1,6-Nucleophilic Di- and Trifluoromethylation of para-Quinone Methides with Me3SiCF2H/Me3SiCF3 Facilitated by CsF/18-Crown-6
Abstract
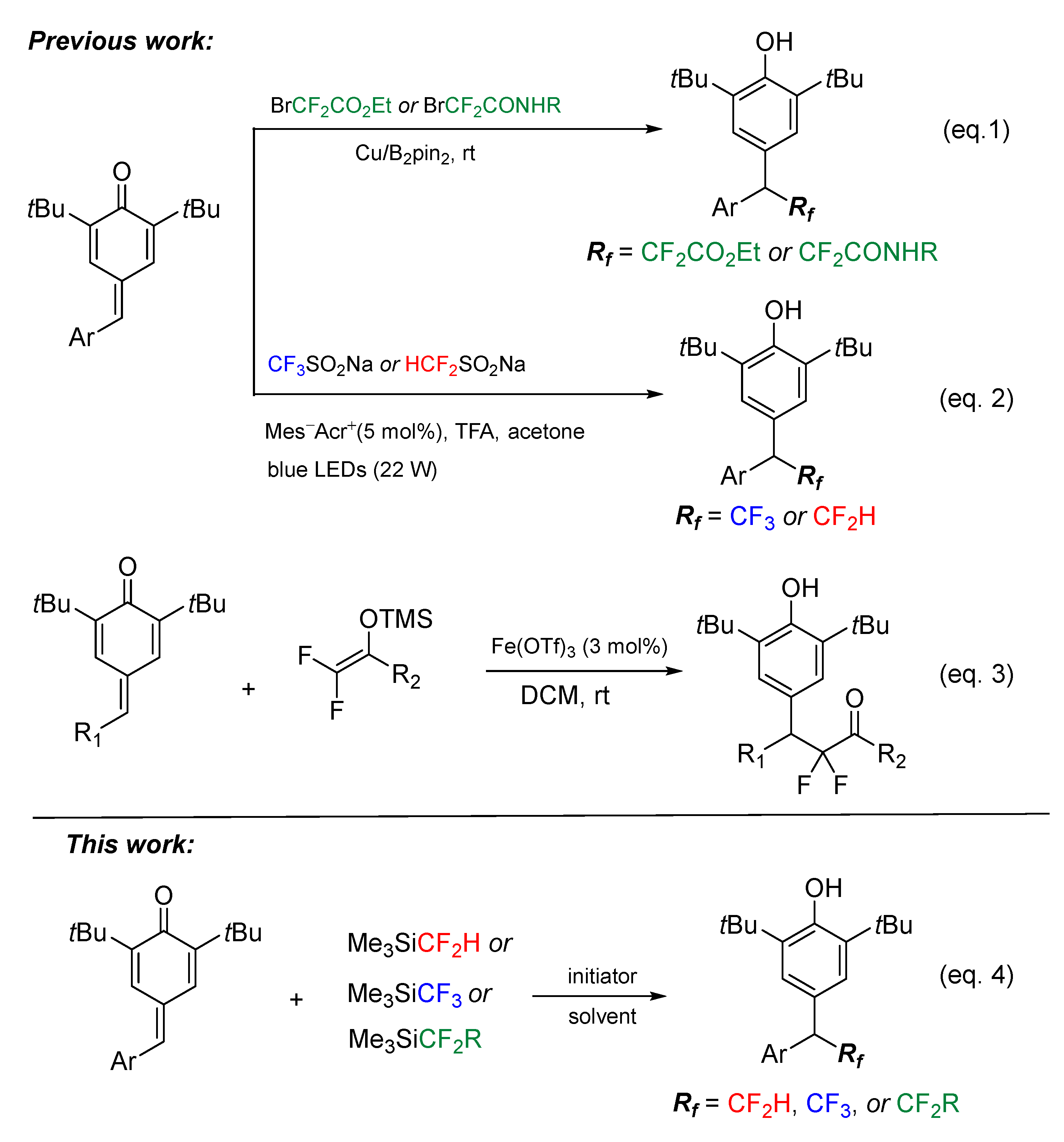
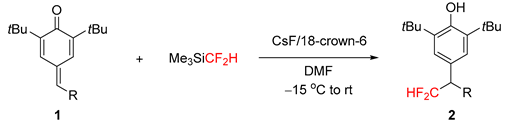
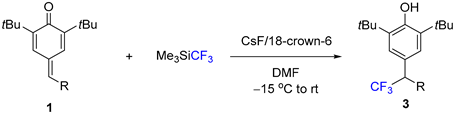
1. Introduction
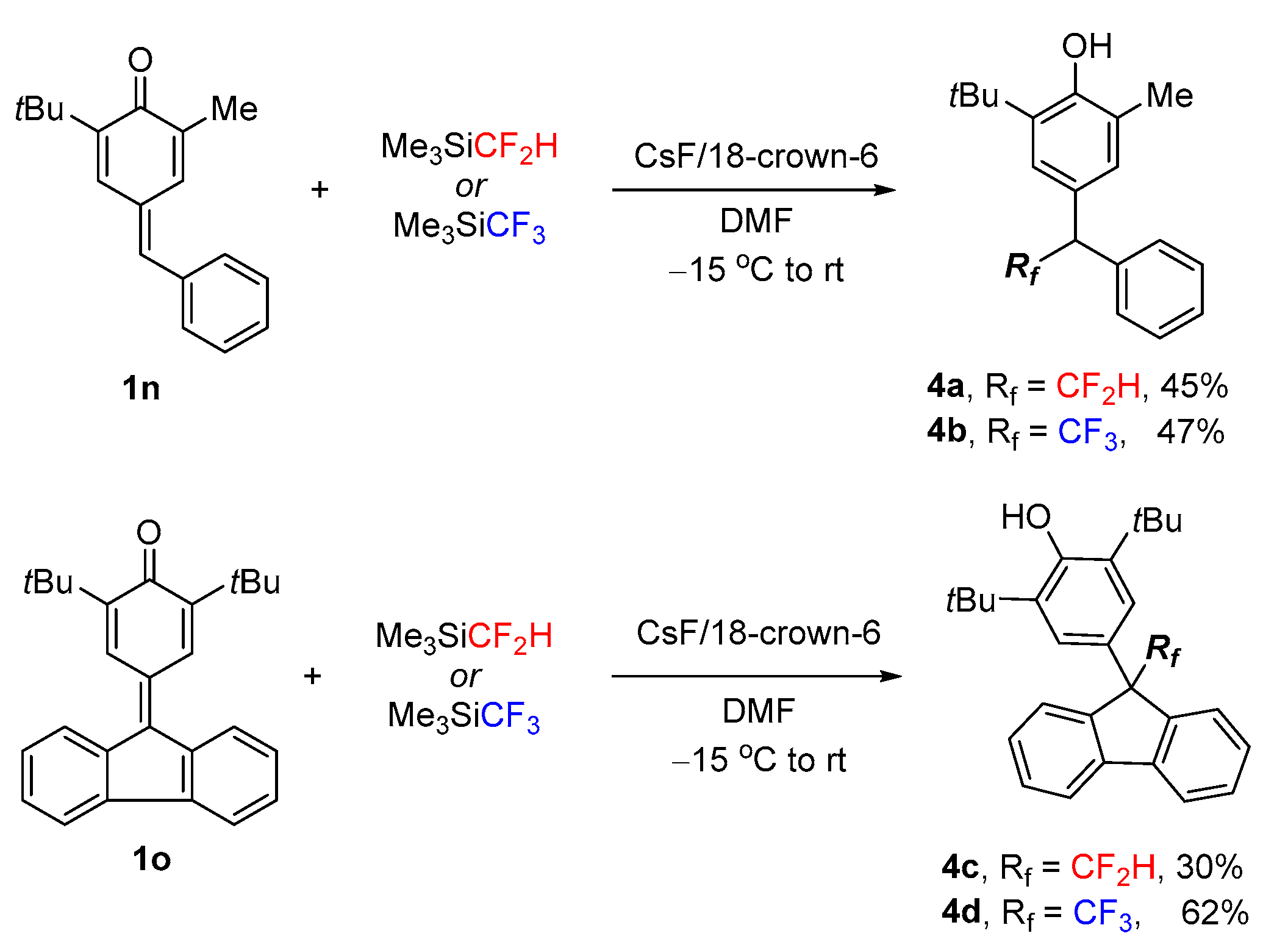
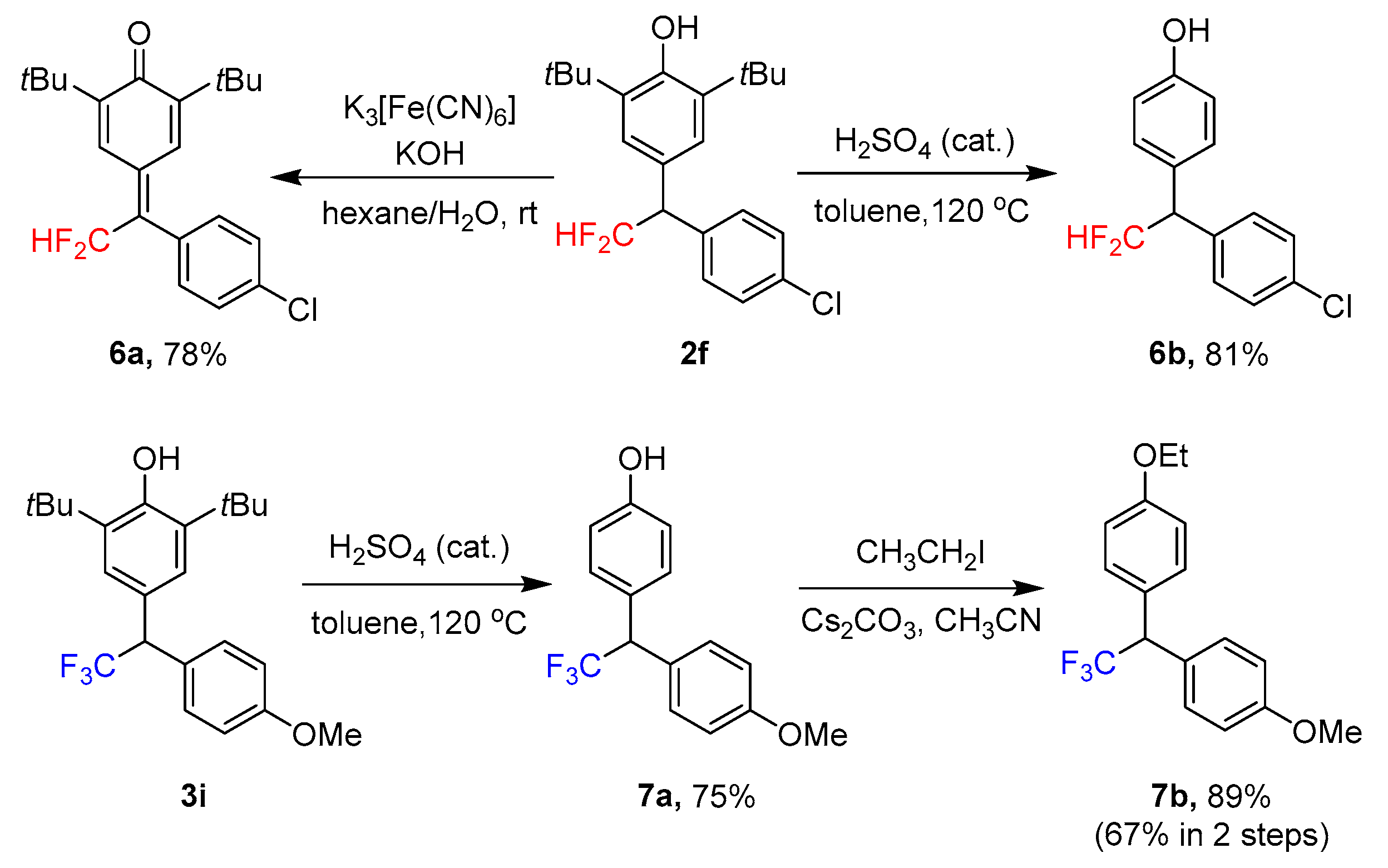
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure
3.2.1. Experimental Procedures for the Synthesis of 2–5
3.2.2. Experimental Procedures for the Synthesis of 2,6-Di-tert-butyl-4-(1-(4-chlorophenyl)-2,2-difluoroethylidene)cyclohexa-2,5-dien-1-one (6a)
3.2.3. Experimental Procedures for the Synthesis of 4-(1-(4-Chlorophenyl)-2,2-difluoroethyl) Phenol (6b)
3.2.4. General Experimental Procedure for the Synthesis of 1-Ethoxy-4-(2,2,2-trifluoro-1-(4-methoxyphenyl)ethyl)benzene (7b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uneyama, K. Organofluorine Chemistry; Blackwell: Oxford, UK, 2006; pp. 206–219. [Google Scholar]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- He, J.; Li, Z.; Dhawan, G.; Zhang, W.; Sorochinsky, A.E.; Butler, G.; Soloshonok, V.A.; Han, J. Fluorine-containing drugs approved by the FDA in 2021. Chin. Chem. Lett. 2023, 34, 107578. [Google Scholar] [CrossRef]
- Li, W.-B.; Cheng, Y.-Z.; Yang, D.-H.; Liu, Y.-W.; Han, B.-H. Fluorine-Containing Covalent Organic Frameworks: Synthesis and Application. Macromol. Rapid Commun. 2023, 44, 2200778. [Google Scholar] [CrossRef]
- Upadhyay, C.; Chaudhary, M.; De Oliveira, R.N.; Borbas, A.; Kempaiah, P.; Rathi, B. Fluorinated scaffolds for antimalarial drug discovery. Expert Opin. Drug Dis. 2020, 15, 705–718. [Google Scholar] [CrossRef]
- Fang, Z.; Peng, Y.; Zhou, X.; Zhu, L.; Wang, Y.; Dong, X.; Xia, Y. Fluorinated Carbon Materials and the Applications in Energy Storage Systems. ACS Appl. Energy Mater. 2022, 5, 3966–3978. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C.J.; Whittaker, A.K. Biological Utility of Fluorinated Compounds: From Materials Design to Molecular Imaging, Therapeutics and Environmental Remediation. Chem. Rev. 2022, 122, 167–208. [Google Scholar] [CrossRef]
