Efficient Trifluoromethylation of Halogenated Hydrocarbons Using Novel [(bpy)Cu(O2CCF2SO2F)2] Reagent
Abstract
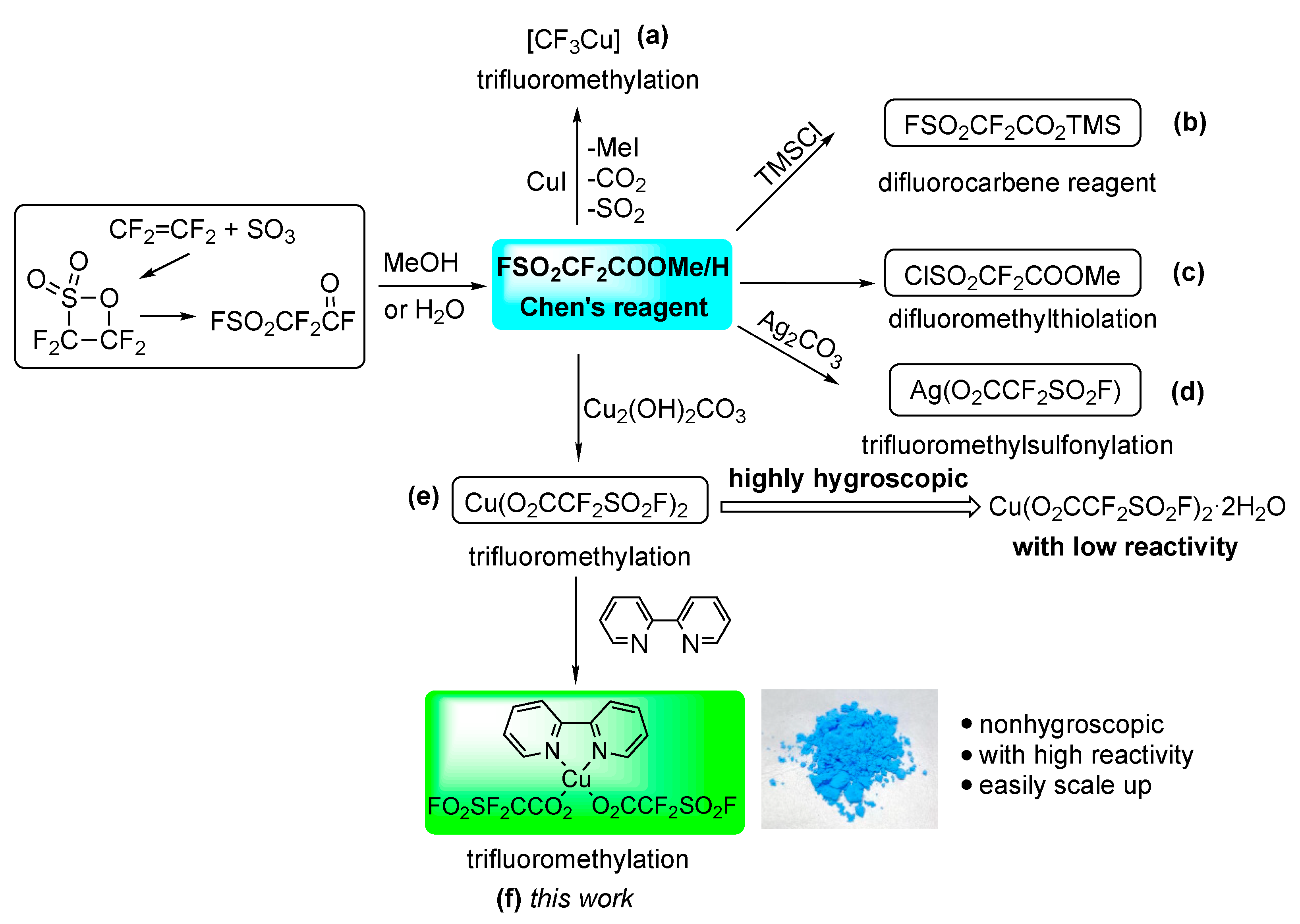
1. Introduction
2. Results
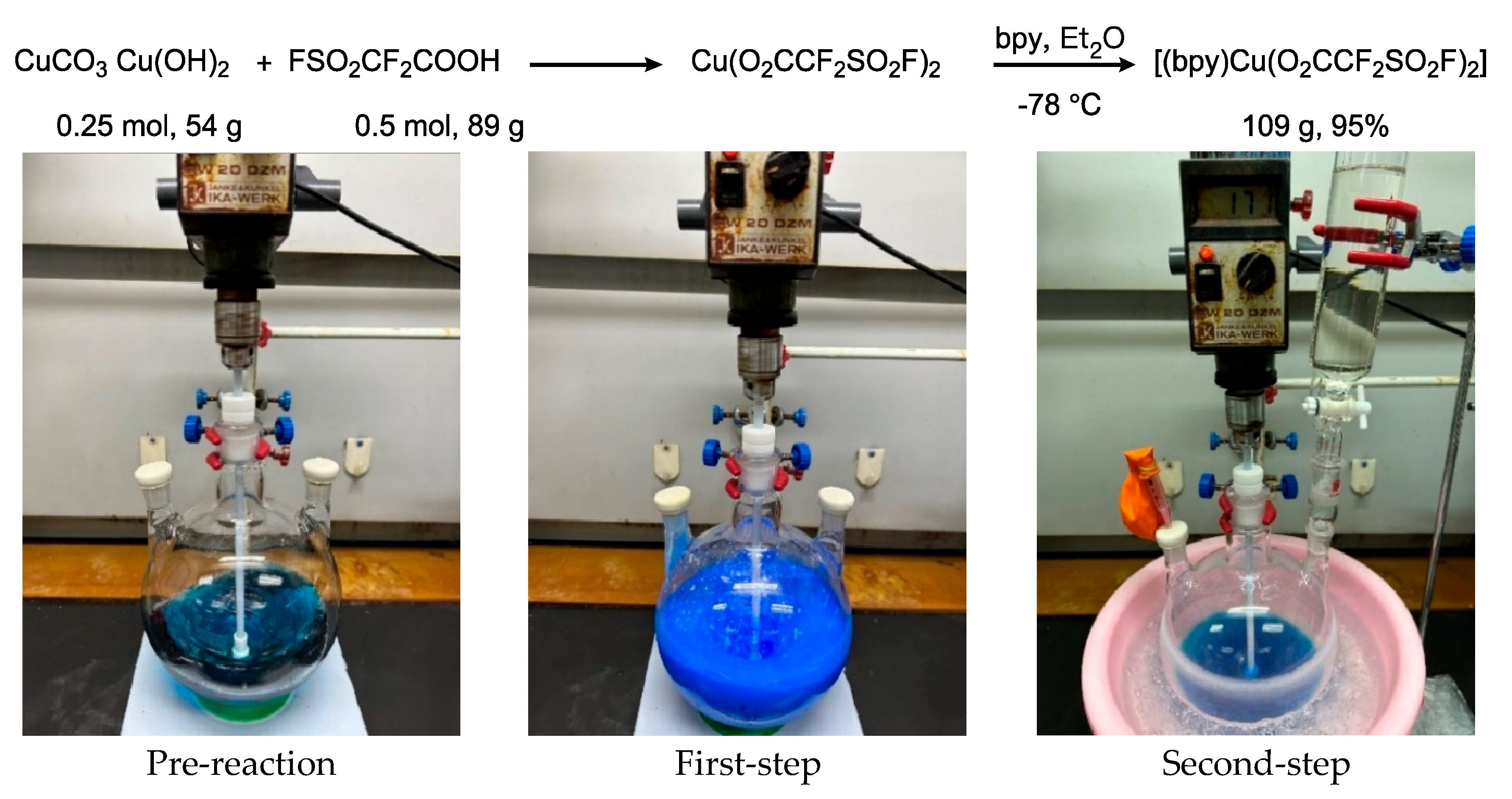
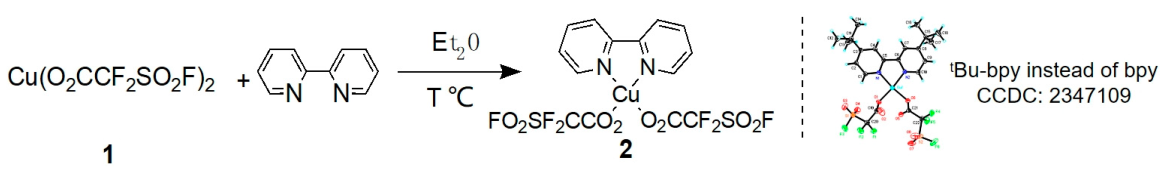
2.1. Synthesis of Fluorosulfonyl Difluoroacetic Acid Copper Complex [(bpy)Cu(O2CCF2SO2F)2]
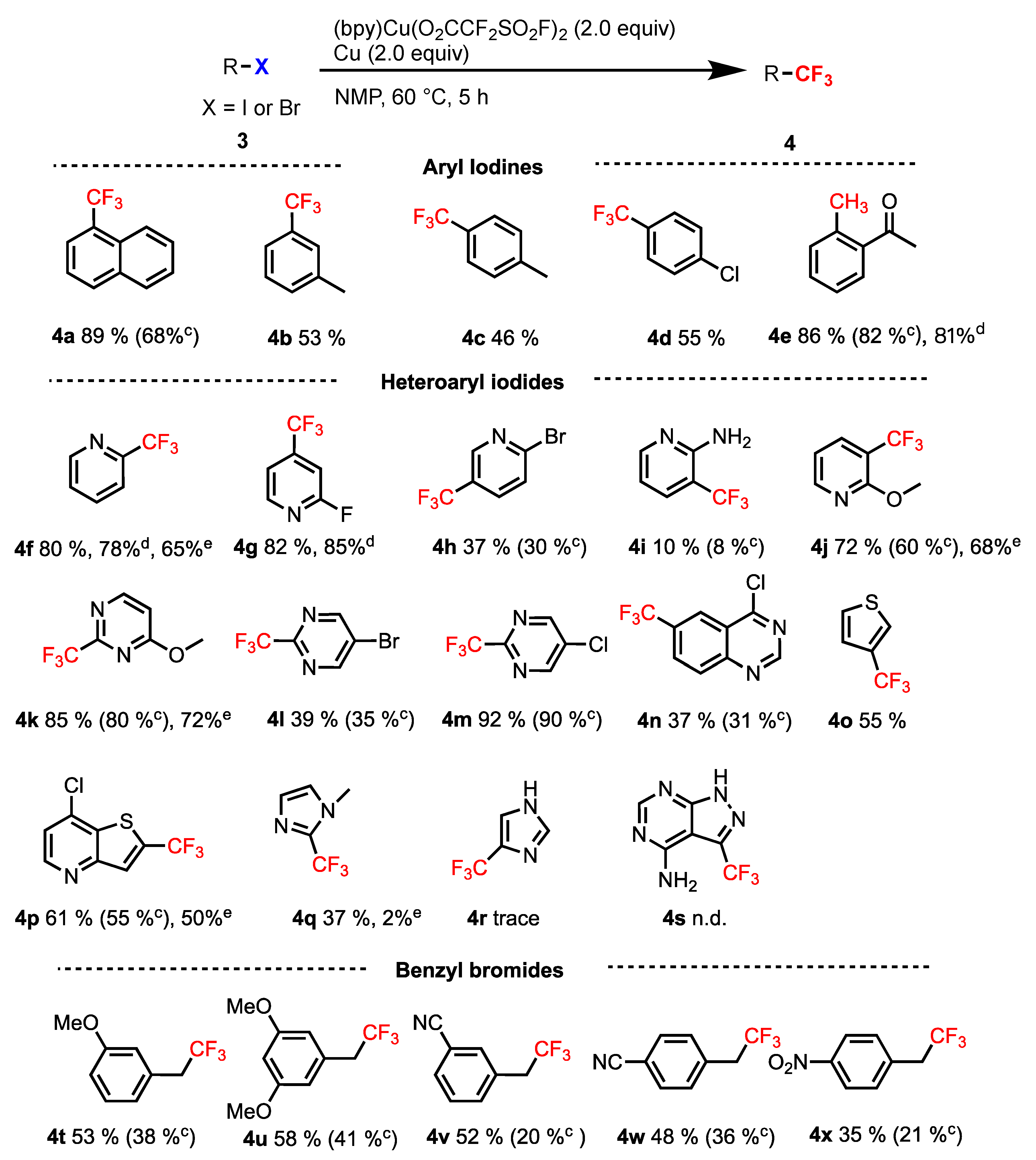
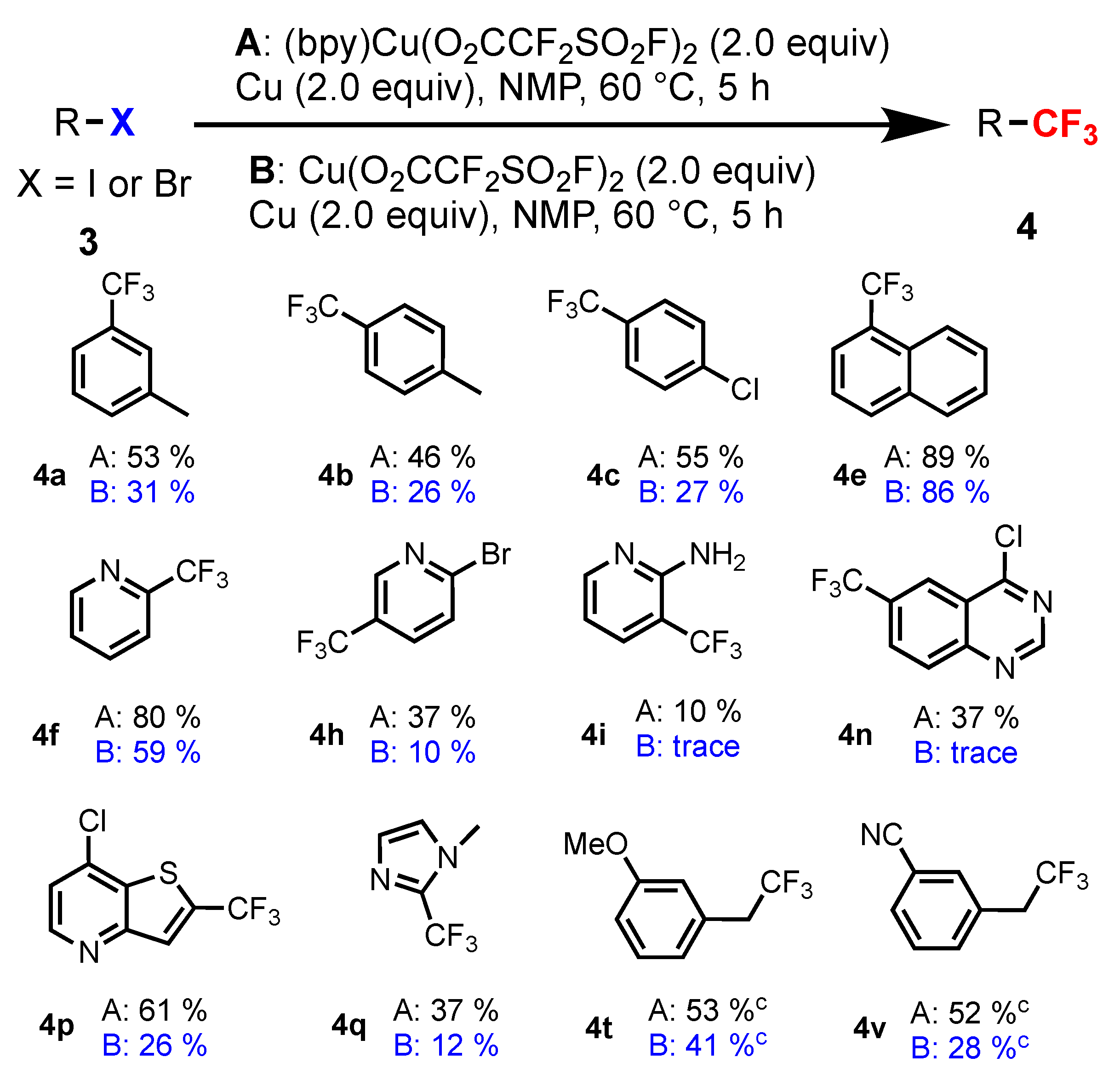
2.2. [(bpy)Cu(O2CCF2SO2F)2] as a Trifluoromethylating Reagent
3. Materials and Methods
3.1. General Information of Materials and Instruments
3.2. General Procedure for the Synthesis of [(bpy)Cu(O2CF2SO2F)2]
3.3. General Procedure for the Synthesis of Compounds (4a–x)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, L.; Qing, F.-L. Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl) trimethylsilane as a nucleophilic CF3 source. Acc. Chem. Res. 2014, 47, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic trifluoromethylation and beyond. Chem. Rev. 2015, 115, 683–730. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, R.P.; Babu, B.P. Progress in electrochemical trifluoromethylation reactions. Adv. Synth. Catal. 2020, 362, 5219–5237. [Google Scholar] [CrossRef]
- Xiao, H.W.; Zhang, Z.Z.; Fang, Y.W.; Zhu, L.; Li, C.Z. Radical trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–6319. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Maji, S.; Pal, T.; Sinha, S.K.; Maiti, D. Recent advances in transition metal-mediated trifluoromethylation reactions. Chem. Commun. 2022, 58, 10442–10468. [Google Scholar] [CrossRef] [PubMed]
