Abstract
This study is the first to investigate the chemical composition and antioxidant, anti-inflammatory, and cytotoxic activities of Peperomia leptostachya leaf oil. A yellow oil was obtained through hydro-distillation, with a yield of 0.1% (w/w). The GC-MS analysis revealed 66 compounds, constituting 99.6% of the oil. Sesquiterpene hydrocarbons predominated (70.4%), followed by monoterpene hydrocarbons (13.2%), oxygenated sesquiterpenes (12.4%), non-terpenic compounds (2.0%), and oxygenated monoterpenes (1.6%). Major constituents included germacrene D (25.1%), (E)-caryophyllene (17.4%), bicyclogermacrene (6.6%), α-pinene (6.2%), and β-pinene (4.7%). The assessment of antioxidant capacity via 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging assay yielded a weak effect, with an IC50 value > 100 µg/mL. The inhibition of lipopolysaccharide-induced nitric oxide production in RAW 264.7 cells was quantified using the MTT assay, showing an IC50 value of 15.15 ± 0.68 µg/mL. Furthermore, cytotoxic effects on SK-LU-1 cell line growth were evaluated using the sulforhodamine B assay, resulting in an IC50 value of 37.45 ± 2.43 μg/mL. The anti-inflammatory activity was notable among the analyzed bioactivities of this oil. By employing a computational model, the predominant secondary metabolites in the essential oil were selected as candidates for interaction analysis with cyclooxygenase-2, an enzyme implicated in the inflammatory response. Our findings suggest that P. leptostachya leaf oil could serve as a potential source of natural compounds with prospective therapeutic effects in treating inflammatory conditions.
1. Introduction
Essential oils (EOs) are a valuable source of plant-derived chemicals with antioxidant, anti-inflammatory, and cytotoxic properties [1]. The antioxidants in EOs reduce oxidative stress by neutralizing damaging radicals, thereby protecting cells and promoting overall health [2]. Additionally, EOs contain secondary metabolites such as monoterpenes, sesquiterpenes, and phenolic compounds, which modulate inflammation and immune response pathways, exhibiting significant anti-inflammatory effects [3]. Chronic inflammation and oxidative stress are two interrelated pathogenic processes contributing to cancer growth and progression [4]. Due to their remarkable biological and pharmacological effects, EOs play a valuable role in treating various diseases. Therefore, research to better understand the therapeutic potential of EOs is essential.
Among EOs from natural sources, the Piperaceae family, a large group within the order of Piperales, comprises over 4000 known species with cultural, medicinal, and economic importance [5,6]. Among the diverse genera in this family, Peperomia is a captivating group of flowering plants, with over 1600 known species distributed across tropical and subtropical regions [5,6]. The unique appearance of Peperomia plants, characterized by slender, erect stems and elongated, lance-shaped leaves with prominent venation, is highlighted for its ornamental appeal [7]. The Peperomia genus has a rich history of traditional use in various cultures for medicinal and therapeutic purposes. Many studies have highlighted the presence of alkaloids, flavonoids, terpenes, and phenolics in Peperomia species, which significantly contribute to these plant’s potential pharmacological effects [8]. Owing to these effects, Peperomia EOs have been used for wound healing and skin applications, gastrointestinal disorders, respiratory health, and antimicrobial and antifungal applications, as well as for their anti-inflammatory, analgesic, diuretic, and antihypertensive effects [9,10]. Due to the numerous medicinal properties and applications of this genus, the chemical composition of Peperomia Eos, such as P. inaequalifolia, P. obtusifolia, P. pellucida, P. hernandiifolia, P. acuminata, P. galioides, P. chalhuapuquiana, P. macrostachyos, P. rotundifolia, P. circinnata, and P. serpens, has been investigated in many studies. However, there are scarce studies on the chemical components and biological effects of P. leptostachya [10,11,12,13,14,15,16,17].
Peperomia leptostachya Hook. & Arn., also known as the slender peperomia or hairy peperomia, is a diminutive succulent herb. Previous research has been conducted on its unique and attractive appearance, growing habits, and distribution in tropical regions of Central and South America [18,19]. As a Peperomia, this species may hold the promise of potential bio-therapeutic properties. Exploring the chemical composition and biological activities of P. leptostachya is necessary to expand the knowledge about this species. The primary objective of this study was to identify the chemical composition and investigate the antioxidant, anti-inflammatory, and cytotoxic activities of P. leptostachya leaf EO. To the best of our knowledge, its components and biological properties were reported for the first time in this study. Furthermore, molecular docking was conducted to elucidate the active components responsible for the observed anti-inflammatory effects, with a primary emphasis on their capacity to inhibit the cyclooxygenase-2 (COX-2) enzyme, which plays a pivotal role in prostaglandin production.