- Masoud, S.M.; Mailyan, A.K.; Dorcet, V.; Roisnel, T.; Dixneuf, P.H.; Bruneau, C.; Osipov, S.N. Metathesis Catalysts with Fluorinated Unsymmetrical NHC Ligands. Organometallics 2015, 34, 2305–2313. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Ganesh, S.K.; Jones, J.P.; Kulkarni, A.; Masood, K.; Swabeck, J.K.; Olah, G.A. Copper-Mediated Difluoromethylation of (Hetero)aryl Iodides and beta-Styryl Halides with Tributyl(difluoromethyl)stannane. Angew. Chem. Int. Ed. 2012, 51, 12090–12094. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Chacko, S. Novel Nucleophilic and Electrophilic Fluoroalkylation Methods. Curr. Opin. Drug Discov. Devel. 2008, 11, 793–802. [Google Scholar]
- Erickson, J.A.; McLoughlin, J.I. Hydrogen Bond Donor Properties of the Difluoromethyl Group. J. Org. Chem. 1995, 60, 1626–1631. [Google Scholar] [CrossRef]
- Levi, N.; Amir, D.; Gershonov, E.; Zafrani, Y. Recent Progress on the Synthesis of CF2H-Containing Derivatives. Synthesis 2019, 51, 4549–4567. [Google Scholar]
- Gewehr, M.; Gladwin, R.J.; Brahm, L. Pesticidal Mixtures. U.S. Patent 2012/0245031 A1, 27 September 2012. [Google Scholar]
- Pérez, R.A.; Sánchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Analytical Methods for the Determination in Soil of Herbicides Used in Forestry by GC–NPD and GC/MS. J. Agric. Food Chem. 1998, 46, 1864–1869. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef]
- Prakash, G.K.; Yudin, S.A.K. Perfluoroalkylation with Organosilicon Reagents. Chem. Rev. 1997, 97, 757–786. [Google Scholar] [CrossRef]
- Fujihira, Y.; Liang, Y.; Ono, M.; Hirano, K.; Kagawa, T.; Shibata, N. Synthesis of trifluoromethyl ketones by nucleophilic trifluoromethylation of esters under a fluoroform/KHMDS /triglyme system. Beilstein J. Org. Chem. 2021, 17, 431–438. [Google Scholar] [CrossRef]
