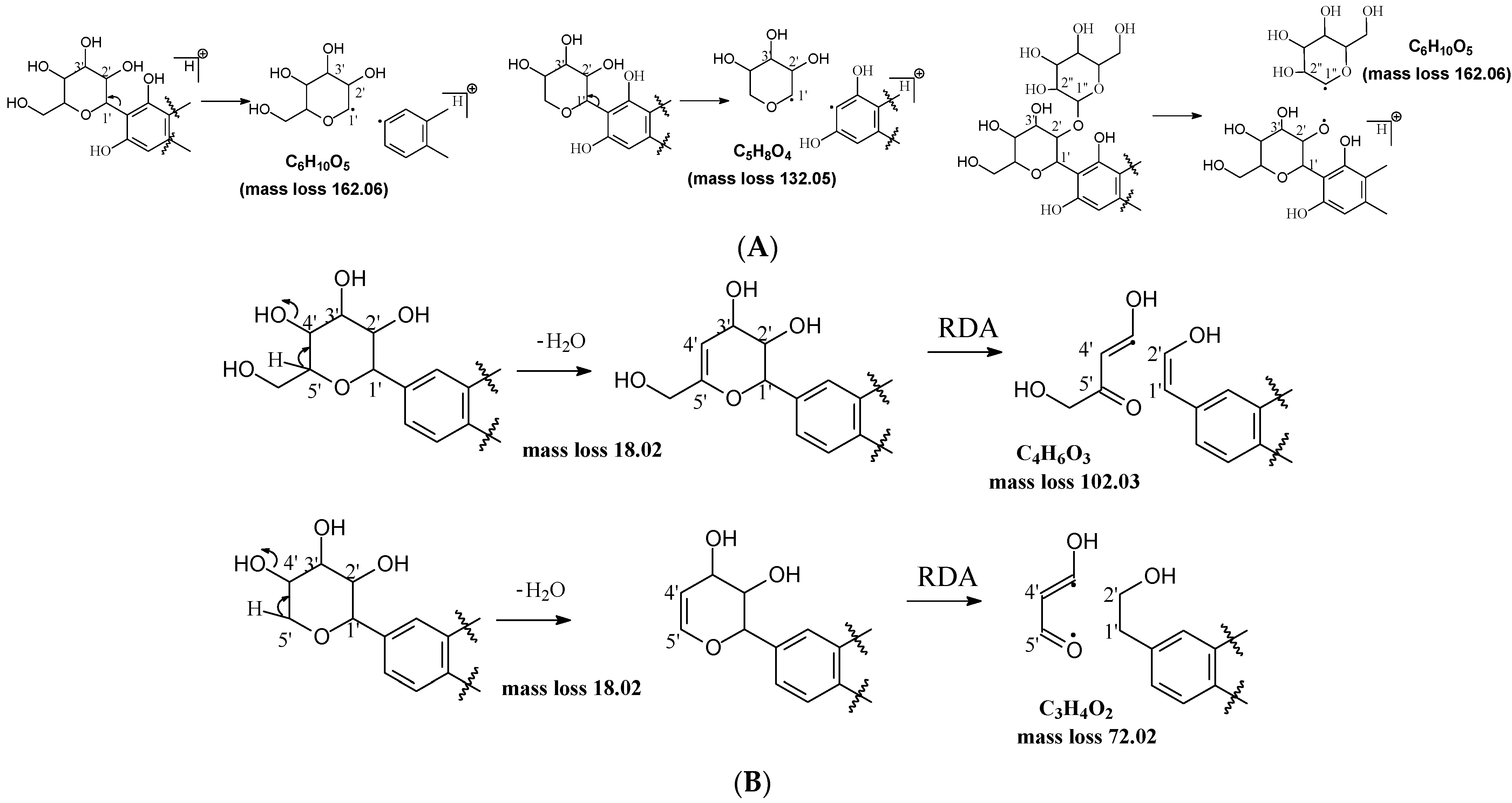
2.2.1. Glycosidic Bond Cleavage
2.2.2. Sugar Cleavage
2.2.3. Aglycone Cleavage
2.3. MS Fragmentation Pathway of Representative Compounds
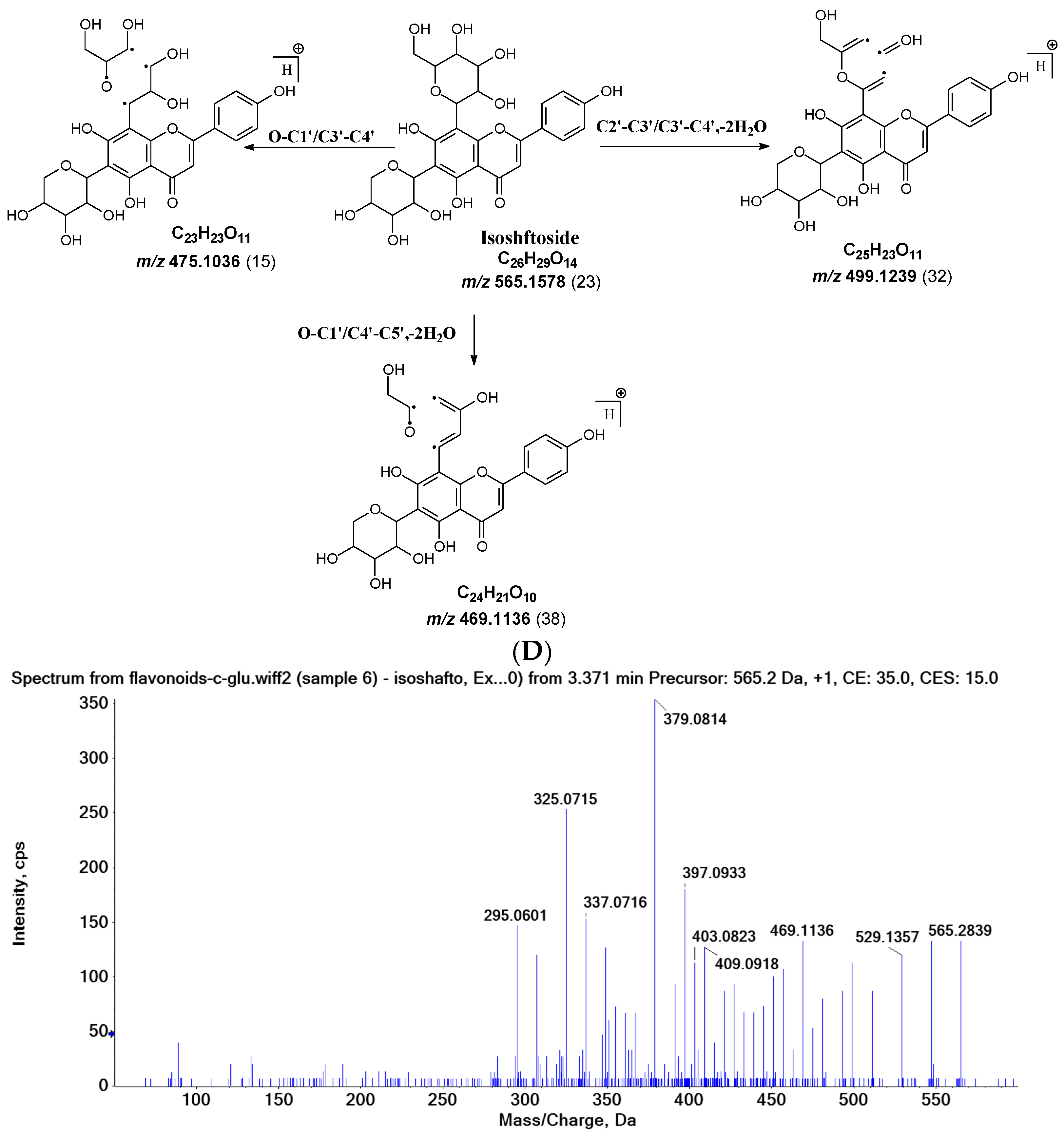
2.3.1. Isoshaftoside MS Fragmentation Pathway
2.3.2. Neomangiferin MS Fragmentation Pathway
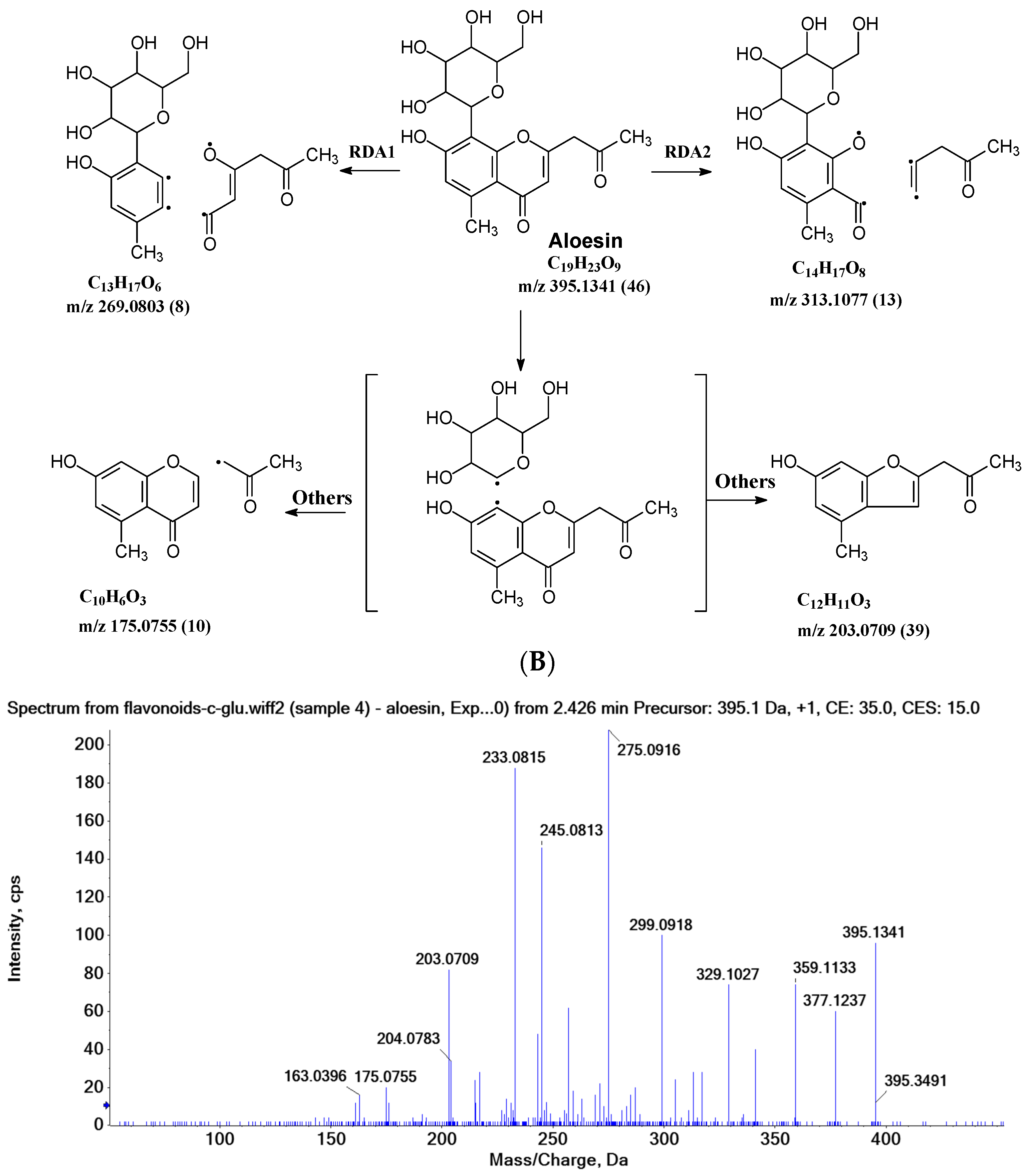
2.3.3. Aloesin MS Fragmentation Pathway

3. Materials and Methods
3.1. Chemical Sand Reagents
3.2. Sample Preparation
3.3. Instrumentation and Analytical Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Q.; Zhang, M.; Wang, Z.L.; Qiao, X.; Ye, M. Advances in plant-derived C-glycosides: Phytochemistry, bioactivities, and biotechnological production. Biotechnol. Adv. 2022, 60, 108030. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F. Recent Advances on Natural Aryl-C-glycoside Scaffolds: Structure, Bioactivities, and Synthesis—A Comprehensive Review. Molecules 2022, 27, 7439. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Y.; Fan, N.L.; Hu, X.G. Recent development in the synthesis of C-glycosides involving glycosyl radicals. Org. Biomol. Chem. 2020, 18, 5095–5109. [Google Scholar] [CrossRef] [PubMed]
- Tegl, G.; Nidetzky, B. Leloir glycosyltransferases of natural product C-glycosylation: Structure, mechanism and specificity. Biochem. Soc. Trans. 2020, 48, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S29–S45. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Zhang, Q.; Jiang, S.; Du, S.; Zhang, W.; Li, Y.; Sun, C.; Niu, Y. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: A double-blind randomized controlled trial. Sci. Rep. 2015, 5, 10344. [Google Scholar] [CrossRef] [PubMed]