2. Results
2.1. GC-MS Profiles
The yellow oil of P. leptostachya leaves was obtained with a yield of 0.1% (w/w). The GC-MS analysis detected 66 compounds in the oil, representing 99.6% of its composition (Figure 1 and Table 1). Sesquiterpene hydrocarbon compounds were present in the highest amounts at 70.4%, followed by monoterpene hydrocarbons (13.2%), oxygenated sesquiterpenes (12.4%), non-terpenic compounds (2.0%), and oxygenated monoterpenes (1.6%). Germacrene D, (E)-caryophyllene, bicyclogermacrene, α-pinene, and β-pinene were revealed as the principal compounds in the oil, with percentages of 25.1, 17.4, 6.6, 6.2, and 4.7%, respectively. Some compounds, including δ-cadinene (3.1%), (E)-nerolidol (2.6%), α-humulene (1.9%), β-bourbonene (1.8%), valerianol (1.8%), allo-aromadendrene (1.6%), epi-α-cadinol (1.6%), β-elemene (1.5%), caryophyllene oxide (1.4%), α-copaene (1.3%), and trans-cadina-1(6),4-diene (1.3%), were detected in amounts greater than 1.0%.
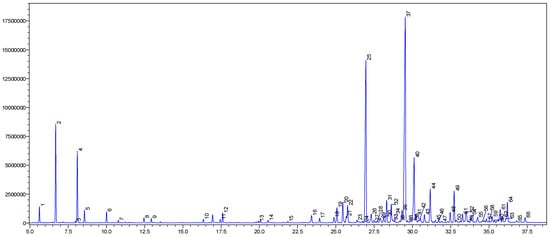
Figure 1.
The GC chromatogram of P. leptostachya leaf EO.

Table 1.
Chemical compounds in P. leptostachya leaf EO.
2.2. Biological Activities of Essential Oil
Antioxidant Activity Detection Using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Scavenging Assays.
The antioxidant activity of the P. leptostachya leaf EO was assessed using DPPH and ABTS assays. Overall, the results indicated that the EO did not exhibit any antioxidant properties, as its IC50 value exceeded 100 µg/mL compared with the positive control, ascorbic acid (IC50 7.37 ± 0.27 µg/mL) for DPPH and (IC50 4.58 ± 0.23 µg/mL) for ABTS assays (Figure 2 and Table 2).
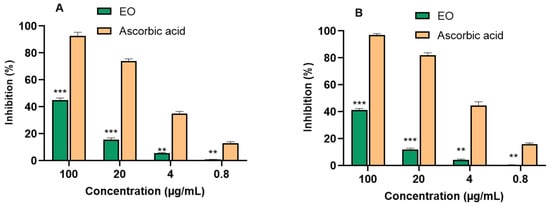
Figure 2.
Free radical scavenging activity ((A) DPPH assay and (B) ABTS assay) from P. leptostachya leaf EO. ** p < 0.01, *** p < 0.001 compared with positive control.

Table 2.
IC50 values of P. leptostachya leaf EO.
The Anti-inflammatory Effects in LPS-Stimulated RAW 264.7 Macrophages.
In exploring the inhibitory effects of P. leptostachya leaf EO on lipopolysaccharide (LPS)-induced NO production in RAW 264.7 cells, the MTT assay revealed that P. leptostachya leaf EO displayed significant anti-inflammatory effects by inhibiting NO production, with an IC50 of 15.15 ± 0.68 µg/mL. This value closely resembled that of the positive control, dexamethasone, which had an IC50 of 12.61 ± 0.98 µg/mL. At a concentration of 20 µg/mL, the EO showed no cytotoxicity, with a cell survival rate of 87.06 ± 1.61% (Figure 3 and Table 2).
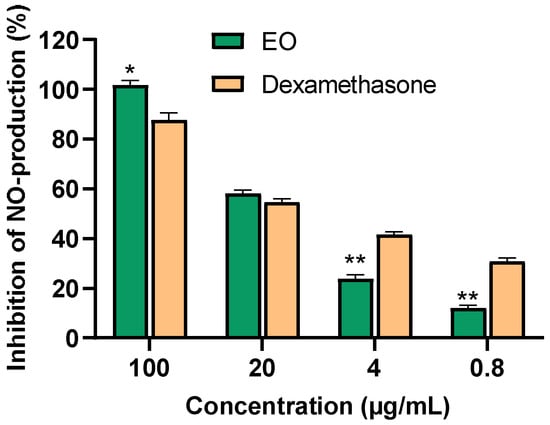
Figure 3.
Inhibition of NO production (%) in LPS-stimulated RAW 264.7 cells of the P. leptostachya leaf EO. * p < 0.05, ** p < 0.01 compared with positive control.
Cytotoxic Activity Against SK-LU-1 Cell Line.
Further investigation into the effect of P. leptostachya leaf oil on the SK-LU-1 cell line was performed using the sulforhodamine B (SRB) assay. The result indicated good cytotoxic effects, with an IC50 value of 37.45 ± 2.43 μg/mL, while the positive control, ellipticine showed highly significant cytotoxicity, with an IC50 value of 0.35 ± 0.04 µg/mL (Figure 4 and Table 2).