- Mu, B.-S.; Gao, Y.; Yang, F.-M.; Wu, W.-B.; Zhang, Y.; Wang, X.; Yu, J.-S.; Zhou, J. The Bifunctional Silyl Reagent Me2(CH2Cl)SiCF3 Enables Highly Enantioselective Ketone Trifluoromethylation and Related Tandem Processes. Angew. Chem. Int. Ed. 2022, 61, e202208861. [Google Scholar] [CrossRef]
- Chang, W.; Lei, Z.; Yang, Y.; Dai, S.; Feng, J.; Yang, J.; Zhang, Z. Tandem Reaction of Azide with Isonitrile and TMSCnFm(H): Access to N-Functionalized C-Fluoroalkyl Amidine. Org. Lett. 2023, 25, 1392–1396. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev. 2015, 115, 683–730. [Google Scholar] [CrossRef]
- Levin, V.V.; Dilman, A.D. One-pot synthesis of α-trifluoromethylstyrenes from aryl ketones and the Ruppert–Prakash reagent. Mendeleev Commun. 2021, 31, 684–685. [Google Scholar] [CrossRef]
- Hagiwara, T.; Fuchikami, T. Difluoroalkylation of Carbonyl Compounds with (1,1-Difluoroalkyl)silane Derivatives. Synlett 1995, 7, 717–718. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, W.; Zheng, J.; Hu, J. Efficient and Direct Nucleophilic Difluoromethylation of Carbonyl Compounds and Imines with Me3SiCF2H at Ambient or Low Temperature. Org. Lett. 2011, 13, 5342–5345. [Google Scholar] [CrossRef]
- Du, G.-F.; Wang, Y.; Gu, C.-Z.; Dai, B.; He, L. Organocatalytic Direct Difluoromethylation of Aldehydes and Ketones with TMSCF2H. RSC Adv. 2015, 5, 35421–35424. [Google Scholar] [CrossRef]
- Obijalska, E.; Utecht, G.; Kowalski, M.K.; Mlostoń, G.; Rachwalski, M. Nucleophilic Addition of (Difluoromethyl)trimethylsilane to Selected α-Imino Ketones and Aryl Diketones. Tetrahedron Lett. 2015, 56, 4701–4703. [Google Scholar] [CrossRef]
- Michurin, O.M.; Radchenko, D.S.; Komarov, I.V. Direct Nucleophilic Difluoromethylation of Enolizable Ketones with CHF2TMS/HMPA. Tetrahedron 2016, 72, 1351–1356. [Google Scholar] [CrossRef]
- Dong, T.; Nie, J.; Zhang, C.-P. A Convenient, Transition Metal-free Synthesis of Difluoromethyl Selenoethers from Organic Selenocyanates and TMSCF2H. Tetrahedron 2018, 74, 5642–5649. [Google Scholar] [CrossRef]
