Exploring the Antitumor Efficacy of N-Heterocyclic Nitrilotriacetate Oxidovanadium(IV) Salts on Prostate and Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
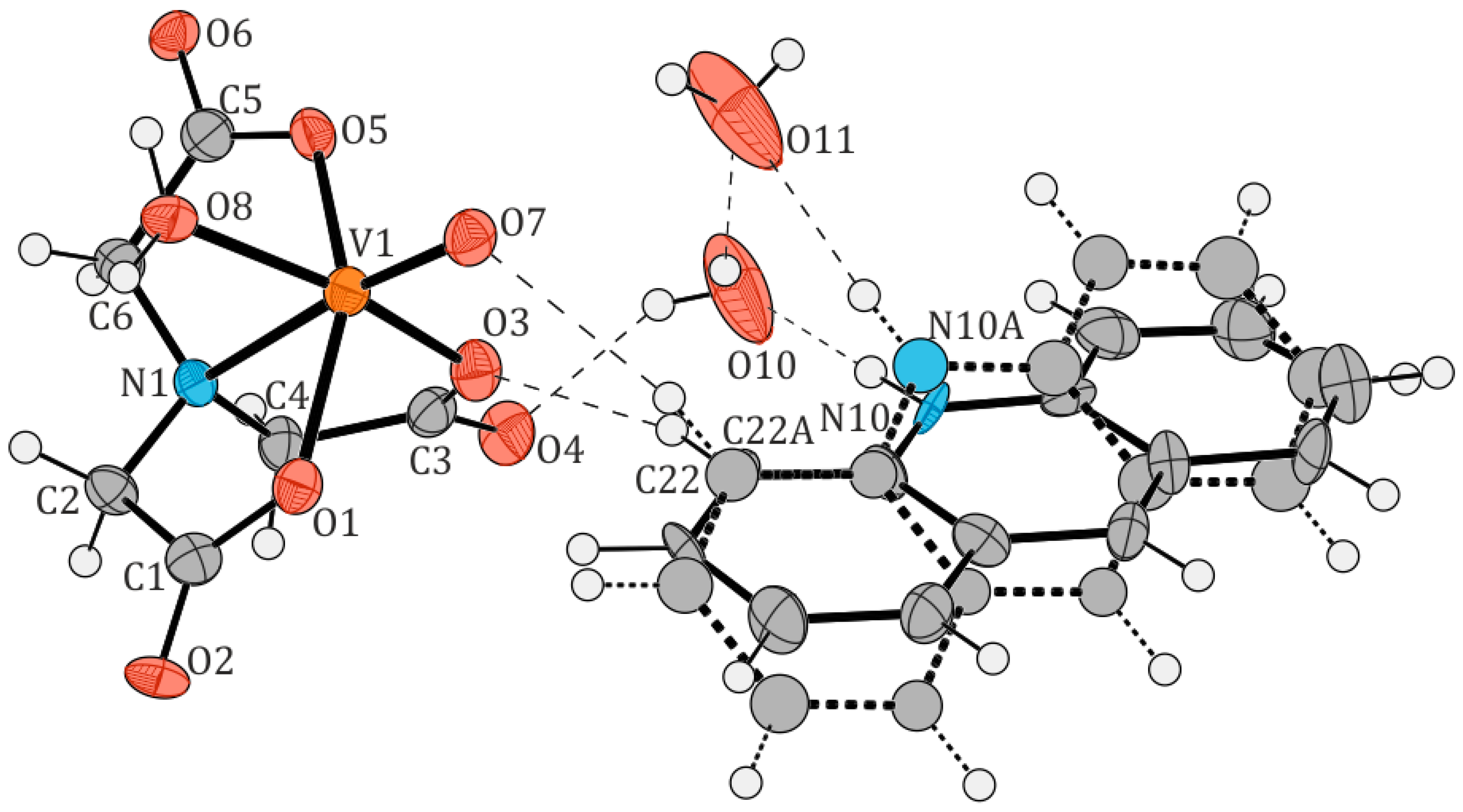
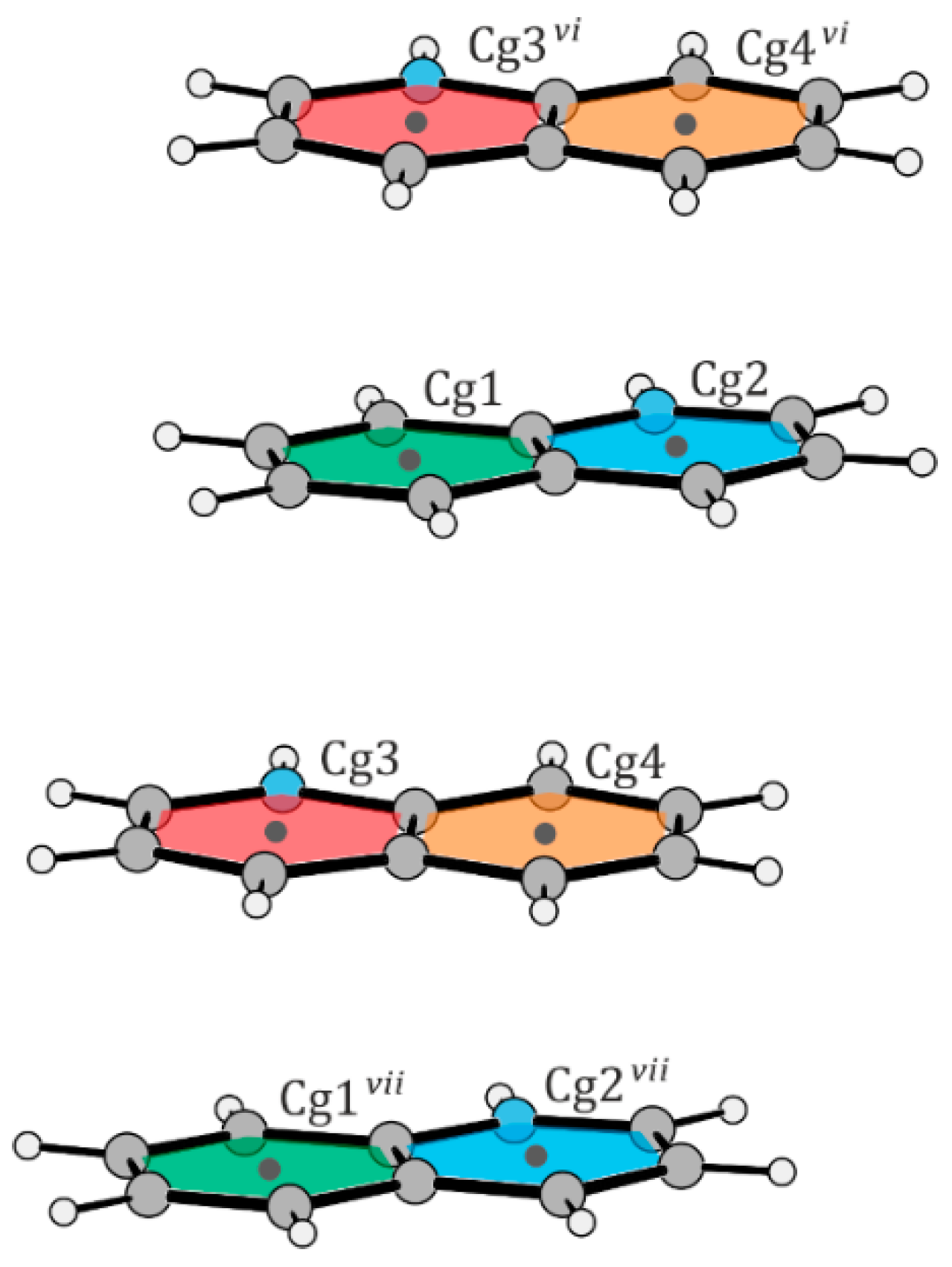
2.1. Structural Description
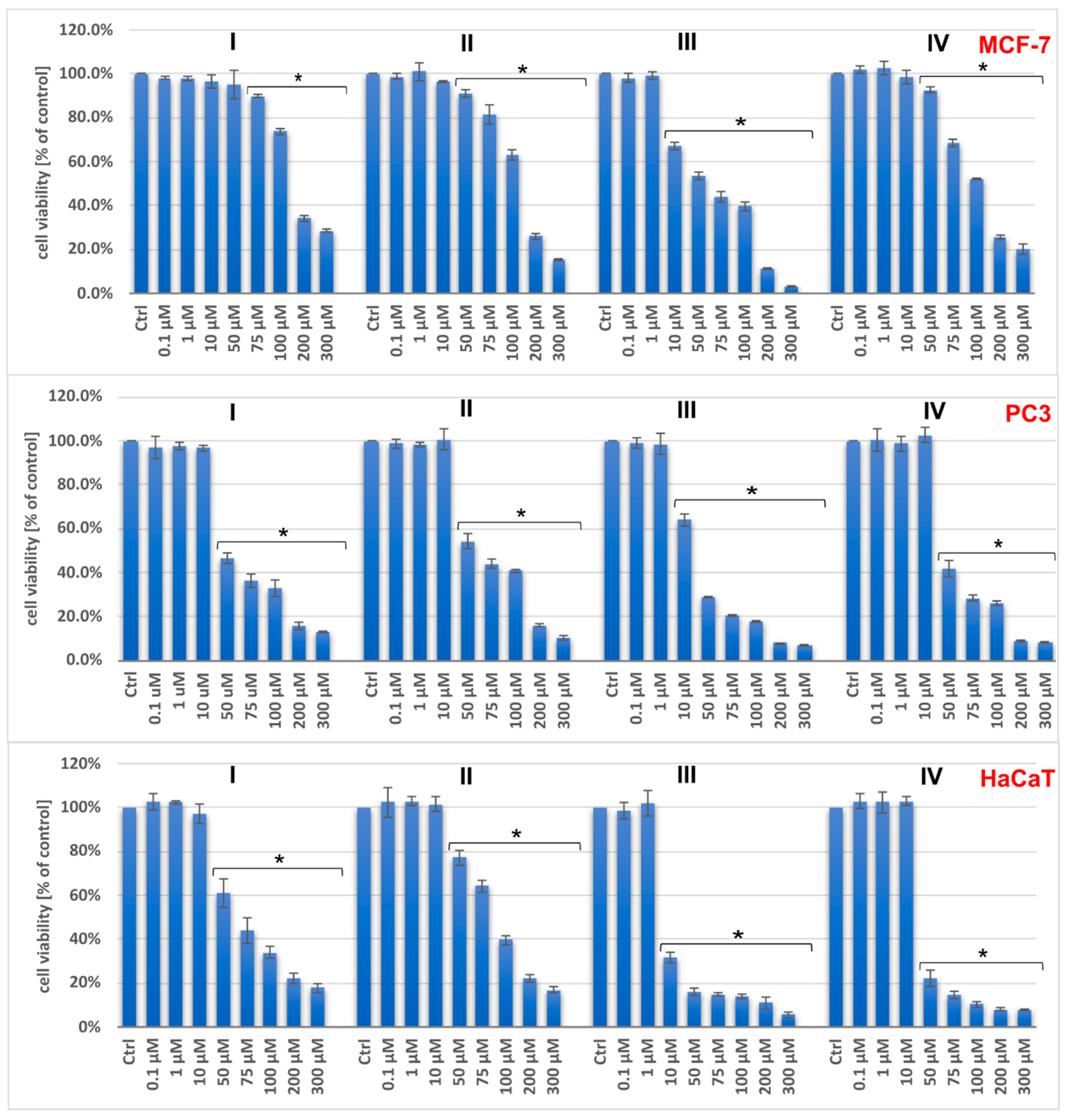
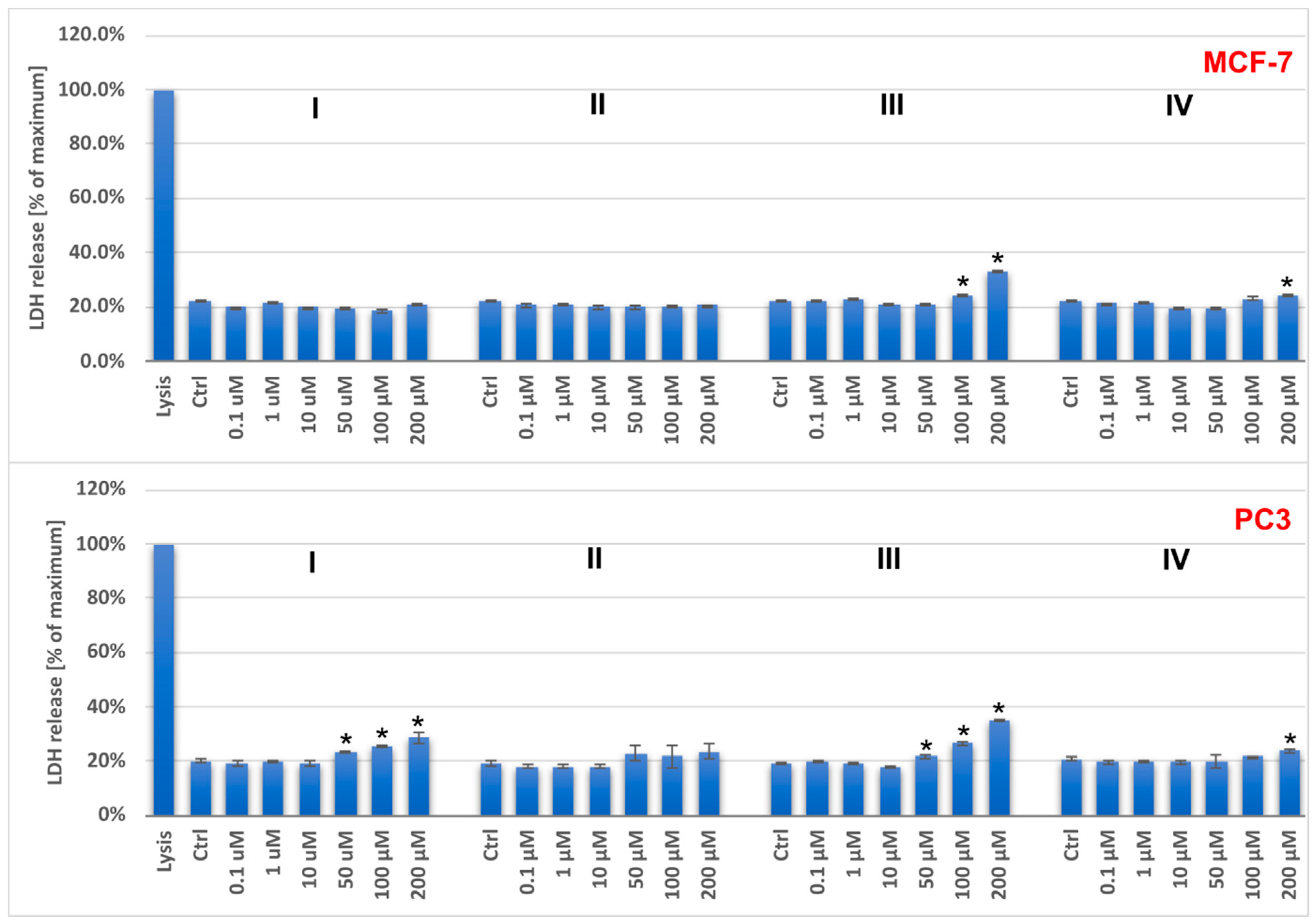
2.2. Cytotoxic Activity of Vanadium Complexes
2.3. Cell Cycle Analysis
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of [QH][VO(nta)(H2O)](H2O)2 (I) and [(acr)H)][VO(nta)(H2O)](H2O)2 (II)
3.3. X-ray Measurements
3.4. IR Spectra
3.5. Biological Studies
3.5.1. MTT Assay
3.5.2. LDH Assay
3.5.3. Cell Cycle Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Lu, C.; Yang, W.; Chen, S.; Yu, Y.; He, X.; Yan, Y.; Liu, J.; Xu, X.; Xia, C.; et al. Synthesis and characterization of vanadium(IV)–M (M = Mn, Zn) and vanadium(IV)–Ln (Ln = La, Nd) complexes with nitrilotriacetate ligands: {(NH4)2[(VIVO)2(μ2-O)(nta)2M(H2O)4]·2H2O}n and NH4[Ln(H2O)9][(VIVO)2 (μ2-O)(nta)2]. J. Inorg. Biochem. 2004, 23, 1975. [Google Scholar] [CrossRef]
- Tesmar, A.; Inkielewicz-Stępniak, I.; Sikorski, A.; Wyrzykowski, D.; Jacewicz, D.; Zięba, P.; Pranczk, J.; Ossowski, T.; Chmurzyński, L. Structure, physicochemical and biological properties of new complex salt of aqua-(nitrilotriacetato-N,O,O′,O″)-oxidovanadium(IV) anion with 1,10-phenanthrolinium cation. J. Inorg. Biochem. 2015, 152, 53. [Google Scholar] [CrossRef] [PubMed]
- Tesmar, A.; Wyrzykowski, D.; Kruszyński, R.; Niska, K.; Inkielewicz-Stępniak, I.; Drzeżdżon, J.; Jacewicz, D.; Chmurzyński, L. Characterization and cytotoxic effect of aqua-(2, 2′, 2′′-nitrilotriacetato)-oxo-vanadium salts on human osteosarcoma cells. BioMetals 2017, 30, 261. [Google Scholar] [CrossRef]
