Controllable Construction of Aptamer-Modified Fe3O4@SiO2-Au Core-Shell-Satellite Nanocomposites with Surface-Enhanced Raman Scattering and Photothermal Properties and Their Effective Capture, Detection, and Elimination of Staphylococcus aureus
Abstract
1. Introduction
2. Results and Discussion
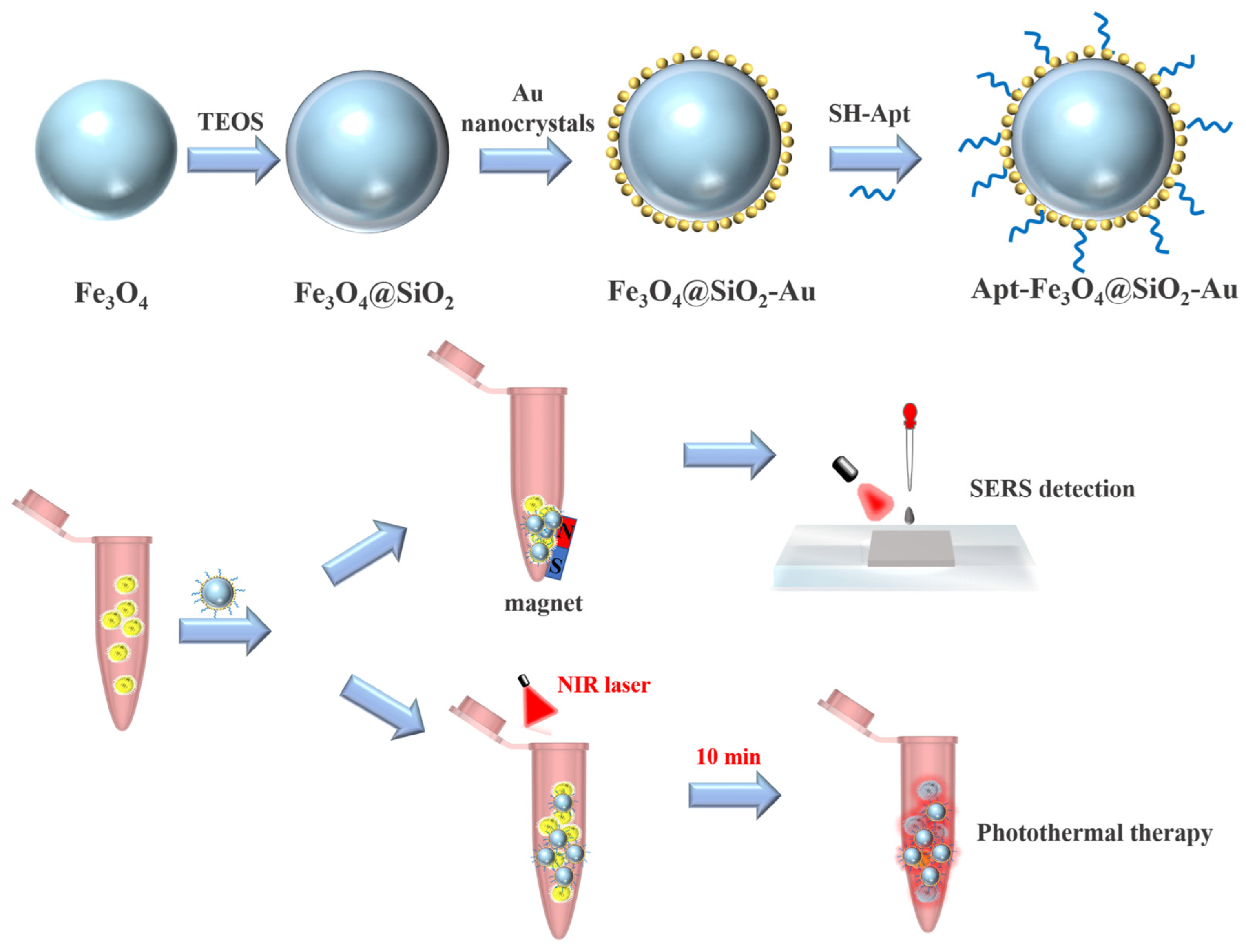
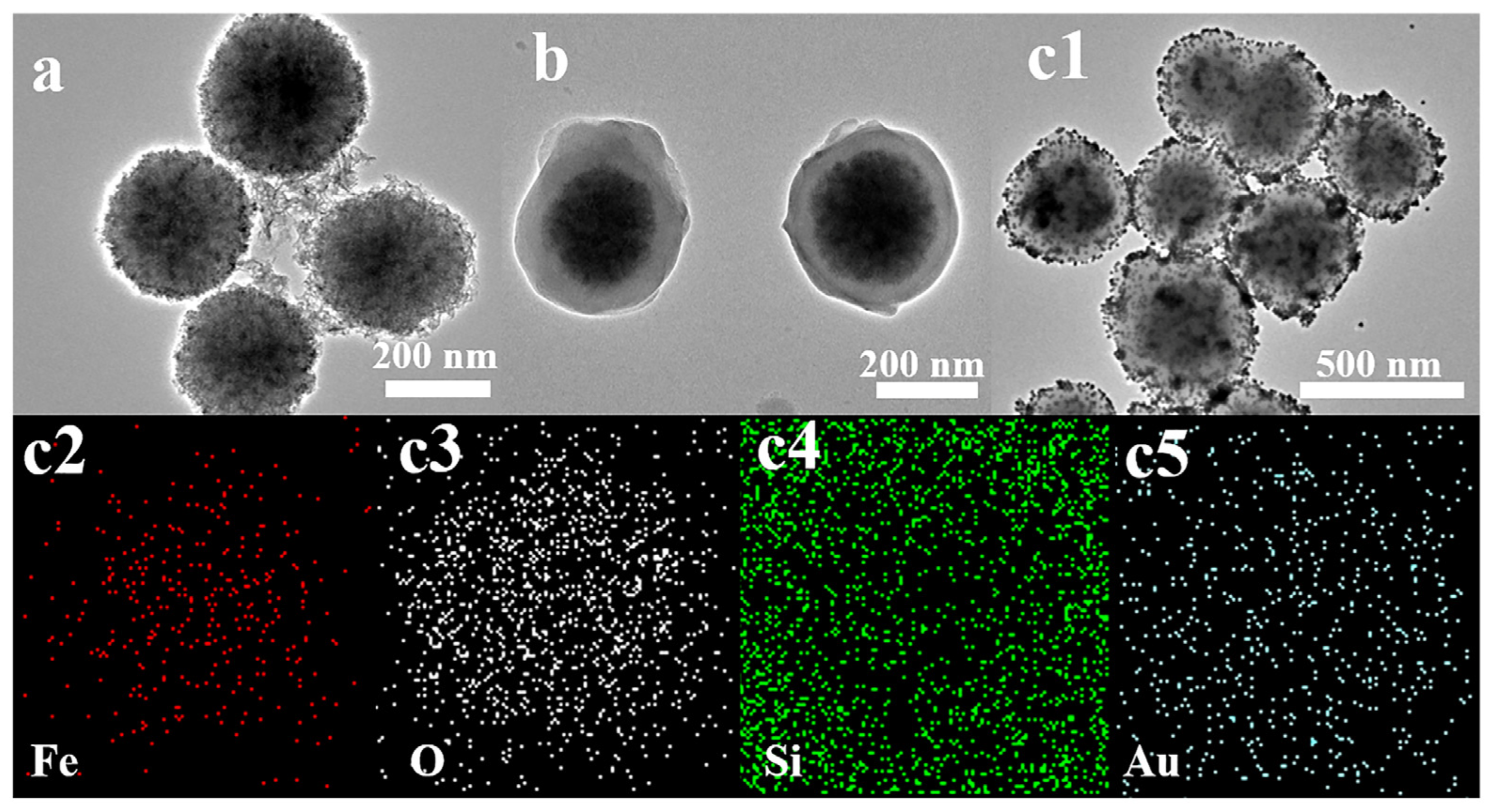
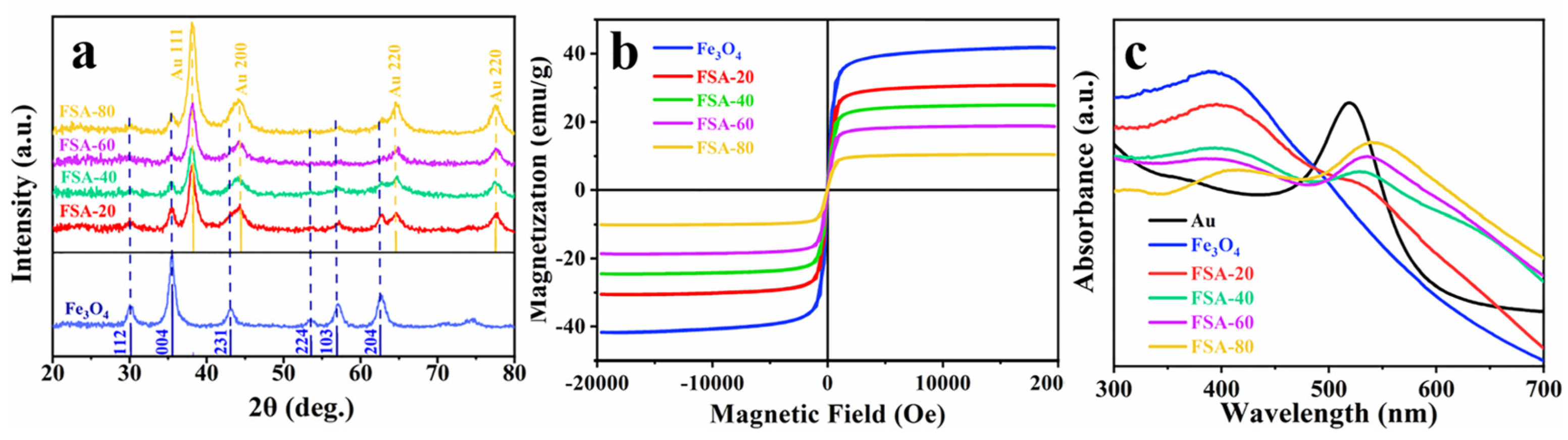
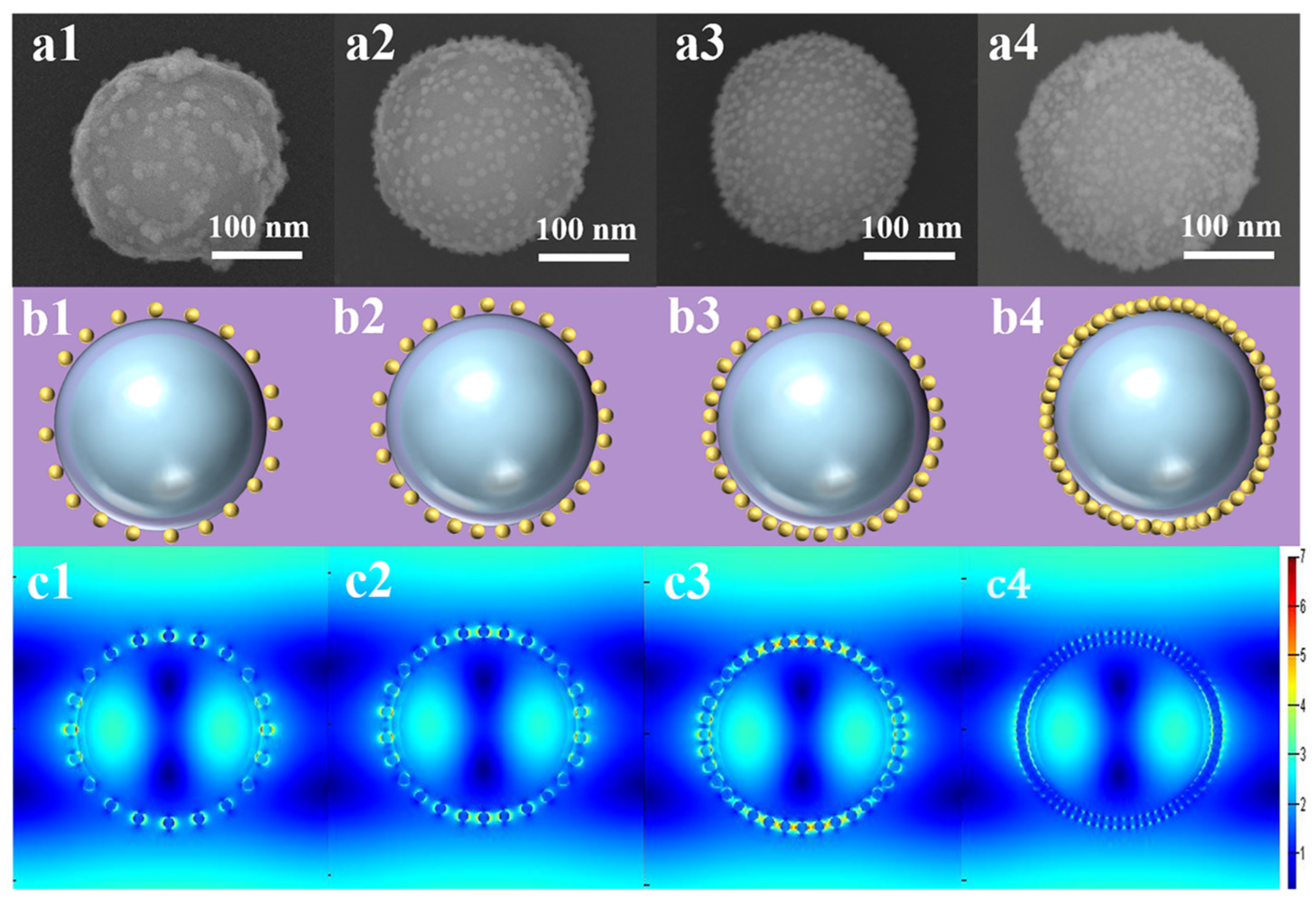
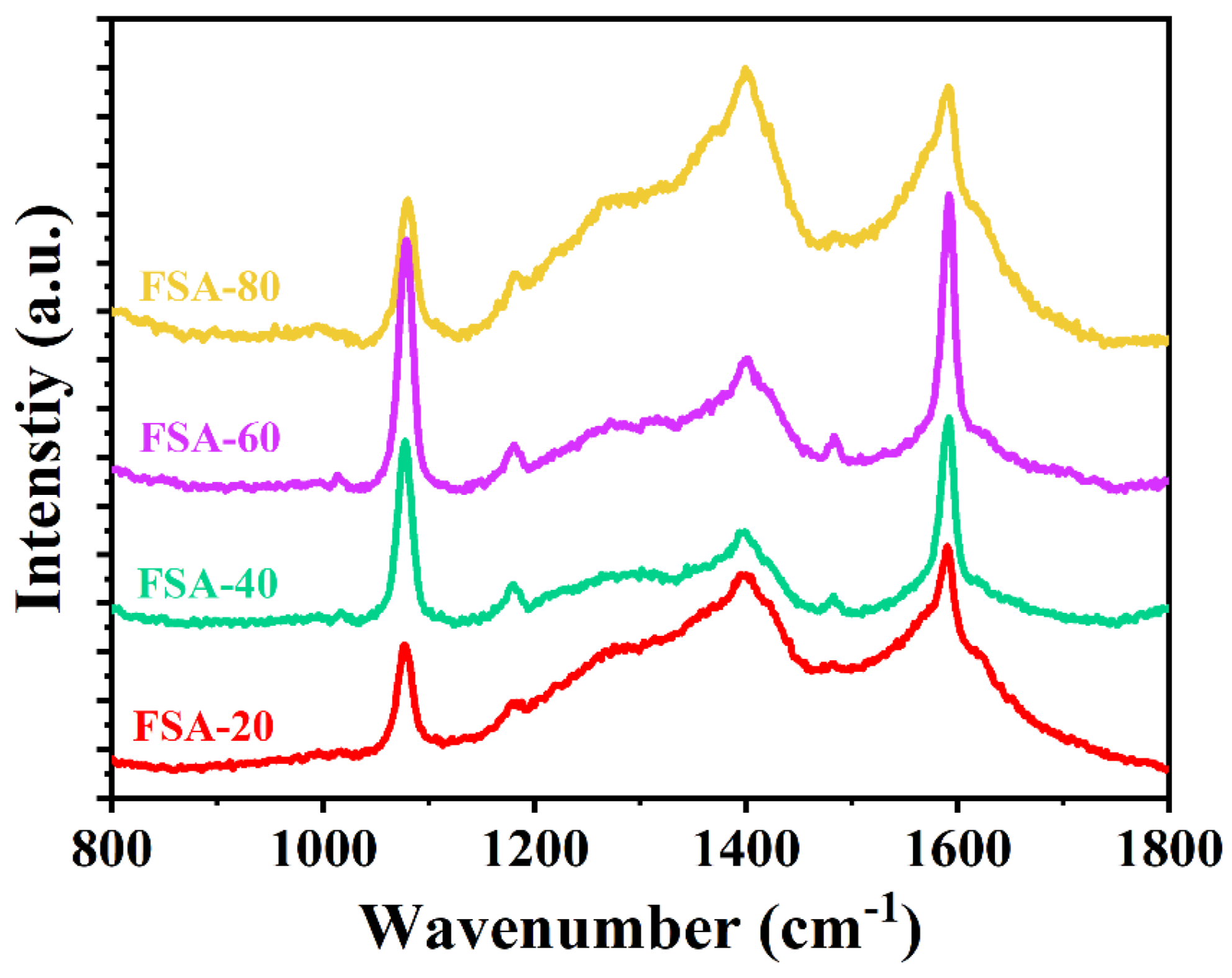
2.1. Characterization and Optimization of Fe3O4@SiO2-Au NCs
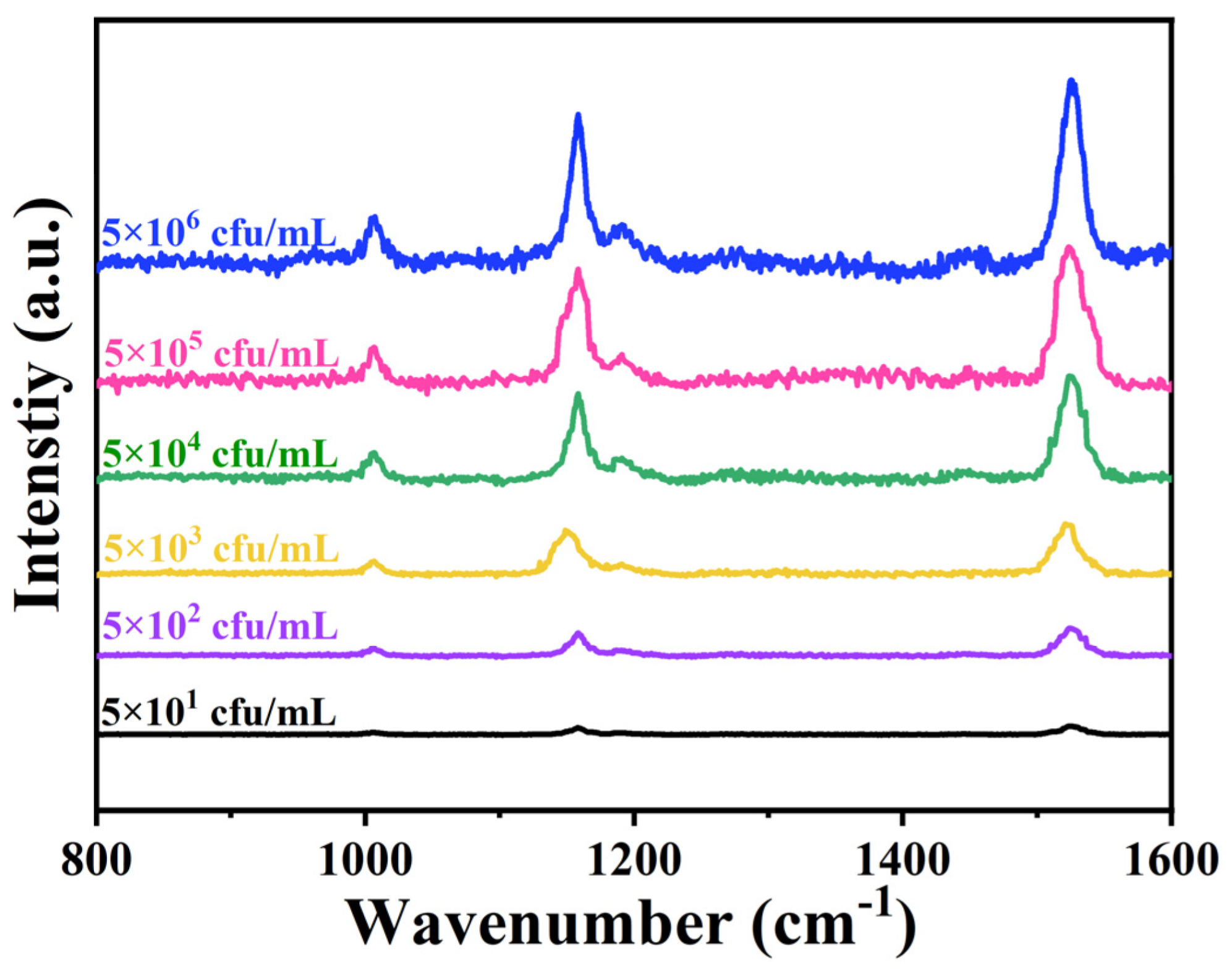
2.2. Capture and Detection of S. aureus by Apt-FSA-60
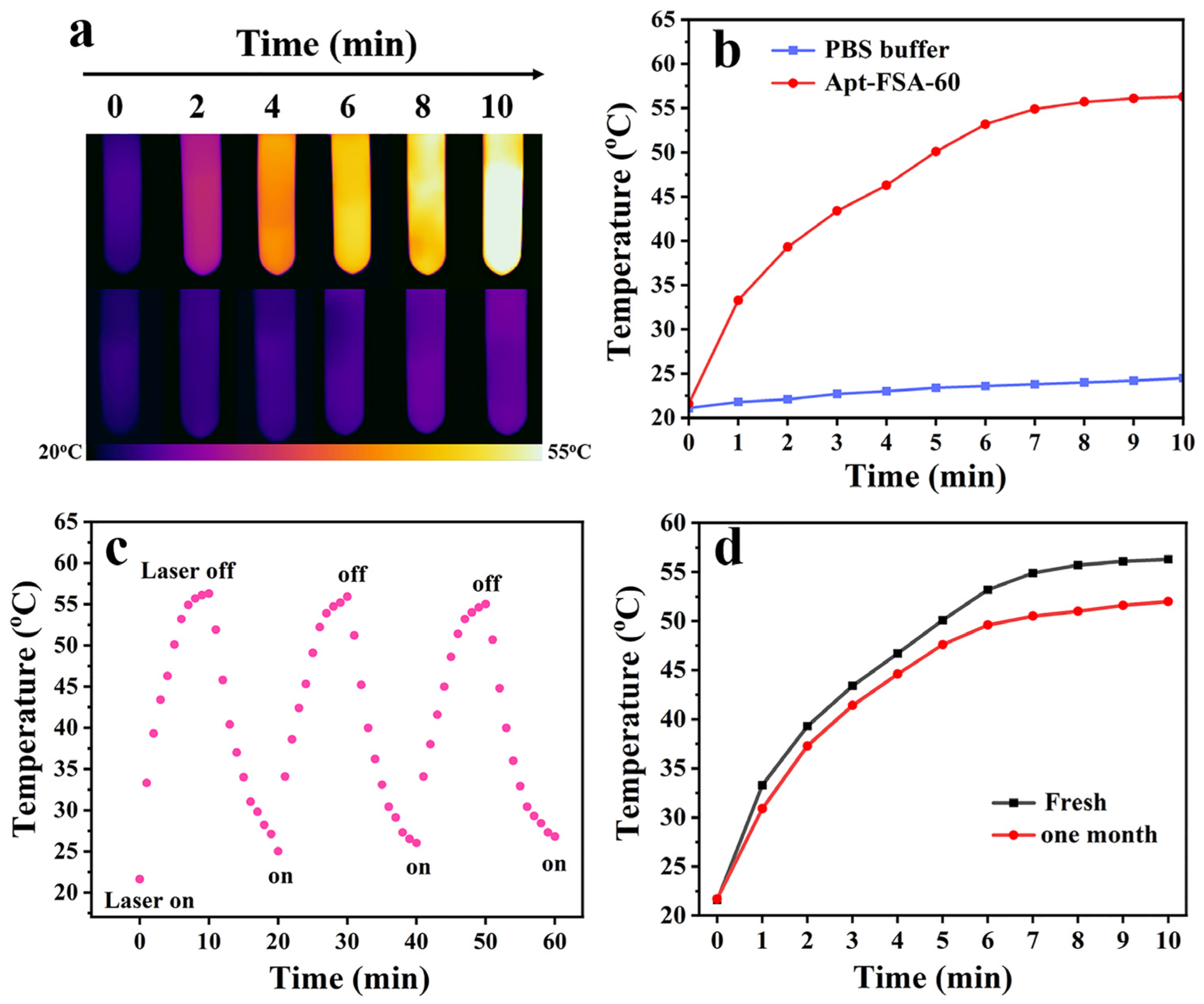
2.3. Investigation of Photothermal Performance of Apt-FSA-60
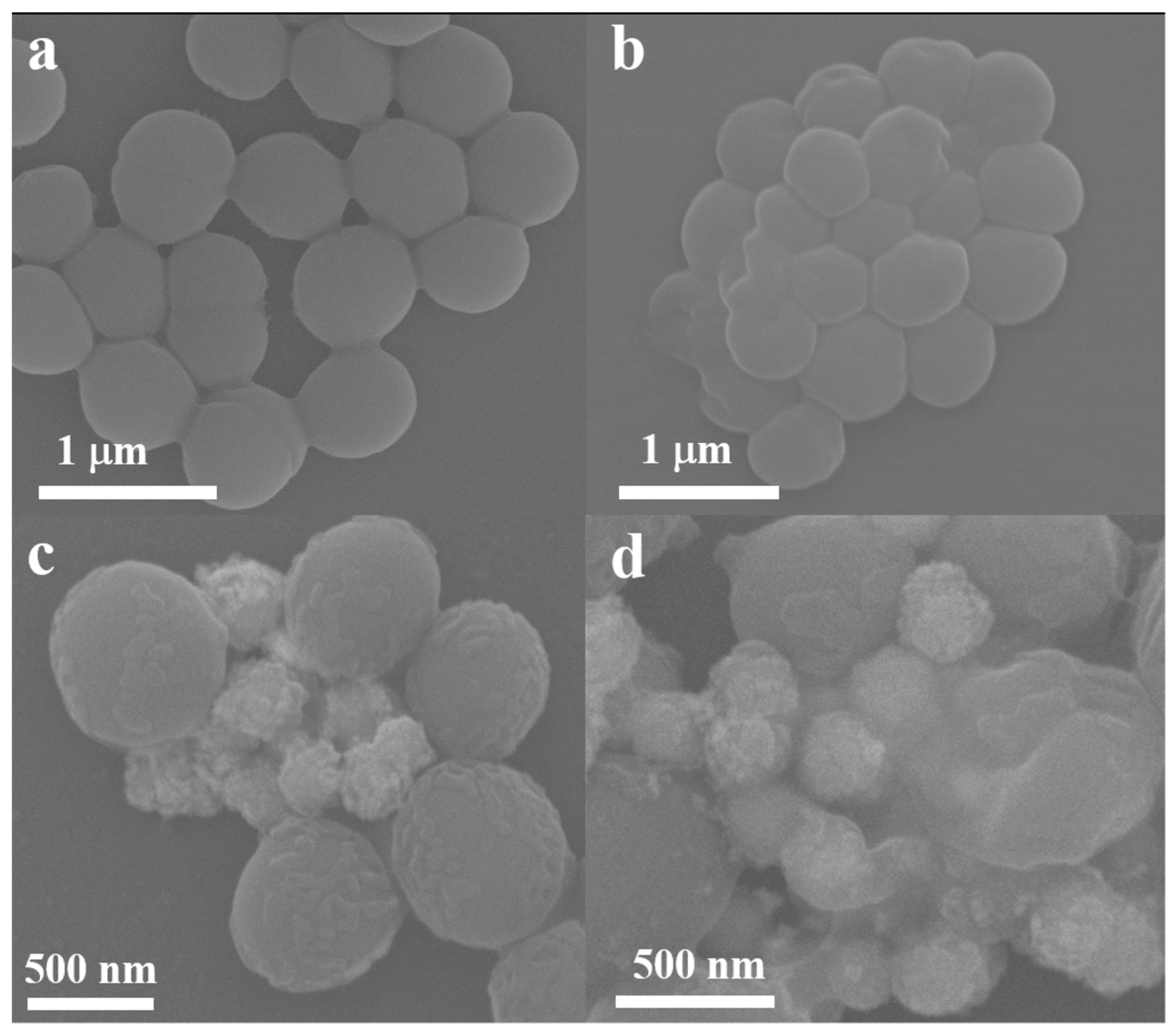
2.4. PTT of S. aureus In Vitro
3. Materials and Methods
3.1. Preparation of Fe3O4@SiO2-Au NCs
3.2. Preparation of Apt-FSA-60
3.3. S. aureus Sample Preparation
3.4. SERS Detection of S. aureus
3.5. Photothermal Conversion Performance of Apt-FSA-60 NCs
3.6. Incubation of S. aureus In Vitro
3.7. Morphology of the Treated S. aureus Determined by SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm formation and control of foodborne pathogenic bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef] [PubMed]
- Lázár, V.; Snitser, O.; Barkan, D.; Kishony, R. Antibiotic combinations reduce Staphylococcus aureus clearance. Nature 2022, 610, 540–546. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Li, Q.; Chen, H.; Lin, Z.; Zhang, S.; Jiang, X.; Qiu, B. Homogeneous electrochemiluminescence aptasensor based on hybridization chain reaction and magnetic separation assistance for Staphylococcus aureus. Microchem. J. 2023, 187, 108377. [Google Scholar] [CrossRef]