- Miele, M.; Citarella, A.; Micale, N.; Holzer, W.; Pace, V. Direct and Chemoselective Synthesis of Tertiary Difluoroketones via Weinreb Amide Homologation with a CHF2-Carbene Equivalent. Org. Lett. 2019, 21, 8261–8265. [Google Scholar] [CrossRef]
- Miele, M.; D’Orsi, R.; Sridharan, V.; Holzer, W.; Pace, V. Highly Chemoselective Difluoromethylative Homologation of Iso(thio) cyanates: Expeditious Access to Unprecedented α,α-Difluoro(thio)amides. Chem. Commun. 2019, 55, 12960–12963. [Google Scholar]
- Howard, J.L.; Schotten, C.; Alston, S.T.; Browne, D.L. Preparation of Difluoromethylthioethers through Difluoromethylation of Disulfides Using TMSCF2H. Chem. Commun. 2016, 52, 8448–8451. [Google Scholar] [CrossRef]
- Hu, J.; Ni, C. (Difluoromethyl)trimethylsilane. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Wang, X.; Pan, S.; Luo, Q.; Wang, Q.; Ni, C.; Hu, J. Controllable Single and Double Difluoromethylene Insertions into C–Cu Bonds: Copper-Mediated Tetrafluoroethylation and Hexafluoropropylation of Aryl Iodides with TMSCF2H and TMSCF2Br. J. Am. Chem. Soc. 2022, 144, 12202–12211. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Prakash, G.K.S. Silicon-Based Reagents for Difluoromethylation and Difluoromethylenation Reactions. Synthesis 2017, 49, 3394–3406. [Google Scholar] [CrossRef]
- Chen, D.; Gao, X.; Song, S.; Kou, M.; Ni, C.; Hu, J. Progress in the study of difluoromethylation reactions with TMSCF2H reagent. Sci. Sin. Chim. 2023, 53, 375–387. [Google Scholar] [CrossRef]
- Chen, D.; Ni, C.; Zhao, Y.; Cai, X.; Li, X.; Xiao, P.; Hu, J. Bis(difluoromethyl)trimethylsilicate Anion: A Key Intermediate in Nucleophilic Difluoromethylation of Enolizable Ketones with Me3SiCF2H. Angew. Chem. Int. Ed. 2016, 55, 12632–12636. [Google Scholar] [CrossRef]
- Besset, T.; Poisson, T.; Pannecoucke, X. 1,4-Addition of the CF3 Group, Perfluoroalkyl Groups and Functionalized Difluoromethylated Moieties: An Overview. J. Fluor. Chem. 2015, 178, 225–240. [Google Scholar] [CrossRef]
- Shen, X.; Ni, C.; Hu, J. Nucleophilic Fluoroalkylation of α,β-Unsaturated Carbonyl Compounds with α-Fluorinated Sulfones: Investigation of the Reversibility of 1,2-Additions and the Formation of 1,4-Adducts. Helv. Chim. Acta 2012, 95, 2043–2051. [Google Scholar] [CrossRef]