- Tesmar, A.; Wyrzykowski, D.; Kazimierczuk, K.; Kłak, J.; Kowalski, S.; Inkielewicz-Stępniak, I.; Drzeżdżon, J.; Jacewicz, D.; Chmurzyński, L. Structure, Physicochemical and Biological Properties of an Aqua (2, 2′, 2′′-Nitrilotriacetato)-oxidovanadium (IV) Salt with 4-Methylpyridinium Cation. Z. Anorg. Allg. Chem. 2017, 643, 501. [Google Scholar] [CrossRef]
- Kowalski, S.; Hać, S.; Wyrzykowski, D.; Zauszkiewicz-Pawlak, A.; Inkielewicz-Stępniak, I. Selective Cytotoxicity of Vanadium Complexes on Human Pancreatic Ductal Adenocarcinoma Cell Line by Inducing Necroptosis, Apoptosis and Mitotic Catastrophe Process. Oncotarget 2017, 8, 60324. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.A.; Murakami, H.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C.; Crans, D.C. Acute toxicity evaluation of non-innocent oxidovanadium (V) schiff base complex. Inorganics 2021, 9, 42. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, V.; Barge, A.; Deagostino, A.; Ghibaudi, E.; Laurenti, E.; Marabello, D.; Diana, E.; Gallicchio, M. Effects of Vanadyl Complexes with Acetylacetonate Derivatives on Non-Tumor and Tumor Cell Lines. Molecules 2021, 26, 5534. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stepniak, I. Molecular and Cellular Mechanisms of Cytotoxic Activity of Vanadium Compounds against Cancer Cells. Molecules 2020, 25, 1757. [Google Scholar] [CrossRef]
- Ni, L.; Zhao, H.; Tao, L.; Li, X.; Zhou, Z.; Sun, Y.; Chen, C.; Wei, D.; Liu, Y.; Diao, G. Synthesis, in vitro cytotoxicity, and structure–activity relationships (SAR) of multidentate oxidovanadium (IV) complexes as anticancer agents. Dalton Trans. 2018, 47, 10035. [Google Scholar] [CrossRef]
- Ferretti, V.; León, I. An overview of vanadium and cell signaling in potential cancer treatments. Inorganics 2022, 10, 47. [Google Scholar] [CrossRef]
- Bishayee, A.; Waghray, A.; Patel, M.A.; Chatterjee, M. Vanadium in the detection, prevention and treatment of cancer: The in vivo evidence. Cancer Lett. 2010, 294, 1. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Xu, B. Breast Cancer: An Up-to-date Review and Future Perspectives. Cancer Commun. 2022, 42, 913. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438. [Google Scholar] [CrossRef] [PubMed]
