Magnetic Titanium Dioxide Nanocomposites as a Recyclable SERRS Substrate for the Ultrasensitive Detection of Histidine
Abstract
1. Introduction
2. Results and Discussion
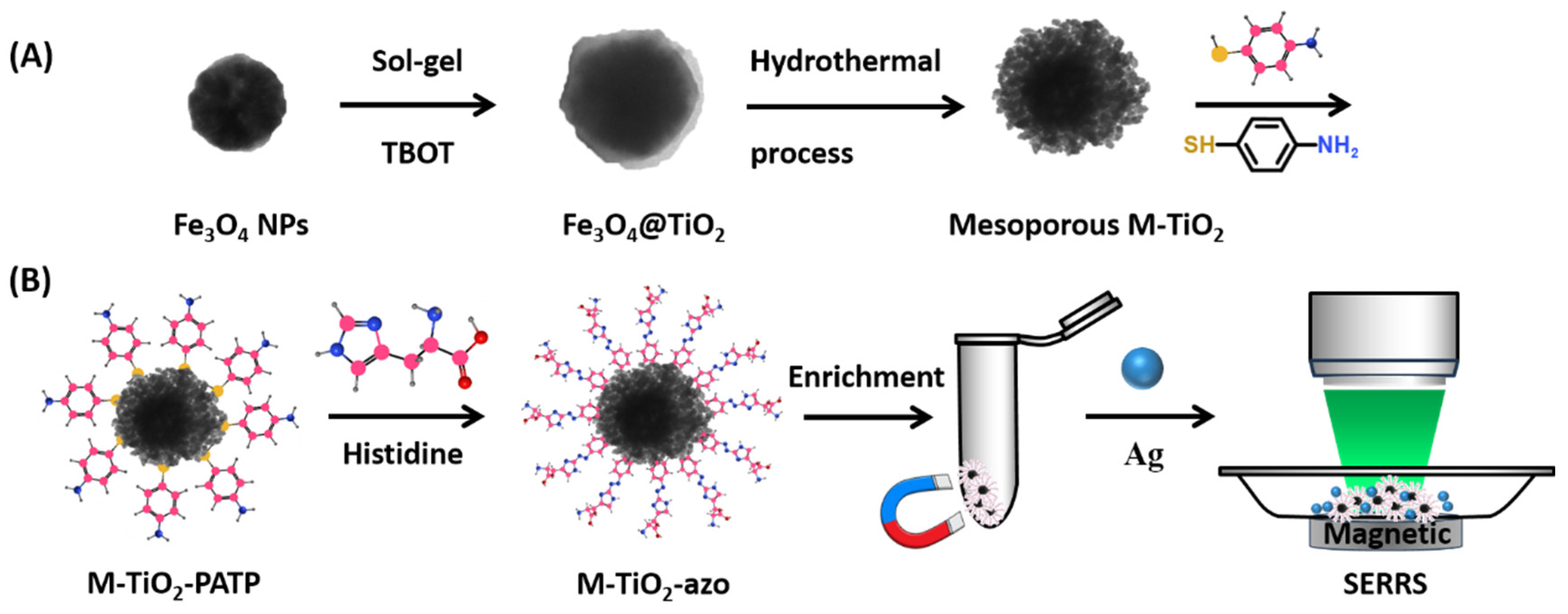
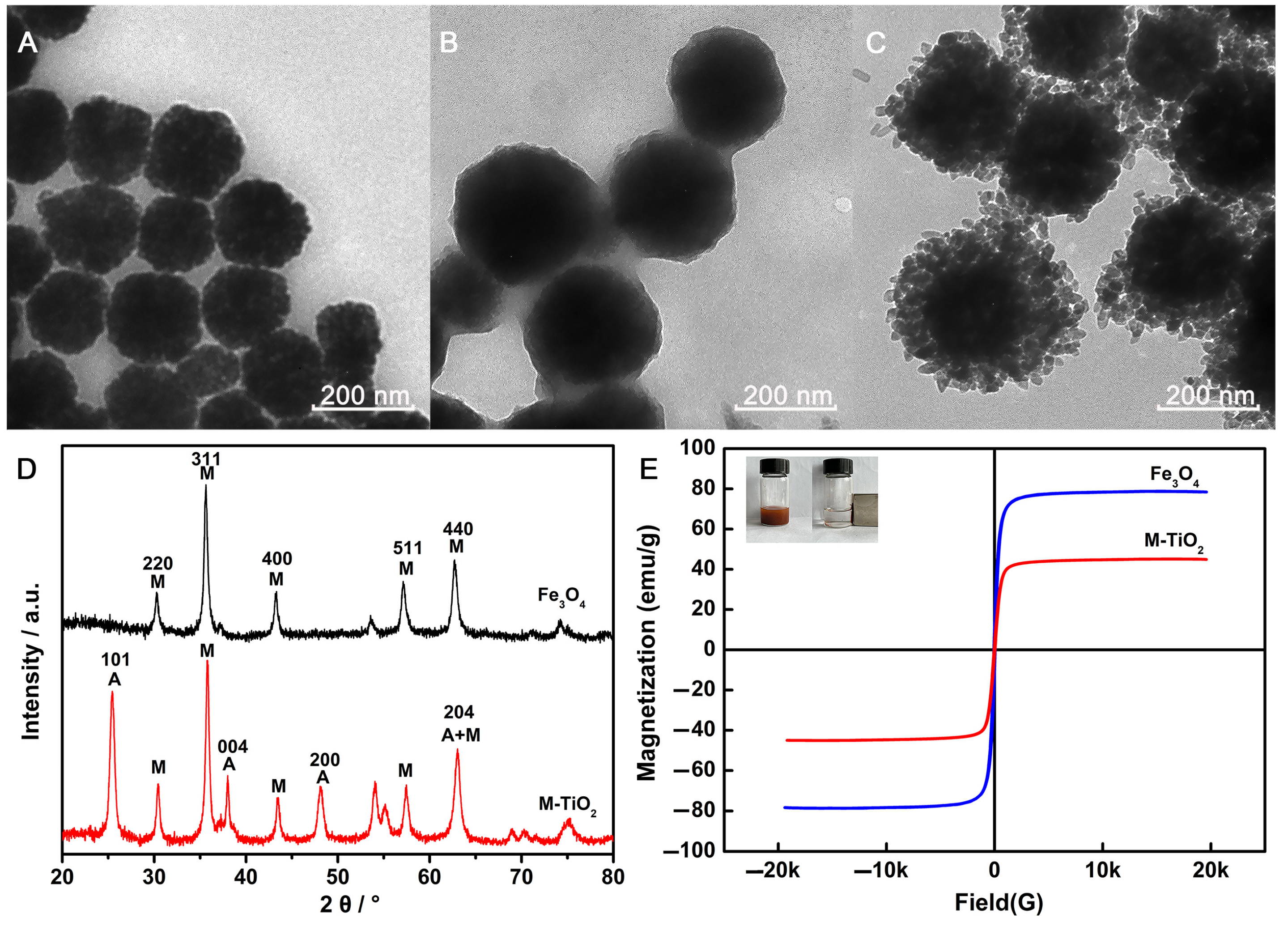
2.1. Characterization of M-TiO2
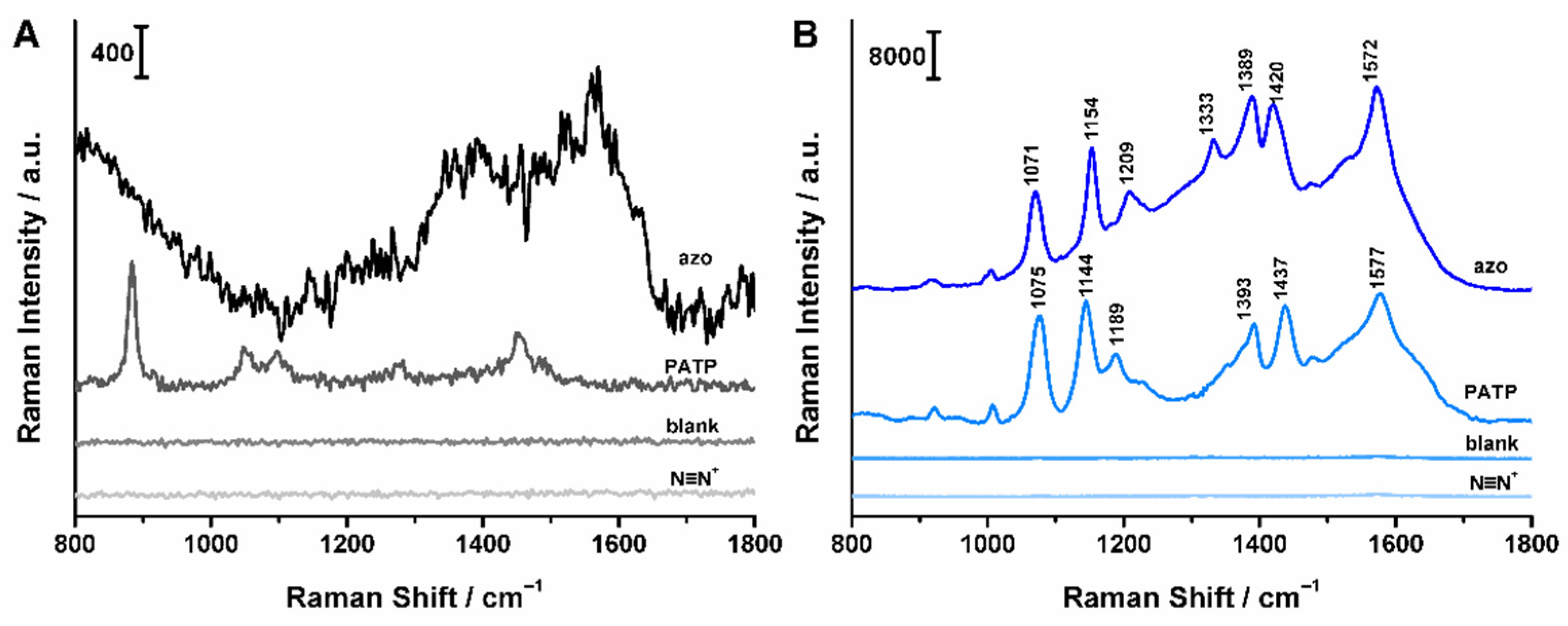
2.2. Azo Coupling of M-TiO2 with Histidine
2.3. UV-Vis Absorption Spectra
2.4. SERRS Spectra
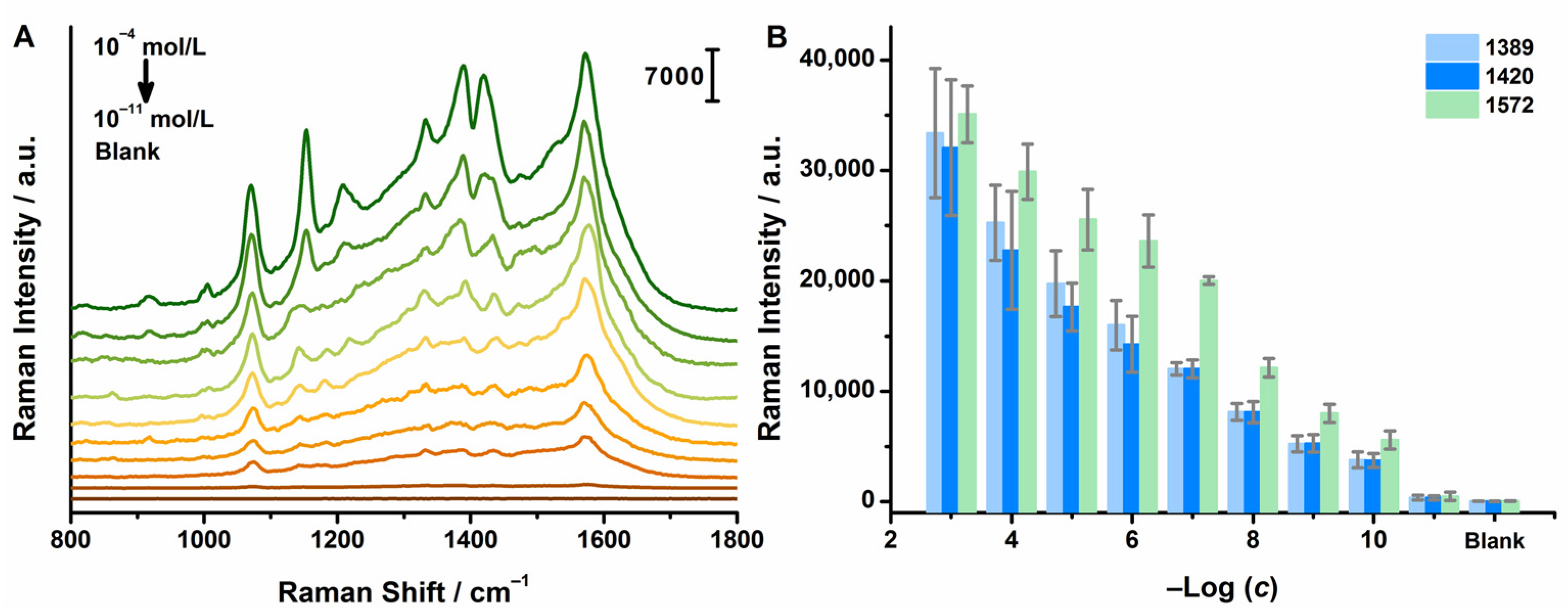
2.5. Sensitivity
2.6. Recycling of M-TiO2 Sensing Device for Histidine
2.7. Interference Study
2.8. Accuracy and Precision
2.9. Comparison with Other Methods for Histidine
2.10. Universality
3. Materials and Methods
3.1. Chemical Reagents
3.2. Apparatus and Measurement
3.3. Synthesis of M-TiO2 Nanocomposites
3.4. Functionalization of M-TiO2 and Azo Coupling
3.5. Preparation of Ag NPs
3.6. Preparation of Histidine Standard Solutions
3.7. Preparation of Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamura, T.; Naganuma, F.; Kudomi, U.; Roh, S.; Yanai, K.; Yoshikawa, T. Oral histidine intake improves working memory through the activation of histaminergic nervous system in mice. Biochem. Biophys. Res. Commun. 2022, 609, 141–148. [Google Scholar] [CrossRef]
- Yu, B.; Li, A.H.; Muzny, D.; Veeraraghavan, N.; de Vries, P.S.; Bis, J.C.; Musani, S.K.; Alexander, D.; Morrison, A.C.; Franco, O.H.; et al. Association of rare loss-of-function alleles in HAL, serum histidine: Levels and incident coronary heart disease. Circ. Cardiovasc. Genet. 2015, 8, 351–355. [Google Scholar] [CrossRef]
- Yang, P.; Deng, F.; Yuan, M.; Chen, M.; Zeng, L.; Ouyang, Y.; Chen, X.; Zhao, B.; Yang, Z.; Tian, Z. Metabolomics reveals the defense mechanism of histidine supplementation on high-salt exposure-induced hepatic oxidative stress. Life Sci. 2023, 314, 121355. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Fang, Y.; Qin, Y.; Xu, S.; Liu, Y.; Wang, E. G-quadruplex-based ultrasensitive and selective detection of histidine and cysteine. Biosens. Bioelectron. 2013, 41, 563–568. [Google Scholar] [CrossRef]