- Zhou, W.; Wen, H.; Hao, G.; Zhang, Y.-S.; Yang, J.; Gao, L.; Zhu, G.; Yang, Z.-Q.; Xu, X. Surface engineering of magnetic peroxidase mimic using bacteriophage for high-sensitivity/specificity colorimetric determination of Staphylococcus aureus in food. Food Chem. 2023, 426, 136611. [Google Scholar] [CrossRef] [PubMed]
- Hadi, J.; Rapp, D.; Dhawan, S.; Gupta, S.K.; Gupta, T.B.; Brightwell, G. Molecular detection and characterization of foodborne bacteria: Recent progresses and remaining challenges. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2433–2464. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Carnazza, S.; De Plano, L.M.; Franco, D.; Nicolò, M.S.; Zammuto, V.; Petralia, S.; Calabrese, G.; Gugliandolo, C.; Conoci, S.; et al. Rapid detection of bacterial pathogens in blood through engineered phages-beads and integrated Real-Time PCR into MicroChip. Sens. Actuators B Chem. 2021, 329, 129227. [Google Scholar] [CrossRef]
- Prucek, R.; Panacek, A.; Gajdova, Z.; Vecerova, R.; Kvitek, L.; Gallo, J.; Kolar, M. Specific detection of Staphylococcus aureus infection and marker for Alzheimer disease by surface enhanced Raman spectroscopy using silver and gold nanoparticle-coated magnetic polystyrene beads. Sci. Rep. 2021, 11, 6240. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, P.; Ahmad, W.; Hassan, M.M.; Ali, S.; Li, H.; Chen, Q. Catalytic hairpin activated gold-magnetic/gold-core-silver-shell rapid self-assembly for ultrasensitive Staphylococcus aureus sensing via PDMS-based SERS platform. Biosens. Bioelectron. 2022, 209, 114240. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, F.; Kołątaj, K.; Steinberger, M.; Liedl, T.; Lohmueller, T. Accessible hotspots for single-protein SERS in DNA-origami assembled gold nanorod dimers with tip-to-tip alignment. Nat. Commun. 2023, 14, 7192. [Google Scholar] [CrossRef]
