Influence of Major Polyphenols on the Anti-Candida Activity of Eugenia uniflora Leaves: Isolation, LC-ESI-HRMS/MS Characterization and In Vitro Evaluation
Abstract
1. Introduction
2. Results
2.1. Spray-Dried Crude Extract and Enriched Fraction
2.2. Screening Tests for Subfractionation
2.2.1. EAF Processing by Reversed-Phase Flash Chromatography (RP-FC)
2.2.2. EAF Processing by Size Exclusion Chromatography
2.3. Chromatographic Strategies for Major Polyphenol Recovery
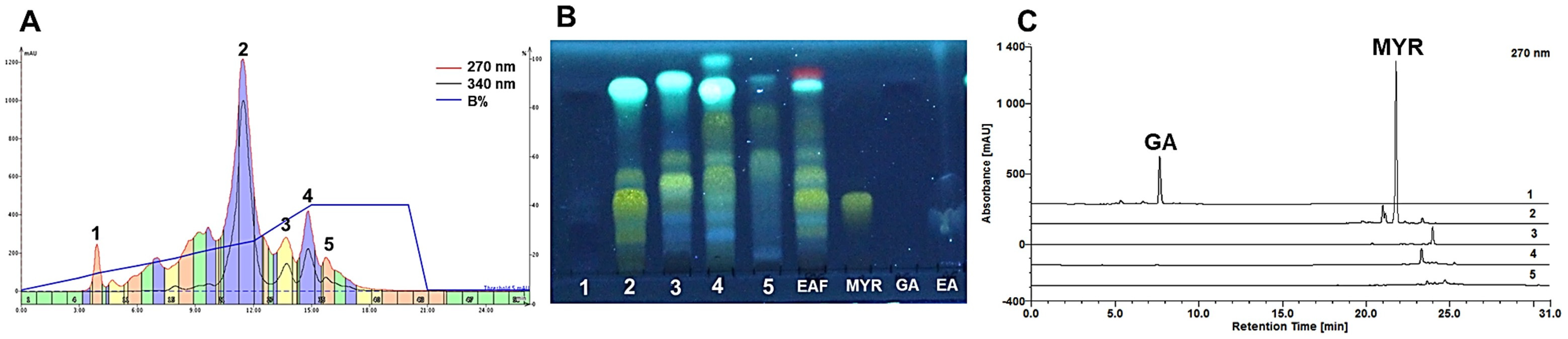
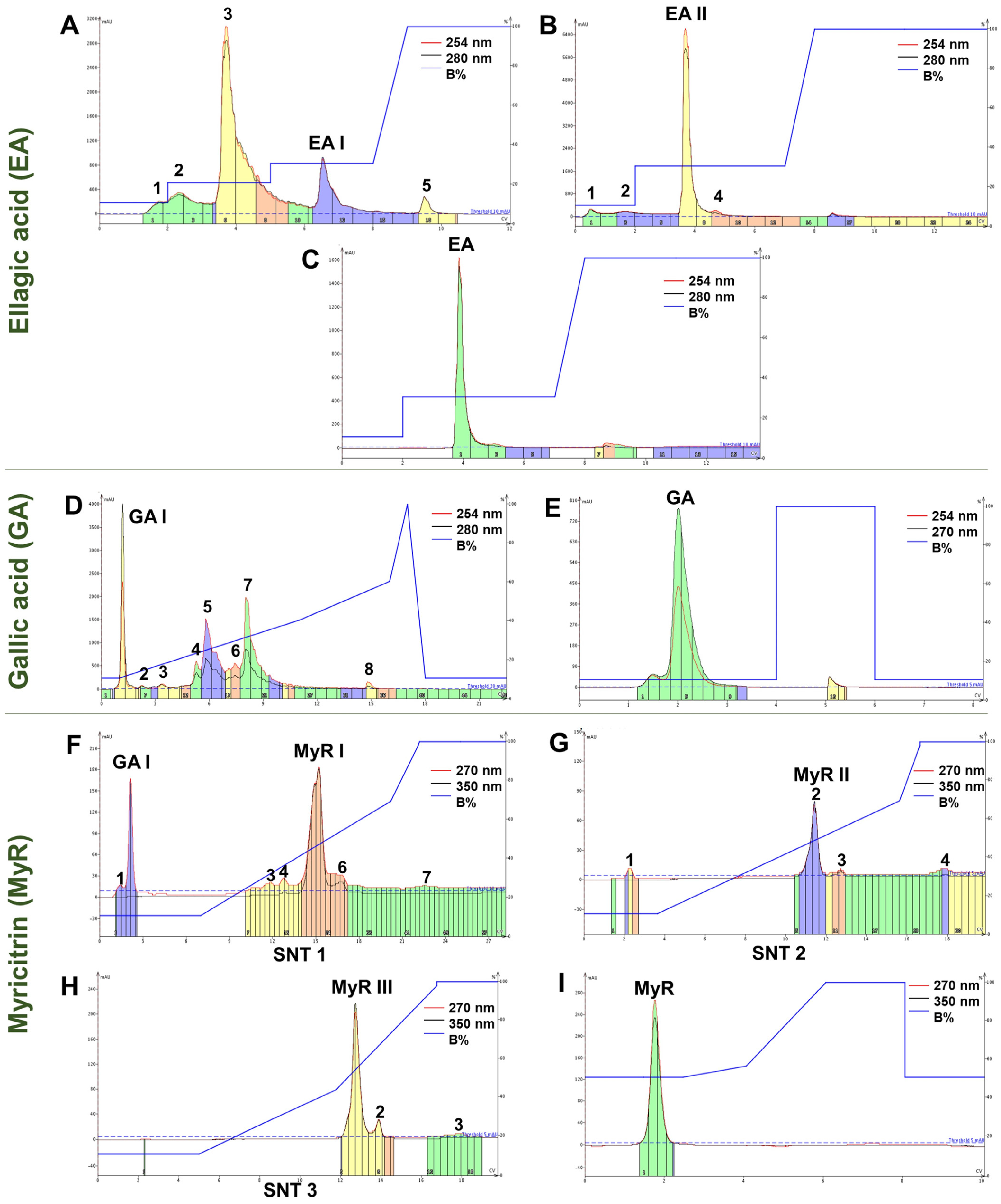
2.3.1. Fractionation by Sephadex® LH-20
2.3.2. Fractionation and Isolation of Major Polyphenols by Flash Chromatography
- Ellagic acid (EA)
- Gallic acid (GA)
- Myricitrin (MyR)
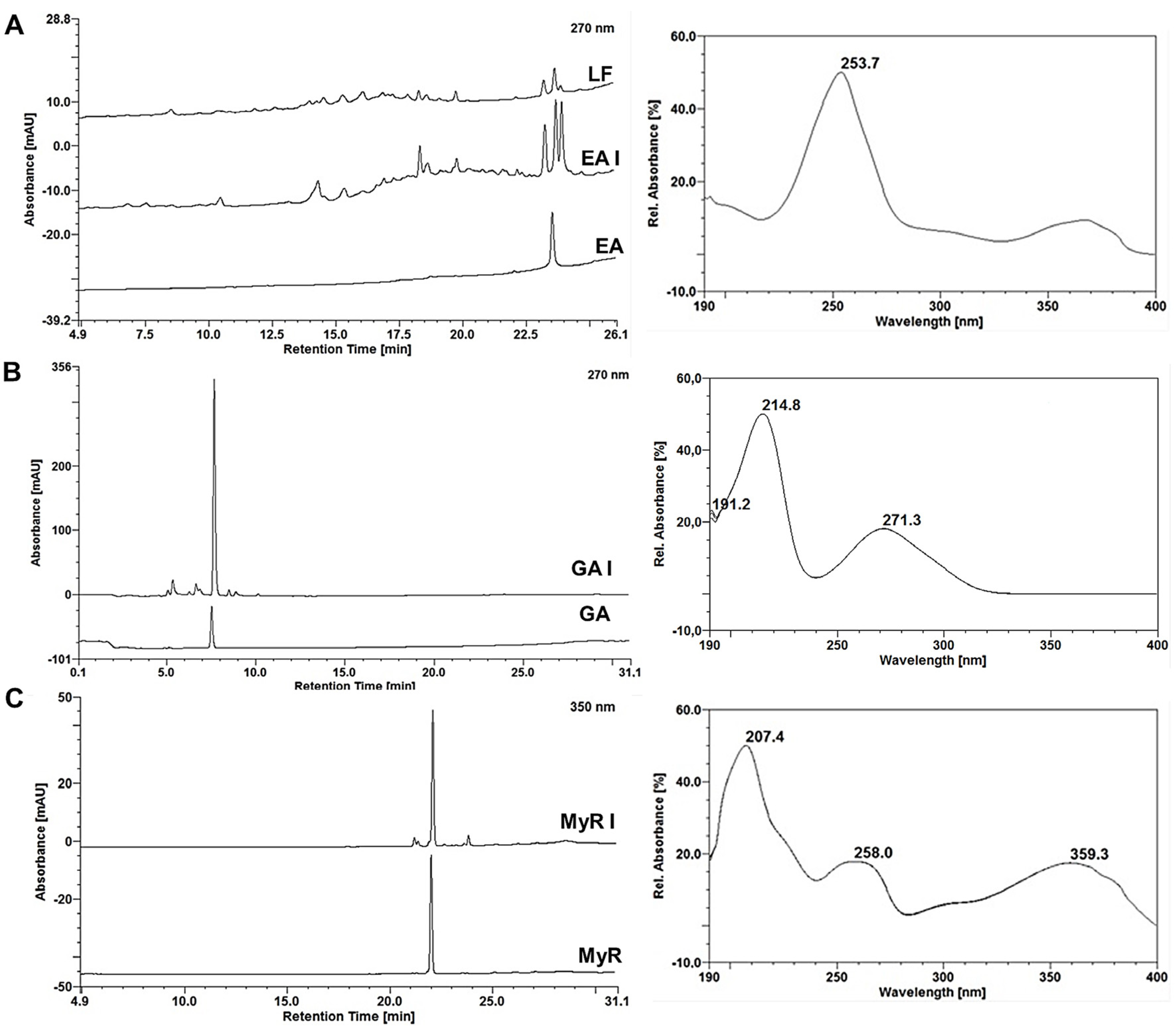
2.4. Monitoring the RP-Flash Subfractionation and Isolation Process by HPLC-DAD
2.5. Phytochemical Characterization of the Extract, Fractions, Subfractions and Isolates
2.5.1. Phytochemical Profile
2.5.2. Identification of Flavonols
2.5.3. Identification of Other Phenolic Compounds
2.5.4. Isolated Compounds from E. uniflora
2.6. Antifungal Activity
3. Material and Methods
3.1. Herbal Material, Extract, and Enriched Fraction
3.2. Screening Tests for Processing the Enriched Fraction
3.2.1. Reversed-Phase Flash Chromatography
3.2.2. Exclusion Chromatography
3.3. Chromatographic Strategies for Major Polyphenol Recovery
3.3.1. EAF Processing by Subfractionation by Sephadex® LH-20
3.3.2. EAF Processing by Subfractionation and Purification by Isolera™
3.3.3. Yields
3.4. Monitoring the Sub-Fractionation and Isolation Process
3.4.1. Thin Layer Chromatography (TLC)
3.4.2. High Performance Liquid Chromatography (HPLC-DAD)
3.5. Characterization LC-ESI-HRMS/MS
3.6. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu Khalaf, R.; Alhusban, A.A.; Al-Shalabi, E.; Al-Sheikh, I.; Sabbah, D.A. Chapter 10—Isolation and structure elucidation of bioactive polyphenols. Stud. Nat. Prod. Chem. 2019, 63, 267–337. [Google Scholar]
- Oldoni, T.L.C.; Merlin, N.; Bicas, T.C.; Prasniewski, A.; Carpes, S.T.; Ascari, J.; de Alencar, S.M.; Massarioli, A.P.; Bagatini, M.D.; Morales, R.; et al. Antihyperglycemic activity of crude extract and isolation of phenolic compounds with antioxidant activity from Moringa oleifera Lam. leaves grown in Southern Brazil. Food Res. Int. 2021, 141, 110082. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Long, P.; Meng, Q.; Ho, C.-T.; Zhang, L. An emerging strategy for evaluating the grades of Keemun black tea by combinatory liquid chromatography-Orbitrap mass spectrometry-based untargeted metabolomics and inhibition effects on α-glucosidase and α-amylase. Food Chem. 2018, 246, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Cui, Y.; Zhang, S.; Li, L.; Li, Y.; Zhou, P.; Sun, B. Preparative separation of grape skin polyphenols by high-speed counter-current chromatography. Food Chem. 2016, 212, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P. Chapter 10—High-Performance Liquid Chromatography for Analysis of Herbal Drugs. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 421–458. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Chapter 19—Applications of High Performance Liquid Chromatography in the Analysis of Herbal Products. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 405–425. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Silva-Rocha, W.P.; de Azevedo, M.F.; Ferreira, M.R.A.; da Silva, J.d.F.; Svidzinski, T.I.E.; Milan, E.P.; Soares, L.A.L.; Rocha, K.B.F.; UchÔa, A.F.; Mendes-Giannini, M.J.S.; et al. Effect of the Ethyl Acetate Fraction of Eugenia uniflora on Proteins Global Expression during Morphogenesis in Candida albicans. Front. Microbiol. 2017, 8, 1788. [Google Scholar] [CrossRef] [PubMed]
- Silva-Rocha, W.P.; de Brito Lemos, V.L.; Ferreira, M.R.A.; Soares, L.A.L.; Svidzisnki, T.I.E.; Milan, E.P.; Chaves, G.M. Effect of the crude extract of Eugenia uniflora in morphogenesis and secretion of hydrolytic enzymes in Candida albicans from the oral cavity of kidney transplant recipients. BMC Complement. Altern. Med. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.d.C.O.; Freires, I.A.; Lazarini, J.G.; Infante, J.; de Alencar, S.M.; Rosalen, P.L. Unexplored endemic fruit species from Brazil: Antibiofilm properties, insights into mode of action, and systemic toxicity of four Eugenia spp. Microb. Pathog. 2017, 105, 280–287. [Google Scholar] [CrossRef]
- Franzon, R.C.; Carpenedo, S.; Viñoly, M.D.; Raseira, M.d.C.B. Pitanga—Eugenia uniflora L. In Exotic Fruits; Rodrigues, S., Silva, E.d.O., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 333–338. [Google Scholar] [CrossRef]
- Denardin, C.C.; Hirsch, G.E.; da Rocha, R.F.; Vizzotto, M.; Henriques, A.T.; Moreira, J.C.F.; Guma, F.T.C.R.; Emanuelli, T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J. Food Drug Anal. 2015, 23, 387–398. [Google Scholar] [CrossRef]
- Sobeh, M.; El-Raey, M.; Rezq, S.; Abdelfattah, M.A.O.; Petruk, G.; Osman, S.; El-Shazly, A.M.; El-Beshbishy, H.A.; Mahmoud, M.F.; Wink, M. Chemical profiling of secondary metabolites of Eugenia uniflora and their antioxidant, anti-inflammatory, pain killing and anti-diabetic activities: A comprehensive approach. J. Ethnopharmacol. 2019, 240, 111939. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.A.-O.; Hamza, M.S.; Ashour, M.A.-O.; Elkhatieb, M.A.-O.X.; El Raey, M.A.-O.; Abdel-Naim, A.A.-O.; Wink, M.A.-O. A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities in vivo. Pharm. 2020, 13, 84. [Google Scholar] [CrossRef]
- Sobral-Souza, C.E.; Silva, A.R.P.; Leite, N.F.; Rocha, J.E.; Costa, J.G.M.; Menezes, I.R.A.; Cunha, F.A.B.; Rolim, L.A.; Sousa, A.K.; Coutinho, H.D.M. The role of extracts from Eugenia uniflora L. against metal stress in eukaryotic and prokaryotic models. S. Afr. J. Bot. 2020, 131, 360–368. [Google Scholar] [CrossRef]