- Wei, P.; Xiao, L.; Gou, Y.; He, F.; Wang, P. A novel fluorescent probe based on a tripeptide-Cu (II) complex system for detection of histidine and its application on test strips and smartphone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 290, 122290. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Immanuel David, C.; Shanmugam, S.; Ramanagul, K.; Rajendran, R.; Gothandapani, V.; Kannan, V.R.; Prabhu, J.; Nandhakumar, R. Positional isomeric symmetric dipodal receptors dangled with rotatable binding scaffolds: Fluorescent sensing of silver ions and sequential detection of L-histidine and their multifarious applications. J. Agric. Food Chem. 2022, 71, 802–814. [Google Scholar] [CrossRef]
- Chabok, A.; Shamsipur, M.; Yeganeh-Faal, A.; Molaabasi, F.; Molaei, K.; Sarparast, M. A highly selective semiconducting polymer dots-based “off–on” fluorescent nanoprobe for iron, copper and histidine detection and imaging in living cells. Talanta 2019, 194, 752–762. [Google Scholar] [CrossRef]
- Tang, S.; Wu, X.; Zhao, P.; Tang, K.; Chen, Y.; Fu, J.; Zhou, S.; Yang, Z.; Zhang, Z. A near-infrared fluorescence capillary imprinted sensor for chiral recognition and sensitive detection of L-histidine. Anal. Chim. Acta 2022, 1206, 339794. [Google Scholar] [CrossRef]
- Shen, R.; Zou, L.; Wu, S.; Li, T.; Wang, J.; Liu, J.; Ling, L. A novel label-free fluorescent detection of histidine based upon Cu2+-specific DNAzyme and hybridization chain reaction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 42–47. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, J.; Liu, C.; Yang, S.; Zhang, Y.; Liu, A. Histidine-triggered turning-on of gold/copper nanoclusters fluorescence for sensitive and selective detection of histidine. Chem. Commun. 2020, 56, 11637–11640. [Google Scholar] [CrossRef]
- Che, Y.; Pang, H.; Li, H.; Yang, L.; Fu, X.; Liu, S.; Ding, L.; Hou, J. Microwave-assisted fabrication of copper-functionalized carbon quantum dots for sensitive detection of histidine. Talanta 2019, 196, 442–448. [Google Scholar] [CrossRef]