- Shi, F.; Xu, J.; Hu, Z.; Ren, C.; Xue, Y.; Zhang, Y.; Li, J.; Wang, C.; Yang, Z. Bird nest-like zinc oxide nanostructures for sensitive electrochemical glucose biosensor. Chin. Chem. Lett. 2021, 32, 3185–3188. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, L.; Liu, Y.; Chen, L. Photocatalytic Deposition of Au Nanoparticles on Ti3C2Tx MXene Substrates for Surface-Enhanced Raman Scattering. Molecules 2024, 29, 2383. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Rao, Y.; Shi, F.; Yu, S.; Yang, H.; Min, L.; Yang, Z. Synthesis of highly ordered AgNPs-coated silica photonic crystal beads for sensitive and reproducible 3D SERS substrates. Chin. Chem. Lett. 2021, 32, 150–153. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Wang, R.; Waterhouse, G.I.N.; Xu, Z. SERS-tag technology in food safety and detection: Sensing from the “fingerprint” region to the “biological-silent” region. J. Future Foods 2024, 4, 309–323. [Google Scholar] [CrossRef]
- Xie, M.; Zhu, Y.; Li, Z.; Yan, Y.; Liu, Y.; Wu, W.; Zhang, T.; Li, Z.; Wang, H. Key steps for improving bacterial SERS signals in complex samples: Separation, recognition, detection, and analysis. Talanta 2023, 268, 125281. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, G.; Wang, W.; Ren, Z.; Zhang, C.; Gong, Y.; Zhao, M.; Qu, Y.; Li, W.; Zhou, H.; et al. Bioinspired hot-spot engineering strategy towards ultrasensitive SERS sandwich biosensor for bacterial detection. Biosens. Bioelectron. 2023, 237, 115497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Mao, D.; Wen, J.; Gao, R.; Wang, Y. 3D SERS Substrate of Z-Shaped Ag Nanorod Array for Thiabendazole Detection. Molecules 2023, 28, 7078. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Pisano, F.; Collard, L.; Balena, A.; Pisanello, M.; Spagnolo, B.; Mach-Batlle, R.; Tantussi, F.; Carbone, L.; De Angelis, F.; et al. Toward Plasmonic Neural Probes: SERS Detection of Neurotransmitters through Gold-Nanoislands-Decorated Tapered Optical Fibers with Sub-10 nm Gaps. Adv. Mater. 2023, 35, e2200902. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Q.; Ma, Y.; Huang, C.; Zhi, W.; Li, J.; Zeng, R.; Pi, J.; Xu, J.-F.; Xu, J.; et al. Chiral Au nanostars for SERS sensing of enantiomers discrimination, multibacteria recognition and photothermal antibacterial application. Chem. Eng. J. 2024, 479, 147528. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Zhou, T.; Huang, J.; Wang, Y.; Li, B.; Chen, L.; Yang, J.; Liu, Y. Aptamer-conjugated magnetic Fe3O4@Au core-shell multifunctional nanoprobe: A three-in-one aptasensor for selective capture, sensitive SERS detection and efficient near-infrared light triggered photothermal therapy of Staphylococcus aureus. Sens. Actuators B Chem. 2022, 350, 130879. [Google Scholar] [CrossRef]
- Tu, J.; Wu, T.; Yu, Q.; Li, J.; Zheng, S.; Qi, K.; Sun, G.; Xiao, R.; Wang, C. Introduction of multilayered magnetic core–dual shell SERS tags into lateral flow immunoassay: A highly stable and sensitive method for the simultaneous detection of multiple veterinary drugs in complex samples. J. Hazard. Mater. 2023, 448, 130912. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, R.; Cheng, S.; Wang, S.; Shi, L.; Wang, C.; Qi, K.; Wang, S. A universal SERS-label immunoassay for pathogen bacteria detection based on Fe3O4@Au-aptamer separation and antibody-protein A orientation recognition. Anal. Chim. Acta 2021, 1160, 338421. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, A.; Wang, D.; Wang, J.; Chen, P.; Sun, H. Cube-like Fe3O4@SiO2@Au@Ag magnetic nanoparticles: A highly efficient SERS substrate for detection of pesticide. Nanotechnology 2018, 29, 165302. [Google Scholar] [CrossRef]