- Ni, C.; Zhang, L.; Hu, J. Fluoroalkylation of α,β-Enones, Arynes, and Activated Alkynes with Fluorinated Sulfones: Probing the Hard/Soft Nature of Fluorinated Carbanions. J. Org. Chem. 2008, 73, 5699–5713. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J. Highly Enantioselective Organocatalytic Asymmetric Mukaiyama-aldol Reaction of Difluoroenoxysilanes with β,γ-Unsaturated α-Ketoesters. Acta Chim. Sin. 2012, 70, 1451–1456. [Google Scholar] [CrossRef]
- Chu, W.-D.; Zhang, L.-F.; Bao, X.; Zhao, X.-H.; Zeng, C.; Du, J.-Y.; Zhang, G.-B.; Wang, F.-X.; Ma, X.-Y.; Fan, C.-A. Asymmetric Catalytic 1,6-Conjugate Addition/Aromatiztion of para-Quinone Methides: Enantioselective Introduction of Functionalized Diarylmethine Stereogenic Centers. Angew. Chem. Int. Ed. 2013, 52, 9229–9233. [Google Scholar] [CrossRef]
- Caruana, L.; Kniep, F.; Johansen, T.K.; Poulsen, P.H.; Jørgensen, K.A. A New Organocatalytic Concept for Asymmetric α-Alkylation of Aldehydes. J. Am. Chem. Soc. 2014, 136, 15929–15932. [Google Scholar] [CrossRef]
- Venkatesh, R.; Shankar, G.; Narayanan, A.C.; Modi, G.; Sabiah, S.; Kandasamy, J. Multicomponent Synthesis of S-Benzyl Dithiocarbamates from para-Quinone Methides and Their Biological Evaluation for the Treatment of Alzheimer’s Disease. J. Org. Chem. 2022, 87, 6730–6741. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Recent Developments in 1,6-Addition Reactions of para-Quinone Methides (p-QMs). Org. Chem. Front. 2020, 7, 1743–1778. [Google Scholar] [CrossRef]
- Singh, T.; Upreti, G.C.; Arora, S.; Chauhan, H.; Singh, A. Visible Light-Mediated Carbamoylation of para-Quinone Methides. J. Org. Chem. 2023, 88, 2784–2791. [Google Scholar] [CrossRef]