- Gu, Z.; Cao, Z. Molecular switch-modulated fluorescent copper nanoclusters for selective and sensitive detection of histidine and cysteine. Anal. Bioanal. Chem. 2018, 410, 4991–4999. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Hu, L.; Zhang, B. Fluorescence sensors for the detection of L-histidine based on silver nanoclusters modulated bby copper ions. Molecules 2024, 29, 2167. [Google Scholar] [CrossRef]
- Amiri, M.; Haji Shabani, A.M.; Dadfarnia, S.; Shokoufi, N.; Hajipour-Verdom, B.; Sadjadi, S. Carbon dots doped by nitrogen and sulfur for dual-mode colorimetric and fluorometric determination of Fe3+ and histidine and intracellular imaging of Fe3+ in living cells. Microchim. Acta 2020, 187, 562. [Google Scholar] [CrossRef]
- Wu, C.; Fan, D.; Zhou, C.; Liu, Y.; Wang, E. Colorimetric strategy for highly sensitive and selective simultaneous detection of histidine and cysteine based on G-quadruplex-Cu(II) metalloenzyme. Anal. Chem. 2016, 88, 2899–2903. [Google Scholar] [CrossRef]
- Kugimiya, A.; Takamitsu, E. Spectrophotometric detection of histidine and lysine using combined enzymatic reactions. Mater. Sci. Eng. C 2013, 33, 4867–4870. [Google Scholar] [CrossRef]
- Sun, S.K.; Tu, K.X.; Yan, X.P. An indicator-displacement assay for naked-eye detection and quantification of histidine in human urine. Analyst 2012, 137, 2124–2128. [Google Scholar] [CrossRef]
- Bae, D.R.; Han, W.S.; Lim, J.M.; Kang, S.; Lee, J.Y.; Kang, D.; Jung, J.H. Lysine-functionalized silver nanoparticles for visual detection and separation of histidine and histidine-tagged proteins. Langmuir 2010, 26, 2181–2185. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Q.; Qiang, H.; Chen, Z. Colorimetric detection of L-histidine based on the target-triggered self-cleavage of swing-structured DNA duplex-induced aggregation of gold nanoparticles. Microchim. Acta 2018, 185, 452. [Google Scholar] [CrossRef]