- Im, A.E.; Eom, S.; Seong, H.J.; Kim, H.; Cho, J.Y.; Kim, D.; Lee, J.H.; Yang, K.Y.; Nam, S.H. Enhancement of debitterness, water-solubility, and neuroprotective effects of naringin by transglucosylation. Appl. Microbiol. Biotechnol. 2023, 107, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Shafreen, M.M.; Achudhan, A.B.; Gupta, A.; Saleena, L. A review on applications of β-glucosidase in food, brewery, pharmaceutical and cosmetic industries. Carbohydr. Res. 2023, 530, 108855. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kumano, T.; He, H.; Watanabe, S.; Senda, M.; Moriya, T.; Adachi, N.; Hori, S.; Terashita, Y.; Kawasaki, M.; et al. C-Glycoside metabolism in the gut and in nature: Identification, characterization, structural analyses and distribution of C-C bond-cleaving enzymes. Nat. Commun. 2021, 12, 6294. [Google Scholar] [CrossRef]
- Yao, C.L.; Yang, W.Z.; Si, W.; Shen, Y.; Zhang, N.X.; Chen, H.L.; Pan, H.Q.; Yang, M.; Wu, W.Y.; Guo, D.A. An enhanced targeted identification strategy for the selective identification of flavonoid O-glycosides from Carthamus tinctorius by integrating offline two-dimensional liquid chromatography/linear ion-trap-Orbitrap mass spectrometry, high-resolution diagnostic product ions/neutral loss filtering and liquid chromatography-solid phase extraction-nuclear magnetic resonance. J. Chromatogr. A 2017, 1491, 87–97. [Google Scholar]
- Liu, R.; Meng, C.; Zhang, Z.; Ma, H.; Lv, T.; Xie, S.; Liu, Y.; Wang, C. Comparative metabolism of schaftoside in healthy and calcium oxalate kidney stone rats by UHPLC-Q-TOF-MS/MS method. Anal. Biochem. 2020, 597, 113673. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kong, Y.; Zu, Y.; Fu, Y.; Luo, M.; Zhang, L.; Li, J. Determination and quantification of active phenolic compounds in pigeon pea leaves and its medicinal product using liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 4723–4731. [Google Scholar] [CrossRef]
- Shao, S.-Y.; Ting, Y.; Wang, J.; Sun, J.; Guo, X.-F. Characterization and Identification of the Major Flavonoids in Phyllostachys edulis Leaf Extract by UPLC–QTOF–MS/MS. Acta Chromatogr. 2020, 4, 228–237. [Google Scholar] [CrossRef]
