Abstract
Organic arsenic compounds such as p-aminophenylarsine oxide (p-APAO) are easier for structural optimization to improve drug-like properties such as pharmacokinetic properties, therapeutic efficacy, and target selectivity. In order to strengthen the selectivity of 4-(1,3,2-dithiarsinan-2-yl) aniline 7 to tumor cell, a thiourea moiety was used to strengthen the anticancer activity. To avoid forming a mixture of α/β anomers, the strategy of 2-acetyl’s neighboring group participation was used to lock the configuration of 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate from 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide. 1-(4-(1,3,2-dithiarsinan-2-yl) aniline)-2-N-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranos-1-yl)-thiourea 2 can increase the selectivity of human colon cancer cells HCT-116 (0.82 ± 0.06 μM vs. 1.82 ± 0.07 μM) to human embryonic kidney 293T cells (1.38 ± 0.01 μM vs. 1.22 ± 0.06 μM) from 0.67 to 1.68, suggesting a feasible approach to improve the therapeutic index of arsenic-containing compounds as chemotherapeutic agents.
1. Introduction
Arsenic trioxide (As, +III) and its derivatives have been used for various purposes for more than 2000 years [1,2,3]. In the 1970s, arsenic trioxide was used to heal acute promyelocytic leukemia (APL) in clinical application by Zhang Tingdong [1]. Subsequently, arsenic trioxide (Trade Name: Trisenox) has been approved for the treatment of relapsed or refractory APL by the U.S. Food and Drug Administration since 2000 and European Medicines Agency since 2017, respectively. The mechanisms of action of arsenic trioxide have revealed that the drug is able to promote the catabolic degradation of an oncogenic fusion protein [4,5,6], disrupt the mitochondrial function [7], downregulate Bcl-2 expression [8], and reactivate mutant p53 for tumor suppression [9]. Despite the remarkable success of arsenic trioxide in the treatment of APL, limitations of inorganic arsenic compound as a chemotherapeutic was the systemic toxicity, associated with its poor pharmacokinetic properties. This may be attributed to the rapid renal clearance of arsenic trioxide metabolites [2,10].
In comparison with inorganic arsenic compounds, organic arsenic compounds are easier for structural optimization to improve drug-like properties such as pharmacokinetic properties, therapeutic efficacy, and target selectivity. Organic arsenic compounds—p-aminophenylarsine oxide (p-APAO) [4,11,12] and others [3,13,14,15]—were investigated either as preclinical and clinical experimental drugs or as molecular probes in cancer cells. Arsenic sulfide and its derivatives showed antitumor activity in solid tumor cell lines such as HCT 116 [16,17,18] (Figure 1).
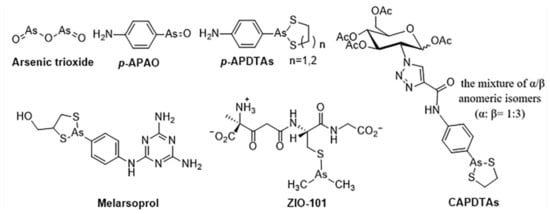
Figure 1.
Representative arsenic-containing compounds with anticancer activities [3,18].
Urea cycle dysregulation (UCD) in cancer is a prevalent phenomenon in multiple cancers. It is also associated with a worse prognosis but a better response to immune therapy [19]. Small organic molecules with a thiourea moiety have been widely used in the treatment of anticancer [20,21,22,23].
Combined with the previous basis of the design and synthesis of small molecules containing urea structure and based on the principle of bioisosterism, we proposed the research topic of the design, synthesis and bioactivity of small-molecule compounds containing a thiourea structure. Here, the moiety of thiourea-conjugating organic arsenic was induced to anomeric carbon of D-glucose derivatives.
Based on the derivatives of D-glucosamine, it could reduce the toxicity of trivalent arsenic compound and other pharmacophore [24,25]. Actually these D-glucosamine linker trivalent arsenic compounds and other pharmacophore a the mixture of α/β anomeric isomers in dynamic equilibrium. To avoid the effect of enantiomer on biological activity, here, a novel glucose conjugated arsenic compound was designed as the follows: thiourea was used to strengthen the anticancer biological activities; the anomeric group’s steric configuration of D-glucosamine derivative was locked by the reaction of 2-acetyl’s neighboring group participation via five-membered glycosyl oxocarbenium ion (Figure 2) to avoid the effect of enantiomer on biological activity [25,26]. 2,3,4, 6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate was designed as precursor of 1-amine-β-d-glucose to avoid the effect of enantiomer on biological activity. Isothiocyanate’s strong electrophilicity was used to react with amine in compound 6 or 7 to form derivatives of 1-amine β-d-glucose 1 and 2, respectively.