- Sumii, Y.; Shibata, N. Current State of Microflow Trifluoromethylation Reactions. Chem. Rec. 2023, 23, e202300117. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Banerjee, D. Recent advances on non-precious metal-catalysed fluorination, difluoromethylation, trifluoromethylation, and perfluoroalkylation of N-heteroarenes. Org. Biomol. Chem. 2023, 21, 9298–9315. [Google Scholar]
- Shaw, R.; Sihag, N.; Bhartiya, H.; Yadav, M.R. Harnessing photocatalytic and electrochemical approaches for C–H bond trifluoromethylation and fluoroalkylation. Org. Chem. Front. 2024, 11, 954–1014. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemica l Industry. iScience 2020, 23, 101467–101490. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of organofluorine compounds to pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, T.; Spinnato, D.; Moon, H.W.; Leutzsch, M.; Cornella, J. Bi-Catalyzed Trifluoromethylation of C(sp2)-H Bonds under Light. J. Am. Chem. Soc. 2023, 145, 25538–25544. [Google Scholar] [CrossRef] [PubMed]
- Dang-Nguyen, A.; Legaspi, K.C.; McCarty, C.T.; Smith, D.K.; Gustafson, J. A Light-Promoted Innate Trifluoromethylation of Pyridones and Related N-Heteroarenes. Org. Lett. 2023, 25, 4898–4902. [Google Scholar] [CrossRef]
- Wang, W.X.; Chamoreau, L.M.; Izzet, G.; Proust, A.; Orio, M.; Blanchard, S. Multi-Electron Visible Light Photoaccumulation on a Dipyridylamine Copper(II)-Polyoxometalate Conjugate Applied to Photocatalytic Generation of CF3 Radicals. J. Am. Chem. Soc. 2023, 145, 12136–12147. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Yang, H.; Zhan, B.; Zhang, X. Photoinduced Bi-catalyzed C–H trifluoromethylation of (hetero)arenes. Chem. Catal. 2024, 4, 100877. [Google Scholar] [CrossRef]