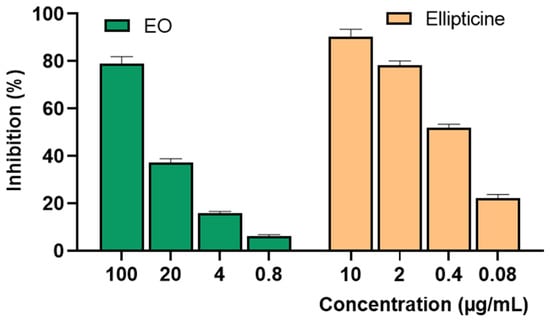
Figure 4.
Inhibition of SK-LU-1 human cancer cell lines of P. leptostachya leaf EO.
2.3. Molecular Docking Analysis
In the computational investigation, a docking analysis was conducted to evaluate the interactions of the five predominant secondary metabolites extracted from P. leptostachya germacrene D (a), (E)-caryophyllene (b), bicyclogermacrene (c), α-pinene (d), and β-pinene (e), with the crystallographic structure of COX-2 complexed with diclofenac (PDB ID: 1PXX), retrieved from the Protein Data Bank. If a compound demonstrates strong binding affinity to the active site of the COX-2 enzyme, it suggests that the compound may inhibit the enzyme’s activity, thereby reducing inflammation. To validate the docking method, the cocrystallized ligand, diclofenac, was re-docked into the active site of the target protein, and the root-mean-square deviation (RMSD) between the re-docked pose and the original crystal structure was calculated. The obtained RMSD value of 1.890 fell within the acceptable range (RMSD ≤ 2.0 Å), indicating that our docking protocol was able to reproduce the experimental binding pose [21,22]. This validation confirmed the reliability of our docking results and supported the credibility of our in silico predictions (Figure 5).
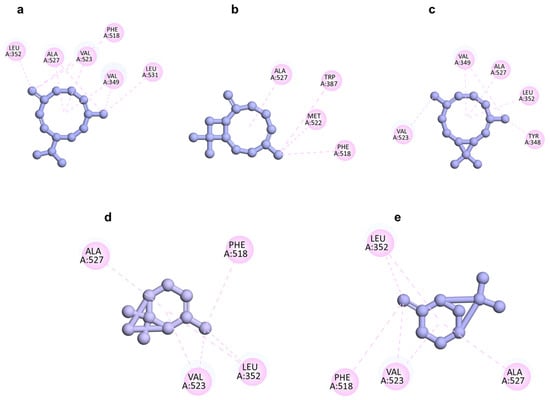
Figure 5.
Two-dimensional docking poses showing interactions of germacrene D (a), (E)-caryophyllene (b), bicyclogermacrene (c), α-pinene (d), and β-pinene (e) in the binding sites of COX-2 (PDB ID: 1PXX).
A high binding energy indicates stronger interaction between the tested compounds and the protein. The five compounds, characterized by a lipophilic carbon framework, demonstrated moderate docking scores ranging from −8.6 to −6.0 kcal/mol compared with diclofenac as a co-ligand (−8.4 kcal/mol). Given their specific chemical structure, the interactions between these five docked compounds and the protein binding site primarily involved hydrophobic interactions. Residues Leu352, Val523, and Ala527 within the COX-2 binding pocket were identified as crucial contributors to this nonpolar interface formation. Among a–e, compound a exhibited the lowest docking score (−8.6 kcal/mol) against COX-2, which was comparable to the positive control diclofenac (−8.4 kcal/mol). It suggested that a had the strongest interaction with the COX-2 enzyme. Following this, c showed a docking score of −7.7 kcal/mol, followed by b (−6.6 kcal/mol), while d (−6.0 kcal/mol) was similar to e (−6.1 kcal/mol). In summary, the lowest docking scores observed for a–e correlated with the significant anti-inflammatory activity of the EO (Figure 5 and Figure 6 and Table 3). This suggested that these compounds may play a key role in mediating the anti-inflammatory effects of P. leptostachya leaf EO by interacting strongly with the COX-2 enzyme, thus prompting further investigation into their therapeutic potential.
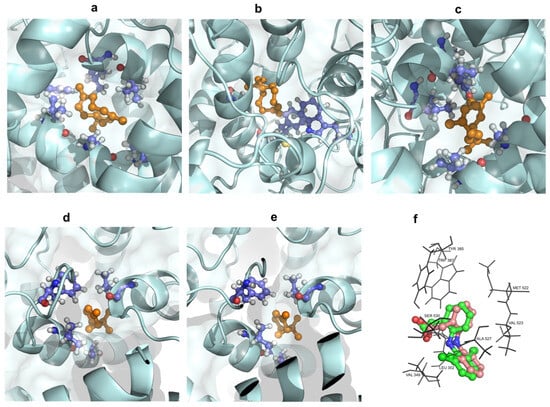
Figure 6.
Three-dimensional docking poses showing interactions of germacrene D (a), (E)-caryophyllene (b), bicyclogermacrene (c), α-pinene (d), and β-pinene (e) in the binding sites of COX-2 (PDB ID: 1PXX); (f) Overlapped structures of native ligand crystal structure of diclofenac (green) and re-docked ligand (wheat).

Table 3.
Docking scores (kcal/mol) of predominant constituents from P. leptostachya leaf EO.