- Liang, J.; Chen, Z.; Guo, L.; Li, L. Electrochemical sensing of L-histidine based on structure-switching DNAzymes and gold nanoparticle–graphene nanosheet composites. Chem. Commun. 2011, 47, 5476–5478. [Google Scholar] [CrossRef]
- Yu, H.; Xu, L.; You, T. Indirect electrochemiluminescence detection of lysine and histidine separated by capillary electrophoresis based on charge displacement. J. Biol. Chem. Lumin. 2013, 28, 217–221. [Google Scholar] [CrossRef]
- Gkantiri, A.M.; Tsiasioti, A.; Zacharis, C.K.; Tzanavaras, P. HPLC method with post-column derivatization for the analysis of endogenous histidine in human saliva validated using the total-error concept. Amino Acids 2022, 54, 399–409. [Google Scholar] [CrossRef]
- Privitera, A.; Cardaci, V.; Weerasekara, D.; Saab, M.W.; Diolosà, L.; Fidilio, A.; Jolivet, R.B.; Lazzarino, G.; Amorini, A.M.; Camarda, M.; et al. Microfluidic/HPLC combination to study carnosine protective activity on challenged human microglia: Focus on oxidative stress and energy metabolism. Front. Pharmacol. 2023, 14, 1161794. [Google Scholar] [CrossRef]
- Pretorius, C.J.; McWhinney, B.C.; Sipinkoski, B.; Wilce, A.; Cox, D.; McWhinney, A.; Ungerer, J.P.J. Rapid amino acid quantitation with pre-column derivatization; ultra-performance reverse phase liquid chromatography and single quadrupole mass spectrometry. Clin. Chim. Acta Int. J. Clin. Chem. Appl. Mol. Biol. 2018, 478, 132–139. [Google Scholar] [CrossRef]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Automated GC-MS analysis of free amino acids in biological fluids. J. Chromatogr. 2008, 870, 222–232. [Google Scholar] [CrossRef]
- Savych, A.; Marchyshyn, S.; Mosula, L.; Bilyk, O.; Humeniuk, I.; Davidenko, A. Analysis of amino acids content in the plant components of the antidiabetic herbal mixture by GC-MS. Pharmacia 2022, 69, 69–76. [Google Scholar] [CrossRef]