- Wu, X.; Ding, W.; Zhong, J.; Wan, J.; Xie, Z. Simultaneous qualitative and quantitative determination of phenolic compounds in Aloe barbadensis Mill by liquid chromatography-mass spectrometry-ion trap-time-of-flight and high-performance liquid chromatography-diode array detector. J. Pharm. Biomed. Anal. 2013, 80, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Krskova, Z.; Goncalves, R.F.; Valentao, P.; Pereira, J.A.; Dusek, J.; Martin, J.; Andrade, P.B. Free water-soluble phenolics profiling in barley (Hordeum vulgare L.). J. Agric. Food Chem. 2009, 57, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zhang, M.; Wang, Q.; Xie, J. Ultrasonic-assisted extraction of swertisin from sour Jujube seed and comprehensive revelation of its antioxidant activity. J. Food Biochem. 2022, 46, e14433. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.A.E.; Milenkovic, D.; Borges, T.K.; Oliveira, L.L.; Costa, A.M. Brazilian passion fruit as a new healthy food: From its composition to health properties and mechanisms of action. Food Funct. 2021, 12, 11106–11120. [Google Scholar] [CrossRef]
- Luyen, B.T.; Tai, B.H.; Thao, N.P.; Cha, J.Y.; Lee, Y.M.; Kim, Y.H. A new phenolic component from Triticum aestivum sprouts and its effects on LPS-stimulated production of nitric oxide and TNF-alpha in RAW 264.7 cells. Phytother. Res. 2014, 28, 1064–1070. [Google Scholar] [CrossRef]
- Jung, E.S.; Lee, S.; Lim, S.H.; Ha, S.H.; Liu, K.H.; Lee, C.H. Metabolite profiling of the short-term responses of rice leaves (Oryza sativa cv. Ilmi) cultivated under different LED lights and its correlations with antioxidant activities. Plant Sci. 2013, 210, 61–69. [Google Scholar] [CrossRef]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Q-TOF LC/MS identification and UHPLC-Online ABTS antioxidant activity guided mapping of barley polyphenols. Food Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef]