- da Cunha, F.A.B.; Waczuk, E.P.; Duarte, A.E.; Barros, L.M.; Elekofehinti, O.O.; Matias, E.F.F.; da Costa, J.G.M.; Sanmi, A.A.; Boligon, A.A.; da Rocha, J.B.T.; et al. Cytotoxic and antioxidative potentials of ethanolic extract of Eugenia uniflora L. (Myrtaceae) leaves on human blood cells. Biomed. Pharmacother. 2016, 84, 614–621. [Google Scholar] [CrossRef]
- de Souza, C.E.S.; da Silva, A.R.P.; Rocha, J.E.; Vega Gomez, M.C.; Rolóm, M.; Coronel, C.; Martins da Costa, J.G.; Netto, M.L.C.; Rolim, L.A.; Coutinho, H.D.M. LC–MS characterization, anti-kinetoplastide and cytotoxic activities of natural products from Eugenia jambolana Lam. and Eugenia uniflora. Asian Pac. J. Trop. Biomed. 2017, 7, 836–841. [Google Scholar] [CrossRef]
- Bezerra, I.C.F.; Ramos, R.T.d.M.; Ferreira, M.R.A.; Soares, L.A.L. Chromatographic profiles of extractives from leaves of Eugenia uniflora. Rev. Bras. Farmacogn. 2018, 28, 92–101. [Google Scholar] [CrossRef]
- Ramos, R.T.M.; Bezerra, I.C.F.; Ferreira, M.R.A.; Soares, L.A.L. Spectrophotometric Quantification of Flavonoids in Herbal Material, Crude Extract, and Fractions from Leaves of Eugenia uniflora Linn. Pharmacogn. Res. 2017, 9, 253. [Google Scholar] [CrossRef]
- Souza, O.A.; Furlani, R.P.; Ramalhão, V.G.d.S.; Borges, M.S.; Funari, C.S.; Bolzani, V.d.S.; Rinaldo, D. Eco-friendly and inexpensive food grade bioethanol for Eugenia uniflora L. chromatographic fingerprinting: A trade-off between separation and sustainability. Phytochem. Lett. 2021, 43, 200–207. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Quinta Barbosa Bittante, A.M.; Sobral, P.J.d.A.; Dalla Rosa, M. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Donadel, G.; Dalmagro, M.; de Oliveira, J.A.; Zardeto, G.; Pinc, M.M.; Hoscheid, J.; Alberton, O.; Belettini, S.T.; Jacomassi, E.; Gasparotto Junior, A.; et al. Safety Investigations of Two Formulations for Vaginal Use Obtained from Eugenia uniflora L. Leaves in Female Rats. Pharmaceuticals 2022, 15, 1567. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Zimenkina, N.y.I. HPLC Determination of Myricitrin in Juglans nigra L. Bark. Pharm. Chem. J. 2021, 55, 881–885. [Google Scholar] [CrossRef]
- Agrawal, O.D.; Kulkarni, Y.A. Mini-Review of Analytical Methods used in Quantification of Ellagic Acid. Rev. Anal. Chem. 2020, 39, 31–44. [Google Scholar] [CrossRef]
- Silva, L.; de Oliveira, M.; Martins, C.; Borges, L.; Fiuza, T.; da Conceição, E.; de Paula, J. Validation HPLC-DAD Method for Quantification of Gallic and Ellagic Acid from Eugenia punicifolia Leaves, Extracts and Fractions. J. Braz. Chem. Soc. 2023, 34, 401–413. [Google Scholar] [CrossRef]
- Nantarat, N.; Mueller, M.; Lin, W.-C.; Lue, S.-C.; Viernstein, H.; Chansakaow, S.; Sirithunyalug, J.; Leelapornpisid, P. Sesaminol diglucoside isolated from black sesame seed cake and its antioxidant, anti-collagenase and anti-hyaluronidase activities. Food Biosci. 2020, 36, 100628. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Hałasa, R.; Dudek, M.K.; Majdan, M.; Jankowska, K.; Granica, S. Antibacterial and anti-inflammatory activity of bistort (Bistorta officinalis) aqueous extract and its major components. Justification of the usage of the medicinal plant material as a traditional topical agent. J. Ethnopharmacol. 2020, 260, 113077. [Google Scholar] [CrossRef] [PubMed]