Statistical Analysis: The results were expressed as mean ± standard deviation (SD), n = 3. The results were analyzed using GraphPad Prism software (version 9.3.0, GraphPad Software, San Diego, CA, USA) and Microsoft Excel software (Excel 2010 version, Redmond, Washington, DC, USA). Statistical differences were determined busing t-Student test with * p < 0.05; ** p < 0.01; *** p < 0.001.
3. Discussion
The chemical compositions of EOs from various species of Peperomia had previously been explored. In the following, we compare and contrast the chemical compositions of EOs among different Peperomia species. To date, the EOs from various Peperomia species have exhibited diverse chemical profiles. P. inaequalifolia from Quito, Ecuador, contains significant amounts of safrole and 11αH-himachal-4-en-1β-ol [23], while in specimens from Guayllabamba parish, elemicin and α-bisabolol predominate [10]. Similarly, P. obtusifolia EOs are dominated by β-caryophyllene in Pakistan and valerianol in Brazil [11,24]. P. pellucida features linalool and limonene in southwestern Nigeria and β-fernesene in Kwara State [12,13]. Dillapiole predominates in Brazilian P. pellucida [14]. P. acuminata EOs from Venezuela primarily contain (2E)-dodecenal [15]. P. borbonensis from Réunion Island contains myristicin and elemicin [16]. Investigating the volatile oil composition of fresh and dried aerial parts of P. galioides and P. chalhuapuquiana reveals different components [18]. P. galioides EO has also been found to contain globulol and β-caryophyllene [25], while P. macrostachya contains epi-α-bisabolol and caryophyllene oxide [13]. P. rotundifolia is rich in decanal and dihydro-β-santalol [14]. P. circinnata EOs from the Amazon contain myrcene and β-phellandrene [26], while those from the Pará state contain myrcene and β-phellandrene [27]. In addition, the EO of P. serpens features (E)-nerolidol and ledol [28]. We addressed the slight differences in retention indices between our study and the literature. These differences can be attributed to several factors, including instrumental variability, differences in experimental conditions, sample preparation methods, and intrinsic variability of the compounds. Instrumental variability involves differences in GC-MS systems and columns, such as column dimensions and stationary phases, which can affect retention times. Variations in experimental conditions, like temperature programming and carrier gas flow rates, also play a significant role. Additionally, differences in sample preparation techniques, such as extraction methods and solvent purity, can lead to changes in the chemical composition and relative abundance of compounds. Furthermore, the chemical components may result in variability in their interactions with the column’s stationary phase, impacting retention indices. Our data analysis revealed that β-caryophyllene predominates as the main compound in EOs derived from numerous species within Peperomia. However, it was noteworthy that germacrene D was the primary compound detected in the EO of P. leptostachya in this study, which distinguished it from other species within the genus. The presence of germacrene D in significant amounts in P. leptostachya EO, while scarce or undetected in other species, suggests a species-specific chemical profile influenced by environmental factors, geographical location, and the inherent characteristics of each species. Furthermore, there is limited research exploring the biological effects of Peperomia EOs, including their antioxidant, anti-inflammatory, and cytotoxic properties. For this reason, we investigated the biological activity of the leaf oil of P. leptostachya.
We first assessed the antioxidant capability of P. leptostachya leaf oil using the DPPH scavenging assay. Previous investigations have focused on the antioxidative activity of EOs derived from Peperomia, including for P. inaequalifolia, P. pellucida, and P. inaequalifolia. Assessments utilizing the DPPH, ABTS assays and antioxidant activity studies revealed noteworthy activity in these EOs. The ABTS assay outperformed the DPPH assay in accurately assessing the antioxidant levels of samples containing hydrophilic and lipophilic components. However, the present study found no antioxidant activity in P. leptostachya leaf EO, which showed no activity. The absence of phenolic compounds detected in this study may contribute to this EO’s relatively low antioxidant activities.
EOs have been widely used in traditional medicine to treat respiratory illnesses through inhalation and oral administration. When ingested, essential oils are absorbed through the digestive system into the bloodstream and distributed throughout the body, including in the lungs [29]. Lung cancer is one of the most common diseases worldwide, affecting millions of people each year and acting as a leading cause of cancer-related deaths globally [30]. Therefore, we investigated the inhibitory effects of P. leptostachya leaf oil on the SK-LU-1 cell line using the SRB assay. The key components in P. leptostachya leaf oil contributed to its substantial cytotoxic activity, with an IC50 value of 37.45 ± 2.43 µg/mL. Additionally, previous studies suggested that (E)-caryophyllene and germacrene D exhibited notable cytotoxic effects against a range of tumor cell lines, which supports our findings.