- De Beer, D.; Schulze, A.E.; Joubert, E.; De Villiers, A.; Malherbe, C.J.; Stander, M.A. Food ingredient extracts of Cyclopia subternata (Honeybush): Variation in phenolic composition and antioxidant capacity. Molecules 2012, 17, 14602–14624. [Google Scholar] [CrossRef]
- Mei, S.; Perumal, M.; Battino, M.; Kitts, D.D.; Xiao, J.; Ma, H.; Chen, X. Mangiferin: A review of dietary sources, absorption, metabolism, bioavailability, and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 3046–3064. [Google Scholar] [CrossRef]
- Anibarro-Ortega, M.; Pinela, J.; Ciric, A.; Lopes, E.; Molina, A.K.; Calhelha, R.C.; Sokovic, M.O.; Ferreira, I.; Barros, F.L. Extraction of Aloesin from Aloe vera Rind Using Alternative Green Solvents: Process Optimization and Biological Activity Assessment. Biology 2021, 10, 951. [Google Scholar] [CrossRef]
- Kim, B.; Woo, S.; Kim, M.J.; Kwon, S.W.; Lee, J.; Sung, S.H.; Koh, H.J. Identification and quantification of flavonoids in yellow grain mutant of rice (Oryza sativa L.). Food Chem. 2018, 241, 154–162. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Sgroppo, S.C.; Sanchez-Moreno, C.; Ancos, B. Mango ‘criollo’ by-products as a source of polyphenols with antioxidant capacity. Ultrasound assisted extraction evaluated by response surface methodology and HPLC-ESI-QTOF-MS/MS characterization. Food Chem. 2022, 396, 133738. [Google Scholar] [CrossRef] [PubMed]





| Compounds | Structure | Major Sources | References |
|---|---|---|---|
| Vitexin |  | Buckwheat, Hawthorn, Mung beans, Passiflora | [15] |
| Isovitexin | 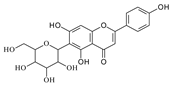 | Barley, Cucumber | [15,16,17] |
| Swertisin | 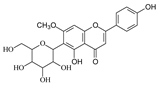 | Jujube | [18] |
| Homoorientin (Isoorientin) | 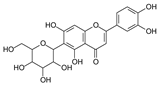 | Passion fruit | [19] |
| Isoshaftoside | 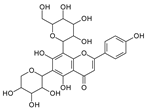 | Barley | [16,20] |
| Isoscoparin |  | Yellow grain rice | [21] |
| Isoscoparin-2″-O-glucoside | 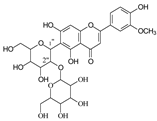 | Yellow grain rice | [19,22] |
| Mangiferin, |  | Mango, Coffea, Honeybush | [23,24] |