- Meucci, E.A.; Nguyen, S.N.; Camasso, N.M.; Chong, E.; Ariafard, A.; Canty, A.J.; Sanford, M.S. Nickel(IV)-Catalyzed C-H Trifluoromethylation of (Hetero)arenes. J. Am. Chem. Soc. 2019, 141, 12872–12879. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Häring, A.P.; Berger, F.; Zhang, L.; Ritter, T. Trifluoromethyl Thianthrenium Triflate: A Readily Available Trifluoromethylating Reagent with Formal CF3+, CF3•, and CF3– Reactivity. J. Am. Chem. Soc. 2021, 143, 7623–7628. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, J.; Jin, Y.X.; Leng, X.B.; Shen, Q.L. Mechanistic Insight into Copper-Mediated Trifluoromethylation of Aryl Halides: The Role of CuI. J. Am. Chem. Soc. 2021, 143, 14367–14378. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Lee, G.S.; Park, B.; Kim, D.; Hong, S.H. Direct C(sp3)-H Trifluoromethylation of Unactivated Alkanes Enabled by Multifunctional Trifluoromethyl Copper Complexes. Angew. Chem. Int. Ed. 2021, 60, 5467–5474. [Google Scholar] [CrossRef]
- Zhang, H.R.; Feng, C.C.; Chen, N.; Zhang, S.L. Direct Arene Trifluoromethylation Enabled by a High-Valent CuIII-CF3 Compound. Angew. Chem. Int. Ed. 2022, 61, e202209029. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Hou, T.T.; Zhang, L.M.; Wang, X.; Hou, J.L.; Liu, Y.P.; Han, G.F.; Song, Y.G. Iron-Catalyzed Trifluoromethylation of Indole-Tethered Alkene Enables Synthesis of CF3-Containing Spiroindolenines and Tetrahydrocarbazoles. Eur. J. Org. Chem. 2023, 26, e202300253. [Google Scholar] [CrossRef]