- De Silva, F.; Alcorn, J. A tale of two cancers: A current concise overview of breast and prostate cancer. Cancers 2022, 14, 2954. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Handbook of Vibrational Spectroscopy; Griffiths, P.R., Ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- León, I.E.; Parajón-Costa, B.S.; Franca, C.A.; Etcheverry, S.B.; Baran, E.J. A New Oxidovanadium (IV) Complex of Oxodiacetic Acid and dppz. Biol. Trace Elem. Res. 2015, 164, 198. [Google Scholar]
- León, I.E.; Etcheverry, S.B.; Parajón-Costa, B.S.; Baran, E.J. Spectroscopic characterization of an oxovanadium (IV) complex of oxodiacetic acid and o-phenanthroline. Bioactivity on osteoblast-like cells in culture. Biol. Trace Elem. Res. 2012, 147, 403. [Google Scholar] [CrossRef] [PubMed]
- Yodoshi, M.; Odoko, M.; Okabe, N. Structures and DNA-binding and cleavage properties of ternary copper (II) complexes of glycine with phenanthroline, bipyridine, and bipyridylamine. Chem. Pharm. Bull. (Tokyo) 2007, 55, 853. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, A.R. Photocleavage of DNA by copper (II) complexes. J. Chem. Sci. 2006, 118, 443. [Google Scholar] [CrossRef]
- Erkkila, K.E.; Odom, D.T.; Barton, J.K. Recognition and reaction of metallointercalators with DNA. Chem. Rev. 1999, 99, 2777. [Google Scholar] [CrossRef]
- Liu, H.-K.; Sadler, P.J. Metal complexes as DNA intercalators. Acc. Chem. Res. 2011, 44, 349. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Liu, Y.-J.; Wang, Q.; Yang, X.