| Neomangiferin | 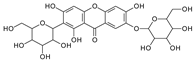 | Mango | [24] |
| Aloesin | 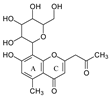 | Aloe | [25] |
| Sugar Moiety | Glycosidic Bond | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | -H2O (−18) | -2H2O (−36) | -3H2O (−54) | -H2O/RDA (−120 or 90) | Alpha (−150 or 120) | C2–C3 and C3–C4, -2H2O, (−66) | O–C1 and C3–C4 (−90) | O–C1 and C4–C5 -2H2O (−96) | −162 or 132 |
| Isovitexin | 415.1024 (11) | 397.0918 (19) | 379.0816 (21) | 313.0707 (86) | 283.0601 (100) | 367.1024 (14) | 337.0711 (45) | 271.0601 (8) | |
| Homoorientin | 431.0973 (8) | 413.0867 (20) | 329.0656 (80) | 299.0550 (100) | 383.0766 (17) | 353.0660 (42) | 287.0550 (9) | ||
| Isoscoparin | 445.1136 (16) | 427.1024 (21) | 409.0925 (21) | 343.0814 (76) | 313.0712 (100) | 397.0930 (19) | 367.0820 (45) | 301.0717 (6) | |
| Vitexin | 415.1024 (39) | 397.0918 (23) | 379.0820 (8) | 313.0707 (39) | 283.0601 (16) | 367.1024 (10) | 343.0812 (7) | 337.0725 (9) | |
| Swertisin | 429.1180 (6) | 411.1074 (12) | 393.0988 (12) | 327.0863 (39) | 297.0757 (100) | 381.1180 (14) | 351.0869 (33) | ||
| Isoshaftoside | 547.1444 (38) | 529.1357 (34) | 511.1247 (25) | 475.1036 (15), 445.1131 (21) 457.1131 (30, -2H2O) | 445.1131 (21), 415.1022 (11), 397.0933 (51, -2H2O) 427.1004 (26), 295.0601 (42, -2H2O) 379.0814 (100, -3H2O) 325.0715 (71, RDA+alpha) | 499.1239 (32) | 475.1036 (15) | 469.1136 (38) | 403.0823 (32) 433.0932 (19) |
| Isoscoparin-2′′-O-glucoside | 445.1127 (47, -O-glu -H2O) | 427.1027 (34, -O-glu -2H2O) | 409.1341 (31, -O-glu) | 343.0812 (100, -O-glu,) | 313.0707 (65, -O-glu,) | 367.0812 (69, -O-glu) | 463.1231 (75, -O-glu) 301.0707 (9, -O glu, -C-glu) | ||
| Mangiferin | 405.0816 (6) | 387.0711 (15) | 369.0609 (18) | 303.0499 (61) | 273.0394 (100) | 357.0609 (8) | 327.0507 (33) | 261.0394 (8) | |