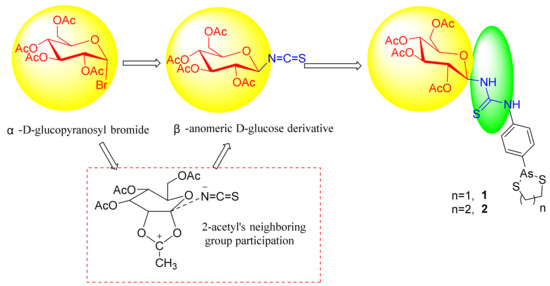
Figure 2.
Design of target compounds 1 and 2.
2. Results and Discussion
Our target compounds, the derivatives of 4-aminophenylarsenous acid (As, +III) linking to the anomer of D-glucose to form β-configuration D-glucose-arsenate compounds were designed as follows: the synthesis routes were started from commercial chemical 4-aminophenylarsonic acid (As, +V) 3. It was reduced to 4-aminophenylarsenic acid (As, +III) 4 by sulfur dioxide under a catalyst of minor iodine. The solution of 4 in ethanol was heated to reflux with propane-1,3-dithiol to obtain compound 7 [27,28,29]. Compound 4 was also dissolved in concentrated HCl to produce intermediate 5. It further reacted with ethane-1,2-dithiol at room temperature to obtain compound 6 under aqueous sodium carbonate [18]. 2,3,4,6-O-tetraacetyl-α-D-glucopyranosyl bromide 8 reacted with potassium thiocyanate through nucleophilic substitution to form β-anomer of D-glucose derivative 9 under the catalyst of a 4 Å molecular sieve and (n-Bu)4NBr as a phase transfer catalyst. With the aid of 2-acetyl’s neighboring group participation, the configuration of SCN formed the β-anomer via a five-membered glycosyl oxocarbenium ion to replace the original α-anomer [30]. The chemical shift of anomeric H in compound 8 is 6.62–6.61 ppm. J1,2 (coupling constant) of anomeric H of compound 8 is 4.0 Hz (Figure 3A), which is in the J1,2 range of α-D-glucose (3–5 Hz), while the peak at 5.14–5.12 ppm is anomeric H of compound 9. Anomeric H’s J1,2 (coupling constant) of compound 9 is 8.0 Hz, which is in the J1,2 range of β-D-glucose (6–9 Hz) in 1H NMR (Figure 3B). The anomeric H’s coupling constants (J1,2) of compound 1 and 2 are 8.0 Hz and 8.0 Hz, respectively. They are also in the anomeric H’s J1,2 of β-D-glucose (6–9 Hz) range in 1H NMR [31]. These results showed that the anomeric H has been changed from α configuration (compound 8) to β one (compound 9, 1 and 2). In the following steps, compounds 6 and 7 reacted with 9 involving a nucleophilic attack to reach our target compounds 1 and 2 at room temperature with yields of 81.9% and 71.5%, respectively (Scheme 1).
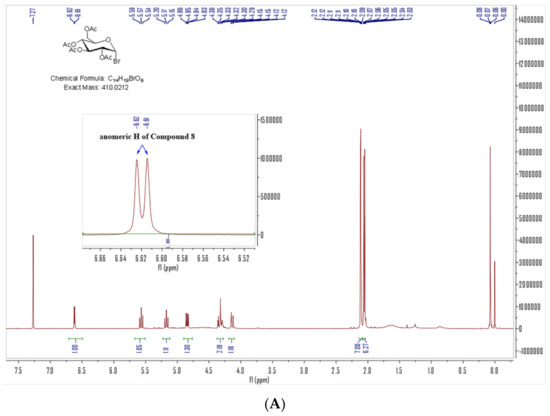
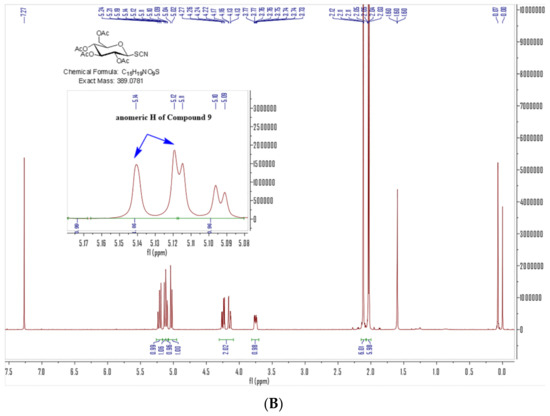
Figure 3.
1H NMR of Compound 8 (A) and 9 (B) (400 MHz, CDCl3) (Blue arrows are anomeric H).