-G.; Wang, K. Reactive-oxygen-species-mediated Cdc25C degradation results in differential antiproliferative activities of vanadate, tungstate, and molybdate in the PC-3 human prostate cancer cell line. J. Biol. Inorg. Chem. 2012, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wang, X.S.; Fang, W.; Cai, X.Y.; Li, H.Z.; Mao, J.W.; Jin, X.B.; Bai, Y.L.; Lu, J.Z. In vitro study of the cytotoxicities of two mixed-ligand oxovanadium complexes on human hepatoma cells. Pharmazie 2013, 68, 827. [Google Scholar] [PubMed]
- X-Area 1.75, STOE & Cie GmbH, Software Package for Collecting Single-Crystal Data on STOE Area-Detector Diffractometers, for Image Processing, Scaling Reflection Intensities and for Outlier Rejection; STOE: Darmstadt, Germany, 2015.
- Sheldrick, G.M. SHELXL-2014; University of Gottingen and Bruker AXS: Karlsruhe, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Koziskova, J.; Hahn, F.; Richter, J.; Kozisek, J. Comparison of different absorption corrections on the model structure of tetrakis(µ2-acetato)- diaqua-di-copper(II). Acta Chim. Slovaca 2016, 9, 136–140. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. 2013, B69, 249–259. [Google Scholar] [CrossRef]


| Structure | Antitumor Activities | References |
|---|---|---|
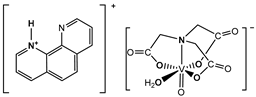 | Cell lines: Human HepG2 and SMMC-7721 hepatocellular carcinoma cell lines Activity: Inhibition of cell proliferation. The IC50 values (the concentration for a 50% growth inhibition) are 42.46 μM for SMMC-7721 cells and 101.62 μM for HepG2 cells A relatively high cytotoxic effect (36.11 μM for SMMC-7721 and 107.79 μM for HepG2) was reported for the free phen ligand. The nta and bpy ligands did not reveal significant cytotoxicity (IC50 > 400 μM for HepG2 and SMMC-7721 cell lines) | [10] |
 and 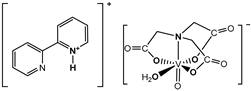 and 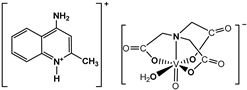 | Cell lines: Human pancreatic ductal adenocarcinoma cell line (PANC-1), non-tumor human immortalized pancreas duct epithelial cells (hTERT-HPNE) Activity: Selective cytotoxicity of the complexes was observed for PANC-1 cells but their