| Neomangiferin | 567.1354(30) 405.0816 (26, -O-glu, -H2O) | 549.1233 (23) 387.0711 (44, -O-glu, -2H2O) | 531.1140 (12) 369.0605 (41, -O-glu) | 465.1028 (36) 303.0507 (89) | 435.0922 (31); 273.0969 (100, -O-glu) | 519.1147 (32) 357.0613 (15) | 327.0497 (79, -O-glu) 489.1026 (29) | 261.0400 (9), 405.0812 (26, -O-glu-H2O) | |
| Aloesin | 377.1237 (29) | 359.1133 (36) | 341.1023 (19) | 275.0912 (100) | 245.0813 (70) | 329.1027 (36) | 305.1024 (12) | 299.0918 (48) | 233.0815 (90) |
| Aglycone | |||||||||
| Compounds | B-ring loss | Loss H2O and B ring | Loss sugar and B ring | C-ring cleavage | Others | ||||
| Isovitexin | 323.0761 (21) | ||||||||
| Homoorientin | 339.0711 (19) | 311.0761 (11) | |||||||
| Isoscoparin | 339.0867 (19) | ||||||||
| Vitexin | |||||||||
| Swertisin | 337.1076 (17) | 323.0761 (22, Loss B-ring and CH3O) 267.0652 (10, Loss CH3O after sugar alpha cleavage) | |||||||
| Isoshaftoside | 307.0607 (34) 337.0716 (43) | ||||||||
| Isoscoparin-2′′-O-glucoside | 339.0872 (6) | ||||||||
| Mangiferin | 299.0761 (6), 313.0554 (13) | ||||||||
| Neomangiferin | 299.0761 (24), 313.0554 (10) | ||||||||
| Aloesin | 269.0803 (8), 313.1077 (13) | 175.0755 (10), 203.0709 (39) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, T.; Wang, Y.; Xie, H.; Liang, D.; Gao, S. Fragmentation Patterns of Phenolic C-Glycosides in Mass Spectrometry Analysis. Molecules 2024, 29, 2953. https://doi.org/10.3390/molecules29132953
Du T, Wang Y, Xie H, Liang D, Gao S. Fragmentation Patterns of Phenolic C-Glycosides in Mass Spectrometry Analysis. Molecules. 2024; 29(13):2953. https://doi.org/10.3390/molecules29132953
Chicago/Turabian StyleDu, Ting, Yang Wang, Huan Xie, Dong Liang, and Song Gao. 2024. "Fragmentation Patterns of Phenolic C-Glycosides in Mass Spectrometry Analysis" Molecules 29, no. 13: 2953. https://doi.org/10.3390/molecules29132953
APA StyleDu, T., Wang, Y., Xie, H., Liang, D., & Gao, S. (2024). Fragmentation Patterns of Phenolic C-Glycosides in Mass Spectrometry Analysis. Molecules, 29(13), 2953. https://doi.org/10.3390/molecules29132953