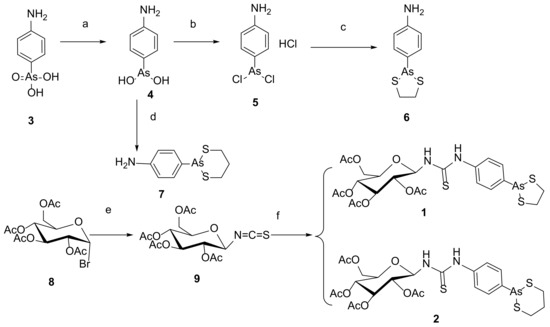
Scheme 1.
Reaction conditions: (a) SO2, KI, HCl, MeOH; (b) HCl(c); (c) ethane-1,2-dithiol, NaHCO3, MeOH, 82%; (d) ethanol, propane-1,3-dithiol, reflux; (e) (i) KSCN, 4 Å MS, (n-Bu)4NBr, CH3CN, r.t. for 4 h; (ii) 8, N2, reflux for 4 h; (f) 6, CH2Cl2, r.t. overnight, 81.9% for 1; 7, CH2Cl2, r.t. overnight, 71.5% for 2.
The synthesized compounds were evaluated for their antiproliferation activities against Hela, HepG2, HCT-116 and 293T via a MTT assay [25]. Hela, HepG2 and HCT-116 were chosen as solid tumor cells. 293T was used as a normal cell. The cytotoxicity results shown in Table 1 indicated that compound 2 with a six-membered ring of 1,3,2-dithiarsinane (As, +III) modified has higher cytotoxicity activities on Hela (11.05 μM vs. 14.4 μM), HepG2 cells (2.81 μM vs. 7.62 μM) and HCT-116 (0.82 μM vs. 1.77 μM) than five-membered ring of 1,3,2-dithiarsolane (As, +III) modified compound 1. Similarly, compound 7 has better anticancer activities than compound 6.

Table 1.
The cytotoxicity of compounds 1 and 2 on 293T, HCT116, Hela and HepG2 cells.
Compounds 1, 2, and 6 showed some selectivity against HCT-116 cells over 293T cells (in Table 1); among them, 293T cells were chosen as normal cells, except for compound 7. The cytotoxicity of compounds 1, 2, 6 and 7 on 293T was generally maintained at the same level. In comparison with non-2,3,4,6-O-tetraacetyl-β-D-glucopyranosyl modification compound 7, the 2,3,4,6-O-tetraacetyl-β-D-glucopyranosyl modification compound 2 showed that the selectivity index (HCT-116 over 293T) was increased by a factor of 1.5. On the contrary, the selectivity index of compound 1 reduced, compared with that of non-2,3,4,6-O-tetraacetyl-β-D-glucopyranosyl modification compound 6 (1.77 vs. 2.18) (Figure 4).
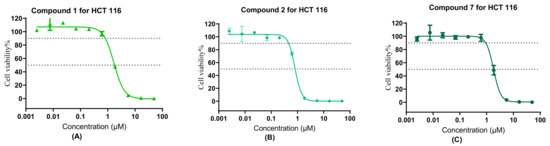
Figure 4.
Cytotoxicity of compound 1 (A), 2 (B) and 7 (C) in HCT-116 cells measured by the MTT assay (means ± SD).
3. Materials and Methods
All the chemical reagents and solvents were purchased from Sinopharm Group Company limited (Shanghai, China). They were used without further purification, unless specified otherwise. 4-Aminophenylarsonic acid was bought from Tokyo Chemical Industry (Shanghai) Development Co., Ltd. (Shanghai, China). All anhydrous reactions were performed under nitrogen atmosphere. Organic phases were dried over anhydrous Na2SO4 and removed under reduced pressure during work-up.
Purities of the intermediates were established by silica gel (200–300 mesh) column chromatography. Thin-layer chromatography (TLC) was carried out by silica gel GF254, both of which were obtained from Qingdao Ocean Chemicals (Qingdao, China). In all experiments, water used was distilled and purified by the Milli-Q system (Millipore, Mississauga, Canada). 1H NMR and 13C NMR spectra of final compounds were recorded on a Bruker Ultra-shield 400 MHz Plus spectrometer (Bruker, Rheinstetten, Germany) using TMS as the internal standard (see Supplementary Materials). All chemical shifts are reported in the standard δ notation of parts per million. High-Resolution Mass Spectra were obtained using Waters UPLC Class I/XevoG2Q-Tof (Waters, Milford, MA, USA).