- Liao, S.; Ding, H.; Wu, Y.; Wu, Z.; Shen, G.; Yu, R. Label-free liquid crystal biosensor for L-histidine: A DNAzyme-based platform for small molecule assay. Biosens. Bioelectron. 2016, 79, 650–655. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, S.; Fu, C.; Ji, S.; Luo, F.; Lin, C.; Qiu, B.; Lin, Z. Single nanoparticle identification coupled with auto-identify algorithm for rapid and accurate detection of L-histidine. Anal. Chim. Acta 2021, 1187, 339162. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Long, F.; Zhu, A. Applications of magnetic nanoparticles in surface-enhanced Raman scattering (SERS) detection of environmental pollutants. J. Environ. Sci. 2019, 80, 14–34. [Google Scholar] [CrossRef]
- Kamal, S.; Yang, T.C.K. Silver enriched silver phosphate microcubes as an efficient recyclable SERS substrate for the detection of heavy metal ions. J. Colloid Interface Sci. 2022, 605, 173–181. [Google Scholar] [CrossRef]
- Tian, M.; Wang, J.; Li, C.; Wang, Z.; Liu, G.; Lv, E.; Zhao, X.; Li, Z.; Cao, D.; Liu, H.; et al. Qualitative and quantitative detection of microcystin-LR based on SERS-FET dual-mode biosensor. Biosens. Bioelectron. 2022, 212, 114434. [Google Scholar] [CrossRef]
- Li, C.; Man, B.; Zhang, C.; Yu, J.; Liu, G.; Tian, M.; Li, Z.; Zhao, X.; Wang, Z.; Cui, W.; et al. Strong plasmon resonance coupling in micro-extraction SERS membrane for in situ detection of molecular aqueous solutions. Sens. Actuat. B Chem. 2024, 398, 134767. [Google Scholar]
- Haroon, M.; Tahir, M.; Nawaz, H.; Majeed, M.I.; AI-Saadi, A.A. Surface-enhanced Raman scattering (SERS) spectroscopy for prostate cancer diagnosis: A review. Photodiagnosis Photodyn. Ther. 2022, 37, 102690. [Google Scholar] [CrossRef]
- Roh, J.Y.; Matecki, M.K.; Svoboda, S.A.; Wustholz, K.L. Identifying pigment mixtures in art using SERS: A treatment flowchart approach. Anal. Chem. 2016, 88, 2028–2032. [Google Scholar] [CrossRef]
- Eskandari, V.; Sahbafar, H.; Zeinalizad, L.; Sabzian-Molaei, F.; Abbas, M.H.; Hadi, A. A surface-enhanced Raman scattering (SERS) biosensor fabricated using the electrodeposition method for ultrasensitive detection of amino acid histidine. J. Mol. Struct. 2023, 1274 Pt 1, 134497. [Google Scholar] [CrossRef]