- Seong, C.M.; Roberts, C.C. Redox-Neutral Decarboxylative and Desulfonylative C(sp3) Trifluoromethylation: Method Development and Mechanistic Inquiry. J. Org. Chem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-Q.; Yang, C.-J.; Li, Z.-L.; Gu, Q.-S.; Liu, L.; Liu, X.-Y. Copper-catalyzed radical trifluoromethylalkynylation of unactivated alkenes with terminal alkynes. J. Fluor. Chem. 2023, 267, 110107. [Google Scholar] [CrossRef]
- Ge, C.; Qiao, L.; Zhang, Y.; Sun, K.; An, J.; Peng, M.; Chen, X.; Qu, L.; Yu, B. Metal-Free Electrochemical Trifluoromethylation of Imidazole-Fused Heterocycles with Trifluoromethyl Thianthrenium Triflate. Chin. J. Chem. 2024, 42, 1679–1685. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, C.; Yu, T.; Xu, C.; Wang, M. O-Trifluoromethylation of Carboxylic Acids via the Formation and Activation of Acyloxy(phenyl)trifluoromethyl-λ3-Iodanes. Angew. Chem. Int. Ed. 2024, 63, e202400449. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Jose, J.; Loudet, A.; Pérez-Bolívar, C.; Anzenbacher, P., Jr.; Burgess, K. Encapsulated Energy-Transfer Cassettes with Extremely Well Resolved Fluorescent Outputs. J. Am. Chem. Soc. 2011, 133, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, A.I.; Lishchynskyi, A.; Grushin, V.V. Mechanism of Trifluoromethylation of Aryl Halides with CuCF3 and the Ortho Effect. J. Am. Chem. Soc. 2014, 136, 13410–13425. [Google Scholar] [CrossRef]