3.1. Synthesis of Compound 1, 2, 6, 7 and 9
3.1.1. 1-(4-(1,3,2-Dithiarsolan-2-yl) aniline)-2-N-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranose-1-yl)-thiourea (1)
Compound 9 (200 mg, 0.5136 mmol) and compound 6 (134 mg, 0.5136 mmol) were dissolved in 10 mL anhydrous dichloromethane. It was stirred overnight. The reaction was monitored by TLC (thin-layer chromatography (PE:EA = 2:1, Rf = 0.28)). When the reaction was over, the solvent was removed under reduced pressure. The residue was purified by preparation of thin-layer chromatography with eluant (PE:EA = 1:1). The product was obtained as a white solid (273.6 mg, 81.9%). The product was characterized by IR, 1H NMR, 13C NMR and HRMS. IR (KBr): 3434 (NH), 2922 (CH3 or CH2), 2853 (CH3 or CH2), 1748 (C=O),1626, 1530, 1379, 1227 (C-S), 1034 (C-O-C), 825 (Ph-H) cm−1; 1H NMR (400 MHz, chloroform-d) δ: 7.92 (s, 1H, NH-Ph), 7.66 (d, J = 8.0 Hz, 2H, Ph-H), 7.10 (d, J = 8.0 Hz, 2H, Ph-H), 6.56 (d, J1,2 = 8.0 Hz, 1H, anomer-H), 5.74–5.70 (t, J = 8.0 Hz, 1H, CH in glucosamine), 5.31–5.26 (t, J = 12 Hz, 8 Hz,1H, CH in glucosamine), 4.99–4.94 (t, J = 12 Hz, 8 Hz, 1H, CH in glucosamine), 4.86–4.81 (t, J = 12 Hz, 8 Hz, 1H, CH in glucosamine), 4.29–4.24 (dd, J = 12 Hz, 8.0 Hz, 1H, O-CH2), 4.03 (d, J = 12 Hz, 1H, O-CH2), 3.81–3.77 (m, 1H, NH-anomer), 3.37–3.29 (m, 2H, CH2-S), 3.15–3.07 (m, 2H, CH2-S), and 2.01–1.94 (m, 12H, CH3C=O). 13C NMR (101 MHz, chloroform-d) δ: 181.1(C=S), 170.0(C=O), 169.6(C=O), 168.8 (C=O), 168.6 (C=O), 142.8 (NH-Ph), 134.9 (AS-Ph), 131.5 (CH in Ph), 123.8 (CH in Ph), 82.3 (anomeric CH), 72.7 (CH in glucoamine), 71.6 (CH in glucoamine), 69.5 (CH in glucoamine), 67.2 (CH in glucoamine), 60.6 (CH2-OAc), 41.0 (CH2-As), 19.8 (CH3 in acetyl), 19.7 (CH3 in acetyl), and 19.6 (CH3 in acetyl); HRMS: [M + H]+: C23H30AsN2O9S3, calculated: 649.0324, and found: 649.0306.
3.1.2. 1-(4-(1,3,2-Dithiarsinan-2-yl) aniline)-2-N-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranose-1-yl)-thiourea (2)
Compound 9 (200 mg, 0.5136 mmol) and compound 7 (140.4 mg, 0.5136 mmol) were dissolved in 10 mL anhydrous dichloromethane. This was stirred overnight. The reaction was monitored by TLC (thin-layer chromatography (PE:EA = 2:1, Rf = 0.48)). When the reaction was over, the solvent was removed under reduced pressure. The residue was purified by preparation of thin-layer chromatography with eluant (PE:EA = 1:1). The product was obtained as a white solid (243 mg, 71.5%). The product was characterized by IR, 1H NMR, 13C NMR and HRMS. IR (KBr): 3493 (NH), 2924 (CH3 or CH2), 1746 (C=O), 1631, 1539, 1378, 1214 (C-S), 1043 (C-O-C), 801 (Ph-H) cm−1; 1H NMR (400 MHz, chloroform-d) δ: 7.99 (s, 1H, NH-Ph), 7.93 (d, J = 8.0 Hz, 2H, Ph-H), 7.24 (d, J = 8.0 Hz, 2H, Ph-H), 6.69 (d, J1,2 = 8.0 Hz, 1H, anomer-H), 5.75–5.70 (t, J = 12 Hz, 8.0 Hz, 1H, CH in glucoamine), 5.33–5.28 (t, J = 12 Hz, 8.0 Hz, 1H, CH in glucoamine), 5.01–4.96 (t, J = 12 Hz, 8.0 Hz, 1H, CH in glucoamine), 4.89–4.84 (t, J = 12 Hz, 8.0 Hz, 1H, CH in glucoamine), 4.32–4.28 (dd, J = 12 Hz, 4.0 Hz, 1H, O-CH2), 4.08–4.02 (m, 1H, O-CH2), 3.83–3.79 (m, 1H, NH-anomer), 2.84–2.75 (m, 2H, CH2-S), 2.67–2.61 (m, 2H, CH2-S), 2.17–2.07 (m, 1H, H-methylene), 2.01–1.94 (m, 12H, CH3C=O), 1.91–1.87 (m, 1H, H-methylene). 13C NMR (101 MHz, chloroform-d) δ: 181.1 (C=S), 170.0 (C=O), 169.6 (C=O), 168.8 (C=O), 168.6 (C=O), 137.8 (NH-Ph), 134.9 (AS-Ph), 133.3 (CH in Ph), 124.6 (CH in Ph), 82.3 (anomeric CH), 72.7 (CH in glucoamine), 71.6 (CH in glucoamine), 69.5 (CH in glucoamine), 67.2 (CH in glucoamine), 60.6 (CH2-OAc), 27.1 (CH2-As), 24.8 (CH2-As), 24.7 (CH2), 19.8 (CH3 in acetyl), 19.7 (CH3 in acetyl), 19.6 (CH3 in acetyl); HRMS: [M + H]+: C24H32AsN2O9S3, calculated: 663.0480, and found: 663.0461.