- Žuvela, P.; Skoczylas, M.; Jay Liu, J.; Ba̧czek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef] [PubMed]
- LaCourse, M.E.; LaCourse, W.R. Chapter 17—General instrumentation in HPLC. In Liquid Chromatography, 2nd ed.; Fanali, S., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 417–429. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Iriti, M. Sephadex® LH-20, Isolation, and Purification of Flavonoids from Plant Species: A Comprehensive Review. Molecules 2020, 25, 4146. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, A.; Shoji, T.; Shibusawa, Y. Separation of proanthocyanidins by degree of polymerization by means of size-exclusion chromatography and related techniques. J. Biochem. Biophys. Methods 2003, 56, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Tanaka, N.; Aung, M.M.; Devkota, H.P.; Mizuno, T. Phenolic compounds from parasitic Sapria himalayana f. albovinosa and Sapria myanmarensis (Rafflesiaceae) in Myanmar. Biochem. Syst. Ecol. 2020, 93, 104179. [Google Scholar] [CrossRef]
- Wen, C.; Song, D.; Zhuang, L.; Liu, G.; Liang, L.; Zhang, J.; Liu, X.; Li, Y.; Xu, X. Isolation and identification of polyphenol monomers from celery leaves and their structure-antioxidant activity relationship. Process Biochem. 2022, 121, 69–77. [Google Scholar] [CrossRef]
- Ovchinnikov, D.V.; Bogolitsyn, K.G.; Druzhinina, A.S.; Kaplitsin, P.A.; Parshina, A.E.; Pikovskoi, I.I.; Khoroshev, O.Y.; Turova, P.N.; Stavrianidi, A.N.; Shpigun, O.A. Study of Polyphenol Components in Extracts of Arctic Brown Algae of Fucus vesiculosus Type by Liquid Chromatography and Mass-Spectrometry. J. Anal. Chem. 2020, 75, 633–639. [Google Scholar] [CrossRef]
- Stanek, N.; Jasicka-Misiak, I. HPTLC Phenolic Profiles as Useful Tools for the Authentication of Honey. Food Anal. Methods 2018, 11, 2979–2989. [Google Scholar] [CrossRef]
- Striegel, A.M. Chapter 10—Size-exclusion chromatography. In Liquid Chromatography, 2nd ed.; Fanali, S., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 245–273. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021, 11, 8276. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Puganen, A.; Alakomi, H.-L.; Sinkkonen, J.; Yang, B. Sephadex LH-20 fractionation and bioactivities of phenolic compounds from extracts of Finnish berry plants. Food Res. Int. 2018, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Giner, R.M.; Marin, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, K.; Wang, Y.; Ma, R. Effects of Myricitrin and Relevant Molecular Mechanisms. Curr. Stem Cell Res. Ther. 2020, 15, 11–17. [Google Scholar] [CrossRef]
- PUBCHEM. PubChem Compound Summary for CID 5281673, Myricitrin: National Center for Biotechnology Information. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Myricitrin (accessed on 30 January 2023).