- Bai, Q.; Luo, H.; Shi, S.; Liu, S.; Wang, L.; Du, F.; Yang, Z.; Zhu, Z.; Sui, N. AuAg nanocages/graphdiyne for rapid elimination and detection of trace pathogenic bacteria. J. Colloid Interface Sci. 2022, 613, 376–383. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Y.; Liu, J.; Liu, L.; Pi, F. Progress on aptamer-based SERS sensors for food safety and quality assessment: Methodology, current applications and future trends. Crit. Rev. Food Sci. Nutr. 2024, 64, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, R.; Zhang, Y.; Zhao, J.; Wang, Y.; Xu, Z. Dual-aptamer-assisted ratiometric SERS biosensor for ultrasensitive and precise identification of breast cancer exosomes. ACS Sens. 2023, 8, 875–883. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Chen, W.; Wang, Y.; Song, G.; He, H.; Wang, H.; Li, P.; Wang, G.P. Tri-functional SERS nanoplatform with tunable plasmonic property for synergistic antibacterial activity and antibacterial process monitoring. J. Colloid Interface Sci. 2022, 608, 2266–2277. [Google Scholar] [CrossRef]
- Kong, L.; Li, J.; Zhang, Y.; Wang, J.; Liang, K.; Xue, X.; Chen, T.; Hao, Y.; Ren, H.; Wang, P.; et al. Biodegradable Metal Complex-Gated Organosilica for Dually Enhanced Chemodynamic Therapy through GSH Depletions and NIR Light-Triggered Photothermal Effects. Molecules 2024, 29, 1177. [Google Scholar] [CrossRef]
- Qi, C.; Wang, W.; Wang, P.; Cheng, H.; Wang, X.; Gong, B.; Xie, A.; Shen, Y. Facile Synthesis of Fe3O4@Au/PPy-DOX Nanoplatform with Enhanced Glutathione Depletion and Controllable Drug Delivery for Enhanced Cancer Therapeutic Efficacy. Molecules 2022, 27, 4003. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, J.; Zhao, H.; Liu, Y. Synthesis of Multi-Stimuli Responsive Fe3O4 Coated with Diamonds Nanocomposite for Magnetic Assisted Chemo-Photothermal Therapy. Molecules 2023, 28, 1784. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.; Wang, Y.; Huang, J.; Wang, H.; Li, H.; Chang, F.; Jiang, Y. Plasmonic nanobipyramids with photo-enhanced catalytic activity under near-infrared II window for effective treatment of breast cancer. Acta Biomater. 2023, 170, 496–506. [Google Scholar] [CrossRef]
- He, J.; Hua, S.; Zhang, D.; Wang, K.; Chen, X.; Zhou, M. SERS/NIR-II Optical Nanoprobes for Multidimensional Tumor Imaging from Living Subjects, Pathology, and Single Cells and Guided NIR-II Photothermal Therapy. Adv. Funct. Mater. 2022, 32, 2208028. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, X.; Qian, J.; Yu, Y.; Li, J.; He, Z.; Duan, S.; Guo, H.; Shen, X.; Guo, Q. Au Nanorods Coated with pH-Responsive Polymers for Photothermal Therapy Against Multidrug-Resistant Bacteria. ACS Appl. Nano Mater. 2022, 5, 16884–16895. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Zhao, W.; Cui, S.; Qian, S.; Chen, Q.; Li, X.; Liu, Y.; Zhang, Q. A novel strategy for specific sensing and inactivation of Escherichia coli: Constructing a targeted sandwich-type biosensor with multiple SERS hotspots to enhance SERS detection sensitivity and near-infrared light-triggered photothermal sterilization performance. Talanta 2024, 269, 125466. [Google Scholar] [PubMed]
- Zhao, C.; Fu, H.; Yang, X.; Xiong, S.; Han, D.; An, X. Adsorption and photocatalytic performance of Au nanoparticles decorated porous Cu2O nanospheres under simulated solar light irradiation. Appl. Surf. Sci. 2021, 545, 149014. [Google Scholar] [CrossRef]
- He, J.; Song, G.; Wang, X.; Zhou, L.; Li, J. Multifunctional magnetic Fe3O4/GO/Ag composite microspheres for SERS detection and catalytic degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2022, 893, 162226. [Google Scholar] [CrossRef]