3.1.3. 4-(1,3,2-Dithiarsolan-2-yl) aniline (6) [24,26]
Compound 6 was synthesized with a yield of 87% as described in previous literature. It was characterized by IR, 1H NMR, 13C NMR and HRMS. IR (KBr): 3434 (NH), 3410 (NH), 2959 (CH3 or CH2), 2926 (CH3 or CH2), 2855 (CH3 or CH2), 1618 (Ph), 1579 (Ph), 1408 (Ph), 1276 (C-S), 1060 (C-O-C), 812 (C-H in Ph) cm−1; 1H NMR (400 MHz, CDCl3) δ: 7.45–7.43 (m, 2H, aromatic-H near As), 6.69–6.67 (m, 2H, aromatic-H near NH2), 3.80 (br, 2H, NH2), 3.40–3.33 (m, 2H, CH2), 3.27–3.21 (m, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ: 147.6 (NH2-C-Ph), 132.1 (CH of Ph near As), 131.3 (As-C-Ph), 114.9 (CH of Ph near NH2), 41.6 (CH2), 30.91 (CH2).
3.1.4. 4-(1,3,2-Dithiarsinan-2-yl) aniline 7 [18]
Compound 4 (1 g, 5.4654 mmol) and propane-1,3-dithiol (615 µL mg, 6.1376 mmol) were dissolved in ethanol (15 mL). The solution was refluxed under N2 for 5 h. It was monitored by TLC (PE: EA = 4:1, Rf = 0.45). The reaction was cooled to room temperature. The solution was evaporated under reduced pressure to concentrated residue. Then, it was transferred to a refrigerator overnight. It was filtered. The filtered cake was washed with 5 mL cold ethanol twice. Compound 7 was obtained as an off-white solid (1.1761 g, 78.8%). It was characterized by IR, 1H NMR, 13C NMR and HRMS. IR (KBr): 3435 (NH), 2924 (CH2), 2878 (CH2), 2853 (CH2), 1620 (C=C in Ph), 1588 (C=C in Ph), 1403 (C=C in Ph), 1295 (C-S), 1266 (C-S), 1076 (C-O-C), 812 (C-H in Ph) cm−1; 1H NMR (400 MHz, CDCl3) δ: 7.66–7.64 (d, 2H, J = 8.0 Hz, CH at Ph near As), 6.79–6.77 (d, 2H, J = 8.0 Hz, CH at Ph near NH2), 3.85 (br, 2H, NH2), 2.93–2.87 (m, 2H, As-CH2), 2.74–2.68 (m, 2H, As-CH2), 2.19–2.10 (m, 1H, H of CH2), 1.97–1.90 (m, 1H, H of CH2). 13C NMR (101 MHz, CDCl3) δ: 146.5 (NH2-C-Ph), 132.8 (CH of As-Ph), 124.7 (NH2-CH-Ph), 114.6 (As-C-Ph), 27.7 (As-CH2), 25.4 (As-CH2).