action was slightly lower than gemcitabine (a positive control, commonly used in pancreatic cancer treatment) Inhibition of autophagy process in selective cytotoxic concentration. The cell cycle arrest in the G2/M phase is associated with mitotic catastrophe. Induction of a mixed type of cell death in PANC-1 cells, including apoptotic and necroptotic processes | [5] |
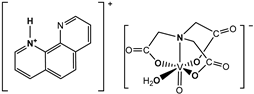
 and 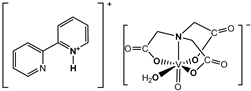 | Cell lines: Human MG-63 and HOS osteosarcoma cell lines and the untransformed (normal) human osteoblast cell line (hFOB1.10) Activity: The compounds showed selectivity for malignant cells. The phenH salt exhibited a higher anti-proliferative activity towards MG-63 and HOS than the bpyH salt and cis-Pt(NH3)2Cl2 (a positive control) | [3] |
 | Cell lines: Human MG-63 and HOS osteosarcoma cell lines and the untransformed (normal) human osteoblast cell line (hFOB1.10) Activity: The cytotoxic effect was observed only at the higher concentration (>10 μM). Very low antiproliferative activity (MG-63 and HOS cells). Lack of selectivity for normal and malignant cells | [4] |
| Distance/Angle | Å, ° | Distance/Angle | Å, ° |
|---|---|---|---|
| V1A—O7A | 1.611(3) | V1B—O7B | 1.600(4) |
| V1A—O8A | 2.001(3) | V1B—O8B | 2.006(4) |
| V1A—O1A | 2.001(3) | V1B—O1B | 2.015(3) |
| V1A—O3A | 1.984(3) | V1B—O3B | 1.990(3) |
| V1A—O5A | 2.002(3) | V1B—O5B | 1.998(3) |
| V1A—N1A | 2.332(3) | V1B—N1B | 2.321(3) |
| O1A—V1A—O3A | 90.57(1) | O1B—V1B—O3B | 91.13(1) |
| O1A—V1A—O5A | 150.27(1) | O1B—V1B—O5B | 150.13(1) |
| O1A—V1A—O7A | 101.91(1) | O1B—V1B—O7B | 106.03(2) |
| O1A—V1A—O8A | 85.02(1) | O1B—V1B—O8B | 84.94(1) |
| O3A—V1A—O5A | 87.37(1) | O3B—V1B—O5B | 89.65(1) |
| O3A—V1A—O7A | 96.19(2) | O3B—V1B—O7B | 92.97(2) |
| O3A—V1A—O8A | 161.96(1) | O3B—V1B—O8B | 165.83(1) |
| O5A—V1A—O7A | 107.80(1) | O5B—V1B—O7B | 103.71(2) |