- Sui, H.; Wang, Y.; Yu, Z.; Cong, Q.; Han, X.X.; Zhao, B. A rapid and ultrasensitive SERRS assay for histidine and tyrosine based on azo coupling. Talanta 2016, 159, 208–214. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Ma, W.F.; Zhang, Y.; Li, L.L.; You, L.J.; Zhang, P.; Zhang, Y.T.; Li, J.M.; Yu, M.; Guo, J.; Lu, H.J. Tailor-made magnetic Fe3O4@mTiO2 microspheres with a tunable mesoporous anatase shell for highly selective and effective enrichment of phosphopeptides. ACS Nano 2012, 6, 3179–3188. [Google Scholar] [CrossRef]
- Han, X.X.; Chen, L.; Kuhlmann, U.; Schulz, C.; Weidinger, I.M.; Hildebrandt, P. Magnetic titanium dioxide nanocomposites for surface-enhanced resonance Raman spectroscopic determination and degradation of toxic anilines and phenols. Angew. Chem. Int. Ed. 2014, 53, 2481–2484. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A unified view of surface-enhanced Raman scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef]
- Alessandri, I. 4-Aminothiophenol photodimerization without plasmons. Angew. Chem. Int. Ed. 2022, 61, e202205013. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhu, H.P.; Liu, G.K.; Wu, D.Y.; Ren, B. When the signal is not from the original molecule to be detected: Chemical transformation of para-aminothiophenol on Ag during the SERS measurement. J. Am. Chem. Soc. 2010, 132, 9244–9246. [Google Scholar] [CrossRef]
- Alessandri, I. Enhancing Raman scattering without plasmons: Unprecedented sensitivity achieved by TiO2 shell-based resonators. J. Am. Chem. Soc. 2013, 135, 5541–5544. [Google Scholar] [CrossRef]
- Han, X.X.; Pienpinijtham, P.; Zhao, B.; Ozaki, Y. Coupling reaction-based ultrasensitive detection of phenolic estrogens using surface-enhanced resonance Raman scattering. Anal. Chem. 2011, 83, 8582–8588. [Google Scholar] [CrossRef]