3.1.5. 2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate (9) [30]
Potassium thiocyanate (473 mg, 4.863 mmol), a 4Å molecular sieve (3.48 g) and tetrabutylammonium bromide (784 mg, 2.432 mmol) were added to 30 mL anhydrous acetonitrile. It was stirred under N2 at room temperature for 3 h. 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide 8 (1000 mg, 2.432 mmol) was added to the solution. The reaction was heated to reflux under N2 for 4 h. It was monitored by thin-layer chromatography with eluant (PE:EA = 3:1). When the reaction was over, the solution was cooled to the room temperature. The solvent was removed under reduced pressure. The residue was purified by silica gel chromatography with eluant (PE:EA = 3:1). The product was obtained as a off-white solid with a yield of 76.3% (690.8 mg). It was characterized by IR, 1H NMR, 13C NMR and HRMS. IR (KBr): 3434, 2985 (CH3), 2940 (CH3), 2883 (CH3), 2113 (SCN), 1746 (C=O), 1626, 1383 (C-H in CH3), 1237 (C-S), 1219 (C-S), 1061 (C-O-C), 1030 (C-O-C), 911 cm−1; 1H NMR (400 MHz, CDCl3) δ: 5.24–5.19 (t, J = 12.0 Hz, 8.0 Hz, 1H, CH in glucoamine), 5.14–5.12 (d, J1,2 = 8.0 Hz, 1H, anomer-H), 5.11–5.09 (t, J = 4 Hz, 1H, CH in glucoamine), 5.03 (d, J1,2 = 8 Hz, 1H, CH in glucoamine), 4.27–4.22 (m, 2H, CH2-O), 3.77–3.73 (m, 1H, CH in glucoamine), 2.12–2.11 (m, 6H, CH3 in acetyl), 2.05–2.03 (m, 6H, CH3 in acetyl).
3.2. Cell Lines and Cell Culture [25]
HeLa and HepG2 cells were obtained from Stem Cell Bank, Chinese Academy of Sciences. The human colon cancer HCT-116 cells and human embryonic kidney cells 293T were purchased from the American Type Culture Collection (ATCC). Cells were cultured in high-glucose DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% (v/v) FBS (Gibco, Grand Island, NE, USA) and 1% (v/v) penicillin–streptomycin (PS) (Gibco, Grand Island, NE, USA), and maintained at 37 °C under 5% CO2 humidified atmosphere. Only cells in logarithmic phase were used in all following experiments.
3.3. MTT Assay [25]
The cytotoxicity of compounds 1, 2, 6 and 7 in Hela, HepG2, HCT 116 and 293T cells was evaluated by MTT assay. Cells were seeded onto a 96-well plate (Corning, Corning, NY, USA) with a density of 3000 cells/well for 24 h in high-glucose DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% (v/v) FBS (Gibco, Grand Island, NE, USA) and 1% (v/v) penicillin–streptomycin (PS) (Gibco, Grand Island, NE, USA), and then treated with tested compounds at various concentrations for 72 h. MTT solution was added to each well for 4 h, and the absorbance was measured at 495 nm by using Varioskan Flash Multimode Reader (Thermo, Waltham, MA, USA). The values of IC50 were calculated by GraphPad Prism Software 9.0.
4. Conclusions
In this investigation, to avoid the form mixture of α/β anomers, the strategy of 2-acetyl’s neighboring group participation was used to lock the β anomeric configuration of 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate 9 based on 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide through a five-membered glycosyl oxocarbenium ion transition state. In the following steps, compounds 6 and 7 reacted with 9 involving a nucleophilic attack to form our target compounds 1 and 2 at room temperature, while their anomeric configurations were still β. The results of 1H NMR identified these cases. 1-(4-(1,3,2-dithiarsinan-2-yl)aniline)-2-N-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranos-1-yl)-thiourea 2 can increase the selectivity of human colon cancer cells HCT-116 (0.82 ± 0.06 μM vs. 1.82 ± 0.07μM) to human embryonic kidney 293T cells (1.38 ± 0.01 μM vs. 1.22 ± 0.06 μM) from 0.67 to 1.68, while the cytotoxicity towards normal cells (293T) was still maintained, which was less than what we expected. These results suggested a new method for the discovery and development of arsenic-containing compounds as novel chemotherapeutics for the treatment of solid tumors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29122850/s1, Figure S1: 1H NMR of Compound 8; Figure S2: 1H NMR of Compound 9; Figure S3: 1H NMR of Compound 6; Figure S4: 13C NMR of Compound 6; Figure S5: 1H NMR of Compound 7; Figure S6: 13C NMR of Compound 7; Figure S7: 1H NMR of Compound 1; Figure S8: 13C NMR of Compound 1; Figure S9: HRMS of Compound 1; Figure S10: 1H NMR of Compound 2; Figure S11: 13C NMR of Compound 2; Figure S12: HRMS of Compound 2; Figure S13: IR of Compound 1; Figure S14: IR of Compound 2; Figure S15: IR of Compound 6; Figure S16: IR of Compound 7; Figure S17: IR of Compound 9; Figure S18: IC50 of Compound 1, 2 and 7 for HCT-116; Figure S19: IC50 of Compound 1, 2, 6 and 7 for 293T; Figure S20: IC50 of Compound 6 for Hela and HepG2; Figure S21: IC50 of Compound 7 for Hela and HepG2; Figure S22: IC50 of Compound 1 and 2 for Hela; Figure S23: IC50 of Compound 1 and 2 for HepG2.