- Lishchynskyi, A.; Novikov, M.A.; Martin, E.; Escudero-Adán, E.C.; Novák, P.; Grushin, V.V. Trifluoromethylation of Aryl and Heteroaryl Halides with Fluoroform-Derived CuCF3: Scope, Limitations, and Mechanistic Features. J. Org. Chem. 2013, 78, 11126–11146. [Google Scholar] [CrossRef] [PubMed]
- Tomashenko, O.A.; Escudero-Adán, E.C.; Martínez Belmonte, M.; Grushin, V.V. Simple, Stable, and Easily Accessible Well-Defined CuCF3 Aromatic Trifluoromethylating Agents. Angew. Chem. Int. Ed. 2011, 50, 7655–7659. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Wu, S.-W. Methyl fluorosulphonyldifluoroacetate; a new trifluoromethylating agent. J. Chem. Soc. Chem. Commun. 1989, 11, 705–706. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Chen, Q.-Y.; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Difluoromethylation and trifluoromethylation reagents derived from tetrafluoroethane β-sultone: Synthesis, reactivity and applications. Coord. Chem. Rev. 2014, 261, 28–72. [Google Scholar] [CrossRef]
- Clarke, S.L.; McGlacken, G.P. Methyl fluorosulfonyldifluoroacetate (MFSDA): An Underutilised Reagent for Trifluoromethylation. Chem. Eur. J. 2017, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Hu, J. Chen’s reagent: A versatile reagent for trifluoromethylation, difluoromethylenation, and difluoroalkylation in organic synthesis. Chin. J. Chem. 2020, 38, 202–212. [Google Scholar] [CrossRef]
- Tian, F.; Kruger, V.; Bautista, O.; Duan, J.-X.; Li, A.-R.; Dolbier, W.R.; Chen, Q.-Y. A Novel and Highly Efficient Synthesis of gem-Difluorocyclopropanes. Org. Lett. 2000, 2, 563–564. [Google Scholar] [CrossRef]