- Ke, M.; Song, Q. Copper-Catalyzed 1,6-Hydrodifluoro-acetylation of para-Quinone Methides at Ambient Temperature with Bis(pinacolato)diboron as Reductant. Adv. Synth. Catal. 2017, 359, 384–389. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Luo, Y.-C.; Wang, Z.-Y.; Xu, P.-F. A New Approach to Access Difluoroalkylated Diarylmethanes via Visible-Light Photo- catalytic Cross-Coupling Reactions. Chem. Commun. 2018, 54, 3993–3996. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Ao, G.-Z.; Liu, F. Redox-Neutral Tri-/Difluoromethylation of para-Quinone Methides with Sodium Sulfinates. Org. Chem. Front. 2018, 5, 2061–2064. [Google Scholar] [CrossRef]
- Ghosh, K.G.; Chandu, P.; Mondal, S.; Sureshkumar, D. Visible-Light Mediated Trifluoromethylation of p-Quinone Methides by 1,6-Conjugate Addition Using Pyrylium Salt as Organic Photocatalyst. Tetrahedron 2019, 75, 4471–4478. [Google Scholar] [CrossRef]
- Qu, C.-H.; Song, G.-T.; Tang, D.-Y.; Shao, J.-W.; Li, H.-y.; Xu, Z.-G.; Chen, Z.-Z. Microwave-Assisted Copper Catalysis of α-Difluorinated gem-Diol toward Difluoroalkyl Radical for Hydrodifluoroalkylation of para-Quinone Methides. J. Org. Chem. 2020, 85, 12785–12796. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Hu, X.-S.; Yu, J.-S.; Zhou, F.; Zhou, Y.; Zhou, J. An Efficient Fe(III)-Catalyzed 1,6-Conjugate Addition of para-Quinone Methides with Fluorinated Silyl Enol Ethers toward β,β-Diaryl α-Fluorinated Ketones. Tetrahedron 2018, 74, 7395–7398. [Google Scholar] [CrossRef]
- Naret, T.; Bignon, J.; Bernadat, G.; Benchekroun, M.; Levaique, H.; Lenoir, C.; Dubois, J.; Pruvost, A.; Saller, F.; Borgel, D.; et al. A Fluorine Scan of a Ttubulin Polymerization Inhibitor isoCombretastatin A-4: Design, Synthesis, Molecular Modelling, and Biological Evaluation. Eur. J. Med. Chem. 2018, 143, 473–490. [Google Scholar] [CrossRef]
- Messaoudi, S.; Hamze, A.; Provot, O.; Tréguier, B.; Rodrigo De Losada, J.; Bignon, J.; Liu, J.-M.; Wdzieczak-Bakala, J.; Thoret, S.; Dubois, J.; et al. Discovery of Isoerianin Analogues as Promising Anticancer Agents. ChemMedChem 2011, 6, 488–497. [Google Scholar] [CrossRef]
- Abu-El-Haj, S.; Fahmy, M.A.H.; Fukuto, T.R. Insecticidal Activity of 1,1,1- Trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) analogs. J. Agric. Food Chem. 1979, 27, 258–261. [Google Scholar] [CrossRef]