| O5A—V1A—O8A | 87.85(1) | O5B—V1B—O8B | 87.08(1) |
| O7A—V1A—O8A | 101.84(1) | O7B—V1B—O8B | 101.20(2) |
| Distance | Å | Angle | ° |
|---|---|---|---|
| V1—O8 | 1.594(5) | O1—V1—O3 | 92.25(2) |
| V1—O7 | 2.038(5) | O1—V1—O5 | 150.55(2) |
| V1—O1 | 2.020(5) | O1—V1—O7 | 86.90(2) |
| V1—O3 | 2.016(5) | O1—V1—O8 | 103.57(2) |
| V1—O5 | 2.009(4) | O3—V1—O5 | 87.27(2) |
| V1—N1 | 2.334(6) | O3—V1—O7 | 163.03(2) |
| O3—V1—O8 | 95.20(2) | ||
| O5—V1—O7 | 85.23(2) | ||
| O5—V1—O8 | 105.79(2) | ||
| O7—V1—O8 | 101.48(2) |
| D—H···A | d(D—H) | d(H···A) | d(D···A) | <(D—H···A) |
|---|---|---|---|---|
| O8B—H8BA···O2A | 0.92 | 1.78 | 2.636 (5) | 152.5 |
| O8B—H8BB···O2B i | 0.92 | 1.68 | 2.583 (5) | 164.2 |
| O8A—H8AA···O10C | 0.92 | 1.74 | 2.624 (6) | 158.9 |
| O8A—H8AB···O3B i | 0.92 | 2.36 | 2.928 (6) | 119.5 |
| N11B—H11B···O5B | 0.96 (8) | 1.71 (8) | 2.666 (6) | 172 (7) |
| N11A—H11C···O1A | 0.88 | 2.20 | 2.862 (6) | 131.8 |
| N11A—H11C···O2A | 0.88 | 2.26 | 3.083 (6) | 154.6 |
| D—H···A | d(D—H) | d(H···A) | d(D···A) | <(D—H···A) |
|---|---|---|---|---|
| O8—H8A···O6 ii | 0.84 (3) | 1.80 (4) | 2.597 (6) | 157 (9) |
| O8—H8B···O2 iii | 0.85 (3) | 1.77 (3) | 2.614 (6) | 171 (9) |
| O10—H10B···O11 | 0.85 (3) | 2.05 (10) | 2.777 (10) | 143 (14) |
| O10—H10C···O4 | 0.85 (3) | 2.00 (9) | 2.762 (8) | 149 (15) |
| O11—H11A···O4 iv | 0.85 (3) | 2.13 (11) | 2.839 (9) | 141 (16) |
| O11—H11B···O10 v | 0.85 (3) | 1.90 (5) | 2.735 (13) | 166 (18) |
| N10—H10···O10 | 0.88 | 2.16 | 3.038 (15) | 174.9 |
| C22—H22···O3 | 0.95 | 2.32 | 3.234 (14) | 162.6 |
| N10A—H10A···O11 | 0.88 | 2.19 | 3.069 (14) | 174.3 |
| C22A—H22A···O7 | 0.95 | 2.39 | 3.297 (8) | 160.1 |
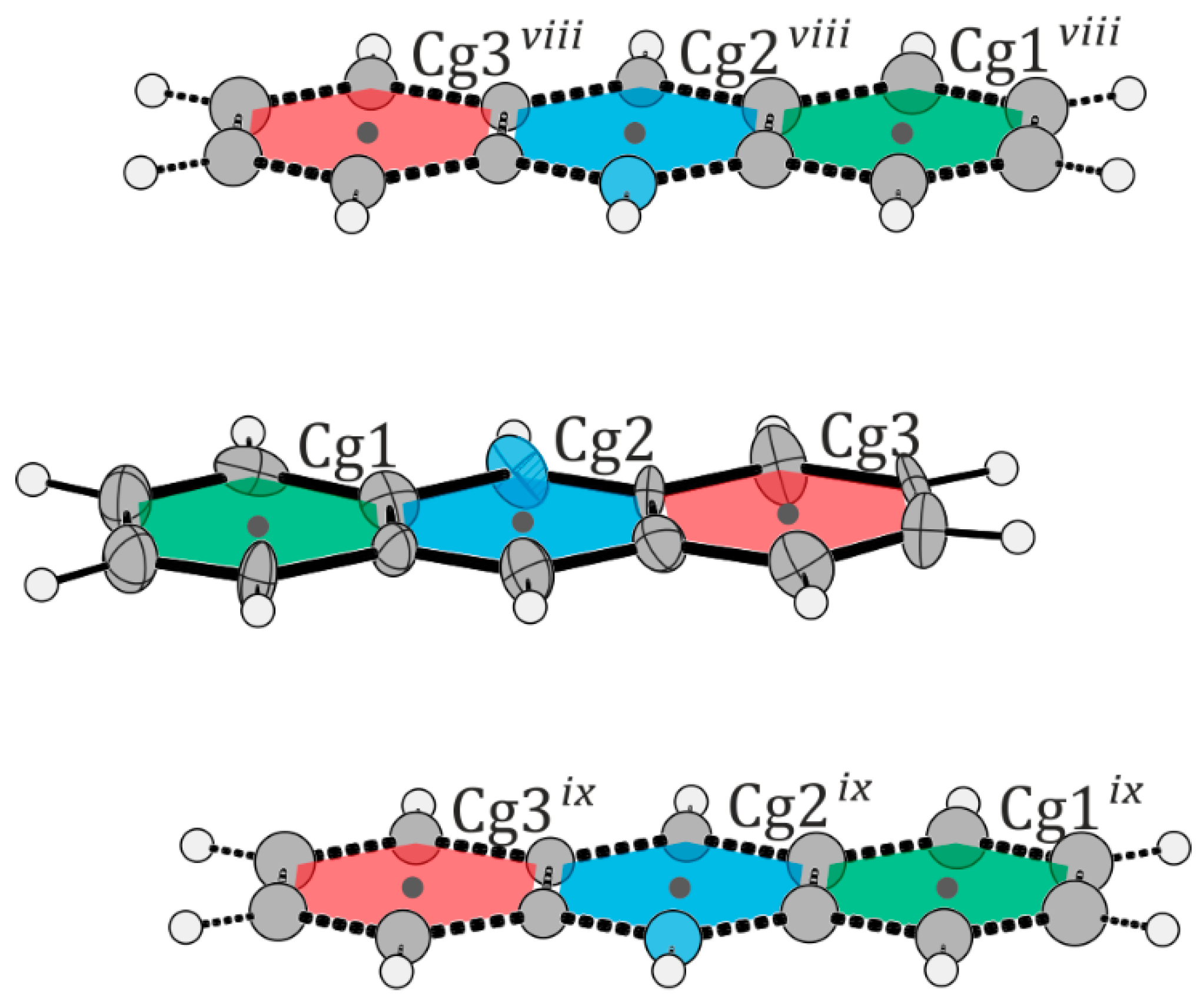
| R(I)‧‧‧R(J) | Cg‧‧‧Cg | α | β | dp |
|---|---|---|---|---|
| Cg1‧‧‧Cg3 vi | 3.691(3) | 177.63(19) | 177.3(6) | −3.432(5) |
| Cg2‧‧‧Cg4 vi | 3.835(4) | 177.3(2) | 179.1(5) | −3.404(6) |
| Cg1‧‧‧Cg3 | 3.634(3) | 2.37(19) | 6.3(6) | 3.415(5) |