- Zheng, L.; Qiu, X.; Xiao, Z.; Ma, X.; Gao, T.; Zhou, X.; Wang, Y.; Guo, Y.; Chen, Q.-Y.; Liu, C. Deoxygenation of ClSO2CF2COOMe with Triphenylphosphine for the Metal-Free Direct Electrophilic Difluoroalkylthiolation of Various Heterocycles. J. Org. Chem. 2023, 88, 7518–7524. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, H.; Xiao, Z.; Chen, Q.-Y.; Liu, C. Trifluoromethylation of haloarenes with a new trifluoro-methylating reagent Cu(O2CCF2SO2F)2. RSC Adv. 2016, 6, 50250–50254. [Google Scholar] [CrossRef]
- Le, B.; Wu, H.; Hu, X.; Zhou, X.; Guo, Y.; Chen, Q.-Y.; Liu, C. Rapid Synthesis of Acyl Fluorides from Carboxylic Acids with Cu(O2CCF2SO2F)2. Tetrahedron Lett. 2020, 61, 152624. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Guo, Y.; Xiao, J.C.; Chen, Q.Y.; Liu, C. Trifluoromethylfluorosulfonylation of Unactivated Alkenes Using Readily Available Ag (O2CCF2SO2F) and N-Fluorobenzenesulfonimide. Angew. Chem. Int. Ed. 2017, 56, 15432–15435. [Google Scholar] [CrossRef]
- Chen, M.; Buchwald, S.L. Rapid and Efficient Trifluoromethylation of Aromatic and Heteroaromatic Compounds Using Potassium Trifluoroacetate Enabled by a Flow System. Angew. Chem. Int. Ed. 2013, 52, 11628–11631. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hammond, G.B.; Xu, B. Cobalt-Catalyzed Aerobic Oxidative Cleavage of Alkyl Aldehydes: Synthesis of Ketones, Esters, Amides, and α-Ketoamides. Chem. Eur. J. 2021, 27, 9737–9741. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, X.; Qing, F. Oxidative Coupling Reactions of Arylboronic Acids and Fluoroform-Derived AgCF3. Chin. J. Org. Chem. 2020, 40, 3426–3430. [Google Scholar] [CrossRef]