Author Contributions
Conceptualization, B.F.; methodology, B.F. and G.L.; formal analysis, B.F.; investigation, B.F., Y.W. (Yufeng Wang), G.L., W.L., X.H. and H.S.; resources, B.F. and C.Q.; data curation, B.F. and Y.W. (Yingsha Wang); writing—original draft preparation, B.F.; writing—review and editing, B.F.; project administration, B.F. and C.Q.; funding acquisition, B.F. and C.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Xiaogan, China (XGKJ2019010046), the Natural Science Foundation of Hubei Province, China (2014CFB570), the Natural Science Foundation of China (21807029) and the Undergraduate Innovation and Entrepreneurship Foundation of Hubei Province, China (S202310528032).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
Thanks to Jing Huang and her group for her kind help in MTT assay. Now she is working at the State Key Laboratory for Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, School of Biomedical Sciences, Hunan University, Changsha, 410082, P. R. China.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Waxman, S.; Anderson, K.C. History of the Development of Arsenic Derivatives in Cancer Therapy. Oncologist 2001, 6, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Swindell, E.P.; Hankins, P.L.; Chen, H.; Miodragović, Đ.U.; O’Halloran, T.V. Anticancer activity of small-molecule and nanoparticulate arsenic (III) complexes. Inorg. Chem. 2013, 52, 12292–12304. [Google Scholar] [CrossRef] [PubMed]
- Dilda, P.J.; Hogg, P.J. Arsenical-based cancer drugs. Cancer Treat. Rev. 2007, 33, 542–564. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Yan, X.-J.; Zhou, Z.-R.; Yang, F.-F.; Wu, Z.-Y.; Sun, H.-B.; Liang, W.-X.; Song, A.-X.; Lallemand-Breitenbach, V.; Jeanne, M. Arsenic Trioxide Controls the Fate of the PML-RARα Oncoprotein by Directly Binding PML. Science 2010, 328, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Gore, S.D. Arsenic trioxide—An old drug rediscovered. Blood Rev. 2010, 24, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lallemand-Breitenbach, V. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission. Oncogene 2001, 20, 7257–7265. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Tallman, M.S.; Gartenhaus, R.B. The potential of arsenic trioxide in the treatment of malignant disease: Past, present, and future. Leuk. Res. 2004, 28, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shen, X.; Zhi, F.; Wen, Z.; Gao, Y.; Xu, J.; Yang, B.; Bai, Y. An overview of arsenic trioxide-involved combined treatment algorithms for leukemia: Basic concepts and clinical implications. Cell Death Discov. 2023, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, J.-L.; Liang, Y.; Tang, Y.-G.; Song, H.-X.; Wu, L.-L.; Xing, Y.-F.; Yan, N.; Li, Y.-T.; Wang, Z.-Y.; et al. Arsenic Trioxide Rescues Structural p53 Mutations through a Cryptic Allosteric Site. Cancer Cell. 2021, 9, 225–239. [Google Scholar] [CrossRef]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Yang, L.; Ling, J.-Y.; Czajkowsky, D.M.; Wang, J.-F.; Zhang, X.-W.; Zhou, Y.-M.; Ge, F.; Yang, M.-K.; Xiong, Q. Systematic identification of arsenic-binding proteins reveals that hexokinase-2 is inhibited by arsenic. Proc. Natl. Acad. Sci. USA 2015, 112, 15084–15089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, F.; Shim, J.-Y.; Kirk, K.L.; Anderson, D.E.; Chen, X. Identification of arsenic-binding proteins in human breast cancer cells. Cancer Lett. 2007, 255, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Hikita, E.; Arai, M.; Tanaka, S.; Onda, K.; Utsumi, H.; Yuan, B.; Toyoda, H.; Hirano, T. Effects of inorganic and organic arsenic compounds on growth and apoptosis of human T-lymphoblastoid leukemia cells. Anticancer Res. 2011, 31, 4169–4178. [Google Scholar] [PubMed]