- de Oliveira, F.M.G.; Romão, W.; Kuster, R.M. Identification of phenolic compounds in Eugenia uniflora leaves by FTICR MS in association with different ionization sources. Anal. Methods 2018, 10, 1647–1655. [Google Scholar] [CrossRef]
- Souza, P.; Santos, M.; Monteiro, R.; Espindola, M.; Souza, H.; Monteiro, A.; Camara, C.; Silva, T. Taninos e Flavonóides das flores de Eugenia uniflora (Myrtaceae). Quim. Nova 2022, 45, 1083–1091. [Google Scholar] [CrossRef]
- Bagatini, L.; Zandoná, G.P.; Hoffmann, J.F.; de Souza Cardoso, J.; Teixeira, F.C.; Moroni, L.S.; Junges, A.; Kempka, A.P.; Stefanello, F.M.; Rombaldi, C.V. Evaluation of Eugenia uniflora L. leaf extracts obtained by pressurized liquid extraction: Identification of chemical composition, antioxidant, antibacterial, and allelopathic activity. Sustain. Chem. Pharm. 2023, 35, 101214. [Google Scholar] [CrossRef]
- García, Y.M.; Ramos, A.A.-O.; de Oliveira Júnior, A.A.-O.; de Paula, A.; de Melo, A.A.-O.; Andrino, M.A.; Silva, M.R.; Augusti, R.A.-O.; de Araújo, R.L.B.; de Lemos, E.A.-O.; et al. Physicochemical Characterization and Paper Spray Mass Spectrometry Analysis of Myrciaria floribunda (H. West ex Willd.) O. Berg Molecules 2021, 26, 7206. [Google Scholar] [CrossRef] [PubMed]
- Rattmann, Y.D.; de Souza Lm Fau-Malquevicz-Paiva, S.M.; Malquevicz-Paiva Sm Fau-Dartora, N.; Dartora N Fau-Sassaki, G.L.; Sassaki Gl Fau-Gorin, P.A.J.; Gorin Pa Fau-Iacomini, M.; Iacomini, M. Analysis of Flavonoids from Eugenia uniflora Leaves and Its Protective Effect against Murine Sepsis. In Evidence-Based Complementary and Alternative Medicine; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.J.; Gunawardena, H.P.; Cornu, A.; Narvekar, A.S.; Richieu, A.; Deffieux, D.; Quideau, S.; Tharayil, N. Rapid Screening of Ellagitannins in Natural Sources via Targeted Reporter Ion Triggered Tandem Mass Spectrometry. Sci. Rep. 2018, 8, 10399. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, A.V.; Yadav, A.; Das, A.M. Antimicrobial and α-Amylase Inhibitory Activities of Constituents from Elaeocarpus floribundus. Rev. Bras. Farmacogn. 2021, 31, 330–334. [Google Scholar] [CrossRef]
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.M.; de Torres, N.W. Activity of Polyphenolic Compounds against Candida glabrata. Molecules 2015, 20, 17903. [Google Scholar] [CrossRef]
- Gadetskaya, A.V.; Tarawneh, A.H.; Zhusupova, G.E.; Gemejiyeva, N.G.; Cantrell, C.L.; Cutler, S.J.; Ross, S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 2015, 104, 80–85. [Google Scholar] [CrossRef]
- Gatto, L.J.; Veiga, A.; Gribner, C.; Moura, P.F.; Rech, K.S.; Murakami, F.S.; Dias, J.d.F.G.; Miguel, O.G.; Miguel, M.D. Myrcia hatschbachii: Antifungal activity and structural elucidation of ellagic and 3-O-methyl ellagic acids. Nat. Prod. Res. 2021, 35, 5540–5543. [Google Scholar] [CrossRef]