Research on the anti-inflammatory activity of EOs from the genus Peperomia was limited. Currently, there is only one study on the anti-inflammatory activity of P. serpens, indicating a significant anti-inflammatory effect in acute inflammation models induced by paw edema from carrageenan, dextran, and croton oil, as well as leukocyte and neutrophil migration into the peritoneal cavity in mice. Additionally, real-time microscopic analysis showed reduced neutrophil rolling and adhesion in the mesenteric microcirculation of mice [28]. Of the three bioactivities tested in the present study, the anti-inflammatory effect of the EO from the plant was particularly remarkable (IC50 15.15 ± 0.68 µg/mL). Docking models were employed to analyze the potential interactions with the target protein of the bioactive compounds. Cyclooxygenase-1 (COX-1) and COX-2 are enzymes involved in the synthesis of prostaglandins, lipid compounds with diverse physiological effects. While COX-1 is constitutively expressed in many tissues and contributes to maintaining normal physiological functions, such as gastric mucosa protection, platelet aggregation promotion, and renal blood flow regulation, COX-2 is typically absent in most tissues but is primarily responsible for increased prostaglandin production during inflammation. Due to its role in increased prostaglandin production, COX-2 was selected as the target for this study [31,32]. The anti-inflammatory activity of P. leptostachya may be attributed to the binding affinity of the majority compounds (e.g., germacrene D, (E)-caryophyllene, bicyclogermacrene, α-pinene, and β-pinene) present in the EOs, highlighting the potential therapeutic significance of these compounds in modulating inflammatory processes and warranting further investigation into their mechanisms of action and clinical applications.
It is important to recognize that the inhibitory properties of the EO cannot only be attributed to its primary components. The anti-inflammatory efficiency might result from additive, synergistic, or antagonistic effects of the EO constituents [33]. Notably, even less common and minor chemicals in trace amounts (<0.5%) may significantly affect the EO’s biological activity. For instance, compounds such as sabinene, linalool, (Z)-cinnamyl alcohol, β-oplopenone, and β-selinene, though present in minute quantities, have been found to play pivotal roles in the anti-inflammatory activity of EOs [34,35,36]. Understanding the synergistic interactions among these compounds, including the minor constituents, is paramount for comprehending the biological effectiveness of the EOs. Another limitation of our study is the unclear mechanism of action underlying the anti-inflammatory effects of P. leptostachya EO. Inflammation can manifest through various pathways. However, the investigation primarily focused on conducting docking simulations to analyze the interaction between the major components of the extracted EO and COX-2, a target protein associated with inflammation inhibition. Therefore, further investigations are needed to specify the signaling pathways in the anti-inflammatory process.
4. Materials and Methods
4.1. General Procedures
The bioactivity measurements were conducted using a BioTex El800 UV-Vis spectrophotometer (Boston, MA, USA). The positive controls and reagents were obtained from Sigma Aldrich (Saint Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were sourced from Life Technologies, Inc. (Gaithersburg, MD, USA). The RAW 264.7 cells were provided by Prof. Domenico Delfino, University of Perugia, Italy.
4.2. Plant Material and Extraction
On 7 July 2023, fresh leaves of P. leptostachya were gathered from Quangtri, Vietnam, situated at coordinates 16°46′56.3″ N 106°35′03.9″ E. Dr. Anh Tuan Le identified the botanical name of the plant. A voucher specimen labeled PLQT-2023 (P. leptostachya leaves) was deposited at the Faculty of Chemistry, University of Education, Hue University in Vietnam.
4.3. Distillation
Each fresh powder sample weighing 1.0 kg underwent hydro-distillation using a Clevenger apparatus. The extraction process was conducted for 4.0 h, facilitating the release of EOs from the plant material. Following hydro-distillation, the oil was obtained by decantation and subsequently dried over Na2SO4 to eliminate any residual water content. The resulting dried EO was then stored in sealed vials under a temperature of −5 °C to safeguard their stability and maintain the integrity of its chemical composition for subsequent analysis [37].
4.4. GC-MS Analysis
The GC-MS analysis was conducted using a Shimadzu Technologies GCMS-QP2010 Plus chromatograph (Shimadzu, Kyoto, Japan), equipped with a fused silica Equity-5 capillary column (30 m × 0.25 mm, film thickness 0.25 µm, Supelco, Bellefonte, PA, USA) [38,39,40,41]. The analytical parameters were set as follows: helium was utilized as the carrier gas at a flow rate of 1.2 mL/min, while the injector and interface temperatures were maintained at 280 °C. The temperature program entailed a ramp from 60 °C (held for 3 min) to 240 °C (held for 15 min) at a rate of 3 °C/min, followed by a final ramp to 280 °C (held for 35 min) at a rate of 5 °C/min for the column. Sample injection utilized a split ratio of 15:1, with an inlet pressure of 93.2 kPa and an injection volume of 1.0 µL. The mass spectrometer settings included an ionization voltage of 70 eV, a detector voltage of 0.82 kV, and data acquisition in the scan mass range of 50–500 amu at a sampling rate of 0.5 scan/s. Identification of chemical constituents was achieved by co-injecting the samples and comparing the retention indices (RIs) to a homologous series of n-alkanes (C7–C40), as well as by referencing the Adams book [20]. Quantification was carried out based on the relative peak area percentage. Comprehensive analysis of the obtained GC-MS data facilitated the identification and characterization of the chemical compounds present in the P. leptostachya leaf EO, laying the groundwork for further investigations and potential applications.