- Jarava-Barrera, C.; Parra, A.; López, A.; Cruz-Acosta, F.; Collado-Sanz, D.; Cárdenas, D.J.; Tortosa, M. Copper-Catalyzed Borylative Aromatization of p-Quinone Methides: Enantioselective Synthesis of Dibenzylic Boronates. ACS Catal. 2016, 6, 442–446. [Google Scholar] [CrossRef]
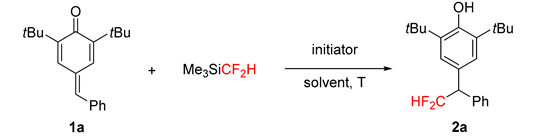 | ||||
|---|---|---|---|---|
| Entry | Initiator (equiv) | T (°C) | Solvent | Yield (%) b |
| 1 | CsF (0.2)/18-crown-6 (0.2) | rt | THF | 0 |
| 2 | TBAF (0.2) | −15 to rt | DMF | 30 |
| 3 | CsF (0.2) | −15 to rt | DMF | 33 |
| 4 | TBAF (0.2) | −30 | DMF | 17 |
| 5 | TMAF (0.2) | −15 to rt | DMF | 30 |
| 6 | KF (0.2) | −30 | DMF | trace |
| 7 | TBAF (0.2) | rt | DMF | 20 |
| 8 | CsF(1.0) | −30 | DMF | 36 |
| 9 | CsF (1.0)/18-crown-6 (0.2) | −30 | DMF | 51 |
| 10 | CsF (0.2)/18-crown-6 (0.1) | −30 | DMF | 42 |
| 11 | CsF (0.2)/18-crown-6 (0.1) | −15 to rt | DMF | 52 |
| 12 | CsF (1.0)/18-crown-6 (1.0) | −15 to rt | DMF | 60 |
| 13 | CsF (1.5)/18-crown-6 (1.5) | −15 to rt | DMF | 70 |
| 14 | CsF (2.0)/18-crown-6 (2.0) | −15 to rt | DMF | 60 |
| 15 | KF (1.5)/18-crown-6 (1.5) | −15 to rt | DMF | 12 |
 | ||||
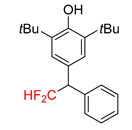 | 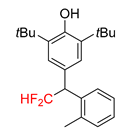 | 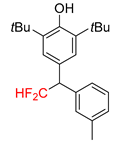 | 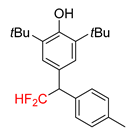 | 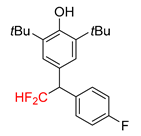 |
| 2a, 70% | 2b, 70% | 2c, 46% | 2d, 49% | 2e, 78% |
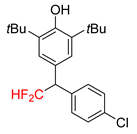 | 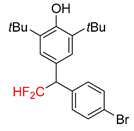 | 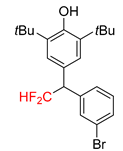 | 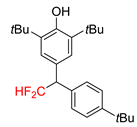 | 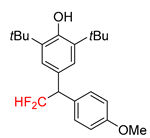 |
| 2f, 86% | 2g, 63% | 2h, 68% | 2i, 60% | 2j, 40% |
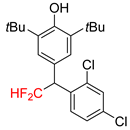 | 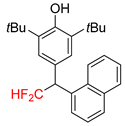 | 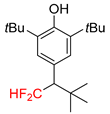 | 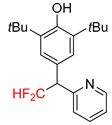 | |
| 2k, 67% | 2l, 61% | 2m, 23% | 2n, 62% | |
 | ||||