- Rossatto, F.C.P.; Tharmalingam, N.; Escobar, I.E.; d’Azevedo, P.A.; Zimmer, K.R.; Mylonakis, E. Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris. J. Fungi 2021, 7, 763. [Google Scholar] [CrossRef]
- Vigbedor, B.Y.; Akoto, C.O.; Neglo, D. Isolation and characterization of 3,3′-di-O-methyl ellagic acid from the root bark of Afzelia africana and its antimicrobial and antioxidant activities. Sci. Afr. 2022, 17, e01332. [Google Scholar] [CrossRef]

| Sample (g) | Yields % | |||||
|---|---|---|---|---|---|---|
| SDCE | AqF | EAF | AqF | EAF/AqF | EAF/SDCE | |
| 1 | 360 | 177 | 7.87 | 49.16 | 4.44 | 2.19 |
| 2 | 270 | 110 | 6.14 | 40.74 | 5.58 | 2.27 |
| 3 | 180 | 78 | 4.27 | 43.33 | 5.47 | 2.37 |
| Mean ± SD (RSD%) | 43.33 ± 4.31 (9.95) | 5.47 ± 0.62 (11.49) | 2.27 ± 0.09 (3.97) | |||
| SP1 | ||||
|---|---|---|---|---|
| nFr | Eluate (mL) | Weight (mg) | Y% | RY% |
| 1 | 50 | 32.90 | 3.21 | 3.26 |
| 2 | 30 | 144.10 | 14.05 | 14.30 |
| 3 | 20 | 63.70 | 6.21 | 6.32 |
| 4 | 20 | 30.00 | 2.92 | 2.98 |
| 5 | 20 | 23.50 | 2.29 | 2.33 |
| 6 | 20 | 51.70 | 5.04 # | 5.13 |
| 7 | 20 | 42.90 | 4.18 # | 4.26 |
| 8 | 20 | 79.20 | 7.72 # | 7.86 |
| 9 | 20 | 47.70 | 4.65 # | 4.73 |
| 10 | 25 | 20.30 | 1.98 # | 2.01 |
| 11 | 20 | 19.80 | 1.93 | 1.96 |
| 12 | 20 | 14.10 | 1.37 | 1.40 |
| 13 | 20 | 11.60 | 1.13 | 1.15 |
| 14 | 20 | 12.00 | 1.17 | 1.19 |
| 15 | 25 | 14.60 | 1.42 | 1.45 |
| 16 | 25 | 15.40 | 1.50 | 1.53 |
| 17 | 25 | 17.10 | 1.67 | 1.70 |
| 18 | 50 | 25.90 | 2.53 | 2.57 |
| 19 | 55 | 53.40 | 5.21 | 5.30 |
| 20 | 65 | 19.00 | 1.85 * | 1.89 |
| 21 | 50 | 8.70 | 0.85 * | 0.86 |
| 22 | 100 | 36.80 | 3.59 | 3.65 |
| Peak No. | tR (min.) | m/z [M − H]− | Molecular Formula | Error (ppm) | MS/MS (Relative Abundance%) | Tentative Assignment | Fraction | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.2 | 191.0562 | C7H12O6 | −0.6 | 173.0954 (23.81); 165.0385 (19.05); 127.0367 (45.24) | Quinic acid | SPF/EAF | [15,44,45,46] |
| 2 | 4.6 | 191.0197 | C6H8O7 | 0.2 | 173.0078 (73.33); 111.0086 (60.00) | Citric acid | SPF/MPF/EAF | [47] |
| 3 | 6.3 | 169.0149 | C7H6O5 | −3.8 | 125.0244 (100.00) | Galic acid* | SPF/MPF/EAF | [15,44,46] |
| 4 | 15.2 | 483.0788 | C20H20O14 | −1.7 | 313.0595 (17.37); 271.0433 (0.47); 211.0230 (2.11); 169.0149 (77.43); 125.0264 (0.43) | Digalloyl-hexoside | SPF | [15,44,45,46] |
| 5 | 15.2 | 353.0889 | C16H18O9 | −3.2 | 191.0543 (100.00) | 5-O-Caffeoylquinic acid | SPF/EAF | [44,46] |
| 6 | 19.2 | 337.0928 | C16H18O8 | 0.1 | 191.0529 (100.00); 163.0380 (14.34); 119.0491(8.93) | 5-O-Coumaroylquinic acid | SPF/EAF | [15,46] |
| 7 | 24.2 | 653.2130 | C30H38O16 | 2.4 | 501.1996 (44.29); 483.1829 (19.36); 313.0577(14.16); 271.0507 (3.87); 211.0194 (7.37); 193.0133 (4.53); 169.0153 (54.58); 125.0216 (6.99) | Digalloyl-hexoside derivative | SPF/EAF | [48] |
| 8 | 25.1 | 479.0848 | C21H20O13 | −3.5 | 317.0245 (24.83); 316.0230 (100.00); 287.0196 (7.65); 271.0269 (13.79); 178.9984 (2.67); 151.0062 (3.39) | Myricetin-O-hexoside | MPF | [15,44,45,46] |