4.5. DPPH and ABTS Radical Scavenging Activities
The assessment of P. leptostachya leaf-derived EO’s capacity to neutralize free radicals generated from DPPH and ABTS adhered to the methodology outlined, with suitable modifications implemented to align with laboratory conditions. The specific protocols for these evaluations were detailed in our prior reports [42,43].
4.6. Anti-Inflammatory Assay
The inhibitory activity of P. leptostachya leaf EO against LPS-induced nitric oxide (NO) production in RAW 264.7 cells was evaluated. The concentration of nitrite, indicating NO presence in the culture medium, was assessed using the Griess reaction. The detailed protocols for these assessments were outlined in our previous reports [42].
4.7. Cytotoxicity Assays
The cytotoxicity of the EO from P. leptostachya leaf was assessed using a sulforhodamine B assay on the SK-LU-1 cell line. Our earlier study described the detailed protocols for these experiments, focusing on cytotoxicity [44].
4.8. In Silico Analysis
The molecular docking analyses were conducted using AutoDock Tools 1.5.7 and AutoDock Vina 1.1.2. The crystal structure of cyclooxygenase-2 (PDB ID: 1PXX) was retrieved from the Protein Data Bank (PDB). Visualization of the compound–protein interactions was performed using BIOVA Discovery Studio Visualizer v24.1.0.23298 [45].
5. Conclusions
Our research provides initial insights into the chemical composition and biological characteristics of P. leptostachya leaf oil, marking its first investigation. Through GC-MS analysis, we identified 66 compounds, with germacrene D emerging as the predominant compound, comprising 25.1% of the oil. The chemical composition of P. leptostachya EO differs significantly from that of the other Peperomia species. Our biological assessments revealed weak antioxidant activity, significant anti-inflammatory effects against NO production in RAW 264.7 cells, and good cytotoxicity against SK-LU-1 cells. The prevalence of five main compounds supported the significant anti-inflammatory impacts of the EOs. These compounds showed potential to interact with COX-2 and mitigate the production of pro-inflammatory prostaglandins. Overall, P. leptostachya leaf EO showed prospective anti-inflammatory and anti-cancer properties, the molecular mechanisms of which should be investigated in future work.
Author Contributions
Conceptualization, T.V.P.; methodology, T.V.P. and B.C.N.; formal analysis H.Q.V. and D.V.H.; investigation, T.V.P., D.V.H., H.M.N. and K.L.C.; resources, T.V.P.; data curation, H.M.N.; T.V.P. and D.V.H.; writing—original draft preparation, L.T.K.N., T.V.P. and D.V.H.; writing—review and editing, H.M.N., D.V.H. and H.T.N.; visualization, T.V.P.; supervision, H.M.N.; project administration, H.M.N. and T.V.P.; funding acquisition, T.V.P. and D.V.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hue University under the Core Research Program, Grant NCM.DHH.2023.02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Neganova, M.; Liu, J.; Aleksandrova, Y.; Klochkov, S.; Fan, R. Therapeutic influence on important targets associated with chronic inflammation and oxidative stress in cancer treatment. Cancers 2021, 13, 6062. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Ghani, N.A. Mini review on botany, traditional uses, phytochemistry and biological activities of Piper amalago (Piperaceae). Malays. J. Chem. 2022, 24, 201–208. [Google Scholar] [CrossRef]
- Alves, N.S.F.; Inoue, S.G.K.; Carneiro, A.R.; Albino, U.B.; Setzer, W.N.; Maia, J.G.; Andrade, E.H.; da Silva, J.K.R. Variation in Peperomia pellucida growth and secondary metabolism after rhizobacteria inoculation. Pandey AK. PLoS ONE 2022, 17, e0262794. [Google Scholar] [CrossRef]
- Ho, K.L.; Yong, P.H.; Wang, C.W.; Kuppusamy, U.R.; Ngo, C.T.; Massawe, F.; Ng, Z.X. Peperomia pellucida (L.) Kunth and eye diseases: A review on phytochemistry, pharmacology and toxicology. J. Integr. Med. 2022, 20, 292–304. [Google Scholar] [CrossRef]
- Gutierrez, Y.V.; Yamaguchi, L.F.; de Moraes, M.M.; Jeffrey, C.S.; Kato, M.J. Natural products from Peperomia: Occurrence, biogenesis and bioactivity. Phytochem. Rev. 2016, 15, 1009–1033. [Google Scholar] [CrossRef]
- Alves, N.S.F.; Setzer, W.N.; Da Silva, J.K.R. The chemistry and biological activities of Peperomia pellucida (Piperaceae): A critical review. J. Ethnopharmacol. 2019, 232, 90–102. [Google Scholar] [CrossRef]