- Ozmen, N.; Erdemoglu, S.; Gungordu, A.; Asilturk, M.; Ozhan, D.; Akgeyik, T.E.; Harper, S.L.; Ozmen, M. Photocatalytic degradation of azo dye using core@shell nano-TiO2 particles to reduce toxicity. Environ. Sci. Pollut. Res. 2018, 25, 29493–29504. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Amal, M.I.; Lee, J.; Cho, M.H. Highly visible light active Ag@TiO2 nanocomposites synthesized using an electrochemically active biofilm: A novel biogenic approach. Nanoscale 2013, 5, 4427–4435. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]



| Found without Spiking (mol/L) | Spiked (mol/L) | Found (mol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1.80 × 10−2 | 1.86 × 10−2 | 102.3 | 8.1 | |
| 1.82 × 10−4 | 1.80 × 10−3 | 1.73 × 10−3 | 95.0 | 5.3 |
| 1.80 × 10−4 | 3.55 × 10−4 | 98.1 | 4.2 |
| Materials | Methods | Samples | Sample Treatment Time | Instrument Test Time | Linear Range | LOD (nmol/L) | Reference |
|---|---|---|---|---|---|---|---|
| NMM/G-4-Cu(II) | Fluorescence | Urine | 2 h 40 min | / | 0.003–10.0 μmol/L | 3 | [4] |
| Probes: SDO, SDM and SDP with Ag+ | Fluorescence | Standard | / | / | / | 3840, 2430, 6640 | [6] |
| PFBT PDs/Cu2+ | Fluorescence | Blood serum | 25 min | / | 0.1–920 μmol/L | 79.6 | [7] |
| CdTe@MIP capillary | Fluorescence | Serum, urine | 15 min | / | 0.1–1.8 pmol/L | 8 × 10−5 | [8] |
| DNAzyme & HCR & Triplex formation | Fluorescence | Urine | 10 h | / | 5.7–455 nmol/L | 2.0 | [9] |
| Au/Cu NCs | Fluorescence | Serum, urine | / | / | 3–10,000 nmol/L | 0.9 | [10] |
| Cu-CDs | Fluorescence | Serum, urine | Serum: 11 min. Urine: / | / | 0.1–15 μmol/L | 30 | [11] |
| CuNCs | Fluorescence | Urine | 20 min | / | 0.05–40.0 μmol/L | 1.6 | [12] |
| DNA-Ag NCs | Fluorescence | Urine | 225 min | / | 0–8 μmol/L | 96 | [13] |
| N, S-CDs/Fe3+ | Dual fluorescence/ colorimetry | Serum, urine | Serum: 25 min. Urine: / | / | 0.1–3.0, 100–375 μmol/L | 30, 24,200 | [14] |
| G-quadruplex-Cu(II) metalloenzyme | Colorimetry | Standard | 2 h 4 min | / | 0.01–1 μmol/L | 10 | [15] |
| Indicator (murexide)-displacement assay (IDA) | Colorimetry | Urine | 22 min | / | 2–30 μmol/L | 400 | [17] |
| Lysine-functionalized Ag NPs | Colorimetry | Standard | 10 min | / | 5.0–30.0 μmol/L | 5000 | [18] |