| Cg2‧‧‧Cg4 | 3.676(4) | 2.7(2) | 7.7(6) | 3.446(5) |
| Cg3‧‧‧Cg1 vii | 3.691(3) | 177.63(19) | 177.3(6) | 3.481(4) |
| Cg4‧‧‧Cg2 vii | 3.835(4) | 177.3(2) | 179.1(5) | 3.483(5) |
| R(I)‧‧‧R(J) | Cg‧‧‧Cg | α | β | dp |
|---|---|---|---|---|
| Cg1‧‧‧Cg3 viii | 3.740(9) | 1.4(5) | 2.5(11) | −3.324(13) |
| Cg2‧‧‧Cg3 viii | 3.833(8) | 0.4(5) | 0.6(11) | −3.352(14) |
| Cg2‧‧‧Cg2 viii | 3.702(8) | 0.1(5) | 0.1(9) | −3.353(14) |
| Cg3‧‧‧Cg2 viii | 3.158(7) | 0.6(5) | 0.8(9) | −3.372(16) |
| Cg3‧‧‧Cg1 viii | 3.645(8) | 0.3(5) | 0.6(12) | −3.364(12) |
| Cg1‧‧‧Cg3 ix | 3.652(9) | 1.4(5) | 2.9(11) | 3.335(17) |
| Cg2‧‧‧Cg3 ix | 3.544(8) | 0.4(5) | 0.9(12) | 3.342(14) |
| Cg2‧‧‧Cg2 ix | 3.673(8) | 0.1(5) | 0.3(12) | 3.340(12) |
| Cg3‧‧‧Cg2 ix | 3.577(7) | 0.6(5) | 1.5(12) | 3.336(11) |
| Cg3‧‧‧Cg1 ix | 3.725(8) | 0.3(5) | 0.2(10) | 3.343(12) |
| Compound | IC50 [µM] | SI | |||
|---|---|---|---|---|---|
| MCF-7 | PC3 | HaCaT | MCF-7 | PC3 | |
| I | 117.03 | 44.16 | 52.11 | 0.46 | 1.18 |
| II | 116.50 | 61.90 | 76.08 | 0.65 | 1.23 |
| III | 56.16 | 18.06 | 6.37 | 0.11 | 0.35 |
| IV | 86.93 | 40.78 | 30.15 | 0.35 | 0.74 |
| Compound | [QH][VO(nta)(H2O)](H2O)2 | [(acr)H][VO(nta)(H2O)](H2O)2 |
|---|---|---|
| Empirical formula | C30H34N4O17V2 [+solvent] | C19H22N2O10V |
| Formula weight | 824.49 | 489.37 |
| Crystal system, space group | orthorhombic, P 21 21 21 | |
| Unit cell dimensions [Å] | a = 31.8596(17) | a = 6.9331(13) |
| b = 31.8596(17) | b = 9.950(3) | |
| c = 7.1243(4) | c = 29.644(7) | |
| V (Å3) | 7231.4 (9) | 2044.9 (8) |
| Z | 8 | 4 |
| Radiation type | Mo Kα | Cu Kα |
| µ (mm−1) | 0.60 | 4.62 |
| Crystal size (mm) | 0.52 × 0.06 × 0.05 | 0.57 × 0.06 × 0.03 |
| Absorption correction | − | Multi-scan STOE LANA, absorption correction by scaling of reflection intensities [30]. Afterwards, a spherical absorption correction was performed within STOE LANA |