- Valarezo, E.; Herrera-García, M.; Astudillo-Dávila, P.; Rosales-Demera, I.; Jaramillo-Fierro, X.; Cartuche, L.; Meneses, M.A.; Morocho, V. Study of the chemical composition and biological activity of the essential oil from Congona (Peperomia inaequalifolia Ruiz and Pav.). Plants 2023, 12, 1504. [Google Scholar] [CrossRef]
- de Araujo Morandim-Giannetti, A.; Pin, A.R.; Santo Pietro, N.A.; de Oliveira, H.C.; Mendes-Giannini, M.J.S.; Alecio, A.C. Composition and antifungal activity against Candida albicans, Candida parapsilosis, Candida krusei and Cryptococcus neoformans of essential oils from leaves of Piper and Peperomia species. J. Med. Plants Res. 2010, 4, 1810–1814. [Google Scholar]
- Okoh, S.; Iweriebor, B.C.; Okoh, O.; Okoh, A. Bioactive constituents, radical scavenging, and antibacterial properties of the leaves and stem essential oils from Peperomia pellucida (L.) Kunth. Pharmacogn Mag. 2017, 13 (Suppl. S3), S392–S400. [Google Scholar] [CrossRef] [PubMed]
- Usman, L.A.; Ismaeel, R.O. Chemical composition of root essential oil of Peperomia pellucida (L.) Kunth. grown in Nigeria. J. Essent. Oil-Bear. Plants 2020, 23, 628–632. [Google Scholar] [CrossRef]
- de Lira, P.N.B.; da Silva, J.K.R.; Andrade, E.H.A.; Sousa, P.J.C.; Silva, N.N.S.; Maia, J.G.S. Essential oil composition of three Peperomia species from the Amazon, Brazil. Nat. Prod. Commun. 2009, 4, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Mora-Vivas, F.D.; Velasco, J.; Díaz, T.; Rojas-Fermín, L.; de Torres, L.D.; Ríos-Tesch, N.; Carmona, J. Composición química y actividad antibacteriana del aceite esencial de Peperomia acuminata de los Andes venezolanos. Rev. Peru. Biol. 2016, 23, 301–304. [Google Scholar] [CrossRef]
- Dorla, E.; Gauvin-Bialecki, A.; Deuscher, Z.; Allibert, A.; Grondin, I.; Deguine, J.; Laurent, P. Insecticidal activity of the leaf essential oil of Peperomia borbonensis MIQ. (Piperaceae) and its major components against the melon fly Bactrocera cucurbitae (Diptera: Tephritidae). Chem. Biodiv. 2017, 14, e1600493. [Google Scholar] [CrossRef] [PubMed]
- Paola, L.L.; Yoni, F.; Catalina, M.B.; Bandoni, A.; Jorge, D.C.; Pastor, D. Composition of the essential oil of two Peperomia from Peru: P. galioides and P. chalhuapuquiana. Rev. Latinoamer. Quim. 2007, 35, 7–12. [Google Scholar]
- Mathieu, G. Peperomia leptostachya (Piperaceae) revived. Candollea 2020, 75, 45. [Google Scholar] [CrossRef]
- de Lange, P.J. Peperomia leptostachya (Piperaceae) on Raoul Island, Kermadec Islands—A name reinstated. Trilepidea 2020, 197, 3–8. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Castro-Alvarez, A.; Costa, A.M.; Vilarrasa, J. The performance of several docking programs at reproducing protein-macrolide-like crystal structures. Molecules 2017, 22, 136. [Google Scholar] [CrossRef]
- da Fonseca, A.M.; Caluaco, B.J.; Madureira, J.M.C.; Cabongo, S.Q.; Gaieta, E.M.; Djata, F.; Colares, R.P.; Neto, M.M.; Fernandes, C.F.C.; Marinho, G.S.; et al. Screening of potential inhibitors targeting the main protease structure of SARS-CoV-2 via molecular docking, and approach with molecular dynamics, RMSD, RMSF, H-Bond, SASA and MMGBSA. Mol. Biotechnol 2023. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.N.; Mosquera, T.; Baldisserotto, A.; Abad, J.; Aillon, C.; Cabezas, D. Chemical composition and in-vitro biological activities of the essential oil from leaves of Peperomia inaequalifolia Ruiz & Pav. Am. J. Essent. Oil Nat. Prod. 2015, 2, 29–31. [Google Scholar]
- Ilyas, S.; Naz, S.; Aslam, F.; Parveen, Z.; Ali, A. Chemical composition of essential oil from in vitro grown Peperomia obtusifolia through GC-MS. Pak. J. Bot. 2014, 46, 667–672. [Google Scholar]
- De Feo, V.; Belaunde, A.J.; Sandoval, J.G.; Senatore, F.; Formisano, C. Antibacterial activity and composition of the essential oil of Peperomia galioides HBK (piperaceae) from Peru. Nat. Prod. Commun. 2008, 3, 933–936. [Google Scholar] [CrossRef]
- Zoghbi, M.; Andrade, E.; Lobato, R.; Tavares, A.; Souza, A.; Conceição, C.; Guimarães, E. Peperomia circinnata Link and Peperomia rotundifolia (L.) Kunth growing on different host-trees in Amazon: Volatiles and relationship with bryophytes. Biochem. Syst. Ecol. 2005, 33, 269–274. [Google Scholar] [CrossRef]