- Dunn, A.D. Nucleophilic displacement in 2-chloro(trifluoromethyl)pyridines with amines and ammonia. J. Fluorine Chem. 1999, 93, 153–157. [Google Scholar] [CrossRef]
- Aikawa, K.; Nakamura, Y.; Yokota, Y.; Toya, W.; Mikami, K. Stable but Reactive Perfluoroalkylzinc Reagents: Application in Ligand-free Coppercatalyzed Perfluoroalkylation of Aryl Iodides. Chem. Eur. J. 2015, 21, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Furukawa, T.; Nomura, Y.; Tokunaga, E.; Shibata, N. Cu-Mediated Chemoselective Trifluoromethylation of Benzyl Bromides Using Shelf-Stable Electrophilic Trifluoromethylating Reagents. Org. Lett. 2011, 13, 3596–3599. [Google Scholar] [CrossRef]
- Kautzky, J.A.; Wang, T.; Evans, R.W.; MacMillan, D.W.C. Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids. J. Am. Chem. Soc. 2018, 140, 6522–6526. [Google Scholar] [CrossRef]
 | ||||
|---|---|---|---|---|
| Entry | [Cu] | Equivalent | T (°C) | Yield (%) b |
| 1 | Cu(OH)2 | 1:1 | r.t. | 0 c |
| 2 | Cu2(OH)2CO3 | 1:1 | r.t. | 0 c |
| 3 | dry Cu(O2CCF2SO2F)2 | 1:1 | r.t. | 0 |
| 4 | dry Cu(O2CCF2SO2F)2 | 1:1 | −196 | 85 |
| 5 | dry Cu(O2CCF2SO2F)2 | 1:1 | −78 | 95 |
| 6 | dry Cu(O2CCF2SO2F)2 | 1:1 | −40 | 96 |
| 7 | dry Cu(O2CCF2SO2F)2 | 1:1 | 0 | 0 |
| 8 | dry Cu(O2CCF2SO2F)2 | 1:1.2 | −78 | 85 |
| 9 | dry Cu(O2CCF2SO2F)2 | 1:1 | −78 | 97 |
| 10 | dry Cu(O2CCF2SO2F)2 | 1:0.9 | −78 | 96 d |