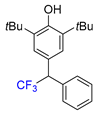 | 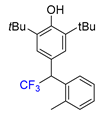 | 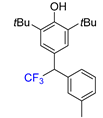 | 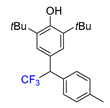 | 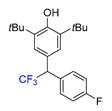 |
| 3a, 66% | 3b, 85% | 3c, 61% | 3d, 82% | 3e, 57% |
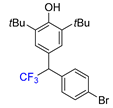 | 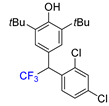 | 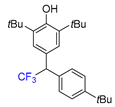 | 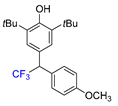 | 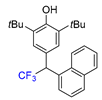 |
| 3f, 86% | 3g, 61% | 3h, 45% | 3i, 55% | 3j, 78% |
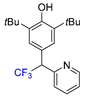 | ||||
| 3k, 30% | ||||
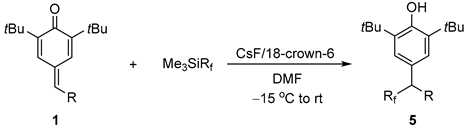 | ||||
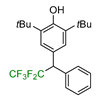 | 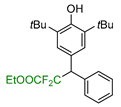 | 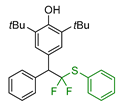 | 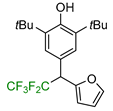 | 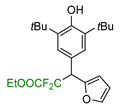 |
| 5a, 88% | 5b, 67% | 5c, 70% | 5d, 73% | 5e, 60% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Huang, L.; Liang, M.; Chen, X.; Cao, D.; Xiao, P.; Ni, C.; Hu, J. 1,6-Nucleophilic Di- and Trifluoromethylation of para-Quinone Methides with Me3SiCF2H/Me3SiCF3 Facilitated by CsF/18-Crown-6. Molecules 2024, 29, 2905. https://doi.org/10.3390/molecules29122905
Chen D, Huang L, Liang M, Chen X, Cao D, Xiao P, Ni C, Hu J. 1,6-Nucleophilic Di- and Trifluoromethylation of para-Quinone Methides with Me3SiCF2H/Me3SiCF3 Facilitated by CsF/18-Crown-6. Molecules. 2024; 29(12):2905. https://doi.org/10.3390/molecules29122905
Chicago/Turabian StyleChen, Dingben, Ling Huang, Mingyu Liang, Xiaojing Chen, Dongdong Cao, Pan Xiao, Chuanfa Ni, and Jinbo Hu. 2024. "1,6-Nucleophilic Di- and Trifluoromethylation of para-Quinone Methides with Me3SiCF2H/Me3SiCF3 Facilitated by CsF/18-Crown-6" Molecules 29, no. 12: 2905. https://doi.org/10.3390/molecules29122905
APA StyleChen, D., Huang, L., Liang, M., Chen, X., Cao, D., Xiao, P., Ni, C., & Hu, J. (2024). 1,6-Nucleophilic Di- and Trifluoromethylation of para-Quinone Methides with Me3SiCF2H/Me3SiCF3 Facilitated by CsF/18-Crown-6. Molecules, 29(12), 2905. https://doi.org/10.3390/molecules29122905