- Negri, C.; Colombo, R.; Bracconi, M.; Atzori, C.; Donazzi, A.; Lucotti, A.; Tommasini, M.; Maestri, M. Operando UV-vis spectroscopy for real-time monitoring of nanoparticle size in reaction conditions: A case study on r WGS over Au nanoparticles. Catal. Sci. Technol. 2024, 14, 1318–1327. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Q.; He, S.; Wang, S.; Nie, W.; Wu, K.; Fan, F.; Li, C. Observation of charge separation enhancement in plasmonic photocatalysts under coupling conditions. Nano Lett. 2023, 23, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Wu, T.; Zheng, H.; Kadasala, N.R.; Yang, S.; Guo, C.; Chen, L.; Liu, Y.; Yang, J. Recyclable Magnetic MIP-Based SERS Sensors for Selective, Sensitive, and Reliable Detection of Paclobutrazol Residues in Complex Environments. ACS Sustain. Chem. Eng. 2020, 8, 14549–14556. [Google Scholar] [CrossRef]
- Yu, D.; Xu, L.; Zhang, H.; Li, J.; Wang, W.; Yang, L.; Jiang, X.; Zhao, B. A new semiconductor-based SERS substrate with enhanced charge collection and improved carrier separation: CuO/TiO2 pn heterojunction. Chin. Chem. Lett. 2023, 34, 107771. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef]
- Cheng, F.; Chang, H.-C.; Chen, T.-Y.; Hu, C.; Yang, F.-L. Rapid (<5 min) Identification of Pathogen in Human Blood by Electrokinetic Concentration and Surface-Enhanced Raman Spectroscopy. Sci. Rep. 2013, 3, 2365. [Google Scholar]
- Ma, X.; Lin, X.; Xu, X.; Wang, Z. Fabrication of gold/silver nanodimer SERS probes for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Microchim. Acta 2021, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Xing, C.; Guo, M.; Xu, F.; Wang, X.; Gruber, H.J.; Zhang, B.; Tang, J. Sodium citrate: A universal reducing agent for reduction/decoration of graphene oxide with au nanoparticles. Nano Res. 2011, 4, 599–611. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, S.; Zou, Y.; Gao, Y.; Ma, B.; Zhang, Y.; Dai, H.; Ma, J.; Zhao, W. Controllable Construction of Aptamer-Modified Fe3O4@SiO2-Au Core-Shell-Satellite Nanocomposites with Surface-Enhanced Raman Scattering and Photothermal Properties and Their Effective Capture, Detection, and Elimination of Staphylococcus aureus. Molecules 2024, 29, 3593. https://doi.org/10.3390/molecules29153593
Wang Y, Wang S, Zou Y, Gao Y, Ma B, Zhang Y, Dai H, Ma J, Zhao W. Controllable Construction of Aptamer-Modified Fe3O4@SiO2-Au Core-Shell-Satellite Nanocomposites with Surface-Enhanced Raman Scattering and Photothermal Properties and Their Effective Capture, Detection, and Elimination of Staphylococcus aureus. Molecules. 2024; 29(15):3593. https://doi.org/10.3390/molecules29153593
Chicago/Turabian StyleWang, Yongdan, Shengyi Wang, Yuhui Zou, Yuze Gao, Boya Ma, Yuhan Zhang, Huasong Dai, Jingmei Ma, and Wenshi Zhao. 2024. "Controllable Construction of Aptamer-Modified Fe3O4@SiO2-Au Core-Shell-Satellite Nanocomposites with Surface-Enhanced Raman Scattering and Photothermal Properties and Their Effective Capture, Detection, and Elimination of Staphylococcus aureus" Molecules 29, no. 15: 3593. https://doi.org/10.3390/molecules29153593
APA StyleWang, Y., Wang, S., Zou, Y., Gao, Y., Ma, B., Zhang, Y., Dai, H., Ma, J., & Zhao, W. (2024). Controllable Construction of Aptamer-Modified Fe3O4@SiO2-Au Core-Shell-Satellite Nanocomposites with Surface-Enhanced Raman Scattering and Photothermal Properties and Their Effective Capture, Detection, and Elimination of Staphylococcus aureus. Molecules, 29(15), 3593. https://doi.org/10.3390/molecules29153593