| 9 | 25.2 | 653.2084 | C30H38O16 | 0.5 | 501.2041 (43.42); 483.1850 (30.92); 313.0568 (23.68); 241.0370 (11.84); 169.0177 (100.00) | Digalloyl-hexoside derivative | MPF | [48] |
| 10 | 25.4 | 501.1983 | C23H34O12 | −1.1 | 451.3264 (25.87); 313.0593 (15.85); 271.0444 (12.63); 211.0243 (9.84); 169.0138 (59.41); 125.0236 (10.19) | Digalloyl-hexoside derivative | SPF | [48] |
| 11 | 25.4 | 539.2142 | C23H36O12 | −1.4 | 501.1896 (9.52); 313.0560 (6.54); 271.0440 (4.93); 169.0147 (46.44); 125.0235 (15.25) | Digalloyl-hexoside derivative | SPF | [48] |
| 12 | 25.5 | 449.0731 | C20H18O12 | −1.2 | 317.0267 (25.26); 316.0229 (100.00); 287.0193 (13.96); 271.0245 (21.05); 178.9980 (4.98); 151.0067 (2.28) | Myricetin-O-pentoside | MPF | [15,44,45,46] |
| 13 | 26.6 | 449.0728 | C20H18O12 | −0.6 | 317.0251 (21.11); 316.0225 (100.00); 287.0193 (14.18); 271.0242 (27.36); 178.9992 (2.01); 151.0019 (3.45) | Myricetin-O-pentoside | MPF | [15,44,45,46] |
| 14 | 27.1 | 463.0900 | C21H20O12 | −3.9 | 317.0265 (20.75); 316.0223 (100.00); 287.00201 (8.96); 271.0243 (19.39); 178.9995 (3.86); 151.0046 (3.38) | Myricetin-O-rhamnoside * (Myricitrin) | MPF | [15,44,46] |
| 15 | 28.0 | 463.0875 | C21H20O12 | 1.6 | 301.0322 (33.21); 300.0263 (100.00); 271.0232 (32.62); 255.0293 (14.11); 179.0010 (4.51); 151.0071 (4.72) | Quercetin-O-hexoside | MPF | [15,45] |
| 16 | 28.3 | 463.0876 | C21H20O12 | 1.3 | 301.0319 (35.81); 300.0264 (100.00); 271.0228 (37.49); 255.0310 (25.03); 178.9933 (10.66); 151.0052 (8.38) | Quercetin-O-hexoside | MPF | [15,45] |
| 17 | 28.9 | 433.0773 | C20H18O11 | 0.8 | 301.0308 (28.23); 300.0270 (100.00); 271.0214 (40.46); 255.0279 (27.53); 178.9937 (3.17); 151.0023 (4.13) | Quercetin-O-pentoside | MPF | [15,45] |
| 18 | 30.1 | 433.0771 | C20H18O11 | 1.2 | 301.0337 (33.29); 300.0274 (100.00); 271.0261 (33.82); 255.0314 (23.84); 179.0014 (3.36); 151.0080 (8.93) | Quercetin-O-pentoside | MPF | [15,45] |
| 20 | 30.5 | 447.0941 | C21H20O11 | −1.8 | 301.0324 (60.54); 300.0266 (100.00); 271.0244 (42.29); 255.0294 (18.59); 178.9983 (6.63); 151.0042 (11.36) | Quercetin-O-deoxyhexoside | MPF | [15,45] |
| 21 | 33.2 | 431.0992 | C21H20O10 | −1.8 | 285.0404 (75.39; 284.0299 (100.00); 255.0314 (86.57); 227.0335 (81.93) | Kaempferol-O-deoxyhexoside | MPF | [15,45] |
| 22 | 33.3 | 471.1305 | C24H24O10 | −1.7 | 285.0368 (10.07); 284.0314 (14.76); 255.0298 (1.50); 227.0376 (2.45) | Kaempferol- derivative | MPF | - |
| Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) (μg/mL) | ||||||
|---|---|---|---|---|---|---|
| Candida albicans | Candida glabrata | Candida auris | ||||
| Samples | MIC | MFC | MIC | MFC | MIC | MFC |
| SDCE | 250 * | 500 | 125 * | ≥1000 | 31.2 * | 1000 |
| Fractions | ||||||
| HF | 1000 | 1000 | 1000 | 1000 | 62.5 * | 1000 |
| EAF | 250* | 500 | 125 * | 1000 | 31.2 * | 1000 |
| rFaq | 1000 | 1000 | 250 | 1000 | 125 | 1000 |
| SPF | 1000 | ≥1000 | 1000 | ≥1000 | 500 | 1000 |
| MPF | 125 * | 125 | 62.5 * | 250 | 500 | 1000 |
| MyR I | 250 * | 500 | 250 * | 500 | 500 | ≥1000 |