| 11 | dry Cu(O2CCF2SO2F)2 | 1:0.8 | −78 | 96 d |
| 12 | undried Cu(O2CCF2SO2F)2 | 1:0.8 | −78 | 96 d |
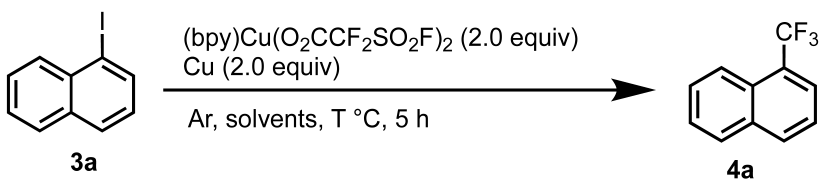 | |||||
|---|---|---|---|---|---|
| Entry | [Cu] (Equiv) | Cu (Equiv) | T (°C) | Solvent | Yield (%) b |
| 1 | 1.5 | 1.5 | −196~RT | dry DMF | 23 |
| 2 | 2.0 | 2.0 | −196~RT | dry DMF | 62 |
| 3 | 2.0 | 2.0 | RT | dry DMF | 53 |
| 4 | 2.0 | 2.0 | RT~60 | dry DMF | 85 |
| 5 | 2.0 | 2.0 | RT~80 | dry DMF | 82 |
| 6 | 2.0 | 2.0 | RT~60 | NMP | 89 |
| 7 | 2.0 | 2.0 | RT~60 | MeCN | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Qiu, X.; Lou, W.; Zhang, S.; Zhang, C.; Ma, X.; Liu, C. Efficient Trifluoromethylation of Halogenated Hydrocarbons Using Novel [(bpy)Cu(O2CCF2SO2F)2] Reagent. Molecules 2024, 29, 2849. https://doi.org/10.3390/molecules29122849
Wu X, Qiu X, Lou W, Zhang S, Zhang C, Ma X, Liu C. Efficient Trifluoromethylation of Halogenated Hydrocarbons Using Novel [(bpy)Cu(O2CCF2SO2F)2] Reagent. Molecules. 2024; 29(12):2849. https://doi.org/10.3390/molecules29122849
Chicago/Turabian StyleWu, Xiong, Xin Qiu, Wenrun Lou, Shengxue Zhang, Chaoyi Zhang, Xiaoyu Ma, and Chao Liu. 2024. "Efficient Trifluoromethylation of Halogenated Hydrocarbons Using Novel [(bpy)Cu(O2CCF2SO2F)2] Reagent" Molecules 29, no. 12: 2849. https://doi.org/10.3390/molecules29122849
APA StyleWu, X., Qiu, X., Lou, W., Zhang, S., Zhang, C., Ma, X., & Liu, C. (2024). Efficient Trifluoromethylation of Halogenated Hydrocarbons Using Novel [(bpy)Cu(O2CCF2SO2F)2] Reagent. Molecules, 29(12), 2849. https://doi.org/10.3390/molecules29122849