- Mesquita, K.D.S.M.; Feitosa, B.d.S.; Cruz, J.N.; Ferreira, O.O.; Franco, C.d.J.P.; Cascaes, M.M.; de Oliveira, M.S.; Andrade, E.H.d.A. Chemical composition and preliminary toxicity evaluation of the essential oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina leach. Molecules 2021, 26, 7359. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.; Silva, A.; Souza, G.; Figueiredo, J.; Cunha, F.; Lahlou, S.; da Silva, J.; Maia, J.; Sousa, P. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (Sw.) Loud. J. Ethnopharmacol. 2011, 138, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. 2015, 30, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A therapeutic target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar] [CrossRef]
- Walduck, A.K.; Weber, M.; Wunder, C.; Juettner, S.; Stolte, M.; Vieth, M.; Wiedenmann, B.; Meyer, T.F.; Naumann, M.; Hoecker, M. Identification of novel Cyclooxygenase-2-dependent genes in Helicobacter pylori infection in vivo. Mol. Cancer 2009, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; De Luca, M.; Toma, C.-C.; Grande, F.; Occhiuzzi, M.A.; Caruso, R.; Conforti, F.; Statti, G. Enhancing the nitric oxide inhibitory activity using a combination of plant essential oils and mixture design approach. Heliyon 2024, 10, e31080. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Kourti, M.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Esters of some non-steroidal anti-inflammatory drugs with cinnamyl alcohol are potent lipoxygenase inhibitors with enhanced anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2015, 25, 5028–5031. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yang, K.W.; Kim, S.S.; Park, S.M.; Park, K.J.; Kim, K.S.; Choi, Y.H.; Cho, K.K.; Lee, N.H.; Hyun, C.G. Chemical composition and anti-inflammatory effects of essential oil from Hallabong flower. EXCLI J. 2013, 12, 933. [Google Scholar] [PubMed]
- Kim, M.G.; Kim, S.M.; Min, J.H.; Kwon, O.K.; Park, M.H.; Park, J.W.; Ahn, H.I.; Hwang, J.Y.; Oh, S.R.; Lee, J.W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.V.; Truong, V.; Dang, N.T.T.; Vo, H.Q.; Ho, D.V. Chemical composition of the essential oils of Distichochlamys citrea leaves collected from central Vietnam. Vietnam J. Chem. 2017, 55, 358–362. [Google Scholar]
- Pham, T.V.; Ngo, H.P.T.; Dang, N.T.T.; Nguyen, H.K.; Hoang, H.T.N.; Pham, T. Volatile constituents and anti-osteoporotic activity of the n-hexane extract from Homalomena gigantea rhizome. Nat. Prod. Commun. 2022, 17, 1934578X221125433. [Google Scholar] [CrossRef]
- Dai, D.N.; Pham, T.V.; Luyen, N.D.; Dung, V.T.; Huong, L.T.; Son, N.T. Essential oils of two Vietnamese plants Piper betle f. densum (Piperaceae) and Disepalum plagioneurum (Annonaceae): Chemical composition, antimicrobial, and cytotoxic activities. Nat. Prod. Commun. 2023, 18, 1934578X231190689. [Google Scholar] [CrossRef]
- Pham, T.V.; Ha, N.X.; Luyen, N.D.; Xuan, T.H.; Le Quoc, T.; Hung, N.H.; The, S.N. Chemical composition, mosquito larvicidal and antimicrobial activities, and molecular docking study of essential oils of Cinnamomum melastomaceum, Neolitsea buisanensis and Uvaria microcarpa from Vietnam. Chem. Biodiv. 2023, 20, e202300652. [Google Scholar] [CrossRef]
- Huong, L.T.; Sam, L.N.; Giang, C.N.; Dai, D.N.; Pham, T.V.; Luyen, N.D.; Setzer, W.N.; Son, N.T. Essential oils of two Zingiber plants Zingiber eberhardtii Gagnep. and Zingiber skornickovae N.S. Lý: Chemical profiles and antimicrobial effects. J. Essent. Oil-Bear. Plants 2023, 26, 761–768. [Google Scholar] [CrossRef]
- Ho, D.V.; Hoang, H.N.T.; Nguyen, N.H.; Do, H.B.; Vo, H.Q.; Le, A.T.; Le, T.Q.; Pham, T.V. GC-MS characterization, in vitro antioxidant and anti-inflammatory activities of essential oil from the leaves of Litsea balansae Lecomte. Nat. Prod. Commun. 2023, 18, 1934578X231214159. [Google Scholar] [CrossRef]
- Buranasudja, V.; Vimolmangkang, S.; Sanookpan, K.; Binalee, A.; Bao Nguyen, A.; Dong Thach, U.; Hang Do, B.; Son Dang, V.; Minh Do, K.; Minh Nguyen, H. Antioxidant, anti-skin-aging, anti-Inflammatory, and anti-acetylcholinesterase activities of Rourea oligophlebia extracts. J Chem. Biodiver. 2023, 20, e202201096. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.V.; Ho, D.V.; Le, A.T.; Ngo, Y.D.; Dang, N.T.T.; Le, T.Q.; Nguyen, B.C. Chemical composition and biological activities of essential oil from Grewia bulot leaves. Nat. Prod. Res. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T. Predictions of the ADMET properties of candidate drug molecules utilizing different QSAR/QSPR modelling approaches. Curr. Drug. Metab. 2010, 11, 285–295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).