| GA I | 500 | 500 | 250 * | 500 | 500 | ≥1000 |
| LF | 125 * | 125 | 125 * | 250 | 125 * | 1000 |
| EA I | 250 | 250 | 62.5 * | 250 | 125 * | 1000 |
| Phytochemicals | ||||||
| GA | 500 | ≥1000 | 250 * | 500 | 500 | 500 |
| MyR | 500 | 1000 | 250 * | 250 * | 500 | 1000 |
| EA | 500 | 1000 | 125 * | 250 * | 1000 | 1000 |
| Synergic samples | ||||||
| GA + MyR | 500 | 500 | 125 * | 500 | 1000 | 1000 |
| MyR + EA | 500 | 1000 | 125 * | 500 | 500 | ≥1000 |
| EA + GA | 1000 | ≥1000 | 1000 | ≥1000 | 1000 | ≥1000 |
| GA + EA + MyR | 1000 | ≥1000 | 500 | ≥1000 | 1000 | ≥1000 |
| Recovery of EA | Recovery of GA | Recovery of MyR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Processed Samples | LF | EA I | MPF | GA I | MPF (SNT1) | MyR I (SNT2) | MyR II (SNT3) | ||||||||
| Chromatographic conditions in RP-FC | Gradient | B% | min | B% | min | B% | min | B% | min | B% | min | B% | min | B% | min |
| 10 | 2 | 10 | 3 | 10 | 4 | 10 | 4 | 10 | 7 | 30 | 1 | 50 | 3 | ||
| 20 | 3 | 30 | 5 | 10–40 | 10 | 100 | 2 | 10–100 | 20 | 30–70 | 6 | 50–56 | 2 | ||
| 30 | 3 | 30–100 | 1 | 40–100 | 6 | 10 | 2 | 100–10 | 1 | 100 | 3 | 56–100 | 2 | ||
| 30–100 | 1 | 100 | 5 | 100–10 | 1 | - | - | 10 | 3 | 30 | 1 | 100 | 2 | ||
| 100 | 3 | - | - | 10 | 4 | - | - | 50 | 2 | ||||||
| Flow (mL/min) | 12 | 12 | 15 | 20 | 20 | 20 | 20 e 15 | ||||||||
| Column (g) | 12 | 12 | 12 | 30 | 30 | 30 | 30 e 12 | ||||||||
| λ (nm) | 254 and 280 | 254 and 270 | 270 and 350 | ||||||||||||
| ST (mAU) | 10 | 10 | 20 | 5 | 10 | 5 | 5 | ||||||||
| max. vol (mL) | 10 | 10 | 15 | 10 | 15 | 10 | 10 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenório, C.J.L.; Dantas, T.d.S.; Abreu, L.S.; Ferreira, M.R.A.; Soares, L.A.L. Influence of Major Polyphenols on the Anti-Candida Activity of Eugenia uniflora Leaves: Isolation, LC-ESI-HRMS/MS Characterization and In Vitro Evaluation. Molecules 2024, 29, 2761. https://doi.org/10.3390/molecules29122761
Tenório CJL, Dantas TdS, Abreu LS, Ferreira MRA, Soares LAL. Influence of Major Polyphenols on the Anti-Candida Activity of Eugenia uniflora Leaves: Isolation, LC-ESI-HRMS/MS Characterization and In Vitro Evaluation. Molecules. 2024; 29(12):2761. https://doi.org/10.3390/molecules29122761
Chicago/Turabian StyleTenório, Camylla Janiele Lucas, Thainá dos Santos Dantas, Lucas Silva Abreu, Magda Rhayanny Assunção Ferreira, and Luiz Alberto Lira Soares. 2024. "Influence of Major Polyphenols on the Anti-Candida Activity of Eugenia uniflora Leaves: Isolation, LC-ESI-HRMS/MS Characterization and In Vitro Evaluation" Molecules 29, no. 12: 2761. https://doi.org/10.3390/molecules29122761
APA StyleTenório, C. J. L., Dantas, T. d. S., Abreu, L. S., Ferreira, M. R. A., & Soares, L. A. L. (2024). Influence of Major Polyphenols on the Anti-Candida Activity of Eugenia uniflora Leaves: Isolation, LC-ESI-HRMS/MS Characterization and In Vitro Evaluation. Molecules, 29(12), 2761. https://doi.org/10.3390/molecules29122761