| Tmin, Tmax | 0.318, 0.818 | |
| No. of measured, independent and observed [I>2σ(I)] reflections | 7652, 5666, 4355 | 17,394, 3485, 3351 |
| Rint | 0.040 | 0.049 |
| (sin θ/λ)max (Å−1) | 0.596 | 0.593 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.043, 0.091, 0.96 | 0.058, 0.155, 1.09 |
| No. of reflections | 5666 | 3485 |
| No. of parameters | 488 | 328 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.33, −0.26 | 0.51, −0.69 |
| Absolute structure | Refined as an inversion twin | Flack x determined using 1321 quotients [(I+) − (I−)]/[(I+) + (I−)] [31] |
| Absolute structural parameter | 0.09 (3) | −0.006 (4) |
| CCDC number | 2341557 | 2341558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmur, K.; Tesmar, A.; Zdrowowicz, M.; Rosiak, D.; Chojnacki, J.; Wyrzykowski, D. Exploring the Antitumor Efficacy of N-Heterocyclic Nitrilotriacetate Oxidovanadium(IV) Salts on Prostate and Breast Cancer Cells. Molecules 2024, 29, 2924. https://doi.org/10.3390/molecules29122924
Chmur K, Tesmar A, Zdrowowicz M, Rosiak D, Chojnacki J, Wyrzykowski D. Exploring the Antitumor Efficacy of N-Heterocyclic Nitrilotriacetate Oxidovanadium(IV) Salts on Prostate and Breast Cancer Cells. Molecules. 2024; 29(12):2924. https://doi.org/10.3390/molecules29122924
Chicago/Turabian StyleChmur, Katarzyna, Aleksandra Tesmar, Magdalena Zdrowowicz, Damian Rosiak, Jarosław Chojnacki, and Dariusz Wyrzykowski. 2024. "Exploring the Antitumor Efficacy of N-Heterocyclic Nitrilotriacetate Oxidovanadium(IV) Salts on Prostate and Breast Cancer Cells" Molecules 29, no. 12: 2924. https://doi.org/10.3390/molecules29122924
APA StyleChmur, K., Tesmar, A., Zdrowowicz, M., Rosiak, D., Chojnacki, J., & Wyrzykowski, D. (2024). Exploring the Antitumor Efficacy of N-Heterocyclic Nitrilotriacetate Oxidovanadium(IV) Salts on Prostate and Breast Cancer Cells. Molecules, 29(12), 2924. https://doi.org/10.3390/molecules29122924