| Au NPs, aptamer | Colorimetry | Serum | 10 min | / | 0–400 nmol/L | 3.6 | [19] |
| GNPs-GNSs, aptamer | Electrochemistry | Standard | / | 30 s | 10 pmol/L–10 μmol/L | 10−4 | [20] |
| Ru(bpy)32+/TPA | CE-ECL | Standard | / | 7 min | 5–35, 35–150 μmol/L | 103 | [21] |
| Post-column derivatization with o-phthalaldehyde | HPLC | Saliva | 10 min | / | 0.5–5.0 μmol/L | 50 | [22] |
| Pre-column AQC derivatization | UPLC-MS | Plasma, urine | 10 min 20 s | 8 min for chromatography | 2–2000 μmol/L | 310 | [24] |
| Pre-column AQC derivatization | GC-MS | Herbal raw materials | 27 h 60 s | / | 1–100 μg/mL | 30 | [25] |
| DNAzyme-based LC biosensor | Optical image | Standard | 1 h | / | / | 50 | [27] |
| Au NPs-CuAAC | Dark-field microscopy | Serum | 36 min | / | 5.0–80 μmol/L | 2100 | [28] |
| M-TiO2 nanocomposites | SERRS | Urine | 15 min | / | 10−4–10−11 mol/L | 8.00 × 10−3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Li, M.; Zhao, C.-Y.; Xu, T.; Fu, S.; Sui, H.; Han, C. Magnetic Titanium Dioxide Nanocomposites as a Recyclable SERRS Substrate for the Ultrasensitive Detection of Histidine. Molecules 2024, 29, 2906. https://doi.org/10.3390/molecules29122906
Wen H, Li M, Zhao C-Y, Xu T, Fu S, Sui H, Han C. Magnetic Titanium Dioxide Nanocomposites as a Recyclable SERRS Substrate for the Ultrasensitive Detection of Histidine. Molecules. 2024; 29(12):2906. https://doi.org/10.3390/molecules29122906
Chicago/Turabian StyleWen, Hailin, Miao Li, Chao-Yang Zhao, Tao Xu, Shuang Fu, Huimin Sui, and Cuiyan Han. 2024. "Magnetic Titanium Dioxide Nanocomposites as a Recyclable SERRS Substrate for the Ultrasensitive Detection of Histidine" Molecules 29, no. 12: 2906. https://doi.org/10.3390/molecules29122906
APA StyleWen, H., Li, M., Zhao, C.-Y., Xu, T., Fu, S., Sui, H., & Han, C. (2024). Magnetic Titanium Dioxide Nanocomposites as a Recyclable SERRS Substrate for the Ultrasensitive Detection of Histidine. Molecules, 29(12), 2906. https://doi.org/10.3390/molecules29122906