- Wimmer, N.; Robinson, J.; Gopisetty-Venkata, A.N.; Roberts-Thomson, S.J.; Monteith, G.R.; Toth, I. Novel Glyco-lipid-arsenicals (III) with Anti-proliferative Effects on MCF-7 Human Breast Cancer Cells. Med. Chem. 2006, 2, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Y.; Chen, X.-Y.; Liu, Y.-J.; Zhong, H.-M.; Jiang, F.-L.; Liu, Y. Oxidative stress-mediated intrinsic apoptosis in human promyelocytic leukemia HL-60 cells induced by organic arsenicals. Sci. Rep. 2016, 6, 29865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tong, Y.; Zhang, X.; Pan, M.; Chen, S. Arsenic sulfide inhibits cell migration and invasion of gastric cancer in vitro and in vivo. Drug Des. Dev. Ther. 2015, 9, 5851–5862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, W.; Tong, Y.; Zhang, X.; Pan, M.; Chen, S. Study of Arsenic Sulfide in Solid Tumor Cells Reveals Regulation of Nuclear Factors of Activated T-cells by PML and p53. Sci. Rep. 2016, 6, 19793. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Wang, X.; Li, Y.; Hu, J.; Lu, D.; Li, W.; Zheng, K.; Qin, C. Carbohydrate-conjugated 4-(1,3,2-dithiarsolan-2-yl) aniline as a cytotoxic agent against colorectal cancer. RSC Adv. 2018, 8, 40760. [Google Scholar] [CrossRef]
- Lee, J.S.; Adler, L.; Karathia, H.; Carmel, N.; Rabinovich, S.; Auslander, N.; Keshet, R.; Stettner, N.; Silberman, A.; Agemy, L.; et al. Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell 2018, 174, 1559–1570. [Google Scholar] [CrossRef]
- Sarkar, K.; Dastidar, P. Rational Approach Towards Designing Metallogels From a Urea-Functionalized Pyridyl Dicarboxylate: Anti-inflammatory, Anticancer, and Drug Delivery. Chem. Asian J. 2019, 14, 194–204. [Google Scholar] [CrossRef]
- Kirishnamaline, G.; Magdaline, J.D.; Chithambarathanu, T.; Aruldhas, D.; Anuf, A.R. Theoretical investigation of structure, anticancer activity and molecular docking of thiourea derivatives. J. Mol. Struct. 2021, 1225, 129118. [Google Scholar] [CrossRef]
- Wael Abdelgayed, A.A.; Amira Atef, G.; Asmaa, K.M. N-Naphthoyl Thiourea Derivatives: An Efficient Ultrasonic-Assisted Synthesis, Reaction, and In Vitro Anticancer Evaluations. ACS Omega 2022, 7, 6210–6222. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, S.P.; Noolvi, M.N.; More, U.A.; Chakraborty, S.; Dash, A.; Aminabhavi, T.M.; Narayan, K.P.; Sutariya, V. Synthesis and Anticancer Activity of Thiadiazole Containing Thiourea, Benzothiazole and Imidazo[2,1-b][1,3,4]thiadiazole Scaffolds. Med. Chem. 2021, 17, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Lv, L.; Xia, C.; Zheng, K.; Zhu, L.; Li, W.; Yan, Y.; Qin, C. Effects of Carbohydrates on the Toxicity of p-Aminophenyl Arsenoxide against S. cerevisiae. J. Med. Chem. Toxicol. 2017, 2, 28–33. [Google Scholar]
- Fu, B.; Li, Y.; Peng, S.; Wang, X.; Hu, J.; Lv, L.; Xia, C.; Lu, D.; Qin, C. Synthesis and pharmacological characterization of glucopyranosyl-conjugated benzyl derivatives as novel selective cytotoxic agents against colon cancer. R. Soc. Open Sci. 2021, 8, 201642. [Google Scholar] [CrossRef] [PubMed]
- Nukada, T.; Berces, A.; Zgierski, M.Z.; Whitfield, D.M. Exploring the Mechanism of Neighboring Group Assisted Glycosylation Reactions. J. Am. Chem. Soc. 1998, 120, 13291–13295. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Yao, J.; Zhang, B.; Peng, S.; Ma, H.; Song, Y.; Fang, J. Dithiaarsanes Induce Oxidative Stress-Mediated Apoptosis in HL-60 Cells by Selectively Targeting Thioredoxin Reductase. J. Med. Chem. 2014, 57, 5203–5211. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, J.; Xu, Q.; Shi, D.; Yao, X.; Fang, J. Structural Modification of Aminophenylarsenoxides Generates Candidates for Leukemia Treatment via Thioredoxin Reductase Inhibition. J. Med. Chem. 2021, 64, 16132–16146. [Google Scholar] [CrossRef]
- Stevenson, K.J.; Hale, G.; Perham, R.N. Inhibition of pyruvate dehydrogenase multienzyme complex from Escherichia coli with mono- and bifunctional arsenoxides. Biochemistry 1978, 17, 2189–2192. [Google Scholar] [CrossRef]
- Camarasa, M.J.; Fernandez-Resa, P.; Garcia-López, M.T.; Heras, G.D.H.; Mendez-Castrillon, P.P.; Felix, A.S. A New Procedure for the Synthesis of Glycosyl Isothiocyanates. Synthesis 1984, 15, 509–510. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Mag. Res. A 2003, 19, 1–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).